Abstract
Gliomas are the most common primary brain tumors either benign or malignant originating from the glial tissue. Glioblastoma multiforme (GBM) is the most prevalent and aggressive form among all gliomas, associated with decimal prognosis due to it's high invasive nature. GBM is also characterized by high recurrence rate and apoptosis resistance features which make the therapeutic targeting very challenging. Mitochondria are key cellular organelles that are acting as focal points in diverse array of cellular functions such as cellular energy metabolism, regulation of ion homeostasis, redox signaling and cell death. Eventual findings of mitochondrial dysfunction include preference of glycolysis over oxidative phosphorylation, enhanced reactive oxygen species generation and abnormal mitochondria mediated apoptotic machinery are frequently observed in various malignancies including gliomas. In particular, gliomas harbor mitochondrial structure abnormalities, genomic mutations in mtDNA, altered energy metabolism (Warburg effect) along with mutations in isocitrate dehydrogenase (IDH) enzyme. Numerous natural compounds have shown efficacy in the treatment of gliomas by targeting mitochondrial aberrant signaling cascades. Some of the natural compounds directly target the components of mitochondria whereas others act indirectly through modulating metabolic abnormalities that are consequence of the mitochondrial dysfunction. The present review offers a molecular insight into mitochondrial pathology in gliomas and therapeutic mechanisms of some of the promising natural compounds that target mitochondrial dysfunction. This review also sheds light on the challenges and possible ways to overcome the hurdles associated with these natural compounds to enter into the clinical market.
Keywords: Apoptosis, cytotoxicity, glioma, mitochondrial dysfunction, mitochondrial membrane potential, natural compounds, oxidative stress, warburg effect
1. INTRODUCTION
Glioma is a broad term that encompasses all the tumors either benign or malignant that arise from glial cells. In 2012, worldwide 256,213 individuals were diagnosed with primary malignant brain tumors with higher incidence rates in developed countries than developing countries [1]. In US, an estimated 68,470 new cases of primary malignant and non-malignant brain and CNS tumors and 13,770 deaths are expected to occur in 2015. Glioma is common primary brain tumor accounting for 28% of all brain tumors and 80% of all malignant tumors [2]. Although its relative incidence is low; poor prognosis, negative impact on quality of life and cognitive function of patients makes its management a challenging task.
Gliomas have different cellular origins, and classified as oligodendroglioma, astrocytoma, ependymoma, glioblastoma, mixed glioma and few other histological types. WHO classified gliomas from grade I-IV with increasing rate of dedifferentiation and malignant nature. Glioblastoma, also known as “grade IV astrocytoma” or gliobalstoma multiforme (GBM) which is highly prevalent (54.7%) of all gliomas and the most aggressive form with poor prognosis [1]. GBM has decimal survival rates with only 4.7% of patients live longer than 5 years [3]. Exposure to high doses of ionizing radiation and presence of few rare genetic syndromes caused by inherited gene mutations are only two accepted risk factors for pathogenesis of gliomas. Numerous reports suggest negative correlation of allergies and atopic diseases to the incidence of gliomas indicating protective effect of allergies [4]. Surgery, radiotherapy, temozolimide based chemotherapy are mainstay treatment options in current clinical management of glioma. However, most of the gliomas are surgically unresectable and diagnosed at later stage and their high level of resistance to conventional chemotherapy, radiotherapy marks the gliomas as one of the numerous cancers that are difficult to treat. Despite of recent developments in these therapies, currently GBM has median survival of 14.6 months from diagnosis. The existence of blood brain barrier, complex network of multiple altered signaling pathways, presence of glioma stem cells are the main causative factors for the poor outcome with current therapies [5]. Hence more efficacious therapies for the treatment of malignant gliomas are urgently warranted. The current review is an attempt to summarize the overall pathophysiology of gliomas with respect to mitochondrial dysfunction. This review also discusses the importance of natural products as potential cytotoxic agents targeting mitochondrial derangements in glioma cells.
1.1. Molecular Pathology of Gliomas
Recent advances in techniques of genomic and proteomic analyses have contributed to our identification and understanding of molecular pathology of malignant gliomas. Based on currently available knowledge in molecular alterations in gliomas, various classification systems have been established which provide a basis for more targeted therapies [6-8]. The cancer genomic atlas (TCGA) has grouped glioblastomas relying on proteomic data into 3 classes: one showing epidermal growth factor receptor (EGFR) mutations or amplifications, second, having ligand driven platelet derived growth factor activation, third with loss of RAS regulator NF1 [6]. Another classification provided by Verhaak et al., categorized glioblastoma into four subtypes: classical, mesenchymal, proneural and neural through genomic profiling [7]. They are defined as follows: Classical- EGFR amplification and amplification of chromosome 7 with loss of chromosome 10; Mesenchymal- increase in frequency of Nf1 mutations/ deletion/ low levels of expression along with loss of Phosphatase and tensin homolog (PTEN); Proneural- focal amplifications in platelet derived growth factor alpha (PDGFRA) and isocitrate dehydrogenase I (IDH1) point mutations, TP53 mutations; Neural- Most similar to normal brain tissue expression patterns.
