Abstract
Parkinson’s disease (PD) is a movement disorder and is associated with some of the intellectual disabilities like cognitive dysfunctions. PD associated cognitive dysfunctions have been proved well in both preclinical and clinical set ups. Like other neurodegenerative diseases, insults to mitochondria have a significant role in the pathobiology of PD associated dementia (PDD). Neurotoxins like MPTP, mutations of the mitochondrial genes, oxidative stress, imbalanced redox mechanisms and dysregulated mitochondrial dynamics have been implicated in mitochondrial dysfunctions and have paramount importance in the pathobiology of PDD. However, the extent of contribution of mitochondrial dysfunctions towards cognitive deficits in PD has not been characterized completely. In this review we highlight on the contribution of mitochondrial dysfunction to PDD. We also highlight different behavioural tests used in nonhuman primate and rodent models for assessing cognitive deficits and some common techniques for evaluation of mitochondrial dysfunction in PDD.
Keywords: Cognitive impairments, mitochondrial biogenesis, mitochondrial dysfunction, mitochondrial transcription factor A, neurodegeneration, Parkinson’s Disease
INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disease reported mainly in sporadic form though few cases are reported as familial form of the disease [1, 2]. A recent meta-analysis of the world-wide data indicated rising trend of occurrence of PD with age with an estimation of 41 subjects in the age groups of 40-49 to 1903 subjects with age more than 80 per 1,00,000 subjects [3]. Apart from intercontinental variations, there exists difference in the prevalence of PD- being more frequent in males than in females in age group of 50-59 years [3]. Among PD patients without dementia, mild cognitive impairment is common and is reported to be in about 20-50% of the cases [4]. On the other hand occurrence of PD associated dementia (PDD) is reported to increase proportionately with age, thus significantly increases morbidity and mortality and touches a cumulative incidence of 80-90% by the age of 90 [5-7].
PD involves progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta and affects movement, emotion, learning and cognitive processes [8, 9]. Out of the two forms of the disease i.e. sporadic and familial forms the former one occurs in about 95% of the cases where as only 5% of the cases have genetic linkage [10]. Mitochondrial complex I inhibition and alpha-synuclein aggregation cause loss of dopaminergic nerves and are the leading cause of sporadic form of the disease [11].
Mutations in genes like α-synuclein, LRRK2, Ubiquitin hydrolase and ligase 1, Parkin, DJ-1 (Parkinson’s Disease protein 7) and Phosphatase and tensin homologue-induced kinase (PINK 1) may present PD in autosomal dominant or autosomal recessive forms [12-14]. In PDD impairment in executive functions, visuospatial dysfunction, impaired verbal fluency, sleep disturbances and delay in retrieving stored information have been noticed [15-17]. PDD also involves cholinergic, noradrenergic and serotonergic deficits during the course of the disease [10, 18]. Underlying causes for selective neurodegeneration in SNpc is always a great area of research and over few decades there is huge unfolding of underlying molecular mechanisms for the same.
There is emerging evidence for link among energy metabolism, bioenergetics and cognitive processes in the brain [19]. Normal functioning of mitochondria is highly essential for energy balance and processing of cognitive processes in the neurons. At the same time there are ample evidences on the involvement of mitochondrial dysfunctions, dysregulated mitochondrial dynamics and mitophagy in PD [20-24]. Many underlying aetiopathological factors aggravate the neurodegenerative processes and influence PDD. Insults by toxic metabolites generated by the dopaminergic neurons [25], genetic mutations, increased susceptibility of neurons to reactive oxygen species (ROS) in PD [26] and defect in oxidative phosphorylation mechanisms [27] aggravates neuro-degeneration. In this context, importance of mitochondria in the neurodegeneration and cognitive processes seems paramount. In this review, we have tried to shed light on involvement of mitochondria in the pathogenesis of PD and associated cognitive impairments.
AETIOPATHOLOGY OF PD ASSOCIATED COGNITIVE IMPAIRMENTS
Mitochondrial Dysfunction in PDD
Imaging techniques like positron emission tomography and single photon emission computed tomography have made it easy to understand the distribution and processes that occur during neurodegeneration in PD [28-30]. As mentioned earlier, PDD involves neurological changes in both dopaminergic and cholinergic neurons. There is a plethora of literature on involvement of dopaminergic system in PD. It is a well-known fact that neurotoxins like MPTP, paraquat, rotenone cause dopaminergic neurodegeneration in substantia nigra. Apart from dopaminergic neurodegeneration in PD, cholinergic projections at basal forebrain, striatal cholinergic interneurons among other cholinergic innervations get affected [29]. Report of deposition of α-synuclein in the cholinergic neurons of basal forebrain, Lewy body formation in the substantia nigra [31] and loss of cholinergic neurons in nucleus basalis of Meynert in PD indicate involvement of cholinergic system in this disease [32, 33]. Cortical cholinergic deficits [34] and improvement of cognitive functions in PD subjects treated with cholinesterase inhibitors [35] confirm definitive role of cholinergic system in PDD.
Inside the neuron, mitochondrion is one of the organelles that is possibly worst affected in PD. These are referred to as “powerhouse” of the cell as they are the main source of chemical energy generated in the form of ATP through aerobic respiration. In addition to their well-known role in oxidative phosphorylation and metabolism, they play central role in various physiological functions like apoptosis, cell differentiation, innate immunity, oxygen sensing and detoxification of reactive-oxygen species, regulation of cytoplasmic and mitochondrial matrix calcium and maintenance of cell quality [36, 37]. Certain mitochondrial proteins and transcription factors play crucial role in proper functioning of mitochondria. To mention few are mitochondrial transcription factor A (mtTFA/TFAM), peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α), nuclear respiratory factory factor (NRF) 1 and NRF-2, uncoupling proteins (UCPs), members of respiratory chain NADH dehydrogenase [ubiquinone] iron-sulfur protein 4 (NDUFS4), succinate dehydrogenase complex, subunit A, flavoprotein (Fp) (SDHA), cytochrome b among others. mtTFA/TFAM play crucial role in mitochondrial transcription and biogenesis [38, 39]. PGC-1α plays a significant role in mitochondrial biogenesis, respiration and imparting protection against the ROS [40, 41]. NRF-1 and NRF-2 are important transcription factors in the biogenesis of mitochondria [42].
Certain UCPs regulate free radical flux from mitochondria by physiologically modulating mitochondrial membrane potential in the brain [43]. Members of respiratory chain NDUFS4, SDHA, Cytochrome b have role in oxidative phosphorylation [44]. Abnormality in any of these processes can have dire consequences and results in diverse human pathologies among which neurodegeneration is of primary concern.
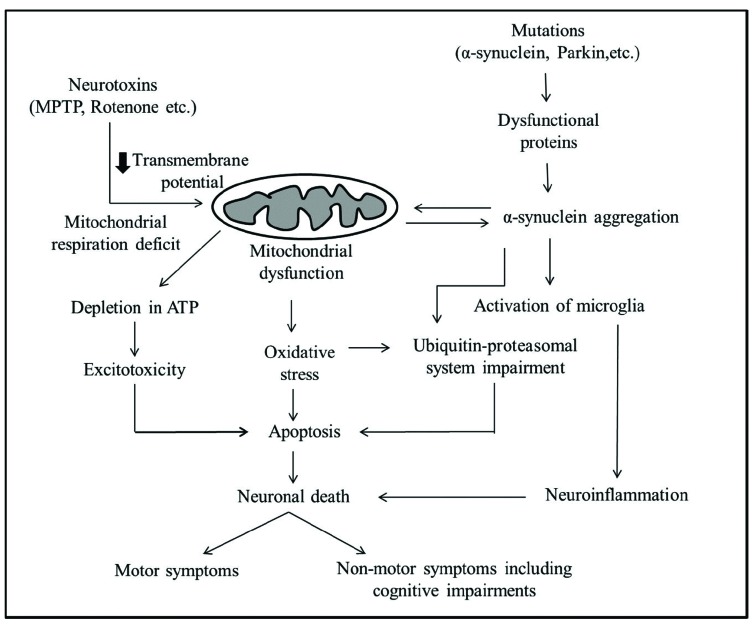
Mitochondrial dysfunction may happen due to certain neurotoxins or mutations in the nuclear as well as mitochondrial DNA (Fig. 1). For example, 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) inhibition of complex I in mitochondria leads to depletion of ATP and excitotoxicity. Decreased intracellular ATP by Complex I inhibition causes partial neuronal depolarisation, due to a reduction in the activity of Na+/K+-ATPase that acts to maintain the resting membrane potential of the cell. With a decreased ATP level in PD, this could impact depolarisation which succeeds with NMDA receptor mediated excitotoxicity that could lead to neuronal death [45]. Increased oxidative stress and generation of free radicals intoxicate the neurons and cause neuronal death. Aggregation of toxic α-synuclein and failure of ubiquitin-proteasomal system may also contribute for the demise of the neurons.
