Abstract
Abstract: Background
Depression is the most debilitating neuropsychiatric disorder with significant impact on socio-occupational and well being of individual. The exact pathophysiology of depression is still enigmatic though various theories have been put forwarded. There are evidences showing that mitochondrial dysfunction in various brain regions is associated with depression. Recent findings have sparked renewed appreciation for the role of mitochondria in many intracellular processes coupled to synaptic plasticity and cellular resilience. New insights in depression pathophysiology are revolving around the impairment of neuroplasticity. Mitochondria have potential role in ATP production, intracellular Ca2+ signalling to establish membrane stability, reactive oxygen species (ROS) balance and to execute the complex processes of neurotransmission and plasticity. So understanding the various concepts of mitochondrial dysfunction in pathogenesis of depression indubitably helps to generate novel and more targeted therapeutic approaches for depression treatment.
Objective
The review was aimed to give a comprehensive insight on role of mitochondrial dysfunction in depression.
Result
Targeting mitochondrial dysfunction and enhancing the mitochondrial functions might act as potential target for the treatment of depression.
Conclusion
Literature cited in this review highly supports the role of mitochondrial dysfunction in depression. As impairment in the mitochondrial functions lead to the generation of various insults that exaggerate the pathogenesis of depression. So, it is useful to study mitochondrial dysfunction in relation to mood disorders, synaptic plasticity, neurogenesis and enhancing the functions of mitochondria might show promiscuous effects in the treatment of depressed patients.
Keywords: Depression, Mitochondrial dysfunction, Reactive oxygen species, neurotransmitter, Mitochondria, electron transport chain
Introduction
Mitochondria have pivotal role in energy production via metabolism of lipids, steroids and proteins, and in maintenance of cellular stability via modulation of Ca2+ levels, maintaining the levels of ROS and regulation of apoptosis [1]. Thus mitochondrial dysfunction not only hampers cells to meet energy requirement but might also be involved in the impairment of neuronal communication and cellular resilience, which prop up mood disorders and psychotic disorders [2, 3]. New hypothesis of mood disorder is revolving around the concept of neuroplasticity mainly depression and bipolar disorder. Neuroplasticity refers to the brain plasticity or brain malleability encompasses both synaptic plasticity and non-synaptic plasticity. It is the process of adapting neurons in response to changing internal or external environment by causing changes in neural pathways and synapses. Synaptic plasticity involves synapto- genesis, growth of axons and dendrite and removal of unnecessary connections between neurons. In neuroplasticity, mitochondria play an important role and it is well studied that stress leads to structural and functional impairment in various brain regions of depressed patients that lead to impaired neuroplasticity [4]. Neurogenesis i.e. the formation of new neurons is believed to be an important mechanism of brain plasticity under physiological conditions and in brain repair after injury [5, 6]. In various studies it has been consistently observed that different types of stresses decrease the adult hippocampal neurogenesis and lead to depression. During neuronal development, mitochondrial biogenesis occurs at higher rate as increased mitochondrial genome and mitochondrial protein is required for neuronal differentiation [7-9]. In this context, it is interesting to note that mitochondrial dysfunction might have an important role in impaired adult hippocampal neurogenesis in depression.
Neuropsychiatric disorders are the “disorders of the brain” affecting the lives of individuals. From the past two decades stronger evidences have become available documenting that brain disorders are not only increasing more frequently as thought before but also contribute to the greater burden of disease globally [10, 11]. Chronic stress related disorder i.e. depression has a life time prevalence rate of 20%, affecting more than 350 million people worldwide with the suicide rate of about 1 million deaths per year. In the present era of modernization, individuals are more prone to psychiatric diseases due to changes in their life style and increasing stress burden in order to cope with personal and professional challenges in life [12]. Correlative studies indicate the role of mitochondria in the aetiology of depression. Dysfunction in monoaminergic neurotransmission is likely to be most accepted pathophysiology of depression that includes disturbances in the serotonin and norepinephrine levels in the brain leading to impairment in the signal transduction required for the proper functioning of the brain in response to stress [13-16]. But still half of the patients among those receiving treatment show relapse and two third of the patients are not getting benefit by available drugs [17]. This shows that more insights are required in the neurobiology of depression. With emerging concept of neuroplasticity in depression many knots have to be revealed. It has been found that there is impaired energy metabolism in depressed patients which indicates that subtle mitochondrial dysfunction might play an important role in various aspects of depression. Chronic stress also induces inhibition of the respiratory chain in the mitochondria [18]. Processes involved in neuroplasticity consume energy in one or other way and impaired oxidative phosphorylation interferes with various intracellular pathways including synaptic plasticity and signal transduction mechanisms. So, it is useful to study mitochondrial dysfunction in relation to mood disorders, synaptic plasticity, neurogenesis and enhancing the functions of mitochondria might show promiscuous effect in the treatment of depressed patients.
Mitochondria and Brain
Mitochondria, “the power house of cell” are the most unique and pivotal organelle. Cells that are involved in intense physiological and functional activities need more energy hence contain more number of mitochondria as compared to the cells that require lower energy for their functions. They have unique property of fission and fusion because of mitochondrial DNA [19]. They divide irrespective of cell division and play many important roles in the normal functioning of neurons. Every cell in our body contains mitochondria and needs energy but the extent of energy demand in not uniform and it is expected that it is higher in the gray matter, as gray matter has high number of synapses and mitochondria, and possesses intense neuronal activity as compared to white matter [20, 21]. Each neuron has thousands of mitochondria in narrow regions i.e. dendrites and synapses. A resting cortical neuron consumes 4.7 billion ATP molecules per second [22-24].
Mitochondria as Power House
Mitochondria are the only organelle in the cell that has its own DNA called mtDNA (mitochondrial DNA). mtDNA codes only 13 protein subunits of electron transport chain (ETC) and rest mitochondrial proteins are coded by cellular DNA genes. It has well known function of producing cellular energy through citric acid cycle and oxidative phosphorylation (OXPHOS). Mitochondria are the power house of the cell producing ATP (adenosine triphosphate) by efficiently utilising oxygen and substrates like glucose and pyruvate. It fulfils 92% energy demand of body via OXPHOS. It consists of two membranes, an outer membrane and an inner membrane. Inner membrane is folded to form cristae on which the machinery for synthesis of ATP i.e. subunits of ETC are assembled in line. ETC consists of five protein complexes which work coherently to produce ATP at complex V. The three ETC chain complexes i.e. I, III and IV transfer H+ to the intermembrane space in mitochondria to produce electrochemical gradient for the synthesis of ATP [25, 26]. Human brain consumes 25% of total body glucose requirement. ATP, the cellular energy fuel is essential for the normal processing of neuronal processes including maintenance of ion gradients across neuronal membrane, accumulation of neurotransmitters in the vesicles, release of neurotransmitter and trafficking of receptors and ion channels to and from the cell surface [27, 28]. Brain stores only small amount of glycogen so it needs constant supply of glucose. Stressed neurons are unable to upregulate glycolysis and on inhibition of mitochondrial respiratory chain, neurons die rapidly and astrocytes use gycolytically derived ATP [29].
Reactive Oxygen Species
Reactive Oxygen Species (ROS) have significant role in pathogenesis of number of diseases; they are also mediators of several physiological processes. Mitochondria are the main intracellular organelle producing ROS [30]. Each complex of mitochondrial ETC has its own function but works in association with others. During electron transfer process O2– is produced at complex I and III. Mitochondrial superoxide dismutase (SOD) converts O2– into H2O2 and O2. •OH plays an essential role in cell physiology by activating guanylate cyclase and formation of second messenger, cGMP and activation of NF-kB by H2O2. H2O2 gets converted into water by glutathione peroxidase and catalase in cytosol [31]. O2–, highly reactive species form highly reactive molecule on interaction with nitric oxide having high oxidant and nitrating property, peroxinitrite which impairs enzymatic function tyrosine residues and results in decreased production of monoaminergic neurotransmitters and other aminergic compounds [32]. H2O2 can also react with Cu2+ and Fe2+ ions, resulting in formation of highly reactive free radical OH‐ that cause oxidative damage to carbohydrates, lipids, proteins, mtDNA and mitochondrial membranes, resulting in functional deficits and death. Monoamine oxidase A and B, located on the outer mitochondrial membrane metabolise serotonin, dopamine and noradrenaline following formation of free radicals [33]. In spite of formation of ROS, mitochondria also produce several protective antioxidant molecules, such as creatinine, coenzyme Q10, niconitamide and glutathione protecting neurons from various deleterious effects of oxidative reactions. Not only this, studies have shown that ROS have modulating role in synaptic plasticity, learning and memory formation [34, 35].