Other researchers used various criteria such as IDH1 mutations, immunohistochemistry to classify the glioblastomas. These classification systems based on molecular markers discern the more appropriate management of the glioblastomas using targeted therapies [8, 9].
In recent years, numerous molecular alterations were identified and added to the core of molecular landscape of gliomas. The most common deregulations include mutations in P53, IDH1, alpha thalassemia/mental retardation syndrome X-linked (ATRX), telomerase reverse transcriptase (TERT), H3 histone, family 3(H3F3) genes, change in methylation patterns of O-6-methylguanine-DNA methyltransferase (MGMT), P16 promoters, Expression of platelet derived growth factor receptor alpha (PDGFRA), oligodendrocyte transcription factor 2 (OLIG2) [10]. Three main core pathways that are frequently altered in >75% of glioblastomas were identified by The Cancer Genome Atlas (TCGA) as i) receptor tyrosine kinase/RAS/phosphatidylinositol 3 kinase (RTK/RAS/PI3K) signaling ii) p53 iii) retinoblastoma (RB) signaling networks [11].
Understanding molecular abnormalities in malignant gliomas has paved a way for the targeted therapies. Aberrations in epidermal growth factor receptor (EGFR), platelet derived growth factor receptor (PDGFR), vascular endothelial growth factor receptor (VEGFR) such as amplifications, mutations are frequently occurring phenomena in glioblastomas resulting in enhanced angiogenesis, migration and survival [12].
2. MITOCHONDRIAL PATHOPHYSIOLOGY IN GLIOMAS
Mitochondria are the key cellular organelles that play essential role in various functions such as regulation of cellular metabolism, redox signaling, ionic homeostasis and cell death. In addition to its well established role as “powerhouse of cell”, recently it has been accepted as “motor of cell death” due to its crucial role in programmed cell death or apoptosis [13]. These are unique double membrane enclosed organelles having their own genetic material and undergo their own biogenesis process with no synchrony with the cell cycle. Due to their fundamental roles in key processes such as the production of energy currency ATP for cellular maintenance, generation of reactive oxygen species (ROS) and execution of cell death pathways, mitochondria has been implicated in numerous pathological processes such as cancer, neurodegenerative diseases, obesity, diabetes and aging.
Mitochondria are considered as semi-autonomous organelles in the eukaryotic cellular system having circular DNA encoding genes for proteins of critical importance [14]. Being a seat for primary cellular ATP and ROS production, changes in the mitochondrial functioning can have dire consequences on cellular fate and thus study of the role and regulation of mitochondria in glioma cancers is of primary concern [15]. Mitochondrial function is found to be altered/ impaired to variable extent in various types of glioblastomas such as astrocytoma and ependymoblastoma. Structural and functional impairment in the glial mitochondria is cumulative effect of altered haemodynamic, mitogenic, apoptotic and bioenergetic signaling, which are the common core features of cancer cells [16]. Structural changes to the mitochondria like partial/total cristolysis, swelling of mitochondria, altered dynamics like fission and fusion cycles of the mitochondria results in a heterogeneous group of mitochondria in various grades of glioma [17]. These structural derangements clearly indicate the compromised oxidative phosphorylation capacity and energy coupling in glioma cell lines.
Malignant glioma cells derived from in vitro cultures and humans primarily depend on cytosolic ATP produced from aerobic glycolysis instead of mitochondria derived ATP, a switch in the energetic preference popularly termed as “Warburg hypothesis” [18]. Their dependence on less energy deriving pathways unlike their healthy counterparts exposes the degree of functional impairment in the mitochondrial function and its inability to utilize ketones and fatty acids for the production of ATP apart from glucose [19]. This property of exclusive dependency on glucose for energy production was harvested for therapeutic targeting of paediatric GBM through ketogenic diet in several patients, where many cases of symptomatic relief and improved tumor management were observed [20]. Some animal models of gliomas have shown pro apoptotic, anti proliferative, anti inflammatory and anti angiogenic profile in the presence of glucose restriction, which strongly warrants the presence of deranged, decoupled mitochondria and inefficiency in ketone metabolism for energy production in the glioblastoma cells [21]. High energy demand along with the lack of proper redox buffering system against mitochondrial ROS generation further makes glioma cells susceptible for apoptotic damage (Fig. 1). Glioma cells attempt for an alternative energy deriving pathways in the absence of glucose, especially from fatty acids, which results in enhanced ROS output. These ROS in the presence of impaired antioxidant defenses, such as reduced GSH levels in gliomas can execute apoptosis and thus prevent tumor progression [22]. This therapeutic benefit of ROS mediated apoptosis in the presence of glucose restriction further warrants the potential of targeting of energy metabolism for glioma therapy.
