Abstract
Previous studies have shown that genetic factors might have an important role in blood pressure (BP) responses to dietary salt or potassium intake. The aim of this study was to assess the association of common genetic variants of the adiponectin gene with BP responses to controlled dietary sodium or potassium interventions. Subjects (n=334) from 124 families in rural areas of Northern China were recruited. After a 3-day baseline observation, participants sequentially maintained a 7-day low-sodium diet (NaCl, 3 g per day; or sodium, 51.3 mmol per day), followed by a 7-day high-sodium diet (NaCl, 18 g per day; or sodium, 307.8 mmol per day) and a 7-day high-sodium plus potassium supplementation intervention (KCl, 4.5 g per day; or potassium, 60 mmol per day). A total of seven single nucleotide polymorphisms (SNPs) in the adiponectin gene were selected as the study sites. After adjustment for multiple testing, the adiponectin SNP rs16861205 was significantly associated with the diastolic BP (DBP) response to low-salt intervention, and the DBP and mean arterial pressure (MAP) responses to high-salt intervention (P=0.028, 0.023 and 0.027, respectively). SNP rs822394 was associated with the DBP and MAP responses to low-salt intervention and the DBP response to high-salt intervention (P=0.023, 0.030 and 0.033 respectively). Meanwhile, significant association also existed between SNP rs16861194 and the systolic BP response to potassium supplementation intervention (P=0.026). In addition, SNP rs822394 was significantly associated with basal DBP after adjustment for multiple testing (P=0.033). Our study indicated that the genetic polymorphisms in the adiponectin gene are significantly associated with BP responses to dietary sodium and potassium intake.
Introduction
Elevated blood pressure (BP) is the leading contributor to the global burden of cardiovascular disease and global mortality.1 As a complex phenotype, hypertension is thought to result from a complex interaction of environmental and genetic factors. A large body of evidence has indicated that BP is positively correlated with dietary sodium intake but negatively associated with dietary potassium intake.2, 3 However, BP responses to dietary sodium intake or potassium supplementation considerably vary among individuals.4, 5 Epidemiological data suggest that genetic predisposition may have an important role in the phenomenon.6, 7 Therefore, discovering novel genetic variants related to BP responses to dietary sodium or potassium intake would help us better understand the biological mechanisms of BP regulation, and might facilitate the development of targeted dietary interventions for hypertension.
Adiponectin (AdipoQ) is a collagen-like adipokine that is exclusively produced by white adipose tissue and circulates in relatively high serum concentrations (mg ml−1).8 The human AdipoQ gene (gene ID: 9370) located on chromosome 3q27 spans ~16 kb and encodes an ~30-kDa protein that is composed of 244 amino acids.9 Recent researches demonstrated that the protein with insulin-sensitising anti-inflammation and anti-atherogenic properties have a protective role in several diseases such as type 2 diabetes, obesity and coronary artery disease.10, 11, 12 Apart from the abovementioned functions, adiponectin is also involved in BP protection. Several cross-sectional and prospective studies have shown that hypoadiponectinaemia is an independent risk factor for hypertension and the increased risk of incident hypertension.13, 14, 15 Otherwise, population-based intervention trials and animal studies have showed that plasma AdipoQ levels could be affected by changes in salt intake.16, 17 Meanwhile, our previous study suggested that the disturbance of adiponectin expression may be involved in the pathogenesis of salt-sensitive hypertension.18
Recently, the association between AdipoQ gene polymorphisms and hypertension was studied by several genetic investigations, but the results were inconsistent.19, 20 Ong et al.19 reported that the single nucleotide polymorphism (SNP) rs266729 of the adiponectin gene was significantly associated with hypertension and adiponectin levels in 1616 Hong Kong Chinese. However, the meta-analysis by Zhao et al.20 did not detect a significant association of this SNP with BP. The conflicting results may be attributed to ethnic differences, environmental factors and study power. However, a few of them have fully considered gene–environment interactions on BP, particularly with regard to dietary sodium or potassium intake. Failure to measure the gene–environment interactions may lead to the inability to fully discover the genetic contribution to BP variability. Therefore, our study aimed to assess the association between common variants in the adiponectin gene and BP responses to controlled dietary sodium or potassium intervention.
Subjects and Methods
Subjects
In northern China, potential probands were determined by community-based BP screening among individuals aged 18–60 years old in the selected villages. Persons who had a mean systolic BP (SBP) between 130 and 160 mm Hg and/or a diastolic BP (DBP) between 85 and 100 mm Hg, and no use of antihypertensive medications were identified as the proband. Probands and their siblings, spouses and offspring were recruited for the dietary intervention study. All of them were Han ethnic. The exclusion criteria were having secondary hypertension, stage 2 hypertension, a history of clinical cardiovascular disease or diabetes, used antihypertensive medications, and being pregnant, heavy alcohol drinkers, currently on a low-sodium diet or unable to sign the informed consent form. A total of 334 subjects underwent the dietary intervention trial and were included in the current analysis.
The Institutional Ethics Committee of Xi'an Jiaotong University Medical School approved the study protocol, and written informed consent for the baseline observation and for the intervention programme was obtained from each participant. All of the procedures were performed in accordance with institutional guidelines.