Fig. (1).
Neurotoxins like MPTP and mutation in mitochondrial genes cause mitochondrial dysfunction. Multiple intermediate events like decrease in ATP production, neuronal depolarization and excitotoxicity, oxidative stress and failure of ubiquitin-proteasomal system all converge to apoptosis. Aggregation of toxic α-synuclein may cause mitochondrial dysfunction and vice versa. Further, aggregated α-synuclein may activate microglia which can lead to neuroinflammation and subsequently demise of the neurons. Neuronal death may ultimately lead to motor and non-motor deficits in PD.
PD complications are broadly classified into motor and nonmotor symptoms (NMS) during the course of the disease progression. While motor symptoms in PD arise mainly due dopaminergic neurodegeneration, other systems and symptoms and processes in the brain are involved for cognitive impairments [46]. PDD comes in the domain of NMS which may often arise earlier to the motor dysfunctions [47, 48]. Dopaminergic neuronal death in SNPc mainly causes motor symptoms. Cognitive impairment in PD mainly affects the executive functions like attention, planning, working memory, visual perception, object recognition and learning [46, 49]. Neurons of hippocampus, amygdala, prefrontal cortex, locus coeruleus, the raphe nuclei, cholinergic complex in basal forebrain are involved in cognition decline and were observed in different studies [6, 49].
Preclinical and Clinical Evidences for Mitochondrial Dysfunctions in PDD
With all the evidences converging into a point that there is involvement of mitochondrial dysfunction in PD; it would now be interesting to find a relationship between mitochondrial dysfunction and PD-associated cognitive imparments. During our literature search we found some useful preclinical and clinical information on the involvement of mitochondria in PDD which we have summarized in Table 1.
Table 1.
Mitochondrial dysfunctions: preclinical and clinical implications in PD.
| Sl no | Preclinical/clinical set up | Observation | Refs. |
|---|---|---|---|
| 1 | Transmitochondrial mice (Mito-mice) with heteroplasmy for wild-type and pathogenically deleted mtDNA. |
The group having >50% of pathogenically deleted mtDNA showed severe impairment in retention and consolidation of memory along with mitochondrial respiration deficiencies in the visual cortex and dentate gyrus. | [50] |
| 2 | Analysis of nucleotide sequences of mitochondrial DNA in the brains of patients with idiopathic PD | Several point mutations observed in the brain samples. It was speculated that these mutations could lead to increased production of oxygen radicals or could increase susceptibility of the respiratory chain components to oxidative damage. | [51] |
| 3 | Immunohistochemical analysis of complex I-IV of electron transport system in PD patients | Reduced staining against the complex I antibody was observed in the fair proportion of the nigral neurons. | [52] |
| 4 | Substantia nigra neurons from both aged controls and individuals with PD. | High levels of deleted mtDNA were observed in both groups and were associated with respiratory chain deficiency. | [53] |
| 5 | Prospective evaluation of low frequency, amino acid changing, heteroplasmic mutations in a narrow region of ND5 (mitochondrial gene encoding a complex I subunit) in brain tissue from PD and controls. | Heteroplasmic mutations in a specific region of ND5 largely segregated PD from controls. |
[54] |
Preclinical Assessment of Cognitive Impairments
PDD is well demonstrated in nonhuman primates and rodent models of PD. A variety of these animal models help us to understand the cellular mechanisms underlying associated cognitive impairments in PD. Nonhuman primates like cyanomologus monkey, rhesus monkey and rodents like rats and mice are most commonly used animals for assessment of behavioural parameters in PDD. Some common behavioural tests and their principles are described in Table 2.
Table 2.
Preclinical assessment of cognitive impairments-Behavioural tests.
| Sl no | Animal Model | Test | Basic Principle and End Point | Refs. |
|---|---|---|---|---|
| 1 | Nonhuman primates (Monkey) |
Variable Delayed response (VDR) | ▪ Choosing ability of the monkey to uncover the food in the hidden well. ▪ Assessment of short-term spatial memory. |
[56] |
| Modified delayed response | ▪ A variant of VDR test with attentional cueing. ▪ Assessment of attention. |
[57] | ||
| Object detour retrieval | ▪ Test of detour ability of monkeys to get food in a transparent chamber open through one random side. ▪ Measures perseverative responses and problem solving skills through reasoning. |
[49, 58] | ||
| 2 | Rodents (Rat/Mice) |
Passive avoidance | ▪ Tests the latency to enter into the darker compartment. ▪ Emotional memory is evaluated based on fear conditioning learning. |
[59-61] |
| Active avoidance | ▪ Tests the number of correct responses to the cue presentation. ▪ Assessment of associative and emotional learning. |
[49, 62] | ||
| Morris water maze | ▪ Based on latency and number of entries into the hidden platform zone. ▪ Assessment of spatial memory. |
[63, 64] | ||
| Operant task conditioning | ▪ Animals trained with an operant behavior perform assigned tasks to get reward or avoid punishment. ▪ Assessment of incentive learning. |
[65, 66] | ||
| Novel object recognition and Spatial object recognition | ▪ Based on the calculation of percentage of time spent exploring the novel (NOR)/relocated object (SOR). ▪ Assessment of attention, recognition and spatial memory. |
[67-70] | ||
| Y-maze/T-maze | ▪ Determination of spontaneous alterations. ▪ Assessment of reference and working memory. |
[71-73] | ||
| Attentional set-shifting | ▪ Measures prefrontal cortex mediated cognitive flexibility. | [74] |
ASSESSMENT OF MITOCHONDRIAL DYSFUNCTIONS
Beyond doubt, mitochondria take center stage in the pathology of PDD. Hence assessing the mitochondrial dysfunction has its own importance unraveling molecular aspects of the disease. It may further help in digging out the underpinning pathological events and for designing newer therapeutic targets for PD as well as other neurodegenerative diseases. Usually mitochondria produce ATP in response to the physiological demands. Mitochondrial dysfunction occurs with disrupted energy metabolism which includes damage to electron transport chain, decreased oxygen consumption rate, defective ATP synthesis, dysregulated calcium homeostasis and exaggerated free radical production due to loss of antioxidant defenses all of which may culminate to apoptosis [55]. Therefore to know about the mitochondrial dysfunction; assessment need to be done to evaluate the disruption of energy metabolism, free radical production and apoptosis induction. Assessment can be done with various techniques which involve intact cells or isolated mitochondria- both methods possessing certain advantages and disadvantages as well [37]. In subsequent section various screening and mechanistic assays have been described.
Screening assays may include measurement of oxidative phosphorylation (OXPHOS) activity, ATP production, oxygen consumption rate, mitochondrial membrane potential and ROS production. Mechanistic assays may include protein expression studies for mitochondrial biogenesis, quality control, estimation of antioxidant levels and also levels of apoptosis inducing markers. To assess the mitochondrial dysfunction in tissues, mitochondria need to be isolated and suspended in respiratory medium for assays [75]. In common practice for isolation of mitochondria from the tissue; it (tissue) has to be isolated, chopped and homogenized with specific buffer followed by multiple centrifugation and reconstitution steps (See protocol by Wieckowski et al. for details) [76].