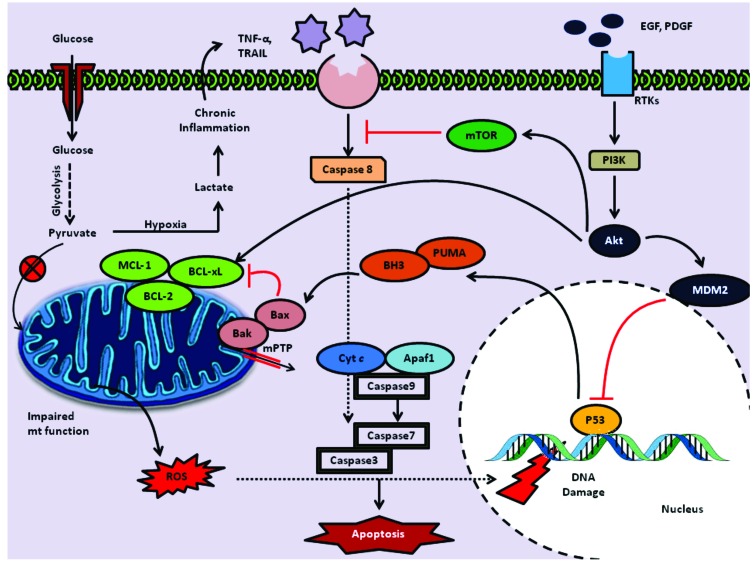
Fig. (1).
Mitochondrial dysfunction in gliomas- Evasion of Apoptosis: Impaired mitochondrial function due to the genomic mutations in mtDNA leads to impaired function of electron transport chain thus enhancing generation of ROS. This elevated cellular oxidative stress leads to the damage of nuclear genome resulting in activation of p53. This process in turn promotes P53 mediated generation of apoptotic response through release of p53 up regulated modulator of apoptosis (PUMA) and Bcl-2 homology domain 3 proteins (BH3) which acts on Bcl2 family apoptotic proteins Bax, Bak and mitochondria mediated apoptotic events. However, gliomas with enhanced growth factor signaling through RTKs results in PI3K/ Akt pathway activation that causes MDM2 mediated degradation of P53. Glioma cells harboring P53 mutations are also resistant to the ROS mediated apoptotic pathway. As glioma cells preferentially generate energy through the glycolysis over krebs cycle, they generate large amounts of pyruvate, and it becomes lactate under hypoxic conditions generating chronic inflammatory state that leads to upregulation of TNF-alpha, TRAIL. These ligands trigger apoptosis through activating caspase 8 by death receptor pathway. Enhanced PI3K/Akt activated signaling blocks this extrinsic pathway through mTOR mediated inhibition.
Impaired mitochondrial metabolic capacity in glioma cells was evident through identification of mutations in genes coding for Isocitrate dehydrogenase (IDH) [23]. This enzyme is responsible for reductive carboxylation of α-keto glutarate to isocitrate, which subsequently get isomerized to citrate, the principal component of Krebs cycle. It has been identified that most of the glioblastomas show a mutation in the gene encoding the cytosolic form of IDH1. IDH1 mutations occurring at nucleotides that code for arginine, R132 are frequent in most of the diffuse and secondary gliomas [24]. Mutations in IDH2, the mt homolog of IDH1 also found to occur at R172 and known to be associated with loss of enzymatic activity [25]. Both the type of mutations leads to accumulation of 2-hydroxy glutarate, an oncometabolite which is used as a biomarker [26]. Mutations in the NADPH linked mitochondrial isoforms of IDH1 and IDH2 leads to impaired energy production in the mitochondria and thus provides an evidence for mitochondrial dysfunction in gliomas and other tumor types [16].