Dietary intervention
The dietary intervention programme was performed among all participants except for the parents of each proband. As described in a previous study, this programme consisted of a 3-day baseline examination during which a questionnaire survey and measurements of BP, height, weight and blood biochemical parameters were conducted, a 7-day low-salt diet (3 g of NaCl or 51.3 mmol of sodium per day), a 7-day high-salt diet (18 g of NaCl or 307.8 mmol of sodium per day) followed by a 7-day high-salt diet plus potassium supplementation (4.5 g of KCl or 60 mmol of potassium per day). All foods were cooked without salt, and prepackaged salt was added to the individual participant's meal when it was served by the study staff. During the entire study period, other dietary nutrient intake remained unchanged. To ensure participants' compliance to the intervention programme, they were required to have their three meals (breakfast, lunch and dinner) in the study kitchen. The participants were also given detailed instructions to avoid consuming any other foods that were not provided by the study.21
Determination of 24-h urinary sodium and potassium excretions
To assess the compliance of subjects to this dietary intervention, one 24-h urinary specimen was collected at baseline and at the end of each intervention period. The concentrations of sodium and potassium in the urine sample were measured with a flame photometer. The content of 24-h urinary sodium or potassium ions of each subject was calculated as the concentration of urinary sodium or potassium multiplied by the 24-h urine volume of each individual.
BP measurements and definition of BP response to dietary intervention
For each subject, three BP measurements were obtained using a random-zero sphygmomanometer (Hawksley & Sons Ltd., Lancing, UK) at each morning of the 3-day baseline examination, as well as on days 5, 6 and 7 of each dietary period. BP was measured by trained and certified observers according to a common protocol.22 All observers were blinded to the dietary intervention. Study participants were required to avoid cigarette smoking, alcohol, coffee/tea and strenuous exercise for at least 30 min before their BP measurement. BP measurement was taken in a sitting position after a 5-min rest. The first and fifth phases of the Korotkoff sounds were taken as SBP and DBP, respectively. Three BP measurements each day were performed with an interval of 1-min, and the mean values of nine BP measurements during the baseline survey and each intervention period were recorded as the BP levels at baseline and each intervention period, respectively. In addition, MAP was calculated according to the following formula: MAP=DBP+1/3(SBP−DBP). In the present study, BP responses were considered as continuous variables and calculated as follows: BP response to low sodium=BP on low-sodium diet minus BP at baseline; BP response to high sodium=BP on high-salt diet minus BP on low-salt diet; and BP response to potassium supplementation=BP on high-salt diet with potassium supplementation minus BP on high-salt diet.
Adiponectin SNP selection and genotyping
We selected seven tagSNPs from the adiponectin gene as the study sites; each SNP had a minor allele frequency of more than 5% in the Chinese Han of the Beijing HapMap databank (www.hapmap.org/). Genomic DNA was extracted from whole-blood specimen drawn from each participant using the GOLDMAG Whole Blood Genomic DNA Purification Kit (Golden Magnetic Nano-Biotechnology Co., Ltd. Xi'an, China). PCR primers and single-base extension primers required for SNP genotyping were designed by MassARRAY Assay Design software (version 3.0; Sequenom, San Diego, CA, USA) and synthesised by Shanghai Biological Technology Co., Ltd. (http://www.sangon.com/). The genotyping experiments were performed by Xi'an BaiMei genetic testing centre (http://www.lifegen.com/) with a MassARRAY system based on the matrix-assisted laser desorption ionisation time-of-flight mass spectrometry, according to the manufacturer's instructions. Genotype calling was performed in real-time with the MassARRAY RT software (version 3.1) and analysed with the MassARRAY Typer software version 4.0 (Sequenom).
Statistical analysis
Continuous data are presented as mean±s.e. Categorical data are expressed as frequency with percentage. Differences in the repeated measures were analysed by a paired samples t-test. The Mendelian consistency of the SNP genotype data was assessed with PLINK.23 Haploview software (Broad Institute, Cambridge, MA, USA) was used to test Hardy–Weinberg equilibrium on parental SNP data and estimate the extent of pairwise linkage disequilibrium (LD) between SNPs. LD blocks were defined by the solid spine LD method implemented in Haploview software.24 The association of single markers with adjusted phenotypes was performed using the family-based association test, and three genetic models (additive, dominant and recessive) were assumed for each SNP analysis.25 To assess the effect of genetic variants on the trait value, a univariate family-based association test was performed for each allele. This test provides a Z-statistic with its corresponding P-value. In the present study, a positive Z-statistic for an allele or a haplotype indicates a decreased response to the low-sodium intervention and the high-sodium plus potassium supplementation intervention, as well as an increased response to high-sodium intervention and an increase in the basal BP. The false discovery rate method was used to correct for multiple testing.26 A P-value of 0.05 was used as the threshold for statistical significance in our study.
Results
Baseline characteristics and BP responses to dietary intervention
According to the inclusion criteria, a total of 515 subjects from 124 pedigrees were recruited in our study. Baseline characteristics and BP responses to low-sodium, high-sodium and high-sodium plus potassium supplementation intervention among study participants are shown in Table 1. The mean levels of baseline SBP, DBP and MAP in the proband group were higher than that in the sibling, spouse and offspring groups, whereas the baseline SBP level of the parent group was the highest among all of the groups. Overall, BP levels were, respectively, reduced and increased in response to the low-sodium and high-sodium intervention, and BP levels were again reduced in response to the high-sodium plus potassium supplementation intervention.