Measurement of OXPHOS Activity
All the assays to screen the activity of ETC complexes (I-IV) can be performed by manual method using spectrophotometer [77] or directly with commercial respirometers. Complex I (NADH: Ubiquinone Oxidoreductase) activity can be measured by following the decrease in absorbance due to the oxidation of NADH at 340 nm [78, 79]. Complex II (Succinate: Ubiquinone Oxidoreductase) activity is measured by estimating decreased absorbance resulting from the reduction of 2,6-dichlorophenolindophenol at 600 nm [80]. Similarly, complex III (Ubiquinol: ferricytochrome c oxidoreductase) activities are measured by monitoring the reduction of cytochrome c (III) at 550 nm and complex IV (cytochrome c Oxidase) activity is measured by following the oxidation of reduced cytochrome c at 550 nm [80].
Measurement of ATP Production
Mitochondrial fraction can be assayed for basal and stimulated production of ATP using biochemiluminiscent method following the principle shown below. The assay uses luciferase to catalyze the reaction [81],
ATP + D-Luciferin + O2 → Oxyluciferin + AMP + PPi + Light.
When ATP is the limiting component in the luciferase reaction, the intensity of the emitted luminescence light is proportional to concentration of ATP. Measurement of the light intensity using a luminometer gives direct quantitation of ATP [81].
Measurement of Oxygen Consumption Rate (OCR)
OCR can be measured in cells using commercial oxygen flux analyzers or respirometers directly. But in vivo the isolated mitochondrial is used to measure the OCR by phosphorescent oxygen-sensitive probe under basal and stimulated conditions with substrate and ADP supplements [82, 83]. Measurement of OCR complements assays of ATP production, extent of uncoupling, membrane potential [84].
Measurement of Mitochondrial Membrane Potential
Mitochondrial dysfunction leads to loss of inner membrane potential that indicates the susceptibility of cell to apoptosis. The integrity of the inner mitochondrial membrane may be measured by observing the potential gradient over this membrane which can be achieved by measuring the uptake of the cationic carbocyanine dye JC-1 (5,5’,6,6’-tetrachloro-1,1’,3,3’-tetra ethyl benzimidazol carbocyanine iodide) into the mitochondrial matrix [85]. It can be determined in isolated mitochondria from the tissues by measuring the uptake of JC-1 with formation of the J-aggregates with time using a kinetic programme [86]. If there is a loss in mitochondrial membrane potential, a drastic decrease in the JC-1 fluorescence is observed [87]. Other dyes like Tetramethylrhodamine, methyl ester (TMRM) is also used to distinguish between apoptotic cells from non-apoptotic cells (with polarized mitochondria). TMRM fluoresces bright red (em max: 573 nm) in non-apoptotic cells and shows diminished fluorescence or no signal in cells having apoptosis [88-90].
Measurement of Free Radical Generation
The main source of cellular free radical generation is mitochondrial electron transport chain which aggravates when there is damage to the complexes [91]. An increased free radical generation indicates mitochondrial dysfunction and can be measured by dye based assays of superoxide generation. Mitosox red is a mitochondrial superoxide indicator, a novel fluorogenic dye used for highly selective detection of superoxide in the mitochondria of live cells [92]. It is live-cell permeant and is rapidly and selectively targeted to the mitochondria. Once in the mitochondria, reagent is oxidized by superoxide and exhibits red fluorescence whose intensity is directly proportional to the extent of superoxide generation [92]. Amplex Red, a non-fluorescent substrate in conjunction with horseradish peroxidase (HRP) is used to estimate H2O2 efflux from mitochondria. The fluorescent product, resorufin formed during the reaction is measured [93, 94]. Similarly mitochondrial nitric oxide can be detected by using 4,5-Diaminofluorescein diacetate [95, 96].
Measurement of Mitochondrial Lipid Peroxidation Levels
Cardiolipin is the primary lipid molecule of inner mitochondrial membrane which helps in maintaining the structural integrity of mitochondria [97]. Free radical-induced damage can cause membrane peroxidation resulting in formation or opening of the transition pore that initiates apoptosis. 10-N-nonyl acridine orange (NAO) is a fluorescent probe which binds to the cardiolipin selectively and produces fluorescence [98]. Decrease in the fluorescence intensity is directly proportional to the extent of mitochondrial lipid peroxidation.
Estimation of Citrate Synthase Levels
Citrate synthase is a mitochondrial marker enzyme which regulates the production of citric acid in citric acid cycle. Its level directly indicates the quantity of mitochondria. It is measured spectrophotometrically based on absorption at 412 nm by its product thionitrobenzoic acid (TNB) [99]. TNB is formed when a saturating concentration of acetyl CoA, oxaloacetic acid and dithionitrobenzoic acid reacts in the presence of the enzyme [99].
Estimation of Mitochondrial Antioxidant Levels
Levels of the mitochondrial non-enzymatic antioxidant, reduced glutathione (GSH) and enzymatic antioxidants glutathione reductase (GR), glutathione-s-transferase (GST), catalase (CAT) and superoxide dismutase (SOD) in mitochondrial fraction of tissue homogenates can be analyzed to estimate the extent of loss of antioxidant defense that indicate mitochondrial dysfunction. SOD activity can be measured by biochemical method, in which xanthine–xanthine oxidase (XO) is used to generate O2•− and reduction of nitro blue tetrazolium (NBT) is used as an indicator of O2•− production [100]. SOD and NBT compete for O2•− and the percentage inhibition of NBT reduction indicates of the amount of SOD present in the sample [100]. Catalase can be measured spectrophotometrically by measuring peroxide removal. The rate of removal of peroxide by catalase is exponential. A decrease in the absorbance of hydrogen peroxide is directly proportional to the levels of catalase [100]. Glutathione peroxidase activity is measured by Tert-butyl and cumene hydroperoxides [100, 101]. Levels of GSH can be estimated by measuring the reduction of the DTNB reagent at 412 nm. Among these antioxidant enzymes SOD and catalase can also be estimated by protein expression studies like western blotting or immunohistochemistry [100].
Other Mechanistic Approaches
Mechanistic investigation mainly includes assessment of mitochondrial biogenesis, quality control and apoptosis levels through protein expression studies like western blotting and immunohistochemistry. They can also be estimated using PCR and RT-PCR techniques [102]. Estimation of the levels of mitochondrial proteins like PGC-1α, NRF-1, NRF-2 and mtTFA helps to assess mitochondrial biogenesis under both physiological and pathological conditions. Estimating the levels of proteins like dynamin-related protein 1, Mfn1/2, Tim/Tom, PINK1, PARKIN, Beclin etc. can also yield valuable information about mitochondrial dynamics and mitophagy processes. Estimating the levels of proteins like PARP, Caspases, Cyt c, Bcl-2, Bax etc. indicates the levels of apoptosis induction at different stages of neurodegeneration.
Treatment Strategies
Main therapeutic targets for the symptomatic relief in PD are mainly dopaminergic drugs like dopamine agonists, dopamine precursors, dopa decaboxylase inhibitors, catechol-O-methyl transferase inhibitors and MAO-B inhibitors. However, anticholinergics like trihexyphenidyl and antiglutamatergics like amantadine are also used [103]. Different formulations of ropinirole, pramipexole, rotigotine are used during initial phase but can also be used along with levodopa or carbidopa during later phase of the therapy. Selegiline and Rasagiline are used as MAO-B inhibitors for symptomatic relief in PD. Combination of Levodopa, a dopadecarboxylase inhibitor (e.g. carbidopa, benserazide etc.) and a catechol-O-methyl transferase inhibitor like entacapone, tolcapone are also used. The routes of administration of these drugs vary. Most drugs are given orally but certain other routes of administration like transdermal (e.g. rotigotine), intraduodenal (e.g. infusion of leveodopa) [104] are used for continuous delivery therapies [103]. Adenosine receptor antagonists, Coenzyme Q10, creatine, Isradipine, protein aggregation inhibitors, stem cell therapy and gene therapy are under preclinical and clinical investigations in PD [103].