Apoptosis is a type of primary cellular death mechanism characterized by nuclear condensation, formation of apoptotic bodies followed by phagocytosis. Several anti cancer agents work predominantly by executing apoptosis and thus halt malignant progression of tumors [27]. Impairment in the apoptotic machinery either through genetic mutations or altered cellular signaling is the ploy mechanism employed by cancer cells responsible for chemo resistance [28]. Glioma cells have been found to have impaired apoptotic signaling primarily due to mutations in the genes of p53 and B-cell lymphoma like 2 (BCL-2) homologue of proteins. P53 is considered as “the guardian of the genome”, known for its active role in DNA repair, apoptosis and cell cycle regulation. P53 through promotion of p53 up regulated modulator of apoptosis (PUMA), phorbol-12-myristate-13-acetate-induced protein 1 (NOXA) and Bcl-2 homology domain 3 proteins (BH3) and apoptotic peptidase activating factor-1 (Apaf-1), helps in executing apoptosis [29]. Inactivating mutations in the p53 gene were found to be common in 30-50% of human gliomas [30]. Along with p53 mutations, mutations in the genes coding for Bcl-2 homologue family of proteins are also common feature of malignant gliomas. Bcl-2 family proteins have variable effect on mitochondrial membrane modulation via stabilization or permeabilization and thus classified into anti apoptotic and pro apoptotic proteins respectively. Immunohistochemical studies have shown elevated expression of anti-apoptotic Bcl-2 protein in human glioma tumor samples [31]. Indeed there is a paradoxical elevation of the pro-apoptotic Bcl-2-associated X protein (Bax), which suggests the abnormal interaction among the Bcl-2 family member proteins corresponding to occurence of mitochondrial membrane dysfunction in malignant gliomas. Mutations in the other anti apoptotic members such as Bcl-2, Bcl-xl and Mcl-1 were also responsible for resistance to radiotherapy and chemotherapy in GBM [32]. In addition to the aforementioned mutations in the intrinsic apoptotic machinery, mutations in the upstream cellular pathways regulating apoptosis were also found to be observed in gliomas. Human gliomas were identified to be characterized by enhanced receptor tyrosine kinase (RTKs) expression on the cellular surfaces [33]. Enhanced growth factor signaling through RTKs leads to phosphoinositide 3- kinase (PI3K) recruitment and corresponding activation of protein kinase Akt, which exerts multitude of cellular effects. Akt through its ability to phosphorylate various proteins that potentiate the action of anti-apoptotic BCL-2 family members, by negative regulation of p53 by murine double minute 2 (MDM2) inhibits intrinsic pathway of apoptosis [34]. It also indirectly inhibits extrinsic pathway of apoptosis through mTOR activation (Fig. 1).
Cardiolipin is an important phospholipid constituent present in inner mitochondrial membrane. It is a dimeric phospholipid with four different acyl chains combined to give variants of cardiolipin molecular species [35]. It is highly concentrated at the contact sites of outer and inner mitochondrial membranes and also at electron transport chain (ETC). Its association with supra molecular complexes of respirasome components is highly essential for mitochondrial function [36]. Several experimental and clinical evidences clearly have shown improper cardiolipin biosynthesis and corresponding mitochondrial functional ETC impairment is associated with gliomas. Molecular lipidomic technologies have clearly indicated asymmetric distribution of different molecular species of cardiolipin corresponds to suboptimal functioning of respiratory complexes [37]. Cardiolipin is also found to affect the opening of mitochondrial permeability transition pore (MPTP), a multimeric channel responsible for release of apoptosis executioners such as cytochrome c, SMAC and Omi. Cardiolipin rich sites of mitochondrial membrane help to recruit pro apoptotic BCL-2 family of proteins and interaction of Bax with MPTP [34]. Thus the degree of faulty cardiolipin synthesis and its function indirectly affects the mitochondrial metabolic capacity and intrinsic apoptosis execution which were found to be impaired/ malfunctioned in certain grades of glioma cells.
It has been also identified that mitochondrial membrane permeability is regulated by cytosolic structural proteins especially those from tubulin family. Class III β-tubulin isotype (βIII-tubulin) is found to be associated with voltage dependent anion channel (VDAC) on outer mitochondrial membrane and its regulation [38]. Tubulin is found to block VDAC by phosphorylation and reduces metabolite movement across the membrane and thus halts mitochondrial function in tumor cells [39]. Indeed βIII-tubulin is identified to be an important pro survival factor for cancer cells which acts in concert with other cellular pro survival factors and is hence responsible for cancer chemo resistance [40]. The pro survival effect mediated by βIII-tubulin is mediated through adoption of cells to oxidative stress and energy deprivation. This way of VDAC regulation by tubulin offers another mechanism of mitochondrial dysfunction in gliomas [16]. Along with mitochondrial tubulins, alterations in other members of the tubulin family are also found to be associated with mitochondrial association with endoplasmic reticulum, cancer cell migration and metastasis [41, 42].