Table 1. Characteristics of participants.
| Probands | Siblings | Spouses | Offspring | Parents | Non-parentsa | |
|---|---|---|---|---|---|---|
| No. of subjects | 99 | 167 | 19 | 49 | 181 | 334 |
| Age, year | 41.8±8.4 | 39.8±7.4 | 47.4±6.1 | 23.3±6.9 | 66.1±8.3 | 38.4±9.9 |
| Male, % | 69.7 | 49.1 | 26.3 | 49.0 | 48.4 | 53.5 |
| BMI, kg m−2 | 23.0±2.8 | 22.2±2.9 | 23.1±4.7 | 20.1±2.7 | — | 22.2±3.1 |
| Baseline BP, mm Hg | ||||||
| SBP | 120.9±12.5 | 107.6±11.1 | 108.6±12.2 | 102.7±10.7 | 123.2±21.3 | 110.9±13.3 |
| DBP | 78.9±8.3 | 70.1±8.1 | 70.6±6.9 | 63.4±8.9 | 70.5±10.5 | 71.8±9.7 |
| MAP | 93.0±9.0 | 82.6±8.7 | 83.3±7.9 | 76.5±9.2 | 88.0±13.1 | 84.8±10.4 |
| BP response to low-sodium intervention, mm Hg | ||||||
| SBP | 111.7±10.0* | 103.4±9.1* | 102.5±7.7* | 100.3±9.4* | — | 105.4±10.2* |
| DBP | 72.8±9.3* | 66.4±7.7* | 67.1±5.8# | 60.7±8.3* | — | 67.5±9.1* |
| MAP | 85.7±9.0* | 78.7±7.6* | 78.9±5.4# | 73.9±8.3# | — | 80.1±9.0* |
| SBP change | −8.65±9.52 | −3.90±5.41 | −6.15±7.88 | −2.38±4.79 | — | −5.20±7.31 |
| DBP change | −6.00±6.71 | −3.64±4.83 | −3.48±6.36 | −2.70±5.21 | — | −4.19±5.69 |
| MAP change | −6.88±7.07 | −3.73±4.55 | −4.37±6.52 | −2.59±4.56 | — | −4.53±5.73 |
| BP response to high-sodium intervention, mm Hg | ||||||
| SBP | 118.9±11.2 | 108.5±11.1# | 108.4±10.9 | 102.0±10.0 | — | 110.6±12.4 |
| DBP | 76.2±8.1* | 68.7±9.3* | 68.6±7.5 | 60.9±8.3* | — | 69.8±10.0* |
| MAP | 90.4±8.5* | 82.0±9.5 | 81.9±8.0 | 74.6±8.4* | — | 83.4±10.4* |
| SBP change | 7.16±7.40 | 5.09±6.50 | 5.93±7.90 | 1.72±4.07 | — | 5.25±6.77 |
| DBP change | 3.49±7.33 | 2.29±5.73 | 1.51±4.69 | 0.22±4.52 | — | 2.30±6.12 |
| MAP change | 4.71±6.86 | 3.22±5.60 | 2.98±5.61 | 0.72±3.79 | — | 3.28±5.90 |
| BP response to high-sodium plus potassium intervention, mm Hg | ||||||
| SBP | 112.2±8.6* | 103.1±8.9* | 102.8±9.4* | 101.0±9.6# | — | 105.5±10.0* |
| DBP | 73.0±7.7* | 66.1±7.9* | 65.9±7.1 | 59.9±8.0* | — | 67.2±8.9* |
| MAP | 86.1±7.4* | 78.4±7.8* | 78.2±7.2* | 73.6±8.1* | — | 79.9±8.8* |
| SBP change | −6.54±5.65 | −5.48±5.86 | −5.59±7.10 | −1.02±4.15 | — | −5.13±5.90 |
| DBP change | −3.21±4.76 | −2.63±4.88 | −2.69±4.42 | −1.02±4.25 | — | −2.56±4.76 |
| MAP change | −4.32±4.40 | −3.58±4.77 | −3.66±5.05 | −1.02±3.35 | — | −3.42±4.60 |
Abbreviations: BP, blood pressure; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure.
Variables are indicated as mean±s.d.
All subjects except for parents.
*P<0.01 and #P<0.05 vs the value of baseline period.
Influence of dietary intervention on 24-h urinary sodium and potassium excretions
The influence of dietary intervention on 24-h urinary sodium and potassium excretion is shown in Table 2. To evaluate the subjects' compliance to the dietary intervention programme, the 24-h urinary sodium and potassium excretion values of each subject were calculated at the end of each dietary intervention period. Overall, the 24-h urinary sodium and potassium excretions at baseline indicated that the diet of the subjects featured high salt and insufficient potassium intake. Compared with the baseline values, urinary excretion of sodium significantly decreased during low-salt intervention but increased during high-salt intervention. In addition, potassium supplementation can not only increase urinary potassium excretion but also slightly promote urinary sodium excretion. The results indicated excellent compliance with the dietary intervention.
Table 2. Influence of dietary intervention to urinary sodium and potassium excretions.
| Probands | Siblings | Spouses | Offspring | Total | |
|---|---|---|---|---|---|
| Baseline, mmol | |||||
| 24-h urinary sodium | 225±11.6 | 213.8±16.4 | 218.3±20.8 | 205.4±23.4 | 215.7±19.2 |
| 24-h urinary potassium | 36.8±10.8 | 38.1±9.1 | 39.4±12.3 | 35.8±15.7 | 37.6±12.1 |
| Low-sodium intervention, mmol | |||||
| 24-h urinary sodium | 54.9±11.3 | 53.6±9.8 | 52.8±13.5 | 58.1±7.3 | 54.9±11.2* |
| 24-h urinary potassium | 35.4±8.9 | 39.8±7.6 | 34.2±6.7 | 40.5±9.8 | 37.0±9.8 |
| High-sodium intervention, mmol | |||||
| 24-h urinary sodium | 317.0±21.8 | 304.4±28.6 | 318.6±20.5 | 298.4±25.1 | 310.0±27.0* |
| 24-h urinary potassium | 43.4±13.9 | 39.6±9.1 | 40.2±8.3 | 41.1±14.5 | 41.1±11.9* |
| High-sodium plus potassium supplementation intervention, mmol | |||||
| 24-h urinary sodium | 319.8±24.2 | 331.2±25.9 | 324.7±28.4 | 327.3±21.8 | 325.8±27.4* |
| 24-h urinary potassium | 86.8±9.6 | 79.3±11.5 | 94.6±12.9 | 87.5±10.7 | 87.1±12.9* |
Variables are indicated as mean±s.d. *P<0.01 vs the value of baseline period.