Ca2+regulation
Mitochondria play an important role in calcium homeostasis, a principal second messenger that is involved in the regulation of neurotransmission and short and long term neuroplasticity in the brain. Both mitochondria and endoplasmic reticulum (ER) are involved in sequestering of intracellular Ca2+. Outer mitochondrial membrane is permeable to Ca2+ and inner mitochondrial membrane has Ca2+uniporters that facilitate inward movement of Ca2+ and Na+/ Ca+ and H+/ Ca2+ antiporters that facilitate outward movement of Ca2+ [36]. The basal intracellular Ca2+ levels is very low compared to the extracellular space and ER, and even multiple, small changes in the concentration level result in repetitive partial mitochondrial depolarisation. Mitochondria have several mechanism for regulation and restoration of changes in Ca2+ levels. Mitochondrial matrix Ca2+ concentration is increased equivalent to increase in cytosolic Ca2+ levels and in situation of high energy demand and gets lowered in low cytosolic Ca2+ levels and high ATP/ADP ratios. In addition to the sequestering of cytocylic Ca2+, mitochondria also contribute to the time, rate and amount of neurotransmitter release from presynaptic sites. A high concentration of Ca2+ in the mitochondrial matrix stimulate the activity of dehydrogenases i.e. pyruvate dehydrogenase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase of the krebs cycle and complex V resulting in increased production of ATP [37]. At extremely increased Ca2+ uptake into mitochondrion results in cessation of ATP formation whereas increased rate of activation of mitochondrial permeability transition pore (mPTP). The increase permeability of the mitochondrial membranes to molecules of molecular weight less than 1,500 Daltons leads to mitochondrial swelling, cytochrome c release, and apoptosis activation [38-39].
Apoptosis
There are three forms of cell death i.e. apoptosis, necrosis and autophagic cell death. Apoptosis is the programmed cell death occurs in the neurons during development and adult cell turnover [40]. Apoptosis is essential to prevent growth of cancerous cells but uncontrolled apoptosis leads to neurodegeneration in post-mitotic cells such as neurons. Apoptosis is complex and energy dependent process in which dying cell itself involved in it. Morphologically, it is characterised by cytoplasmic shrinkage, chromatin condensation and rapid phagocytosis by neighbouring cells [41]. In normal physiological conditions apoptosis removes those neurons and glia which are unable to make neuronal connections. New neurons are produced in large number during adult neurogenesis and these neurons compete for making connections with other neurons and those neurons which have neither electrical activity nor neurotrophic support die by apoptosis [42]. Two biochemical pathways are involved in apoptosis i.e. intrinsic and extrinsic pathway, mitochondria play an important part in both the apoptotic pathways. Both pathways are activated by different stimuli but ultimately result in activation of proteases and cell death [43]. In extrinsic pathway, engagement of death receptors such as cluster of differentiation 95 (CD95), TNF-α1, on the cell surface recruits Fas-associated death domain protein (FADD) which further triggers caspase-8 activation. Activated caspase-8 upon release in cytosol cleaves and activates other caspases and cleaves BH3 interacting domain death agonist (BID), which then translocates to the mitochondria and initiates the caspase cascade which in turn triggers the executioner caspases. This ultimately leads to outer mitochondrial membrane permeabilization, leakage of apoptogenic factors and ultimately apoptosis [44]. In intrinsic pathway, mitochondria have significant role and is predominant in neurons. High levels of Ca2+ or ROS, damage caused by irradiation and activation of pro-apoptotic B-cell lymphoma protein 2 (BCL-2) family proteins, oxidative stress, over activation of glutamate receptors are the examples of stimuli that trigger activation of various kinases that lead to the loss of mitochondrial membrane potential. This occurs due to release of intermembrane cytochrome c into the cytoplasm. Cytochrome c then binds to cytosolic protein, apoptotic-protease-activating factor 1 (Apaf-1) which further recruits and activates caspase 9 and results in the formation of a multisubunit enzyme complex known as the apoptosome [45, 46]. Activated caspase 9 then activates other executioner caspases like caspase 3 and 7 [47]. Similarly to cytochrome c, two intermembrane proteins, Smac/ DIABLO and Omi/HtrA2 inactivate IAP (inhibitors of apoptosis proteins), preventing caspase -3 and -9 inhibition. Once mitochondrial membrane integrity is impaired i.e. on mitochondrial membrane permeabilization (MMP), apoptosis inducer factor (AIF) and endonuclease G move to the nucleus and mediate chromatinolysis. All these processes lead to apoptosis by disabling repair processes, terminating cell cycle progression, inactivating inhibitors of apoptosis, mediating cytoskeletal and nuclear disassembly and activates cell phagocytosis [48-50]. Indeed neuronal apoptosis is essential in the early stages of development and during adult neurogenesis as it leads to the removal of damaged, unresponsive networks and also facilitates the synaptic plasticity and long-term potentiation.
Mitochondria and Neurogenesis
Neurogenesis is the process of formation of new neurons in which neural stem cells proliferate and then differentiate into neurons. Clear evidences from studies have shown that neurogenesis is prominent in the dentate gyrus of hippocampal region [51-55]. In depression, it has been shown that there is decreased hippocampal neurogenesis which leads to the formation of faulty neuronal networks and hence impairs adaptation to the changed milieu. During neuronal development, mitochondrial biogenesis occur at higher rate which leads to increase mitochondria number per neuron. Vayssiere, Cordeau-Lossouarn, Larcher, Basseville, Gros and Croizat [56] showed that inhibitor of mitochondrial protein synthesis, chloramphenicol prevents neuronal differentiation whereas oligomycin, mitochondrial ATP synthase inhibitor have no effect on the differentiation process. This suggests that increased mitochondrial genome and mitochondrial protein is required for neuronal differentiation [7-9]. Mitochondria can freely translocate with in the neuron. During neurite outgrowth the velocity at which mitochondria move decrease as the neurite outgrowth slows down and synaptogenesis occurs [57]. In the process of axogenesis, number of mitochondria increase in the neurite destined to become axon and decrease in neurite destined to become dendrite [33]. The increased mitochondrial mass differentiation synthesize large amount of ATP to meet increased energy demand and fundamental cellular processes involved in neurite outgrowth and synaptogenesis.