Autophagy is a vital cell death pathway carried out by lysosomes for recycling the damaged cellular components. Various cancers have been shown to have impaired or aberrant autophagic signaling which results in tumor progression and pathogenesis [43]. Recent studies on in vitro cultures of glioma cells have found that certain cytotoxic agents such as selenite and inhibitor of growth (ING4) can execute autophagy through mitochondrial ROS generation and mitochondrial membrane hyperpolarization and corresponding activation of mitophagy [44, 45]. The absence of endogenous activation of mitophagy in response to enormous generation of cancer cells suggests an impaired homeostatic regulation of autophagic/mitophagic pathways in glioblastoma cells.
3. PHYTOCHEMICALS TARGETING MITOCHONDRIAL DYSFUNCTION IN GLIOMAS
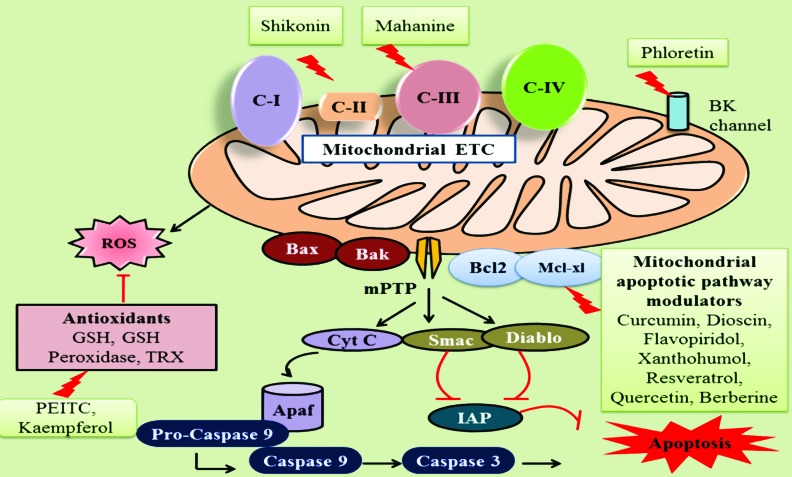
The main goal in the drug discovery lies in the discovery and development of efficacious, safe and affordable chemotherapeutic agents. Bioactive natural compounds are of great importance in the drug discovery as they are in agreement with these objectives. Recent studies in the last decades have revealed that anticancer activity of numerous natural compounds in gliomas by specifically targeting mitochondrial dysfunction. Some of these compounds act on various components of mitochondria directly, affecting its oxidative phosphorylation and apoptotic signaling and others act indirectly by modulation of metabolic abnormalities occurred as a result of mitochondrial dysfunction (Fig. 2). Table 1 represents the list of natural compounds, their model systems, cytotoxic range and mechanisms identified responsible for their observations.
Fig. (2).
Natural compounds targeting mitochondrial dysfunction in gliomas: Phytochemicals are known to exert anti cancer effect in several gliomas through affecting multitude functions of mitochondria at various levels. Shikonin, Mahanine were identified to inhibit ETC complexes II, III respectively and thus create cellular oxidative stress. Cellular antioxidants such as GSH, thioredoxin, GSH peroxidase are necessary to regulate the ROS levels and create normal redox balance. PEITC, Kaempferol disrupts these antioxidant systems in cancer cells. BK channels (Functional big potassium channels) exist in membranes of mitochondria, endoplasmic reticulum and maintain normal osmotic balance in cell. Phloretin causes opening of these BK channels which leads to osmotic damage to mitochondria and thus induces swelling and vacuolization which consequently triggers paraptosis like death. Bcl2 family apoptotic proteins, mitochondrial permeability transition pore are important players in mitochondria mediated apoptosis. Natural compounds such as Curcumin, Dioscin, Resveratrol and Berberine triggers generation of ROS and loss of mitochondrial membrane potential to release cytochrome c, Smac, Diablo in to cytosol. The released Cytocrome c in cytosol forms complex with Apaf-1 and promotes the activation of caspase 9 which in turn activates final executioner caspase 3 to induce apoptotic cell death.
Table 1.
Natural compounds targeting mitochondrial dysfunction by in various experimental models of gliomas.
|
S.