The information on genotyped SNPs of AdipoQ
Seven tagging SNPs were selected in AdipoQ: rs16861194; rs182052; rs16861205; rs822394; rs12495941; rs2241767; and rs2082940. The genomic position, minor allele frequency and Hardy–Weinberg equilibrium test for each SNP are shown in Table 3. None of the alleles significantly deviated from Hardy–Weinberg equilibrium (P>0.05), except for rs2082940, which was excluded in the subsequent analysis.
Table 3. Information on genotyped SNPs of adiponectin.
| SNP | Region in gene | Position | Allelesa | MAFb | HW P-valuesb |
|---|---|---|---|---|---|
| rs16861194 | Promoter | 186559425 | A/G | 0.140884 | 0.8024 |
| rs182052 | Intron1 | 186560782 | G/A | 0.433702 | 0.2208 |
| rs16861205 | Intron1 | 186561634 | G/A | 0.167582 | 0.9529 |
| rs822394 | Intron1 | 186566728 | C/A | 0.127778 | 0.9674 |
| rs12495941 | Intron1 | 186568180 | G/T | 0.403846 | 0.1494 |
| rs2241767 | Intron2 | 186571196 | A/G | 0.252747 | 0.8834 |
| rs2082940 | 3′-UTR | 186574164 | C/T | 0.200549 | 0.0305 |
Abbreviations: HW, Hardy–Weinberg equilibrium test; MAF, minor allele frequency; SNP, single nucleotide polymorphism; UTR, untranslated region.
Major allele/minor allele.
Parental generation.
AdipoQ and BP responses to dietary intervention as well as basal BP
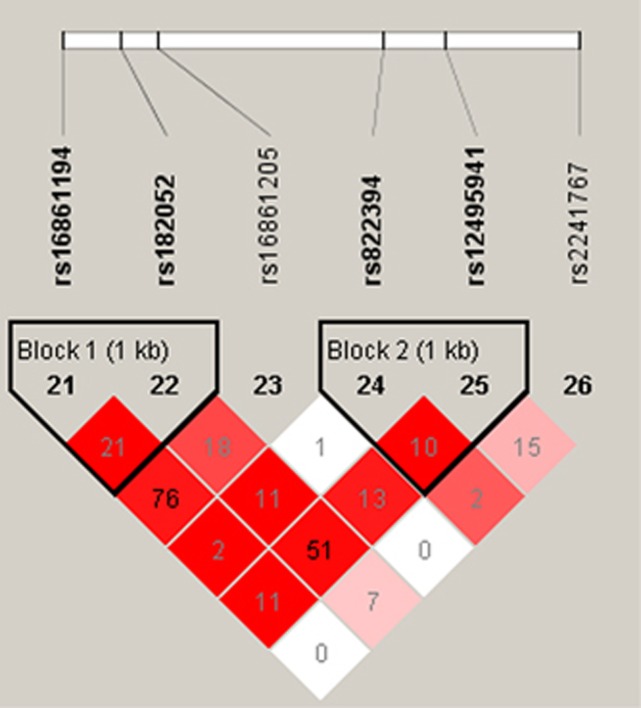
The associations of AdipoQ SNPs with the BP response to dietary intervention after correcting for multiple testing (false discovery rate <0.05) are listed in Table 4. Concretely speaking, SNP rs16861205 was significantly associated with the DBP response to low-salt intervention and the DBP and MAP responses to high-salt intervention (P=0.028, 0.023 and 0.027, respectively). SNP rs822394 was associated with the DBP and MAP responses to low-salt intervention and the DBP responses to high-salt intervention (P=0.023, 0.030 and 0.033, respectively). In addition, a significant association was found between SNP rs16861194 and the SBP response to potassium supplementation intervention (P=0.026). Furthermore, two haplotype blocks of these SNPs were constructed with Haploview software. However, further analysis revealed that none of the AdipoQ haplotypes were associated with BP responses to dietary intervention (Figure 1 and Table 5).