None of the current PD therapies is found to significantly improve cognitive symptoms without any adverse effects. Antiparkinsonian drug levodopa is reported to benefit on spatial working memory but in chronic use it is associated with abnormal movements, visual hallucinations, psychosis and fluctuation in motor performance [105-107]. Rivastigmine is the only drug approved by US FDA for PDD treatment but it is associated with side effects like nausea, vomiting, tremor, diarrhea, anorexia, and dizziness [108-110]. Donepezil, a cholinesterase inhibitor and Memantine, an NMDA receptor antagonist used in AD but are yet to get recommendation for PDD [109]. Thus treatment of PDD is an unmet medical need and that should be addressed with newer and alternate therapeutics.
CONCLUSION AND FUTURE PERSPECTIVES
Being a less addressed concept, treatment of PDD remains an unmet medical need. The preclinical studies done in different animal models for PDD have proven the involvement of mitochondria in the disease pathology. Beyond doubt, mithochondrial dysfunction is one of the underlying causes in the cognitive impairment process in PD and with a proper animal model one can assess the cognitive impairment process. Further, evaluation of mitochondrial dysfunction with different available molecular techniques could give better insight in understanding the process and could help develop new chemical entities targeting mitochondria not only in PDD but also for other neuro- degenerative diseases.
ACKNOWLEDGEMENTs
NRD was working as Senior Research Fellow in DST, New Delhi project # SR/CSI/49/2009 while writing the manuscript.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Celardo I., Martins L.M., Gandhi S. Unravelling mitochondrial pathways to Parkinson’s disease. Br. J. Pharmacol. 2014;171(8):1943–1957. doi: 10.1111/bph.12433. [http://dx.doi.org/10.1111/bph.12433]. [PMID: 24117181]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uppalapati D, Das NR, Gangwal RP, Damre MV, Sangamwar A. SS Neuroprotective Potential of Peroxisome Proliferator Activated receptor-α (PPAR-α) agonist in cognitive impairment in Parkinson’s disease: Behavioral, biochemical and PBPK profile. PPAR Res. 2014;2014 doi: 10.1155/2014/753587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pringsheim T., Jette N., Frolkis A., Steeves T.D. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 2014;29(13):1583–1590. doi: 10.1002/mds.25945. [http://dx.doi.org/10.1002/ mds.25945]. [PMID: 24976103]. [DOI] [PubMed] [Google Scholar]
- 4.Goldman J.G., Litvan I. Mild cognitive impairment in Parkinson’s disease. Minerva Med. 2011;102(6):441–459. [PMID: 22193376]. [PMC free article] [PubMed] [Google Scholar]
- 5.Buter T.C., van den Hout A., Matthews F.E., Larsen J.P., Brayne C., Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008;70(13):1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [http://dx.doi.org/10.1212/01.wnl.0000306632.43729. 24]. [PMID: 18362281]. [DOI] [PubMed] [Google Scholar]
- 6.Gratwicke J., Jahanshahi M., Foltynie T. Parkinson’s disease dementia: a neural networks perspective. Brain. 2015;138(Pt 6):1454–1476. doi: 10.1093/brain/awv104. [http://dx.doi.org/10.1093/brain/awv104]. [PMID: 25888551]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy G., Tang M-X., Louis E.D., Côté L.J., Alfaro B., Mejia H., Stern Y., Marder K. The association of incident dementia with mortality in PD. Neurology. 2002;59(11):1708–1713. doi: 10.1212/01.wnl.0000036610.36834.e0. [http://dx. doi.org/10.1212/01.WNL.0000036610.36834.E0]. [PMID: 12473757]. [DOI] [PubMed] [Google Scholar]
- 8.Barzilai A., Melamed E. Molecular mechanisms of selective dopaminergic neuronal death in Parkinson’s disease. Trends Mol. Med. 2003;9(3):126–132. doi: 10.1016/s1471-4914(03)00020-0. [http://dx.doi.org/10.1016/S1471-4914 (03)00020-0]. [PMID: 12657434]. [DOI] [PubMed] [Google Scholar]
- 9.Eriksen N., Stark A.K., Pakkenberg B. Age and Parkinson’s disease-related neuronal death in the substantia nigra pars compacta. Springer; 2009. [DOI] [PubMed] [Google Scholar]
- 10.Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [http://dx.doi.org/10.1016/ S0896-6273(03)00568-3]. [PMID: 12971891]. [DOI] [PubMed] [Google Scholar]
- 11.Dawson T.M., Dawson V.L. Molecular pathways of neuro- degeneration in Parkinson’s disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [http://dx.doi.org/10.1126/science.1087753]. [PMID: 14593166]. [DOI] [PubMed] [Google Scholar]
- 12.Klein C., Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2(1):a008888. doi: 10.1101/cshperspect.a008888. [http://dx.doi. org/10.1101/cshperspect.a008888]. [PMID: 22315721]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singleton A.B., Farrer M.J., Bonifati V. The genetics of Parkinson’s disease: progress and therapeutic implications. Mov. Disord. 2013;28(1):14–23. doi: 10.1002/mds.25249. [http://dx.doi.org/10.1002/mds.25249]. [PMID: 23389780]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood-Kaczmar A., Gandhi S., Wood N.W. Understanding the molecular causes of Parkinson’s disease. Trends Mol. Med. 2006;12(11):521–528. doi: 10.1016/j.molmed.2006.09.007. [http://dx.doi.org/10.1016/j.molmed.2006.09.007]. [PMID: 17027339]. [DOI] [PubMed] [Google Scholar]
- 15.Bronnick K., Emre M., Lane R., Tekin S., Aarsland D. Profile of cognitive impairment in dementia associated with Parkinson’s disease compared with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2007;78(10):1064–1068. doi: 10.1136/jnnp.2006.108076. [http://dx.doi.org/10.1136/ jnnp.2006.108076]. [PMID: 17287236]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emre M., Aarsland D., Brown R., Burn D.J., Duyckaerts C., Mizuno Y., Broe G.A., Cummings J., Dickson D.W., Gauthier S., Goldman J., Goetz C., Korczyn A., Lees A., Levy R., Litvan I., McKeith I., Olanow W., Poewe W., Quinn N., Sampaio C., Tolosa E., Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [http://dx.doi.org/10.1002/mds.21507]. [PMID: 17542011]. [DOI] [PubMed] [Google Scholar]
- 17.Kumar P., Kaundal R.K., More S., Sharma S.S. Beneficial effects of pioglitazone on cognitive impairment in MPTP model of Parkinson’s disease. Behav. Brain Res. 2009;197(2):398–403. doi: 10.1016/j.bbr.2008.10.010. [http://dx.doi.org/10.1016/j.bbr.2008.10.010]. [PMID: 18983875]. [DOI] [PubMed] [Google Scholar]
- 18.Williams-Gray C.H., Foltynie T., Lewis S.J., Barker R.A. Cognitive deficits and psychosis in Parkinson’s disease: a review of pathophysiology and therapeutic options. CNS Drugs. 2006;20(6):477–505. doi: 10.2165/00023210-200620060-00004. [http://dx.doi.org/10.2165/00023210-200620060-00004]. [PMID: 16734499]. [DOI] [PubMed] [Google Scholar]