No. |
Name of Natural Compound | Experimental Model System | Observed Cytotoxic Range | Probable Mechanisms of Action | Observations | Refs. |
|---|---|---|---|---|---|---|
| 1. | Curcumin | U87MG glioblastoma cell line U87 MG cells along with TRAIL T98G glioblastoma multiforme |
25-50µM Sublethal conc. 25- 50µM |
Enhanced Bax-Bcl2 ratio, caused cytochrome c, Smac, Diablo release, Decreased cIAP2, caspase 9 activation Release of cytochrome c, caspase 9, 3,8 activation Caused cytochrome c, smac, Diablo, AIF release, Enhanced Bax-Bcl2 ratio, decreased cIAP1,cIAP2, caspase 9 activation |
Decrease in cell viability, induction of apoptosis Sensitizes to cytotoxic action of TRAIL, induction of apoptosis Decrease in cell viability, induction of apoptosis |
[48] [49] [50] |
| 2. | Mahanine | U87MG, LN229 In vivo U87 MG xenografts |
5-20 µM | Mitochondrial electron transport chain complex III inhibition, decreased oxygen consumption, elevated ROS, decreased MMP, activation and up regulation of Chk1/chk2, down regulation of CDK4/CDK6, cyclin D1/D3, CDC25A | Decrease in proliferation, G0/G1 arrest, decreased invasion, induction of differentiation in vivo Reduced tumor burden in models |
[54] |
| 3. | PEITC (Phenethyl isothiocyanate) |
GBM 8041 cells U87MG cells Ln229 cells |
4-8 μM 2.5 μM 10, 20 μM |
ROS generation, rise in [ca2+] I, promotion of Fas, FasL, FADD, TRAIL, caspases 8,9,3, increase in apoptotic proteins (Bax, Bak, Bid) inhibition of anti apoptotic proteins(Bcl2, Bcl-xl), release of cytochrome c, AIF, Endo G, decrease in mitochondrial membrane potential, Enhanced ROS generation, up regulation of DR5, enhanced activities of caspase 3, 8, 9, increase in mitochondrial superoxide levels Induction of ROS, inhibition of SOD and glutathione and enhancement of caspase 3 activity |
Inhibition of growth, induction of apoptosis Enhancement of cytotoxicity of TRAIL at sub toxic concentrations of PEITC Inhibition of proliferation, induction of apoptosis, cell cycle arrest |
[61] [62] [63] |
| 4. | Aloe emodin | U87 | 58.6 µg/ml | Collapse of mitochondrial membrane potential, S phase cell cycle arrest | Decrease in cell proliferation, induction of apoptosis | [68] |
| 5. | Dioscin | Rat glioma c6 cells in vitro and in vivo | 1.25-5 µg/ml in vitro and 30mg/kg oral in vivo |
ROS generation, ca++ release, mitochondrial structural changes, mitochondrial permeability changes, release of cytochrome c, PDCD5, increase of Bax, Bak, Bid Decrease of Bcl-2, Bcl-xl, increased activity of caspase 9, 3 | Inhibition of cell proliferation, Induction of apoptosis, in vivo- Increase of survival time of rodents, decrease of tumor volume | [72] |
| 6. | α-bisabolol | U87 | 2.5-10 µM | Dissipation of mitochondrial membrane potential, release of cytochrome c, PARP cleavage | Inhibition of cell viability, induction of apoptosis | [73] |
| 7. | Dantron | C6 | 10-100 µM | Induction of ROS, disruption of mitochondrial membrane potential, release of cytochrome c, AIF, Endo G, increase in caspase 3, 9 activities | Decrease in cell viability, induction of apoptosis | [74] |
| 8. | Flavopiridol | Murine glioma GL261 in vitro and in vivo | 100-400nM in vitro and in vivo 5mg/ kg |
Mitochondrial damage, release of cytochrome c, nuclear translocation of apoptosis inducing factor | Inhibition of cell growth and inhibition of migration In vivo decrease of tumor volume |
[76, 77] |
| 9. | Xanthohumol | T98G cells | 1-50 μM | ROS generation, depolarization of mitochondria, mitochondrial permeability transition, cytochrome c release, decrease in Bcl-2, activation of caspase-9, | Decrease of cell viability, induction of apoptosis | [79] |
| 10. | Shikonin | U87MG cells | 2-8 μM | Induction of ROS, disruption of mitochondrial membrane potential, mitochondrial superoxide generation, inhibition of complex II of mitochondrial ETC, GSH depletion, catalase down regulation, SOD1 up regulation, modulation of Bcl-2 family proteins | Induction of apoptosis | [81, 82] |