Mitochondria and Synaptic Plasticity
The long term changes in strength and activity of neuronal connections in response to stress and other aversive stimuli is called synaptic plasticity that involves structural and functional plasticity. In neurons, mitochondria have major function in regulation of developmental and synaptic plasticity [58]. The mitochondria in presynaptic axon terminals and postsynaptic dendritic spines differ in behaviour and functional properties. More than twice the number of mitochondria in the axon is motile as compared to the mitochondria in dendrites of cultured hippoocampal neurons. Mitochondria in dendrites are highly charged and more metabolically active than in axons [59]. Studies have shown, mitochondria play an active role in synaptic plasticity [60, 61]. During synaptogenesis, the structural plasticity of dendritic spines is dependent on the number of mitochondria present in dendrites. Impairment of dynamin-like GTPases Drp1, mediate mitochondrial fission and OPA1, mediate mitochondrial fusion or over expression of dominant-negative form of Drp1 (A38K) reduces the number of dendritic mitochondria and impairs synaptogenesis whereas on treating cells with creatine increases dendritic mitochondrial mass and activity and approximately double the number of synapses [62-65]. Thus mitochondria have an imperative role in structural synaptic plasticity. The function of presynaptic neuronal axons and postsynaptic neuronal dendrites are highly dependent on the changes in the intracellular Ca2+ levels as a result of metabolic and oxidative stress. Mitochondria act as Ca2+ sensor and take up Ca2+ after synaptic stimulation and thereby playing a role in neuronal transduction [66]. Mitochondria have also been involved in LTP, a form of synaptic plasticity. It has been shown in studies that ROS are essential for the induction of LTP [67, 68]. Various studies have shown that superoxide, hydrogen peroxide and nitric oxide are involved in hippocampal synaptic plasticity. These ROS increase the activity of protein kinase C, extracellular-regulated kinase 2, protein tyrosine kinases ryanodine receptor type 3, and decrease the activity of protein phosphatases 2A and 2B, and a protein tyrosine phosphatase [69-71]. Moreover, it has been found in in-vitro study that on treatment with scavengers of ROS attenuated LTP in hippocampus slices and in in-vivo study where hippocampus of transgenic mice overexpress Cu/Zn superoxide dismutase [72, 73]. Excessive ROS have detrimental effect on the functions of brain. Similarly, excessive Ca2+ levels lead to the activation of pro-apoptotic factors and activation of apoptotic process lead to LTD (long term depression), another form of synaptic plasticity. Thus, a balance between LTD and LTP in the brain is essential for mental health [74]. In addition to the changes in the movement of mitochondria within axons and dendrites, changes in other mitochondrial function such as Ca2+ regulation, energy metabolism and ROS production also play important role in synaptic plasticity. Emerging findings also suggest that mitochondria exhibit some the effects of BDNF
and glutamate on synaptic plasticity. Markham, Cameron, Franklin and Spedding [75] showed increase in glucose utilization in cultured cortical neurons in response to enhanced energy demand and increase in the respiratory control index in rat brain mitochondria on treatment with BDNF [75, 76]. Regulation of various processes involved in synaptic plasticity by mitochondria enlightens its essential role in synaptic plasticity.
Mitochondrial Dysfunction in Depression
Brain is highly active organ with high energy consumption and organs with highest energy demand are more susceptible to the deleterious effect of reduced energy production. Mainly neurons obtain energy from mitochondrial OXPHOS. Neuron obtains energy through glycolysis but brain is unable to store glycogen so constant supply of glucose is needed. When oxidative phosphorylation is compromised, neurons are unable to meet their energy demand and alteration in their various physiological functions occurs due to reduced ATP production. Studies have shown that chronic mild stress inhibits mitochondrial OXPHOS, dissipates mitochondrial membrane potential and damages mitochondrial ultrastructure in various brain regions including hippocampus, cortex and hypothalamus of mice [77, 78]. These observations lead to the conclusion that most probably depression is caused by energy impairment in the brain due to mitochondrial dysfunction [79, 80]. In support of this notion, studies have shown that patient suffering from depression showed reduced glucose utilization in the PFC, anterior cingulated gyrus and caudate nucleus [81-83].
The relationship between social–psychological and physical stress and the development of depression in susceptible individuals has long been established [84]. Rodents subjected to chronic mild stress show depression like symptoms [85]. Major depression is associated with reduced hippocampal volume. Both structural and functional changes within several brain regions, including the hippocampus, amygdala and prefrontal cortex, resulting in faulty connections between anterior cingulate cortex (ACC), PFC, hippocampal, and amygdaloid regions, ends up with constant alterations in neuroplasticity [86, 87].
Hyperactivity of HPA axis (Hypothalamic–pituitary–adrenal axis) in depression is associated with increased glucocorticoid (GC) levels both in CNS and peripherally [88, 89]. GCs play biphasic role in regulating mitochondrial functions. Cultured neurons on acute exposure to either low or high levels of glucocorticoids are markedly increase mitochondrial activity. GCs bound to the glucocorticoid receptors can translocate to mitochondria and inhibit the formation of Bax- containing pores on the mitochondrial outer membrane by forming a complex with the anti-apoptotic protein Bcl-2, and reduce the release of calcium and cytochrome C from the mitochondria and ultimately inhibit apoptosis [31]. Whereas chronic elevation in GCs level cause kanic acid induced toxicity of cortical neurons. Prolonged exposure to GCs also cause respiratory chain dysfunction, increased ROS generation, mitochondrial structural abnormalities, apoptosis and cell death in skeletal muscle cells and hippocampal neurons [90, 91].
Stress also increases the levels of proinflammtory cytokines including IL-1β, IL-6, and TNFα and there are ample evidences showing that debilitating major depression is accompanied by activation of immune, inflammatory, oxidative, increased HPA-axis and nitrosative stress pathways.
Animals subjected to different stresses like psychological stress, immobilization stress, inescapable shock, physical restraint, open field stress or conditioned, aversive stimuli showed increased pro-inflammatory cytokines and nitric oxide in the brain and plasma [92-94]. TNF-α, a pro-inflammatory cytokine, suppresses mitochondrial complexes I and IV and pyruvate dehydrogenase activities and induces mitochondrial damage [95]. IL-6 stimulates increased ROS production in the brain [96] and nitric oxide is the potent inhibitor of mitochondrial cytochrome c oxidase [97]. Cytokines also induce activation of pro-apoptotic members of the BCL-2 family and activation of the caspase cascade. It has been shown that unpredictable stress reduces the expression of Bcl-2 mRNA in the rat limbic structures, an anti-apoptotic endogenous membrane protein that promotes neuritogenesis and axon regeneration [98-100]. BAG-1(Bcl-2 associated athanogene) is a gene that binds to Bcl-2 and enhances the anti-apoptotic effects of BCL-2. Chronic mild stress has been shown to reduce the expression of BAG-1 [101]. This ultimately leads to the activation of caspases and BCL-2-associated X protein (BAX) and BCL-2 antagonist/killer (BAK) in the mitochondria which further alter membrane permeability, ends up with neuronal death. The production of ATP and Ca2+ buffering is also an important function of presynaptic mitochondria in order to maintain neuron-neuron communication by controlling synaptic vesicles mobilization and recycling process. Vesicle cycling and neurotransmission is highly dependent on mitochondrial function as many steps involved in it are ATP consuming. Mobilization of synaptic vesicles to the active sites of synapse and endo-exo cycling pool process is dependent on ATP. Synaptic vesicles propulsion is dependent on the activation of myosin ATPase complex which results in the translocation of actin filaments. Upon activation ATP gets converted into cAMP by phosphodiesterase and adenylatecyclase. cAMP then activates PKA kinase, resulting in the mobilization of synaptic vesicles to the active site [102]. Similarly in a mammalian study, it has been observed that impairment of interaction of syntabulin with kinesin impairs neurotransmitter release due to inhibition of mitochondria translocation at the active site of synapse and is reversed by exogenous ATP addition [103]. Thus, defects in ATP production and disturbed mitochondrial translocation to the active site of synapse lead to the local ATP depletion and defective neuronal transmission [104, 105].
An imbalance between ROS production and antioxidant defence mechanism may represent the one factor influencing the etiology of depression. It is reported that chronic unpredictable mild stress impairs the endogenous antioxidant status in the brain. Various studies showed that chronic mild stress induced depression in rodents lowers the concentration of brain glutathione, total antioxidant capacity, superoxide dismutase and catalase [106-108]. Hence, there is increased oxidative stress in depression. As mitochondria are the main site of ROS production in the cell, they are highly susceptible to oxidative damage. Lipids, proteins, oxidative phosphorylation enzymes and mtDNA in mitochondria are more susceptible to oxidative damage [109]. Thus, mitochondrial functions are impaired and aggravate oxidative stress, increase in intracellular Ca2+ levels, damage or deletions of mtDNA, alterations in fusion/fission and morphology of mitochondria and finally to neuronal death [110]. Further mitochondrial dysfunction is compromised by increasing energy demand for cellular repair process and in this way mitochondria damage causes additional damage [111].