- 19.Kapogiannis D., Mattson M.P. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10(2):187–198. doi: 10.1016/S1474-4422(10)70277-5. [http:// dx.doi.org/10.1016/S1474-4422(10)70277-5]. [PMID: 21147038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imai Y., Lu B. Mitochondrial dynamics and mitophagy in Parkinson’s disease: disordered cellular power plant becomes a big deal in a major movement disorder. Curr. Opin. Neurobiol. 2011;21(6):935–941. doi: 10.1016/j.conb.2011.10.016. [http://dx.doi.org/10.1016/j.conb.2011.10.016]. [PMID: 22048001]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johri A., Beal M.F. Mitochondrial dysfunction in neuro- degenerative diseases. J. Pharmacol. Exp. Ther. 2012;342(3):619–630. doi: 10.1124/jpet.112.192138. [http://dx.doi.org/10.1124/jpet.112.192138]. [PMID: 22700435]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandemakers W., Morais V.A., De Strooper B. A cell biological perspective on mitochondrial dysfunction in Parkinson disease and other neurodegenerative diseases. J. Cell Sci. 2007;120(Pt 10):1707–1716. doi: 10.1242/jcs.03443. [http://dx.doi.org/10.1242/jcs.03443]. [PMID: 17502481]. [DOI] [PubMed] [Google Scholar]
- 23.Van Laar V.S., Berman S.B. Mitochondrial dynamics in Parkinson’s disease. Exp. Neurol. 2009;218(2):247–256. doi: 10.1016/j.expneurol.2009.03.019. [http:// dx.doi.org/10.1016/j.expneurol.2009.03.019]. [PMID: 19332061]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Laar V.S., Berman S.B. The interplay of neuronal mitochondrial dynamics and bioenergetics: implications for Parkinson’s disease. Neurobiol. Dis. 2013;51:43–55. doi: 10.1016/j.nbd.2012.05.015. [http://dx. doi.org/10.1016/j.nbd.2012.05.015]. [PMID: 22668779]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hameed S., Hsiung G-Y. The role of mitochondria in aging, neurodegenerative disease, and future therapeutic options. B. C. Med. J. 2011;53(4):188–192. [Google Scholar]
- 26.Taira T., Saito Y., Niki T., Iguchi-Ariga S.M., Takahashi K., Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5(2):213–218. doi: 10.1038/sj.embor.7400074. [http://dx.doi.org/ 10.1038/sj.embor.7400074]. [PMID: 14749723]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoffner J.M., Watts R.L., Juncos J.L., Torroni A., Wallace D.C. Mitochondrial oxidative phosphorylation defects in Parkinson’s disease. Ann. Neurol. 1991;30(3):332–339. doi: 10.1002/ana.410300304. [http://dx.doi.org/ 10.1002/ana.410300304]. [PMID: 1952821]. [DOI] [PubMed] [Google Scholar]
- 28.Antonini A., Vontobel P., Psylla M., Günther I., Maguire P.R., Missimer J., Leenders K.L. Complementary positron emission tomographic studies of the striatal dopaminergic system in Parkinson’s disease. Arch. Neurol. 1995;52(12):1183–1190. doi: 10.1001/archneur.1995.00540360061017. [http://dx.doi.org/10.1001/archneur.1995.00540360061017]. [PMID: 7492293]. [DOI] [PubMed] [Google Scholar]
- 29.Bohnen N.I., Albin R.L. The cholinergic system and Parkinson disease. Behav. Brain Res. 2011;221(2):564–573. doi: 10.1016/j.bbr.2009.12.048. [http://dx. doi.org/10.1016/j.bbr.2009.12.048]. [PMID: 20060022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brücke T., Djamshidian S., Bencsits G., Pirker W., Asenbaum S., Podreka I. SPECT and PET imaging of the dopaminergic system in Parkinson’s disease. J. Neurol. 2000;247(4) doi: 10.1007/pl00007769. [DOI] [PubMed] [Google Scholar]
- 31.Braak H., Del Tredici K., Rüb U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [http://dx.doi.org/10.1016/S0197-4580(02)00065-9]. [PMID: 12498954]. [DOI] [PubMed] [Google Scholar]
- 32.Liu A.K., Chang R.C., Pearce R.K., Gentleman S.M. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015;129(4):527–540. doi: 10.1007/s00401-015-1392-5. [http://dx.doi.org/10.1007/ s00401-015-1392-5]. [PMID: 25633602]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehouse P.J., Hedreen J.C., White C.L., III, Price D.L. Basal forebrain neurons in the dementia of Parkinson disease. Ann. Neurol. 1983;13(3):243–248. doi: 10.1002/ana.410130304. [http://dx.doi.org/10.1002/ana. 410130304]. [PMID: 6847136]. [DOI] [PubMed] [Google Scholar]
- 34.Bohnen N.I., Kaufer D.I., Hendrickson R., Ivanco L.S., Lopresti B.J., Constantine G.M., Mathis ChA., Davis J.G., Moore R.Y., Dekosky S.T. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. J. Neurol. 2006;253(2):242–247. doi: 10.1007/s00415-005-0971-0. [http://dx.doi.org/10.1007/s00415-005-0971-0]. [PMID: 16133720]. [DOI] [PubMed] [Google Scholar]
- 35.Aarsland D., Mosimann U.P., McKeith I.G. Role of cholinesterase inhibitors in Parkinson’s disease and dementia with Lewy bodies. J. Geriatr. Psychiatry Neurol. 2004;17(3):164–171. doi: 10.1177/0891988704267463. [http://dx.doi.org/10.1177/0891988704267463]. [PMID: 15312280]. [DOI] [PubMed] [Google Scholar]
- 36.Smith R.A., Hartley R.C., Cochemé H.M., Murphy M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012;33(6):341–352. doi: 10.1016/j.tips.2012.03.010. [http://dx.doi.org/10.1016/j.tips.2012.03.010]. [PMID: 22521106]. [DOI] [PubMed] [Google Scholar]
- 37.Brand M.D., Nicholls D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435(2):297–312. doi: 10.1042/BJ20110162. [http://dx.doi.org/ 10.1042/BJ20110162]. [PMID: 21726199]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekstrand M.I., Falkenberg M., Rantanen A., Park C.B., Gaspari M., Hultenby K., Rustin P., Gustafsson C.M., Larsson N.G. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004;13(9):935–944. doi: 10.1093/hmg/ddh109. [http://dx.doi.org/10.1093/hmg/ddh109]. [PMID: 15016765]. [DOI] [PubMed] [Google Scholar]
- 39.Virbasius J.V., Scarpulla R.C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci. USA. 1994;91(4):1309–1313. doi: 10.1073/pnas.91.4.1309. [http://dx.doi.org/ 10.1073/pnas.91.4.1309]. [PMID: 8108407]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Handschin C. The biology of PGC-1α and its therapeutic potential. Trends Pharmacol. Sci. 2009;30(6):322–329. doi: 10.1016/j.tips.2009.03.006. [http://dx.doi.org/ 10.1016/j.tips.2009.03.006]. [PMID: 19446346]. [DOI] [PubMed] [Google Scholar]
- 41.St-Pierre J., Drori S., Uldry M., Silvaggi J.M., Rhee J., Jäger S., Handschin C., Zheng K., Lin J., Yang W., Simon D.K., Bachoo R., Spiegelman B.M. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [http://dx.doi.org/ 10.1016/j.cell.2006.09.024]. [PMID: 17055439]. [DOI] [PubMed] [Google Scholar]
- 42.Escrivá H., Rodríguez-Peña A., Vallejo C.G. Expression of mitochondrial genes and of the transcription factors involved in the biogenesis of mitochondria Tfam, NRF-1 and NRF-2, in rat liver, testis and brain. Biochimie. 1999;81(10):965–971. doi: 10.1016/s0300-9084(99)00223-0. [http://dx.doi. org/10.1016/S0300-9084(99)00223-0]. [PMID: 10575350]. [DOI] [PubMed] [Google Scholar]
- 43.Kim-Han J.S., Dugan L.L. Mitochondrial uncoupling proteins in the central nervous system. Antioxid. Redox Signal. 2005;7(9-10):1173–1181. doi: 10.1089/ars.2005.7.1173. [http://dx.doi.org/10.1089/ars.2005.7.1173]. [PMID: 16115020]. [DOI] [PubMed] [Google Scholar]