| 11. | Resveratrol | U251 cells | 10-100 μM | Release of cytochrome c from mitochondria, activation of caspase 9, up regulation and translocation of Bax to mitochondria | Cytotoxic action, induction of apoptosis, inhibition of proliferation | [84] |
| 12. | Quercetin | U373MG | 25-100 μM | Decrease in mitochondrial membrane potential, upregulation and translocation of P53 to mitochondria, release of cytochrome c, increase in activities of caspase 9, 3. | Inhibition of cell proliferation, induction of apoptosis and cytoprotective autophagy | [86] |
| 13. | Hydroxygenkwanin | c6 glioma | 25 μM | Loss of mitochondrial membrane potential, mitochondrial damage including swelling, over expression of Bak, Bid, reduced expression of Bcl-xl | Inhibition of cell proliferation, apoptosis | [89] |
| 14. | Alantolactone | U87, U373, LN229 Kumming mice |
40 μM 100mg/kg/ day |
ROS generation, dissipation of mitochondrial membrane potential, cardiolipin oxidation, GSH depletion, release of cytochrome c, upregulation of p53, Bax, down regulation of Bcl2, increase in activities of caspase 9, 3 | Induction of cell death, apoptosis induction No hepatotoxicity, nephrotoxicity |
[91] |
| 15. | Kaempferol | T98G, U373MG, LN229 | 50 μM | ROS generation, loss of mitochondrial membrane potential, down regulation of Bcl2 | Inhibition of cell viability, induction of apoptosis, potentiation of doxorubicn’s cytotoxic effects | [93] |
| 16. | Honokiol | DBTRG-05MG | 20-80 μM | Increased ROS accumulation, decrease of mitochondrial membrane potential, release of cytochrome c, increase in activities of caspase 9, 3 and elevation of intracellular calcium | Induction of apoptosis | [94, 95] |
| 17. | Parthenolide | U87MG, U373 Intracerbral glioblastoma xenograft model U138MG, U87, U373 and C6 |
0.1-50 μM 10mg/kg/day i.p daily for 3 weeks 5-50 μM |
Increased expression of Bax, Bak, down regulation of Bcl2, Increased activities of caspase 9, 3 Reduction in VEGF, MMP-9 Loss of mitochondrial membrane potential, release of cytochrome c, decrease in Bcl-xl |
Reduction of proliferation, suppression of invasion and angiogenesis Reduction of tumor growth, angiogenesis Decrease in cell viability, induction of apoptosis |
[97] [97] [98] |
| 18. | Phloretin | U251, T9 glioma | 50, 100 μM | Mitochondrial swelling, loss of ATP, induction of paraptosis (cellular swelling along with vacuolization), BK channel activation, over expression of heat shock proteins(HSP 60, 70,90) | Reduction in cell viability | [101, 102] |
| 19 | Gossypol | U87, U373, MZ-54 U87MG-luc2 xenograft along with TMZ |
10-30 μM 30mg/kg/day |
Induction of autophagic cell death, decrease in mitochondrial membrane potential, release of cytochrome c Decrease in TUNEL positive, Ki67positive cells, decrease in micro vessel density |
Cytotoxic action, autophagic cell death Reduction of tumor burden, increase in apoptosis |
[103] [104] |
| 20 | Berberine | T98G | 50-200 μM 50, 100 mg/kg/day |
Enhanced oxidative stress, Ca2+ levels, loss of mitochondrial membrane potential, enhanced Bax-Bcl2 ratio, increased caspase 9,3 activities Senescence induction, decrease in Ki67 and EGFR |
Decreased cell viability, Induction of apoptosis Inhibition of tumor growth |
[107] [108] |
(AIF- Apoptosis inducing factor; Bax- BCL2-Associated X Protein; Bak- Bcl-2 antagonist/killer-1; Bid- BH3 interacting domain death agonist; BK channel- Big Potassium channel; CDC25A- cell division cycle 25 homolog A; CDK- Cyclin-dependent kinase; cIAP- Cellular inhibitor of apoptosis 2; Diablo- Direct IAP binding protein with low pI; DR5- Death Receptor 5; Endo G- Endonuclease G; ETC-Electron transport chain; Fas- Apoptotsis stimulating fragment; FasL –Fas ligand; FADD- Fas-Associated protein with Death Domain; MMP9- Matrix metallopeptidase 9; PARP- Poly ADP ribose polymerase; PDCD5- Programmed cell death protein 5; ROS-reactive oxygen species; Smac- Second Mitochondria-Derived Activator of Caspases; SOD- Superoxide dismuatase; VEGF- Vascular endothelial growth factor; TRAIL- TNF-related apoptosis-inducing ligand; TUNEL- Terminal deoxynucleotidyl transferase dUTP nick end labeling).