Conclusion
The exact pathogenic mechanisms behind depression are still not perfectly known but increasing evidences suggest that mitochondrial dysfunction may be involved in various psychiatric diseases. Regulatory role of mitochondria in synaptic strength and cellular resilience in neuronal circuits mediate complex, high-order brain functions and implicate mitochondrial dysfunction in the pathogenesis of mood disorders. Thus, enhancing mitochondrial functions appears to be reasonable for increasing mitochondrial function and preventing or alleviating the burden of many stress-related disorders. Targeting mechanisms of mitochondrial dysfunction due to its special impact on energy metabolism, synaptic plasticity and neuronal survival, may be an important avenue for development of new mood-stabilizing agents. The mitochondrially-targeted therapeutics that have reached clinical trials so far have produced encouraging but largely inconclusive results. Uridine showed statistically significant improvement in depressive symptoms in Phase IIa trial whereas Phase IIb study showed negative results [112]. Coenzyme Q10, acetyl L-carnitine and α- lipoic acid are also being studied for treatment of Biplar disorder (ClinicalTrials. gov identifiers NCT01390389 and NCT00719706). Increasing understanding of mitochondrial dysfunction generated exciting preclinical data that show association of mitochondrial dysfunction with neurodegenerative disorders but less clinical evidences are available so further work is needed to shed light on the role of mitochondrial dysfunction in the pathogenesis of neuronal disorders.
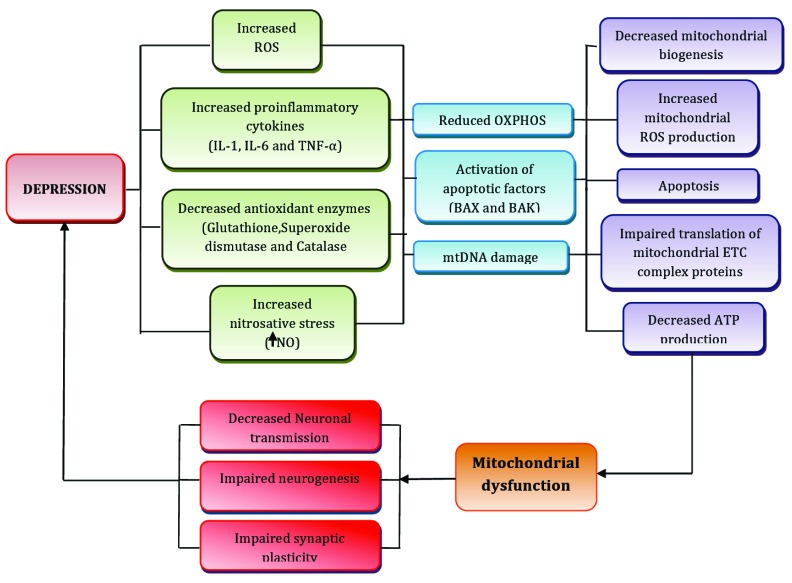
Fig. (1).
Exposure to different stressful situations leads to increase ROS production, increased levels proinflammatory cytokines, increased nitrosative stress and decrease in antioxidant enzymes level which ultimately leads to reduce OXPHOS, activation of apoptotic pathway and causes mtDNA damage as consequence of which there is decreased mitochondrial biogenesis, increased ROS production, apoptosis of neuronal cells, impaired translation of mitochondrial ETC complex proteins and decreased ATP production which results into mitochondrial dysfunction. Neurogenesis, synaptic plasticity and neuronal transmission, the important parameters for successful adaptation to the stressful conditions are also compromised due to mitochondrial dysfunction hence also play an important role in major depression.
ACKNOWLEDGEMENTS
Department of Science & Technology, New Delhi is gratefully acknowledged for providing Inspire Fellowship to Ms Yashika Bansal.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Scheffler I. Mitochondria. 1999. Canada: Wiley-Liss, Inc.; 1999. [Google Scholar]
- 2.Quiroz J.A., Gray N.A., Kato T., Manji H.K. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33(11):2551–2565. doi: 10.1038/sj.npp.1301671. [http://dx.doi.org/10.1038/sj.npp.1301671]. [PMID: 18235426]. [DOI] [PubMed] [Google Scholar]
- 3.Rezin G.T., Gonçalves C.L., Daufenbach J.F., Fraga D.B., Santos P.M., Ferreira G.K., Hermani F.V., Comim C.M., Quevedo J., Streck E.L. Acute administration of ketamine reverses the inhibition of mitochondrial respiratory chain induced by chronic mild stress. Brain Res. Bull. 2009;79(6):418–421. doi: 10.1016/j.brainresbull.2009.03.010. [http://dx.doi.org/10.1016/j.brainresbull.2009.03.010]. [PMID: 19393724]. [DOI] [PubMed] [Google Scholar]
- 4.Sheline Y.I., Gado M.H., Kraemer H.C. Untreated depression and hippocampal volume loss. Am. J. Psychiatry. 2003;160(8):1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [http://dx.doi.org/10.1176/appi.ajp.160.8.1516]. [PMID: 12900317]. [DOI] [PubMed] [Google Scholar]
- 5.Kempermann G., Wiskott L., Gage F.H. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004;14(2):186–191. doi: 10.1016/j.conb.2004.03.001. [http://dx.doi.org/10.1016/j.conb.2004.03.001]. [PMID: 15082323]. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura T., Saitoh Y., Takashima N., Murayama A., Niibori Y., Ageta H., Sekiguchi M., Sugiyama H., Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139(4):814–827. doi: 10.1016/j.cell.2009.10.020. [http://dx.doi.org/10.1016/j.cell.2009.10.020]. [PMID: 19914173]. [DOI] [PubMed] [Google Scholar]
- 7.Kathleen Baxter K., Uittenbogaard M., Yoon J., Chiaramello A. The neurogenic basic helix-loop-helix transcription factor NeuroD6 concomitantly increases mitochondrial mass and regulates cytoskeletal organization in the early stages of neuronal differentiation. ASN Neuro. 2009;1(4):e00016. doi: 10.1042/AN20090036. [http://dx.doi.org/10.1042/ AN20090036]. [PMID: 19743964]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calingasan N.Y., Ho D.J., Wille E.J., Campagna M.V., Ruan J., Dumont M., Yang L., Shi Q., Gibson G.E., Beal M.F. Influence of mitochondrial enzyme deficiency on adult neurogenesis in mouse models of neurodegenerative diseases. Neuroscience. 2008;153(4):986–996. doi: 10.1016/j.neuroscience.2008.02.071. [http://dx.doi.org/10.1016/j.neuroscience.2008. 02.071]. [PMID: 18423880]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirby D.M., Rennie K.J., Smulders-Srinivasan T.K., Acin-Perez R., Whittington M., Enriquez J.A., Trevelyan A.J., Turnbull D.M., Lightowlers R.N. Transmitochondrial embryonic stem cells containing pathogenic mtDNA mutations are compromised in neuronal differentiation. Cell Prolif. 2009;42(4):413–424. doi: 10.1111/j.1365-2184.2009.00612.x. [http://dx.doi.org/10.1111/j.1365-2184.2009.00612.x]. [PMID: 19552636]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray C.J., Lopez A.D. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274(5288):740–743. doi: 10.1126/science.274.5288.740. [http://dx.doi.org/10.1126/science.274.5288.740]. [PMID: 8966556]. [DOI] [PubMed] [Google Scholar]
- 11.Organization W. H. European status report on alcohol and health. 2010 [Google Scholar]
- 12.Hidaka B.H. Depression as a disease of modernity: explanations for increasing prevalence. J. Affect. Disord. 2012;140(3):205–214. doi: 10.1016/j.jad.2011.12.036. [http://dx.doi.org/10.1016/j.jad.2011.12.036]. [PMID: 22244375]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aboul-Fotouh S. Chronic treatment with coenzyme Q10 reverses restraint stress-induced anhedonia and enhances brain mitochondrial respiratory chain and creatine kinase activities in rats. Behav. Pharmacol. 2013;24(7):552–560. doi: 10.1097/FBP.0b013e3283654029. [http://dx.doi.org/ 10.1097/FBP.0b013e3283654029]. [PMID: 23928691]. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto K., Bundo M., Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum. Mol. Genet. 2005;14(2):241–253. doi: 10.1093/hmg/ddi022. [http://dx.doi.org/10.1093/hmg/ddi022]. [PMID: 15563509]. [DOI] [PubMed] [Google Scholar]