- 44.Håkansson J., Eliasson B., Smith U., Enerbäck S. Adipocyte mitochondrial genes and the forkhead factor FOXC2 are decreased in type 2 diabetes patients and normalized in response to rosiglitazone. Diabetol. Metab. Syndr. 2011;3(3):32. doi: 10.1186/1758-5996-3-32. [http://dx. doi.org/10.1186/1758-5996-3-32]. [PMID: 22098677]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keane P, Kurzawa M, Blain P, Morris C. Mitochondrial dysfunction in Parkinson’s disease. Parkinson’s Disease. 2011 doi: 10.4061/2011/716871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kehagia A.A., Barker R.A., Robbins T.W. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9(12):1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [http://dx.doi.org/10.1016/S1474-4422(10)70212-X]. [PMID: 20880750]. [DOI] [PubMed] [Google Scholar]
- 47.Poewe W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008;15(s1) Suppl. 1:14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [http://dx.doi.org/10.1111/ j.1468-1331.2008.02056.x]. [PMID: 18353132]. [DOI] [PubMed] [Google Scholar]
- 48.Tolosa E., Gaig C., Santamaría J., Compta Y. Diagnosis and the premotor phase of Parkinson disease. Neurology. 2009;72(7) Suppl.:S12–S20. doi: 10.1212/WNL.0b013e318198db11. [http://dx.doi.org/10.1212/WNL.0b013e318198db11]. [PMID: 19221308]. [DOI] [PubMed] [Google Scholar]
- 49.Solari N., Bonito-Oliva A., Fisone G., Brambilla R. Understanding cognitive deficits in Parkinson’s disease: lessons from preclinical animal models. Learn. Mem. 2013;20(10):592–600. doi: 10.1101/lm.032029.113. [http://dx.doi.org/10.1101/lm.032029.113]. [PMID: 24049188]. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka D., Nakada K., Takao K., Ogasawara E., Kasahara A., Sato A., Yonekawa H., Miyakawa T., Hayashi J. Normal mitochondrial respiratory function is essential for spatial remote memory in mice. Mol. Brain. 2008;1(1):21. doi: 10.1186/1756-6606-1-21. [http://dx.doi. org/10.1186/1756-6606-1-21]. [PMID: 19087269]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikebe S., Tanaka M., Ozawa T. Point mutations of mitochondrial genome in Parkinson’s disease. Brain Res. Mol. Brain Res. 1995;28(2):281–295. doi: 10.1016/0169-328x(94)00209-w. [http://dx.doi.org/10.1016/0169-328X(94)00209-W]. [PMID: 7723627]. [DOI] [PubMed] [Google Scholar]
- 52.Hattori N., Tanaka M., Ozawa T., Mizuno Y. Immuno-histochemical studies on complexes I, II, III, and IV of mitochondria in Parkinson’s disease. Ann. Neurol. 1991;30(4):563–571. doi: 10.1002/ana.410300409. [http://dx.doi.org/10.1002/ana.410300409]. [PMID: 1665052]. [DOI] [PubMed] [Google Scholar]
- 53.Bender A., Krishnan K.J., Morris C.M., Taylor G.A., Reeve A.K., Perry R.H., Jaros E., Hersheson J.S., Betts J., Klopstock T., Taylor R.W., Turnbull D.M. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006;38(5):515–517. doi: 10.1038/ng1769. [http://dx.doi.org/10. 1038/ng1769]. [PMID: 16604074]. [DOI] [PubMed] [Google Scholar]
- 54.Parker W.D., Jr, Parks J.K. Mitochondrial ND5 mutations in idiopathic Parkinson’s disease. Biochem. Biophys. Res. Commun. 2005;326(3):667–669. doi: 10.1016/j.bbrc.2004.11.093. [http://dx.doi.org/10.1016/j.bbrc.2004.11. 093]. [PMID: 15596151]. [DOI] [PubMed] [Google Scholar]
- 55.Dykens J.A., Will Y. The significance of mitochondrial toxicity testing in drug development. Drug Discov. Today. 2007;12(17-18):777–785. doi: 10.1016/j.drudis.2007.07.013. [http://dx.doi.org/10.1016/j.drudis.2007.07.013]. [PMID: 17826691]. [DOI] [PubMed] [Google Scholar]
- 56.Schneider J.S., Tinker J.P., Decamp E. Clonidine improves attentional and memory components of delayed response performance in a model of early Parkinsonism. Behav. Brain Res. 2010;211(2):236–239. doi: 10.1016/j.bbr.2010.03.040. [http://dx.doi.org/10.1016/j.bbr.2010.03. 040]. [PMID: 20347876]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Decamp E., Tinker J.P., Schneider J.S. Attentional cueing reverses deficits in spatial working memory task performance in chronic low dose MPTP-treated monkeys. Behav. Brain Res. 2004;152(2):259–262. doi: 10.1016/j.bbr.2003.10.007. [http://dx.doi.org/10.1016/j.bbr.2003.10.007]. [PMID: 15196793]. [DOI] [PubMed] [Google Scholar]
- 58.Schneider J.S., Pope-Coleman A. Cognitive deficits precede motor deficits in a slowly progressing model of parkinsonism in the monkey. Neurodegeneration. 1995;4(3):245–255. doi: 10.1016/1055-8330(95)90014-4. [http://dx.doi. org/10.1016/1055-8330(95)90014-4]. [PMID: 8581557]. [DOI] [PubMed] [Google Scholar]
- 59.Das N.R., Gangwal R.P., Damre M.V., Sangamwar A.T., Sharma S.S. PPAR-β/δ agonist is neuroprotective and decreases cognitive impairment in a rodent model of Parkinson’s disease. Curr. Neurovasc. Res. 2014;11(2):114–124. doi: 10.2174/1567202611666140318114037. [http://dx.doi.org/ 10.2174/1567202611666140318114037]. [PMID: 24635117]. [DOI] [PubMed] [Google Scholar]
- 60.Mutlu O., Ulak G., Celikyurt I.K., Akar F.Y., Erden F. Effects of citalopram on cognitive performance in passive avoidance, elevated plus-maze and three-panel runway tasks in naïve rats. Chin. J. Physiol. 2011;54(1):36–46. doi: 10.4077/cjp.2011.amk077. [http://dx.doi.org/10.4077/ CJP.2011.AMK077]. [PMID: 21786537]. [DOI] [PubMed] [Google Scholar]
- 61.Sarkaki A., Rezaiei M., Gharib Naseri M., Rafieirad M. Improving active and passive avoidance memories deficits due to permanent cerebral ischemia by pomegranate seed extract in female rats. Malays. J. Med. Sci. 2013;20(2):25–34. [PMID: 23983574]. [PMC free article] [PubMed] [Google Scholar]
- 62.Da Cunha C., Gevaerd M.S., Vital M.A., Miyoshi E., Andreatini R., Silveira R., Takahashi R.N., Canteras N.S. Memory disruption in rats with nigral lesions induced by MPTP: a model for early Parkinson’s disease amnesia. Behav. Brain Res. 2001;124(1):9–18. doi: 10.1016/s0166-4328(01)00211-x. [http://dx.doi.org/10.1016/S0166-4328(01) 00211-X]. [PMID: 11423161]. [DOI] [PubMed] [Google Scholar]
- 63.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [http://dx.doi.org/10.1016/0165-0270(84)90007-4]. [PMID: 6471907]. [DOI] [PubMed] [Google Scholar]
- 64.Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [http://dx.doi.org/10.1038/nprot. 2006.116]. [PMID: 17406317]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Charbonneau D., Riopelle R.J., Beninger R.J. Impaired incentive learning in treated Parkinson’s disease. Can. J. Neurol. Sci. 1996;23(4):271–278. doi: 10.1017/s031716710003821x. [http://dx.doi.org/10.1017/S031716710003821X]. [PMID: 8951205]. [DOI] [PubMed] [Google Scholar]
- 66.Dowd E., Dunnett S.B. Comparison of 6-hydroxydopamine-induced medial forebrain bundle and nigrostriatal terminal lesions in rats using a lateralised nose-poking task with low stimulus-response compatibility. Behav. Brain Res. 2005;165(2):181–186. doi: 10.1016/j.bbr.2005.06.036. [http://dx.doi.org/10.1016/j.bbr.2005.06.036]. [PMID: 16139374]. [DOI] [PubMed] [Google Scholar]