Table 4. SNPs associated with BP response to dietary intervention.
| SNP | Allelea |
SBP response |
DBP response |
MAP response |
|||
|---|---|---|---|---|---|---|---|
| Z | P-value | Z | P-value | Z | P-value | ||
| Low-sodium intervention | |||||||
| rs16861194 | G | −0.741 | 0.459 | 1.271 | 0.204 | 0.579 | 0.563 |
| rs182052 | A | −1.495 | 0.135 | −0.332 | 0.740 | −0.845 | 0.398 |
| rs16861205 | A | 1.016 | 0.310 | 2.192 | 0.028b | 1.906 | 0.057 |
| rs822394 | A | −0.320 | 0.749 | −2.278 | 0.023c | −2.175 | 0.030c |
| rs12495941 | T | 1.251 | 0.211 | 0.790 | 0.429 | 1.074 | 0.283 |
| rs2241767 | G | −0.639 | 0.523 | −0.087 | 0.931 | −0.320 | 0.749 |
| High-sodium intervention | |||||||
| rs16861194 | G | −0.760 | 0.447 | 0.787 | 0.431 | 0.293 | 0.770 |
| rs182052 | A | −0.052 | 0.958 | 0.723 | 0.470 | 0.498 | 0.618 |
| rs16861205 | A | 1.630 | 0.103 | 2.272 | 0.023b | 2.206 | 0.027b |
| rs822394 | A | 0.089 | 0.929 | −2.127 | 0.033c | −1.198 | 0.231 |
| rs12495941 | T | −0.355 | 0.722 | 0.132 | 0.895 | −0.040 | 0.968 |
| rs2241767 | G | −1.346 | 0.178 | 0.042 | 0.966 | −0.468 | 0.640 |
| High-sodium plus potassium supplementation intervention | |||||||
| rs16861194 | G | −2.224 | 0.026c | −0.822 | 0.411 | −1.206 | 0.228 |
| rs182052 | A | −1.364 | 0.173 | 0.385 | 0.700 | −0.282 | 0.778 |
| rs16861205 | A | 0.083 | 0.934 | −0.003 | 0.997 | 0.028 | 0.977 |
| rs822394 | A | 1.657 | 0.098 | 0.372 | 0.710 | 0.964 | 0.335 |
| rs12495941 | T | −0.098 | 0.922 | −0.782 | 0.434 | −0.606 | 0.545 |
| rs2241767 | G | −0.901 | 0.368 | −0.342 | 0.732 | −0.084 | 0.933 |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure; SNP, single nucleotide polymorphism.
For associations that were not significant under any model (additive model, dominant model and recessive model), Z- and P-values for an additive model are listed. All genetic models are based on the minor allele of each SNP. Z indicates test statistic for family-based association test; P-values are corrected for multiple testing (false discovery rate <0.05).
Minor allele.
Additive model.
Dominant model. Bold entries denote significant P-values.
Figure 1.
LD structure of the adiponectin gene. Pairwise LD coefficients D′ × 100 are shown in each cell (D′ values of 1.0 are not shown) and the r2 colour scheme of Haploview was applied (r2=0 shown in white, 0<r2<1 shown in shades of grey and r2=1 shown in black). The solid spine LD method implemented in the Haploview software was used to define LD blocks.
Table 5. Haplotypes in AdipoQ and BP responses to dietary intervention.
| Haplotype sequence | Haplotype frequency |
SBP response |
DBP response |
MAP response |
|||
|---|---|---|---|---|---|---|---|
| Z | P-value | Z | P-value | Z | P-value | ||
| Low-sodium intervention | |||||||
| Haplotype1 (rs16861194–rs182052) | |||||||
| AG | 0.564 | 1.446 | 0.148 | 0.304 | 0.761 | 0.807 | 0.420 |
| AA | 0.295 | −1.058 | 0.290 | −1.254 | 0.210 | −1.289 | 0.197 |
| GA | 0.138 | −0.688 | 0.492 | 1.078 | 0.281 | 0.461 | 0.645 |
| Haplotype2 (rs822394–rs12495941) | |||||||
| CG | 0.462 | −1.354 | 0.176 | −0.109 | 0.914 | −0.63 | 0.529 |
| CT | 0.406 | 1.207 | 0.227 | 0.776 | 0.438 | 1.048 | 0.295 |
| AG | 0.132 | 0.126 | 0.900 | −1.06 | 0.289 | −0.583 | 0.560 |
| High-sodium intervention | |||||||
| Haplotype1 | |||||||
| AG | 0.564 | 0.137 | 0.891 | −0.701 | 0.484 | −0.457 | 0.647 |
| AA | 0.295 | 0.4 | 0.689 | 0.272 | 0.786 | 0.339 | 0.735 |
| GA | 0.138 | −0.712 | 0.477 | 0.819 | 0.413 | 0.342 | 0.732 |
| Haplotype2 | |||||||
| CG | 0.462 | 0.346 | 0.730 | 0.959 | 0.338 | 0.814 | 0.416 |
| CT | 0.406 | −0.512 | 0.609 | 0.045 | 0.964 | −0.159 | 0.874 |
| AG | 0.132 | 0.245 | 0.807 | −1.691 | 0.091 | −1.07 | 0.285 |
| High-sodium plus potassium supplementation intervention | |||||||
| Haplotype1 | |||||||
| AG | 0.564 | 1.408 | 0.159 | −0.302 | 0.763 | 0.357 | 0.721 |
| AA | 0.295 | −0.355 | 0.722 | 0.947 | 0.344 | 0.539 | 0.590 |
| GA | 0.138 | −1.689 | 0.091 | −0.737 | 0.461 | −1.158 | 0.247 |
| Haplotype2 | |||||||
| CG | 0.462 | −0.642 | 0.521 | 0.672 | 0.502 | 0.223 | 0.824 |
| CT | 0.406 | −0.198 | 0.843 | −0.718 | 0.473 | −0.6 | 0.549 |
| AG | 0.132 | 1.4 | 0.161 | 0.059 | 0.953 | 0.667 | 0.504 |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure; SNP, single nucleotide polymorphism.
Z indicates test statistic for family-based association test; P-values are corrected for multiple testing (false discovery rate <0.05).