- 15.Maes M., Fišar Z., Medina M., Scapagnini G., Nowak G., Berk M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates--Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology. 2012;20(3):127–150. doi: 10.1007/s10787-011-0111-7. [http://dx.doi.org/10.1007/s10787-011-0111-7]. [PMID: 22271002]. [DOI] [PubMed] [Google Scholar]
- 16.Nutt D. J. Relationship of neurotransmitters to the symptoms of major depressive disorder. J. Clin. Psychiatry. 2008;69:4–7. [PubMed] [Google Scholar]
- 17.Thase M.E. Preventing relapse and recurrence of depression: a brief review of therapeutic options. CNS Spectr. 2006;11(12) Suppl. 15:12–21. doi: 10.1017/s1092852900015212. [PMID: 17146414]. [DOI] [PubMed] [Google Scholar]
- 18.Madrigal J.L., Olivenza R., Moro M.A., Lizasoain I., Lorenzo P., Rodrigo J., Leza J.C. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology. 2001;24(4):420–429. doi: 10.1016/S0893-133X(00)00208-6. [http://dx. doi.org/10.1016/S0893-133X(00)00208-6]. [PMID: 11182537]. [DOI] [PubMed] [Google Scholar]
- 19.Liesa M., Palacín M., Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 2009;89(3):799–845. doi: 10.1152/physrev.00030.2008. [http://dx.doi.org/10.1152/physrev.00030.2008]. [PMID: 19584314]. [DOI] [PubMed] [Google Scholar]
- 20.Howarth C., Peppiatt-Wildman C.M., Attwell D. The energy use associated with neural computation in the cerebellum. J. Cereb. Blood Flow Metab. 2010;30(2):403–414. doi: 10.1038/jcbfm.2009.231. [http://dx.doi.org/ 10.1038/jcbfm.2009.231]. [PMID: 19888288]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen Q., Chklovskii D.B. Segregation of the brain into gray and white matter: a design minimizing conduction delays. PLOS Comput. Biol. 2005;1(7):e78. doi: 10.1371/journal.pcbi.0010078. [http://dx.doi.org/10.1371/journal. pcbi.0010078]. [PMID: 16389299]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolfe D.F., Brown G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77(3):731–758. doi: 10.1152/physrev.1997.77.3.731. [PMID: 9234964]. [DOI] [PubMed] [Google Scholar]
- 23.Wong-Riley M.T., Tripathi S.C., Trusk T.C., Hoppe D.A. Effect of retinal impulse blockage on cytochrome oxidase-rich zones in the macaque striate cortex: I. Quantitative electron-microscopic (EM) analysis of neurons. Vis. Neurosci. 1989;2(5):483–497. doi: 10.1017/s0952523800012384. [http://dx.doi.org/10.1017/S0952523800012384]. [PMID: 2562109]. [DOI] [PubMed] [Google Scholar]
- 24.Zhu X.H., Qiao H., Du F., Xiong Q., Liu X., Zhang X., Ugurbil K., Chen W. Quantitative imaging of energy expenditure in human brain. Neuroimage. 2012;60(4):2107–2117. doi: 10.1016/j.neuroimage.2012.02.013. [http://dx. doi.org/10.1016/j.neuroimage.2012.02.013]. [PMID: 22487547]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boekema E.J., Braun H.P. Supramolecular structure of the mitochondrial oxidative phosphorylation system. J. Biol. Chem. 2007;282(1):1–4. doi: 10.1074/jbc.R600031200. [http://dx.doi.org/10.1074/jbc.R600031200]. [PMID: 17102127]. [DOI] [PubMed] [Google Scholar]
- 26.Wallace D.C., Fan W., Procaccio V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [http://dx.doi.org/10.1146/annurev.pathol.4.110807.092314]. [PMID: 20078222]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleichmann M., Mattson M.P. Neuronal calcium homeostasis and dysregulation. Antioxid. Redox Signal. 2011;14(7):1261–1273. doi: 10.1089/ars.2010.3386. [http://dx.doi.org/10.1089/ars.2010.3386]. [PMID: 20626318]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacAskill A.F., Atkin T.A., Kittler J.T. Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur. J. Neurosci. 2010;32(2):231–240. doi: 10.1111/j.1460-9568.2010.07345.x. [http://dx.doi.org/10.1111/j.1460-9568.2010.07345.x]. [PMID: 20946113]. [DOI] [PubMed] [Google Scholar]
- 29.Bolaños J.P., Almeida A., Moncada S. Glycolysis: a bioenergetic or a survival pathway? Trends Biochem. Sci. 2010;35(3):145–149. doi: 10.1016/j.tibs.2009.10.006. [http://dx.doi.org/10.1016/j.tibs.2009.10.006]. [PMID: 20006513]. [DOI] [PubMed] [Google Scholar]
- 30.Massaad C.A., Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal. 2011;14(10):2013–2054. doi: 10.1089/ars.2010.3208. [http://dx.doi.org/10.1089/ars.2010.3208]. [PMID: 20649473]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du J., Wang Y., Hunter R., Wei Y., Blumenthal R., Falke C., Khairova R., Zhou R., Yuan P., Machado-Vieira R., McEwen B.S., Manji H.K. Dynamic regulation of mitochondrial function by glucocorticoids. Proc. Natl. Acad. Sci. USA. 2009;106(9):3543–3548. doi: 10.1073/pnas.0812671106. [http://dx.doi.org/10.1073/pnas.0812671106]. [PMID: 19202080]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckman J.S., Koppenol W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [PMID: 8944624]. [DOI] [PubMed] [Google Scholar]
- 33.Mattson M.P., Gleichmann M., Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60(5):748–766. doi: 10.1016/j.neuron.2008.10.010. [http://dx.doi.org/10.1016/j.neuron.2008.10.010]. [PMID: 19081372]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu D., Serrano F., Oury T.D., Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. 2006;26(15):3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [http://dx.doi.org/10.1523/JNEUROSCI.5566-05.2006]. [PMID: 16611809]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kishida K.T., Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid. Redox Signal. 2007;9(2):233–244. doi: 10.1089/ars.2007.9.ft-8. [http://dx.doi.org/10.1089/ars.2007.9.233]. [PMID: 17115936]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patergnani S., Suski J.M., Agnoletto C., Bononi A., Bonora M., De Marchi E., Giorgi C., Marchi S., Missiroli S., Poletti F., Rimessi A., Duszynski J., Wieckowski M.R., Pinton P. Calcium signaling around Mitochondria Associated Membranes (MAMs). Cell Commun. Signal. 2011;9:19. doi: 10.1186/1478-811X-9-19. [http://dx.doi.org/10.1186/ 1478-811X-9-19]. [PMID: 21939514]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzuto R., Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 2006;86(1):369–408. doi: 10.1152/physrev.00004.2005. [http://dx.doi.org/10.1152/physrev.00004. 2005]. [PMID: 16371601]. [DOI] [PubMed] [Google Scholar]
- 38.Chalmers S., Nicholls D.G. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 2003;278(21):19062–19070. doi: 10.1074/jbc.M212661200. [http://dx.doi.org/10.1074/jbc.M212661200]. [PMID: 12660243]. [DOI] [PubMed] [Google Scholar]
- 39.Demaurex N., Distelhorst C. Cell biology. Apoptosis--the calcium connection. Science. 2003;300(5616):65–67. doi: 10.1126/science.1083628. [http://dx.doi.org/10. 1126/science.1083628]. [PMID: 12677047]. [DOI] [PubMed] [Google Scholar]