- 67.Bevins R.A., Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat. Protoc. 2006;1(3):1306–1311. doi: 10.1038/nprot.2006.205. [http://dx.doi.org/10.1038/nprot.2006.205]. [PMID: 17406415]. [DOI] [PubMed] [Google Scholar]
- 68.Grayson B., Leger M., Piercy C., Adamson L., Harte M., Neill J.C. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav. Brain Res. 2015;285:176–193. doi: 10.1016/j.bbr.2014.10.025. [http://dx.doi.org/10.1016/j.bbr.2014.10.025]. [PMID: 25447293]. [DOI] [PubMed] [Google Scholar]
- 69.Sy H-N., Wu S-L., Wang W-F., Chen C.H., Huang Y.T., Liou Y.M., Chiou C.S., Pawlak C.R., Ho Y.J. MPTP-induced dopaminergic degeneration and deficits in object recognition in rats are accompanied by neuroinflammation in the hippocampus. Pharmacol. Biochem. Behav. 2010;95(2):158–165. doi: 10.1016/j.pbb.2009.12.020. [http://dx.doi.org/10.1016/j.pbb.2009.12.020]. [PMID: 20064549]. [DOI] [PubMed] [Google Scholar]
- 70.Wang W-F., Wu S-L., Liou Y-M., Wang A-L., Pawlak C.R., Ho Y-J. MPTP lesion causes neuroinflammation and deficits in object recognition in Wistar rats. Behav. Neurosci. 2009;123(6):1261–1270. doi: 10.1037/a0017401. [http://dx.doi.org/10.1037/a0017401]. [PMID: 20001109]. [DOI] [PubMed] [Google Scholar]
- 71.Braga R., Kouzmine I., Canteras N.S., Da Cunha C. Lesion of the substantia nigra, pars compacta impairs delayed alternation in a Y-maze in rats. Exp. Neurol. 2005;192(1):134–141. doi: 10.1016/j.expneurol.2004.11.006. [http://dx. doi.org/10.1016/j.expneurol.2004.11.006]. [PMID: 15698627]. [DOI] [PubMed] [Google Scholar]
- 72.Chrobak J.J., Hanin I., Walsh T.J. AF64A (ethylcholine aziridinium ion), a cholinergic neurotoxin, selectively impairs working memory in a multiple component T-maze task. Brain Res. 1987;414(1):15–21. doi: 10.1016/0006-8993(87)91322-9. [http://dx.doi.org/10.1016/0006-8993(87) 91322-9]. [PMID: 3620916]. [DOI] [PubMed] [Google Scholar]
- 73.Mihara T., Mihara K., Yarimizu J., Mitani Y., Matsuda R., Yamamoto H., Aoki S., Akahane A., Iwashita A., Matsuoka N. Pharmacological characterization of a novel, potent adenosine A1 and A2A receptor dual antagonist, 5-[5-amino-3-(4-fluorophenyl) pyrazin-2-yl]-1-isopropylpyridine-2(1H)-one (ASP5854), in models of Parkinson’s disease and cognition. J. Pharmacol. Exp. Ther. 2007;323(2):708–719. doi: 10.1124/jpet.107.121962. [http://dx.doi.org/10.1124/jpet.107.121962]. [PMID: 17684118]. [DOI] [PubMed] [Google Scholar]
- 74.Heisler J.M., Morales J., Donegan J.J., Jett J.D., Redus L., O'Connor J.C. The attentional set shifting task: a measure of cognitive flexibility in mice. JoVE. 2015;96:e51944–e44. doi: 10.3791/51944. [http://dx.doi.org/ 10.3791/51944]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beeson C.C., Beeson G.C., Schnellmann R.G. A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal. Biochem. 2010;404(1):75–81. doi: 10.1016/j.ab.2010.04.040. [http://dx.doi.org/10.1016/j.ab. 2010.04.040]. [PMID: 20465991]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wieckowski M.R., Giorgi C., Lebiedzinska M., Duszynski J., Pinton P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat. Protoc. 2009;4(11):1582–1590. doi: 10.1038/nprot.2009.151. [http://dx.doi.org/10.1038/nprot.2009.151]. [PMID: 19816421]. [DOI] [PubMed] [Google Scholar]
- 77.Birch-Machin M.A., Turnbull D.M. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 2001;65:97–117. doi: 10.1016/s0091-679x(01)65006-4. [http:// dx.doi.org/10.1016/S0091-679X(01)65006-4]. [PMID: 11381612]. [DOI] [PubMed] [Google Scholar]
- 78.Fang N., Casida J.E. Anticancer action of cubé insecticide: correlation for rotenoid constituents between inhibition of NADH:ubiquinone oxidoreductase and induced ornithine decarboxylase activities. Proc. Natl. Acad. Sci. USA. 1998;95(7):3380–3384. doi: 10.1073/pnas.95.7.3380. [http://dx.doi.org/10.1073/pnas.95.7.3380]. [PMID: 9520374]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wood E., Latli B., Casida J.E. Fenazaquin acaricide specific binding sites in NADH: ubiquinone oxidoreductase and apparently the ATP synthase stalk. Pestic. Biochem. Physiol. 1996;54(2):135–145. [http://dx.doi.org/10.1006/pest.1996.0017]. [Google Scholar]
- 80.Barrientos A, Fontanesi F, Díaz F. Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Current Protocols in Human Genetics. 2009;19(3):1–193. doi: 10.1002/0471142905.hg1903s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crouch S.P., Kozlowski R., Slater K.J., Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods. 1993;160(1):81–88. doi: 10.1016/0022-1759(93)90011-u. [http://dx. doi.org/10.1016/0022-1759(93)90011-U]. [PMID: 7680699]. [DOI] [PubMed] [Google Scholar]
- 82.Hynes J., Marroquin L.D., Ogurtsov V.I., Christiansen K.N., Stevens G.J., Papkovsky D.B., Will Y. Investigation of drug-induced mitochondrial toxicity using fluorescence-based oxygen-sensitive probes. Toxicol. Sci. 2006;92(1):186–200. doi: 10.1093/toxsci/kfj208. [http://dx.doi.org/10.1093/toxsci/kfj208]. [PMID: 16638925]. [DOI] [PubMed] [Google Scholar]
- 83.Will Y., Hynes J., Ogurtsov V.I., Papkovsky D.B. Analysis of mitochondrial function using phosphorescent oxygen-sensitive probes. Nat. Protoc. 2006;1(6):2563–2572. doi: 10.1038/nprot.2006.351. [http://dx.doi.org/ 10.1038/nprot.2006.351]. [PMID: 17406510]. [DOI] [PubMed] [Google Scholar]
- 84.Dykens J.A., Jamieson J.D., Marroquin L.D., Nadanaciva S., Xu J.J., Dunn M.C., Smith A.R., Will Y. In vitro assessment of mitochondrial dysfunction and cytotoxicity of nefazodone, trazodone, and buspirone. Toxicol. Sci. 2008;103(2):335–345. doi: 10.1093/toxsci/kfn056. [http://dx.doi.org/10.1093/toxsci/kfn056]. [PMID: 18344530]. [DOI] [PubMed] [Google Scholar]
- 85.Sohn I.P., Ahn H.J., Park D.W., Gye M.C., Jo D.H., Kim S.Y., Min C.K., Kwon H.C. Amelioration of mitochondrial dysfunction and apoptosis of two-cell mouse embryos after freezing and thawing by the high frequency liquid nitrogen infusion. Mol. Cells. 2002;13(2):272–280. [PMID: 12018850]. [PubMed] [Google Scholar]
- 86.Reers M., Smiley S.T., Mottola-Hartshorn C., Chen A., Lin M., Chen L.B. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [http://dx.doi.org/10. 1016/0076-6879(95)60154-6]. [PMID: 8592463]. [DOI] [PubMed] [Google Scholar]
- 87.Hassouna A., Loubani M., Matata B.M., Fowler A., Standen N.B., Galiñanes M. Mitochondrial dysfunction as the cause of the failure to precondition the diabetic human myocardium. Cardiovasc. Res. 2006;69(2):450–458. doi: 10.1016/j.cardiores.2005.11.004. [http://dx.doi.org/10.1016/ j.cardiores.2005.11.004]. [PMID: 16330008]. [DOI] [PubMed] [Google Scholar]
- 88.Cottet-Rousselle C., Ronot X., Leverve X., Mayol J.F. Cytometric assessment of mitochondria using fluorescent probes. Cytometry A. 2011;79(6):405–425. doi: 10.1002/cyto.a.21061. [http://dx.doi.org/10.1002/ cyto.a.21061]. [PMID: 21595013]. [DOI] [PubMed] [Google Scholar]