In addition, we analysed the association of six SNPs in AdipoQ with the basal BP. As shown in Table 6, SNP rs822394 was significantly associated with basal DBP after adjustment for multiple testing (P=0.033). Similarly, haplotype analysis showed that no statistical associations exist between haplotypes of AdipoQ and basal BP (Table 7).
Table 6. SNPs significantly associated with basal BP.
| SNP | Allelesa |
SBP |
DBP |
MAP |
|||
|---|---|---|---|---|---|---|---|
| Z | P-value | Z | P-value | Z | P-value | ||
| rs16861194 | G | −0.595 | 0.552 | 0.296 | 0.767 | −0.054 | 0.957 |
| rs182052 | A | −1.131 | 0.258 | −1.478 | 0.140 | −1.419 | 0.156 |
| rs16861205 | A | 0.859 | 0.390 | 1.452 | 0.147 | 1.297 | 0.195 |
| rs822394 | A | 0.519 | 0.604 | 2.132 | 0.033b | 0.909 | 0.363 |
| rs12495941 | T | 0.373 | 0.709 | −0.017 | 0.987 | 0.147 | 0.883 |
| rs2241767 | G | −0.551 | 0.582 | −0.558 | 0.577 | −0.587 | 0.557 |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure; SNP, single nucleotide polymorphism.
Z indicates test statistic for family-based association test; P-values are corrected for multiple testing (false discovery rate <0.05).
Minor allele.
Recessive model. Bold entries denote significant P-values.
Table 7. Haplotypes in AdipoQ and basal BP.
| Haplotype sequence | Haplotype frequency |
SBP |
DBP |
MAP |
|||
|---|---|---|---|---|---|---|---|
| Z | P-value | Z | P-value | Z | P-value | ||
| Block1 (rs16861194–rs182052) | |||||||
| AG | 0.564 | 1.062 | 0.288 | 1.447 | 0.148 | 1.371 | 0.170 |
| AA | 0.295 | −0.796 | 0.426 | −1.601 | 0.109 | −1.352 | 0.177 |
| GA | 0.138 | −0.628 | 0.530 | −0.044 | 0.965 | −0.294 | 0.769 |
| Block2 (rs822394–rs12495941) | |||||||
| CG | 0.462 | −0.951 | 0.341 | −1.092 | 0.275 | −1.101 | 0.271 |
| CT | 0.406 | 0.462 | 0.644 | 0.201 | 0.841 | 0.325 | 0.745 |
| AG | 0.132 | 0.68 | 0.497 | 1.481 | 0.139 | 1.216 | 0.224 |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure; SNP, single nucleotide polymorphism.
Z indicates test statistic for family-based association test; P-values are corrected for multiple testing (false discovery rate <0.05).
Discussion
In the present study, several common variants in the AdipoQ gene were associated with the BP response to dietary intervention and basal BP. SNP rs16861205 was significantly associated with the DBP response to low-salt intervention and the DBP and MAP responses to high-salt intervention. SNP rs822394 was associated with the DBP and MAP responses to low-salt intervention and the DBP response to high-salt intervention. Meanwhile, a significant association also existed between SNP rs16861194 and SBP response to potassium supplementation intervention. In addition, SNP rs822394 was significantly associated with basal DBP. These SNPs might be used as genetic markers to promote the screening of individuals who are sensitive to dietary salt or potassium intake and may provide potential genetic targets for the treatment of hypertension.
It is currently accepted that adiponectin is biologically involved in the pathophysiology of hypertension. Animal studies have shown that adiponectin knockout mice developed hypertension compared with wild mice after a 3-week high-salt diet, whereas adenovirus-mediated adiponectin replenishment could significantly reduce the SBP of genetically hypertensive obese KKAy mice.27 A meta-analysis of 17 598 adults from 43 non-prospective studies and 5 prospective studies concluded that the adiponectin level in hypertensive adults was 1.64 μg ml−1 lower than in normotensive adults, and the risk of hypertension was reduced by 6% per 1 μg ml−1 increase in plasma adiponectin levels.15 However, whether adiponectin may also genetically affect hypertension is still inconclusive.19, 20 In our study, the associations between adiponectin gene polymorphisms and basal BP were explored in the Chinese Han population. The SNP rs822394 in intron 1 of the gene was significantly associated with basal DBP. Two other SNPs (rs182052 and rs12495941) were also detected in this study, but none was associated with BP, which is in line with the findings in the Hong Kong population.19 These results further confirmed that the adiponectin gene might be mechanistically involved in BP regulation. To date, functional studies on SNP rs822394 have not been reported. The Chennai Urban Rural Epidemiological study (CURES) noted that SNPs rs822393 and rs822396 are both located at the first intron of the human adiponectin gene and associated with hypoadiponectinaemia.28 This intron of the human adiponectin gene contains a 34-bp intronic enhancer, which regulates the expression of the adiponectin gene.29 Thus, we speculated that the adjacent variant rs822394 could probably regulate gene expression. However, this locus may not be causal but will still be in high LD with the true causal SNPs, which need to be clarified by further study.