- 40.Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., El-Deiry W.S., Golstein P., Green D.R., Hengartner M., Knight R.A., Kumar S., Lipton S.A., Malorni W., Nuñez G., Peter M.E., Tschopp J., Yuan J., Piacentini M., Zhivotovsky B., Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16(1):3–11. doi: 10.1038/cdd.2008.150. [http://dx. doi.org/10.1038/cdd.2008.150]. [PMID: 18846107]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [http://dx.doi.org/10.1038/bjc. 1972.33]. [PMID: 4561027]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobson M.D., Weil M., Raff M.C. Programmed cell death in animal development. Cell. 1997;88(3):347–354. doi: 10.1016/s0092-8674(00)81873-5. [http://dx.doi.org/ 10.1016/S0092-8674(00)81873-5]. [PMID: 9039261]. [DOI] [PubMed] [Google Scholar]
- 43.Mattson M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000;1(2):120–129. doi: 10.1038/35040009. [http://dx.doi.org/10.1038/ 35040009]. [PMID: 11253364]. [DOI] [PubMed] [Google Scholar]
- 44.Bender T., Martinou J. C. Where killers meet-permeabilization of the outer mitochondrial membrane during apoptosis. Csh. Perspect Biol. 2013;5(1) doi: 10.1101/cshperspect.a011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li P., Nijhawan D., Budihardjo I., Srinivasula S.M., Ahmad M., Alnemri E.S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [http://dx.doi.org/10. 1016/S0092-8674(00)80434-1]. [PMID: 9390557]. [DOI] [PubMed] [Google Scholar]
- 46.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15(22):2922–2933. [PMID: 11711427]. [PubMed] [Google Scholar]
- 47.Jiang X., Wang X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [http://dx.doi.org/10.1146/annurev. biochem.73.011303.073706]. [PMID: 15189137]. [DOI] [PubMed] [Google Scholar]
- 48.Earnshaw W.C., Martins L.M., Kaufmann S.H. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [http://dx.doi. org/10.1146/annurev.biochem.68.1.383]. [PMID: 10872455]. [DOI] [PubMed] [Google Scholar]
- 49.Shoshan-Barmatz V., Ben-Hail D. VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion. 2012;12(1):24–34. doi: 10.1016/j.mito.2011.04.001. [http://dx.doi.org/10.1016/j.mito.2011.04.001]. [PMID: 21530686]. [DOI] [PubMed] [Google Scholar]
- 50.Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [http://dx.doi.org/10.1038/nrm2308]. [PMID: 18097445]. [DOI] [PubMed] [Google Scholar]
- 51.Esposito M.S., Piatti V.C., Laplagne D.A., Morgenstern N.A., Ferrari C.C., Pitossi F.J., Schinder A.F. Neuronal differentiation in the adult hippocampus recapitulates embryonic development (vol 25, pg 10074, 2005). J. Neurosci. 2005;25(49) doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge S., Yang C.H., Hsu K.S., Ming G.L., Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [http://dx.doi. org/10.1016/j.neuron.2007.05.002]. [PMID: 17521569]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kempermann G., Kuhn H.G., Gage F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [http://dx.doi.org/10.1038/386493a0]. [PMID: 9087407]. [DOI] [PubMed] [Google Scholar]
- 54.Ramirez-Amaya V., Marrone D.F., Gage F.H., Worley P.F., Barnes C.A. Integration of new neurons into functional neural networks. J. Neurosci. 2006;26(47):12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [http://dx.doi. org/10.1523/JNEUROSCI.2195-06.2006]. [PMID: 17122048]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [http://dx.doi.org/10.1126/science.1083328]. [PMID: 12907793]. [DOI] [PubMed] [Google Scholar]
- 56.Vayssiere J.L., Cordeaulossouarn L., Larcher J.C., Basseville M., Gros F., Croizat B. 1992.
- 57.Chang D.T., Reynolds I.J. Differences in mitochondrial movement and morphology in young and mature primary cortical neurons in culture. Neuroscience. 2006;141(2):727–736. doi: 10.1016/j.neuroscience.2006.01.034. [http:// dx.doi.org/10.1016/j.neuroscience.2006.01.034]. [PMID: 16797853]. [DOI] [PubMed] [Google Scholar]
- 58.Knott A.B., Perkins G., Schwarzenbacher R., Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008;9(7):505–518. doi: 10.1038/nrn2417. [http://dx.doi.org/10.1038/nrn2417]. [PMID: 18568013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Overly C.C., Rieff H.I., Hollenbeck P.J. Organelle motility and metabolism in axons vs dendrites of cultured hippocampal neurons. J. Cell Sci. 1996;109(Pt 5):971–980. doi: 10.1242/jcs.109.5.971. [PMID: 8743944]. [DOI] [PubMed] [Google Scholar]
- 60.Cheng A., Hou Y., Mattson M.P. Mitochondria and neuroplasticity. ASN Neuro. 2010;2(5):e00045. doi: 10.1042/AN20100019. [http://dx.doi.org/ 10.1042/AN20100019]. [PMID: 20957078]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manji H., Kato T., Di Prospero N.A., Ness S., Beal M.F., Krams M., Chen G. Impaired mitochondrial function in psychiatric disorders. Nat. Rev. Neurosci. 2012;13(5):293–307. doi: 10.1038/nrn3229. [PMID: 22510887]. [DOI] [PubMed] [Google Scholar]
- 62.Cameron H.A., Kaliszewski C.K., Greer C.A. Organization of mitochondria in olfactory bulb granule cell dendritic spines. Synapse. 1991;8(2):107–118. doi: 10.1002/syn.890080205. [http://dx.doi.org/10.1002/syn. 890080205]. [PMID: 1715612]. [DOI] [PubMed] [Google Scholar]
- 63.Detmer S.A., Chan D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007;8(11):870–879. doi: 10.1038/nrm2275. [http://dx.doi.org/10.1038/nrm2275]. [PMID: 17928812]. [DOI] [PubMed] [Google Scholar]
- 64.Li Z., Okamoto K., Hayashi Y., Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–887. doi: 10.1016/j.cell.2004.11.003. [http://dx.doi. org/10.1016/j.cell.2004.11.003]. [PMID: 15607982]. [DOI] [PubMed] [Google Scholar]
- 65.Popov V., Medvedev N.I., Davies H.A., Stewart M.G. Mitochondria form a filamentous reticular network in hippocampal dendrites but are present as discrete bodies in axons: a three-dimensional ultrastructural study. J. Comp. Neurol. 2005;492(1):50–65. doi: 10.1002/cne.20682. [http://dx.doi.org/10.1002/cne.20682]. [PMID: 16175555]. [DOI] [PubMed] [Google Scholar]
- 66.Pivovarova N.B., Pozzo-Miller L.D., Hongpaisan J., Andrews S.B. Correlated calcium uptake and release by mitochondria and endoplasmic reticulum of CA3 hippocampal dendrites after afferent synaptic stimulation. J. Neurosci. 2002;22(24):10653–10661. doi: 10.1523/JNEUROSCI.22-24-10653.2002. [PMID: 12486158]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cooke S.F., Bliss T.V. Plasticity in the human central nervous system. Brain. 2006;129(Pt 7):1659–1673. doi: 10.1093/brain/awl082. [http://dx.doi.org/ 10.1093/brain/awl082]. [PMID: 16672292]. [DOI] [PubMed] [Google Scholar]
- 68.Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J. Neurophysiol. 1998;80(1):452–457. doi: 10.1152/jn.1998.80.1.452. [PMID: 9658063]. [DOI] [PubMed] [Google Scholar]