- 89.Elmore S.P., Nishimura Y., Qian T., Herman B., Lemasters J.J. Discrimination of depolarized from polarized mitochondria by confocal fluorescence resonance energy transfer. Arch. Biochem. Biophys. 2004;422(2):145–152. doi: 10.1016/j.abb.2003.12.031. [http://dx.doi.org/10.1016/ j.abb.2003.12.031]. [PMID: 14759601]. [DOI] [PubMed] [Google Scholar]
- 90.Scaduto R.C., Jr, Grotyohann L.W. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys. J. 1999;76(1 Pt 1):469–477. doi: 10.1016/S0006-3495(99)77214-0. [http://dx.doi. org/10.1016/S0006-3495(99)77214-0]. [PMID: 9876159]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsuzaki S., Szweda P.A., Szweda L.I., Humphries K.M. Regulated production of free radicals by the mitochondrial electron transport chain: Cardiac ischemic preconditioning. Adv. Drug Deliv. Rev. 2009;61(14):1324–1331. doi: 10.1016/j.addr.2009.05.008. [http://dx.doi.org/10.1016/ j.addr.2009.05.008]. [PMID: 19716389]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mukhopadhyay P., Rajesh M., Yoshihiro K., Haskó G., Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem. Biophys. Res. Commun. 2007;358(1):203–208. doi: 10.1016/j.bbrc.2007.04.106. [http://dx.doi.org/10.1016/j.bbrc.2007.04.106]. [PMID: 17475217]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [http://dx.doi.org/10.1042/ BJ20081386]. [PMID: 19061483]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao B., Summers F.A., Mason R.P. Photooxidation of Amplex Red to resorufin: implications of exposing the Amplex Red assay to light. Free Radic. Biol. Med. 2012;53(5):1080–1087. doi: 10.1016/j.freeradbiomed.2012.06.034. [http://dx. doi.org/10.1016/j.freeradbiomed.2012.06.034]. [PMID: 22765927]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghafourifar P., Parihar M.S., Nazarewicz R., Zenebe W.J., Parihar A. Detection assays for determination of mitochondrial nitric oxide synthase activity; advantages and limitations. Methods Enzymol. 2008;440:317–334. doi: 10.1016/S0076-6879(07)00821-X. [http://dx.doi.org/10.1016/S0076-6879(07)00821-X]. [PMID: 18423228]. [DOI] [PubMed] [Google Scholar]
- 96.Räthel T.R., Leikert J., Vollmar A.M., Dirsch V.M. Application of 4,5-diaminofluorescein to reliably measure nitric oxide released from endothelial cells in vitro. Biol. Proced. Online. 2003;5(1):136–142. doi: 10.1251/bpo55. [http://dx.doi.org/10.1251/bpo55]. [PMID: 14569611]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schlame M., Ren M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochimica et Biophysica Acta (BBA)-. Biomembranes. 2009;1788(10):2080–2083. doi: 10.1016/j.bbamem.2009.04.019. [http://dx.doi.org/10.1016/j.bbamem.2009.04.019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jacobson J., Duchen M.R., Heales S.J. Intracellular distribution of the fluorescent dye nonyl acridine orange responds to the mitochondrial membrane potential: implications for assays of cardiolipin and mitochondrial mass. J. Neurochem. 2002;82(2):224–233. doi: 10.1046/j.1471-4159.2002.00945.x. [http://dx.doi.org/10.1046/j.1471-4159.2002.00945.x]. [PMID: 12124423]. [DOI] [PubMed] [Google Scholar]
- 99.Lanza I.R., Nair K.S. Functional assessment of isolated mitochondria in vitro. Methods Enzymol. 2009;457:349–372. doi: 10.1016/S0076-6879(09)05020-4. [http:// dx.doi.org/10.1016/S0076-6879(09)05020-4]. [PMID: 19426878]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weydert C.J., Cullen J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010;5(1):51–66. doi: 10.1038/nprot.2009.197. [http://dx.doi.org/10.1038/nprot. 2009.197]. [PMID: 20057381]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu J., Du J., Zhang Y., Sun W., Smith B.J., Oberley L.W., Cullen J.J. Suppression of the malignant phenotype in pancreatic cancer by overexpression of phospholipid hydroperoxide glutathione peroxidase. Hum. Gene Ther. 2006;17(1):105–116. doi: 10.1089/hum.2006.17.105. [http://dx.doi.org/10.1089/hum.2006.17.105]. [PMID: 16409129]. [DOI] [PubMed] [Google Scholar]
- 102.Medeiros D.M. Assessing mitochondria biogenesis. Methods. 2008;46(4):288–294. doi: 10.1016/j.ymeth.2008.09.026. [http://dx.doi.org/10.1016/j.ymeth.2008. 09.026]. [PMID: 18929661]. [DOI] [PubMed] [Google Scholar]
- 103.Obeso J.A., Rodriguez-Oroz M.C., Goetz C.G., Marin C., Kordower J.H., Rodriguez M., Hirsch E.C., Farrer M., Schapira A.H., Halliday G. Missing pieces in the Parkinson’s disease puzzle. Nat. Med. 2010;16(6):653–661. doi: 10.1038/nm.2165. [http://dx.doi.org/10. 1038/nm.2165]. [PMID: 20495568]. [DOI] [PubMed] [Google Scholar]
- 104.Valldeoriola F., Cámara A. Rev. Neurol. 2010;51(1):41–48. [Intraduodenal infusion of levodopa]. [Intraduodenal infusion of levodopa]. [PMID: 20568067]. [PubMed] [Google Scholar]
- 105.Beato R., Levy R., Pillon B., Vidal C., du Montcel S.T., Deweer B., Bonnet A.M., Houeto J.L., Dubois B., Cardoso F. Working memory in Parkinson’s disease patients: clinical features and response to levodopa. Arq. Neuropsiquiatr. 2008;66(2A):147–151. doi: 10.1590/s0004-282x2008000200001. [http://dx.doi.org/10.1590/S0004-282X2008000200001]. [PMID: 18545772]. [DOI] [PubMed] [Google Scholar]
- 106.Moskovitz C., Moses H., III, Klawans H.L. Levodopa-induced psychosis: a kindling phenomenon. Am. J. Psychiatry. 1978;135(6):669–675. doi: 10.1176/ajp.135.6.669. [http://dx.doi.org/10.1176/ajp.135.6.669]. [PMID: 655276]. [DOI] [PubMed] [Google Scholar]
- 107.Rampello L., Raffaele R., Furnari P., Vecchio I., Malaguarnera M. Psychotic complications of long term levodopa treatment of Parkinson’s disease. Arch. Gerontol. Geriatr. 1996;22(Suppl. 1):63–67. doi: 10.1016/0167-4943(96)86915-9. [http://dx.doi.org/10.1016/0167-4943(96)86915-9]. [PMID: 18653010]. [DOI] [PubMed] [Google Scholar]
- 108.Emre M., Aarsland D., Albanese A., Byrne E.J., Deuschl G., De Deyn P.P., Durif F., Kulisevsky J., van Laar T., Lees A., Poewe W., Robillard A., Rosa M.M., Wolters E., Quarg P., Tekin S., Lane R. Rivastigmine for dementia associated with Parkinson’s disease. N. Engl. J. Med. 2004;351(24):2509–2518. doi: 10.1056/NEJMoa041470. [http://dx.doi.org/10.1056/NEJMoa041470]. [PMID: 15590953]. [DOI] [PubMed] [Google Scholar]
- 109.Goldman J.G., Holden S. Treatment of psychosis and dementia in Parkinson’s disease. Curr. Treat. Options Neurol. 2014;16(3):281. doi: 10.1007/s11940-013-0281-2. [http://dx.doi.org/10.1007/s11940-013-0281-2]. [PMID: 24464490]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moretti R., Torre P., Vilotti C., Antonello R.M., Pizzolato G. Rivastigmine and Parkinson dementia complex. Expert Opin. Pharmacother. 2007;8(6):817–829. doi: 10.1517/14656566.8.6.817. [http://dx.doi.org/10.1517/ 14656566.8.6.817]. [PMID: 17425477]. [DOI] [PubMed] [Google Scholar]