Adiponectin gene polymorphisms are involved in BP regulation and may contribute to the salt sensitivity of BP. Available evidence indicated that adiponectin levels were regulated by salt intake.16, 17, 30 Kamari et al.17 explored the effect of salt intake on plasma adiponectin levels in male Sprague–Dawley rats on 3% high-salt diet for 5 weeks, and found that salt loading increased adiponectin levels independent of BP elevation. Lely et al.16 reported that the concentrations of plasma adiponectin significantly decreased in 35 healthy male subjects maintained on a 7-day low-salt dietary intervention. Another animal study demonstrated that the expression of adiponectin in heart and adipose tissues increased in obese and diabetic db/db mice subjected to a long-term low-salt diet (0.03%, NaCl).30 In addition, our previous study demonstrated that plasma adiponectin levels were significantly increased from the low-sodium to high-sodium diet in normotensive salt-resistant individuals, but not in salt-sensitive individuals, thereby suggesting that the disturbance of adiponectin may be involved in the pathogenesis of salt-sensitive of BP.18 However, the effect of common variants of the adiponectin gene on BP responses to dietary salt intake has not been studied. Our study is the first to explore the association of adiponectin gene polymorphisms with BP responses to dietary salt intake in a Chinese Han population. In the present study, SNPs rs822394 and rs16861205 were significantly associated with BP responses to low-salt and high-salt intervention. The results provided evidence to support the hypothesis that adiponectin genetic variations are involved in the development of salt-sensitive hypertension. To our knowledge, similar to SNP rs822394, rs16861205 is also located at the intron 1 region. This locus was associated with multiple pathophysiological processes, such as the baseline body weight, change of serum adiponectin levels and gastric cancer; however, the exact mechanism of this locus is unknown.31, 32 Further functional studies on this locus are warranted.
In contrast with the dietary salt, dietary potassium supplementation is inversely correlated with the BP level. Dietary intervention studies indicated that the BP response to potassium intake is heterogeneous among individuals; BP may be sensitive to potassium.5 Previous studies suggested that common variations in several genes contribute to BP potassium sensitivity. He and colleagues6, 33 reported that BP response to dietary potassium supplementation was significantly associated with common variants of several genes, such as angiotensin II type 1 receptor, endothelin 1 and E selectin. To date, the relationship between the potassium sensitivity of BP and adiponectin has not been studied. This work is the first to explore the association of adiponectin gene polymorphisms with BP response to dietary potassium supplementation. SNP rs16861194 is associated with SBP response to potassium supplementation, which suggests that the genetic variation of the adiponectin gene may contribute to the development of BP potassium sensitivity. SNP rs16861194 is located in the promoter region of the adiponectin gene. The influence of this locus on gene promoter activity was investigated by Laumen et al.34 Functional studies showed that the adiponectin promoter activity and DNA-binding activity were significantly impaired during adipocyte differentiation, when the minor allele of rs16861194 was present. Their group further analysed the association of rs16861194 with circulating adiponectin levels in the MONICA/KORA S123 cohort (1692 German participants) and the KORA S4 cohort (696 participants); subjects carrying the minor allele of SNP rs16861194 showed lower circulating adiponectin levels in both cohorts. Given the consistency of potassium and adiponectin in the protection of BP, we hypothesised that potassium supplementation may reduce BP by increasing adiponectin gene transcription and protein expression, while SNP rs16861194 has a regulatory role in this process, which needs to be validated by further studies.
This study has several advantages. First, our study was based on family pedigree and conducted in a Chinese Han population; the bias caused by population stratification is unlikely. Second, participants who came from a homogenous environment have basically the same lifestyle and eating habits. Moreover, the dietary intake of sodium and potassium is strictly controlled in our study; the confounding genetic associations caused by intra-individual (day-to-day) and inter-individual variations in dietary sodium and potassium intake are reduced. Finally, the 24-h urinary sodium and potassium excretions in each stage indicated excellent compliance with the dietary intervention. However, this study was only carried out in a Chinese Han population; the findings may not be generalisable to other populations. Therefore, large-sample and multicentre studies are necessary to validate the abovementioned results. Furthermore, the causal polymorphic loci and their functions should be identified. In addition, the circulating adiponectin level was not measured in our study. The relationship between the adiponectin genotype and the adiponectin levels could not be determined.
In conclusion, several genetic variations in the adiponectin gene were associated with BP responses to dietary sodium and potassium intake in the studied Chinese population. The novel findings suggested that the adiponectin gene may contribute to the development of salt sensitivity and potassium sensitivity of BP.

Acknowledgments
This work was supported by the grant 2012CB517804 from the National Program on Key Basic Research Project of China (973 Program) and funds 81370357 and 81570381 from the National Science Foundation of China.
The authors declare no conflict of interest.