- 69.Huddleston A.T., Tang W., Takeshima H., Hamilton S.L., Klann E. Superoxide-induced potentiation in the hippocampus requires activation of ryanodine receptor type 3 and ERK. J. Neurophysiol. 2008;99(3):1565–1571. doi: 10.1152/jn.00659.2007. [http://dx.doi.org/ 10.1152/jn.00659.2007]. [PMID: 18199822]. [DOI] [PubMed] [Google Scholar]
- 70.Klann E., Roberson E.D., Knapp L.T., Sweatt J.D. A role for superoxide in protein kinase C activation and induction of long-term potentiation. J. Biol. Chem. 1998;273(8):4516–4522. doi: 10.1074/jbc.273.8.4516. [http://dx.doi.org/10.1074/jbc.273.8.4516]. [PMID: 9468506]. [DOI] [PubMed] [Google Scholar]
- 71.Lee K.Y., Chung K., Chung J.M. Involvement of reactive oxygen species in long-term potentiation in the spinal cord dorsal horn. J. Neurophysiol. 2010;103(1):382–391. doi: 10.1152/jn.90906.2008. [http://dx.doi.org/10. 1152/jn.90906.2008]. [PMID: 19906875]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thiels E., Urban N.N., Gonzalez-Burgos G.R., Kanterewicz B.I., Barrionuevo G., Chu C.T., Oury T.D., Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. 2000;20(20):7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [PMID: 11027223]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Viggiano A., Serù R., Damiano S., De Luca B., Santillo M., Mondola P. Inhibition of long-term potentiation by CuZn superoxide dismutase injection in rat dentate gyrus: involvement of muscarinic M1 receptor. J. Cell. Physiol. 2012;227(8):3111–3115. doi: 10.1002/jcp.23062. [http://dx.doi.org/10.1002/jcp.23062]. [PMID: 22015651]. [DOI] [PubMed] [Google Scholar]
- 74.Marsden W.N. Stressor-induced NMDAR dysfunction as a unifying hypothesis for the aetiology, pathogenesis and comorbidity of clinical depression. Med. Hypotheses. 2011;77(4):508–528. doi: 10.1016/j.mehy.2011.06.021. [http://dx.doi.org/10.1016/j.mehy.2011.06.021]. [PMID: 21741771]. [DOI] [PubMed] [Google Scholar]
- 75.Markham A., Cameron I., Franklin P., Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur. J. Neurosci. 2004;20(5):1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [http://dx.doi.org/10.1111/j.1460-9568.2004.03578.x]. [PMID: 15341590]. [DOI] [PubMed] [Google Scholar]
- 76.Burkhalter J., Fiumelli H., Allaman I., Chatton J.Y., Martin J.L. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J. Neurosci. 2003;23(23):8212–8220. doi: 10.1523/JNEUROSCI.23-23-08212.2003. [PMID: 12967982]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong Y., Chai Y., Ding J.H., Sun X.L., Hu G. Chronic mild stress damages mitochondrial ultrastructure and function in mouse brain. Neurosci. Lett. 2011;488(1):76–80. doi: 10.1016/j.neulet.2010.11.006. [http://dx.doi.org/ 10.1016/j.neulet.2010.11.006]. [PMID: 21070835]. [DOI] [PubMed] [Google Scholar]
- 78.Rezin G.T., Cardoso M.R., Gonçalves C.L., Scaini G., Fraga D.B., Riegel R.E., Comim C.M., Quevedo J., Streck E.L. Inhibition of mitochondrial respiratory chain in brain of rats subjected to an experimental model of depression. Neurochem. Int. 2008;53(6-8):395–400. doi: 10.1016/j.neuint.2008.09.012. [http://dx.doi.org/10.1016/j.neuint.2008. 09.012]. [PMID: 18940214]. [DOI] [PubMed] [Google Scholar]
- 79.Gardner A., Boles R.G. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(3):730–743. doi: 10.1016/j.pnpbp.2010.07.030. [http:// dx.doi.org/10.1016/j.pnpbp.2010.07.030]. [PMID: 20691744]. [DOI] [PubMed] [Google Scholar]
- 80.Morava E., Gardeitchik T., Kozicz T., de Boer L., Koene S., de Vries M.C., McFarland R., Roobol T., Rodenburg R.J., Verhaak C.M. Depressive behaviour in children diagnosed with a mitochondrial disorder. Mitochondrion. 2010;10(5):528–533. doi: 10.1016/j.mito.2010.05.011. [http://dx.doi.org/10.1016/j.mito.2010.05.011]. [PMID: 20573558]. [DOI] [PubMed] [Google Scholar]
- 81.Baxter L.R., Jr, Schwartz J.M., Phelps M.E., Mazziotta J.C., Guze B.H., Selin C.E., Gerner R.H., Sumida R.M. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry. 1989;46(3):243–250. doi: 10.1001/archpsyc.1989.01810030049007. [http://dx.doi.org/10.1001/archpsyc.1989.01810030049007]. [PMID: 2784046]. [DOI] [PubMed] [Google Scholar]
- 82.Gardner A., Johansson A., Wibom R., Nennesmo I., von Döbeln U., Hagenfeldt L., Hällström T. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J. Affect. Disord. 2003;76(1-3):55–68. doi: 10.1016/s0165-0327(02)00067-8. [http://dx.doi.org/10.1016/S0165-0327(02)00067-8]. [PMID: 12943934]. [DOI] [PubMed] [Google Scholar]
- 83.Videbech P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr. Scand. 2000;101(1):11–20. doi: 10.1034/j.1600-0447.2000.101001011.x. [http://dx.doi.org/10.1034/ j.1600-0447.2000.101001011.x]. [PMID: 10674946]. [DOI] [PubMed] [Google Scholar]
- 84.Caspi A., Sugden K., Moffitt T.E., Taylor A., Craig I.W., Harrington H., McClay J., Mill J., Martin J., Braithwaite A., Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [http://dx.doi.org/10.1126/science.1083968]. [PMID: 12869766]. [DOI] [PubMed] [Google Scholar]
- 85.Rygula R., Abumaria N., Flügge G., Hiemke C., Fuchs E., Rüther E., Havemann-Reinecke U. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav. Pharmacol. 2006;17(1):19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [http://dx.doi.org/10.1097/01.fbp. 0000186631.53851.71]. [PMID: 16377960]. [DOI] [PubMed] [Google Scholar]
- 86.Drevets W.C., Price J.L., Furey M.L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 2008;213(1-2):93–118. doi: 10.1007/s00429-008-0189-x. [http://dx.doi.org/10.1007/s00429-008-0189-x]. [PMID: 18704495]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sacher J., Neumann J., Fünfstück T., Soliman A., Villringer A., Schroeter M.L. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J. Affect. Disord. 2012;140(2):142–148. doi: 10.1016/j.jad.2011.08.001. [http://dx.doi.org/10.1016/ j.jad.2011.08.001]. [PMID: 21890211]. [DOI] [PubMed] [Google Scholar]
- 88.Gillespie C.F., Nemeroff C.B. Hypercortisolemia and depression. Psychosom. Med. 2005;67(Suppl. 1):S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [http://dx.doi.org/ 10.1097/01.psy.0000163456.22154.d2]. [PMID: 15953796]. [DOI] [PubMed] [Google Scholar]
- 89.Pariante C.M., Miller A.H. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry. 2001;49(5):391–404. doi: 10.1016/s0006-3223(00)01088-x. [http://dx.doi.org/10.1016/ S0006-3223(00)01088-X]. [PMID: 11274650]. [DOI] [PubMed] [Google Scholar]
- 90.Duclos M., Gouarne C., Martin C., Rocher C., Mormède P., Letellier T. Effects of corticosterone on muscle mitochondria identifying different sensitivity to glucocorticoids in Lewis and Fischer rats. Am. J. Physiol. Endocrinol. Metab. 2004;286(2):E159–E167. doi: 10.1152/ajpendo.00281.2003. [http://dx.doi.org/10.1152/ajpendo.00281.2003]. [PMID: 12965871]. [DOI] [PubMed] [Google Scholar]