References
- Lawes CM, Vander HS, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet 2008; 371(9623): 1513–1518. [DOI] [PubMed] [Google Scholar]
- Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 2013; 346: f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013; 346: f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft FC, Weinberger MH. Heterogeneous responses to changes in dietary salt intake: the salt-sensitivity paradigm. Am J Clin Nutr 1997; 65(2 Suppl): 612S–617S. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Hum Hypertens 2003; 17(7): 471–480. [DOI] [PubMed] [Google Scholar]
- Montasser ME, Shimmin LC, Gu D, Chen J, Gu C, Kelly TN et al. Blood pressure response to potassium supplementation is associated with genetic variation in endothelin 1 and interactions with E selectin in rural Chinese. J Hypertens 2010; 28(4): 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defago MD, Gu D, Hixson JE, Shimmin LC, Rice TK, Gu CC et al. Common genetic variants in the endothelial system predict blood pressure response to sodium intake: the GenSalt study. Am J Hypertens 2013; 26(5): 643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone. Diabetes Care 2003; 26(8): 2442–2450. [DOI] [PubMed] [Google Scholar]
- Arikoglu H, Ozdemir H, Kaya DE, Ipekci SH, Arslan A, Kayis SA et al. The Adiponectin variants contribute to the genetic background of type 2 diabetes in Turkish population. Gene 2014; 534(1): 10–16. [PubMed] [Google Scholar]
- Lindberg S, Mogelvang R, Pedersen SH, Bjerre M, Frystyk J, Flyvbjerg A et al. Relation of serum adiponectin levels to number of traditional atherosclerotic risk factors and all-cause mortality and major adverse cardiovascular events (from the Copenhagen City Heart Study). Am J Cardiol 2013; 111(8): 1139–1145. [DOI] [PubMed] [Google Scholar]
- Lazra Y, Falach A, Frenkel L, Rozenberg K, Sampson S, Rosenzweig T. Autocrine/paracrine function of globular adiponectin: inhibition of lipid metabolism and inflammatory response in 3T3-L1 adipocytes. J Cell Biochem 2015; 116(5): 754–766. [DOI] [PubMed] [Google Scholar]
- Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes 2015; 64(1): 36–48. [DOI] [PubMed] [Google Scholar]
- Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH et al. Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension 2007; 49(6): 1455–1461. [DOI] [PubMed] [Google Scholar]
- Imatoh T, Miyazaki M, Momose Y, Tanihara S, Une H. Adiponectin levels associated with the development of hypertension: a prospective study. Hypertens Res 2008; 31(2): 229–233. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim C, Ding EL, Townsend MK, Lipsitz LA. Adiponectin levels and the risk of hypertension: a systematic review and meta-analysis. Hypertension 2013; 62(1): 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lely AT, Krikken JA, Bakker SJ, Boomsma F, Dullaart RP, Wolffenbuttel BH et al. Low dietary sodium and exogenous angiotensin II infusion decrease plasma adiponectin concentrations in healthy men. J Clin Endocrinol Metab 2007; 92(5): 1821–1826. [DOI] [PubMed] [Google Scholar]
- Kamari Y, Shimoni N, Koren F, Peleg E, Sharabi Y, Grossman E. High-salt diet increases plasma adiponectin levels independent of blood pressure in hypertensive rats: the role of the renin-angiotensin-aldosterone system. J Hypertens 2010; 28(1): 95–101. [DOI] [PubMed] [Google Scholar]
- Liu F, Mu J, Yuan Z, Wu G, Liu E, Zheng S et al. High salt intake fails to enhance plasma adiponectin in normotensive salt-sensitive subjects. Nutrition 2012; 28(4): 422–425. [DOI] [PubMed] [Google Scholar]
- Ong KL, Li M, Tso AW, Xu A, Cherny SS, Sham PC et al. Association of genetic variants in the adiponectin gene with adiponectin level and hypertension in Hong Kong Chinese. Eur J Endocrinol 2010; 163(2): 251–257. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhao J. Genetic effects of adiponectin on blood lipids and blood pressure. Clin Endocrinol (Oxf) 2011; 74(2): 214–222. [DOI] [PubMed] [Google Scholar]
- Chu C, Wang Y, Wang M, Mu JJ, Liu FQ, Wang L et al. Common variants in serum/glucocorticoid regulated kinase 1 (SGK1) and blood pressure responses to dietary sodium or potassium interventions: a family-based association study. Kidney Blood Press Res 2015; 40(4): 424–434. [DOI] [PubMed] [Google Scholar]
- Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M et al. Human blood pressure determination by sphygmomanometry. Circulation 1993; 88(5 Pt 1): 2460–2470. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81(3): 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21(2): 263–265. [DOI] [PubMed] [Google Scholar]
- Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype–phenotype associations. Eur J Hum Genet 2001; 9(4): 301–306. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001; 125(1–2): 279–284. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 2006; 47(6): 1108–1116. [DOI] [PubMed] [Google Scholar]
- Ramya K, Ayyappa KA, Ghosh S, Mohan V, Radha V. Genetic association of ADIPOQ gene variants with type 2 diabetes, obesity and serum adiponectin levels in south Indian population. Gene 2013; 532(2): 253–262. [DOI] [PubMed] [Google Scholar]
- Qiao L, Maclean PS, Schaack J, Orlicky DJ, Darimont C, Pagliassotti M et al. C/EBPalpha regulates human adiponectin gene transcription through an intronic enhancer. Diabetes 2005; 54(6): 1744–1754. [DOI] [PubMed] [Google Scholar]
- Baudrand R, Lian CG, Lian BQ, Ricchiuti V, Yao TM, Li J et al. Long-term dietary sodium restriction increases adiponectin expression and ameliorates the proinflammatory adipokine profile in obesity. Nutr Metab Cardiovasc Dis 2014; 24(1): 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Ohara-Imaizumi M, Kubota N, Hashimoto S, Eto K, Kanno T et al. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia 2008; 51(5): 827–835. [DOI] [PubMed] [Google Scholar]
- Ye L, Zhang ZY, Du WD, Schneider ME, Qiu Y, Zhou Y et al. Genetic analysis of ADIPOQ variants and gastric cancer risk: a hospital-based case-control study in China. Med Oncol 2013; 30(3): 658. [DOI] [PubMed] [Google Scholar]
- He J, Gu D, Kelly TN, Hixson JE, Rao DC, Jaquish CE et al. Genetic variants in the renin-angiotensin-aldosterone system and blood pressure responses to potassium intake. J Hypertens 2011; 29(9): 1719–1730. [DOI] [PubMed] [Google Scholar]
- Laumen H, Saningong AD, Heid IM, Hess J, Herder C, Claussnitzer M et al. Functional characterization of promoter variants of the adiponectin gene complemented by epidemiological data. Diabetes 2009; 58(4): 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]