- 91.McEwen B.S. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54(5) Suppl. 1:20–23. doi: 10.1016/j.metabol.2005.01.008. [http://dx.doi.org/10.1016/j.metabol.2005.01.008]. [PMID: 15877308]. [DOI] [PubMed] [Google Scholar]
- 92.Maes M. Psychological stress and the inflammatory response system. Clin. Sci. 2001;101(2):193–194. [http://dx.doi.org/10. 1042/cs1010193]. [PMID: 11473495]. [PubMed] [Google Scholar]
- 93.Shintani F., Nakaki T., Kanba S., Sato K., Yagi G., Shiozawa M., Aiso S., Kato R., Asai M. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adreno- corticotropic hormone and in release of hypothalamic monoamines in the rat. J. Neurosci. 1995;15(3 Pt 1):1961–1970. doi: 10.1523/JNEUROSCI.15-03-01961.1995. [PMID: 7891145]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shizuya K., Komori T., Fujiwara R., Miyahara S., Ohmori M., Nomura J. The influence of restraint stress on the expression of mRNAs for IL-6 and the IL-6 receptor in the hypothalamus and midbrain of the rat. Life Sci. 1997;61(10):135–140. doi: 10.1016/s0024-3205(97)00608-5. [http://dx.doi.org/10.1016/S0024-3205(97)00608-5]. [DOI] [PubMed] [Google Scholar]
- 95.Samavati L., Lee I., Mathes I., Lottspeich F., Hüttemann M. Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J. Biol. Chem. 2008;283(30):21134–21144. doi: 10.1074/jbc.M801954200. [http://dx. doi.org/10.1074/jbc.M801954200]. [PMID: 18534980]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Behrens M.M., Ali S.S., Dugan L.L. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J. Neurosci. 2008;28(51):13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [http://dx.doi.org/10. 1523/JNEUROSCI.4457-08.2008]. [PMID: 19091984]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giuffrè A., Sarti P., D’Itri E., Buse G., Soulimane T., Brunori M. On the mechanism of inhibition of cytochrome c oxidase by nitric oxide. J. Biol. Chem. 1996;271(52):33404–33408. doi: 10.1074/jbc.271.52.33404. [http://dx.doi.org/10.1074/jbc.271.52.33404]. [PMID: 8969202]. [DOI] [PubMed] [Google Scholar]
- 98.Chen D.F., Schneider G.E., Martinou J.C., Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385(6615):434–439. doi: 10.1038/385434a0. [http://dx.doi.org/10.1038/ 385434a0]. [PMID: 9009190]. [DOI] [PubMed] [Google Scholar]
- 99.Jiao J., Huang X., Feit-Leithman R.A., Neve R.L., Snider W., Dartt D.A., Chen D.F. Bcl-2 enhances Ca(2+) signaling to support the intrinsic regenerative capacity of CNS axons. EMBO J. 2005;24(5):1068–1078. doi: 10.1038/sj.emboj.7600589. [http://dx.doi.org/10.1038/sj.emboj.7600589]. [PMID: 15719013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kosten T.A., Galloway M.P., Duman R.S., Russell D.S., D’Sa C. Repeated unpredictable stress and antidepressants differentially regulate expression of the bcl-2 family of apoptotic genes in rat cortical, hippocampal, and limbic brain structures. Neuropsychopharmacology. 2008;33(7):1545–1558. doi: 10.1038/sj.npp.1301527. [http://dx.doi. org/10.1038/sj.npp.1301527]. [PMID: 17700647]. [DOI] [PubMed] [Google Scholar]
- 101.Takayama S., Sato T., Krajewski S., Kochel K., Irie S., Millan J.A., Reed J.C. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell. 1995;80(2):279–284. doi: 10.1016/0092-8674(95)90410-7. [http://dx.doi.org/10.1016/0092-8674(95)90410-7]. [PMID: 7834747]. [DOI] [PubMed] [Google Scholar]
- 102.Kuromi H., Kidokoro Y. Exocytosis and endocytosis of synaptic vesicles and functional roles of vesicle pools: lessons from the Drosophila neuromuscular junction. Neuroscientist. 2005;11(2):138–147. doi: 10.1177/1073858404271679. [http://dx.doi.org/10.1177/1073858404271679]. [PMID: 15746382]. [DOI] [PubMed] [Google Scholar]
- 103.Ma H., Cai Q., Lu W., Sheng Z.H., Mochida S. KIF5B motor adaptor syntabulin maintains synaptic transmission in sympathetic neurons. J. Neurosci. 2009;29(41):13019–13029. doi: 10.1523/JNEUROSCI.2517-09.2009. [http://dx.doi. org/10.1523/JNEUROSCI.2517-09.2009]. [PMID: 19828815]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guo X., Macleod G.T., Wellington A., Hu F., Panchumarthi S., Schoenfield M., Marin L., Charlton M.P., Atwood H.L., Zinsmaier K.E. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47(3):379–393. doi: 10.1016/j.neuron.2005.06.027. [http://dx.doi.org/10.1016/j.neuron.2005.06.027]. [PMID: 16055062]. [DOI] [PubMed] [Google Scholar]
- 105.Verstreken P., Ly C.V., Venken K.J., Koh T.W., Zhou Y., Bellen H.J. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47(3):365–378. doi: 10.1016/j.neuron.2005.06.018. [http://dx.doi.org/10.1016/j.neuron. 2005.06.018]. [PMID: 16055061]. [DOI] [PubMed] [Google Scholar]
- 106.Eren I., Naziroğlu M., Demirdaş A. Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem. Res. 2007;32(7):1188–1195. doi: 10.1007/s11064-007-9289-x. [http://dx.doi.org/10.1007/s11064-007-9289-x]. [PMID: 17401662]. [DOI] [PubMed] [Google Scholar]
- 107.Eren I., Naziroğlu M., Demirdaş A., Celik O., Uğuz A.C., Altunbaşak A., Ozmen I., Uz E. Venlafaxine modulates depression-induced oxidative stress in brain and medulla of rat. Neurochem. Res. 2007;32(3):497–505. doi: 10.1007/s11064-006-9258-9. [http://dx.doi.org/10.1007/ s11064-006-9258-9]. [PMID: 17268845]. [DOI] [PubMed] [Google Scholar]
- 108.Zhang D., Wen X.S., Wang X.Y., Shi M., Zhao Y. Anti- depressant effect of Shudihuang on mice exposed to unpredictable chronic mild stress. J. Ethnopharmacol. 2009;123(1):55–60. doi: 10.1016/j.jep.2009.02.029. [http://dx.doi.org/10.1016/j.jep.2009.02.029]. [PMID: 19429340]. [DOI] [PubMed] [Google Scholar]
- 109.Tanaka M., Kovalenko S.A., Gong J.S., Borgeld H.J., Katsumata K., Hayakawa M., Yoneda M., Ozawa T. Accumulation of deletions and point mutations in mitochondrial genome in degenerative diseases. Ann. N. Y. Acad. Sci. 1996;786:102–111. doi: 10.1111/j.1749-6632.1996.tb39055.x. [http://dx.doi.org/10.1111/j.1749-6632.1996.tb39055.x]. [PMID: 8687011]. [DOI] [PubMed] [Google Scholar]
- 110.Hayashi M., Miyata R., Tanuma N. Oxidative stress in developmental brain disorders. Adv. Exp. Med. Biol. 2012;724:278–290. doi: 10.1007/978-1-4614-0653-2_21. [http://dx.doi.org/10.1007/978-1-4614-0653-2_21]. [PMID: 22411250]. [DOI] [PubMed] [Google Scholar]
- 111.Aw T.Y., Jones D.P. Nutrient supply and mitochondrial function. Annu. Rev. Nutr. 1989;9:229–251. doi: 10.1146/annurev.nu.09.070189.001305. [http://dx.doi.org/10.1146/ annurev.nu.09.070189.001305]. [PMID: 2669872]. [DOI] [PubMed] [Google Scholar]
- 112.Jensen J.E., Daniels M., Haws C., Bolo N.R., Lyoo I.K., Yoon S.J., Cohen B.M., Stoll A.L., Rusche J.R., Renshaw P.F. Triacetyluridine (TAU) decreases depressive symptoms and increases brain pH in bipolar patients. Exp. Clin. Psychopharmacol. 2008;16(3):199–206. doi: 10.1037/1064-1297.16.3.199. [http://dx.doi.org/10.1037/1064-1297.16.3.199]. [PMID: 18540779]. [DOI] [PubMed] [Google Scholar]