3.1. Curcumin
Curcumin, a diferuloylmethane, a naturally occurring polyphenolic yellow pigment obtained from the plant turmeric is widely used in medicine and culinary traditions in India. Accumulating evidence suggests its antioxidant, anti-inflammatory, anti-proliferative properties [46, 47]. Curcumin induced mitochondria mediated apoptotic pathway by the release of cytochrome c, elevated Bax-Bcl2 ratio, cleavage of Bid to tBid and activated caspase 8, 9 and 3 in u87MG cells [48]. Curcumin was also found to sensitize the resistant U87MG cells to the TNF-related apoptosis-inducing ligand (TRAIL) mediated apoptosis at sub lethal concentrations by triggering both extrinsic and intrinsic apoptotic pathways through release of cytochrome c form mitochondria and activation of caspases 3,8,9 [49]. In another study in human glioblastoma T98G cells, curcumin enhanced Bax-Bcl2 ratio, induced release of cytochrome c,
apoptosis inducibe Factor (AIF), second mitochondria-derived activator of caspases (Smac) from the mitochondria, activated caspase 9 and indicating role of mitochondria mediated apoptosis [50]. Curcumin increased mitochondrial membrane permeability, resulting in reduced respiration and ATP synthesis in isolated rat liver mitochondria. The observed outcome was found to be due to opening of mitochondrial membrane permeability transition pore (MPTP) by oxidation of membrane thiol groups and low ca++ levels in mitochondria [51].
3.2. Mahanine
Mahanine is a carbazole alkaloid, derived from asian vegetable plants such as Micromelum minutum, Murraya koenigii etc., Recent studies suggest many pharmacological properties of mahanine such as anti mutagenicity, antibacterial activity, and anticancer properties in various tumor models
[52, 53]. Mahanine has shown potent antiproliferative effects in both in vitro and in vivo models of GBM without having effect on normal astrocytes. In U87MG, LN229 cell lines, it demonstrated specific inhibition of mitochondrial ETC complex III thus inducing generation of ROS and its associated DNA damage response. This mediates up regulation and activation of Chk1/Chk2 leading to its G0/G1 arrest. N-acetyl cysteine (NAC) mediated scavenging of ROS and knockdown of Checkpoint kinases 1/2 (chk1/chk2) decreased propensity of mahanine’s inducing ability of G0/G1 arrest. These events clearly establish the essential role of mahanine’s induced ROS generation and its downstream activation of chk1/chk2 in its anti proliferative activity. Furthermore, mahanine also reduced invasion and induced differentiation of glioblastoma cells [54].
3.3. Phenethylisothiocyanate (PEITC)
PEITC is an important member of group of naturally occurring isothiocyanates, a group of chemicals containing isothiocyanate group (N=C=S) that are abundant in many cruciferous vegetable plants such as cabbage, broccoli, watercress etc. Numerous reports suggest chemopreventive and anticancer potential of PEITC in a variety of human cancers with less toxicity towards normal cells [55-59]. The selective cytotoxicity of PEITC at least in part was attributed to its ROS modulating effects. PEITC initially depletes glutathoione (GSH), glutathione-S-S-glutathione (GSSG) and later form adducts with glutathione and exports it from the cell thus completely disrupting total glutathione antioxidant system. Furthermore it also inhibits glutathione peroxidase system [60]. Lee et al., Chou et al., Su et al., reported that PEITC exhibits potential antitumor efficacy against a variety of human glioma cell lines through apoptosis induction. In vitro studies with PEITC in human GBM (GBM8401) cells suggest the possible involvement of both death receptor and mitochondria mediated death pathway in the apoptosis induction. PEITC treatment enhanced ROS generation, disrupted mitochondrial membrane potential leading to the release of various apoptotic modulatory proteins from mitochondria such as cytochrome c, Endo G and apoptosis inducing factor (AIF). It also enhanced pro- apoptotic proteins (Bax, Bid and Bak) and decreased anti- apoptotic protein levels (Bcl-2, Bcl-xl) and increased caspase-9, 8, 3 activities. Furthermore, subtoxic concentrations of PEITC also sensitized glioma cells to cytotoxicity of TRAIL through increased total ROS generation, increased mitochondrial superoxide levels [61, 62]. In another study, PEITC reduced components of cellular antioxidant system such as GSH expression and activity of SOD in LN 229 cells [63]. In a study, chen et al., reported that PEITC suppresses mitochondrial respiration through disruption of its electron transport chain at complex I by an early degradation of NADH dehydrogenase Fe-S-protein-3 and rapidly depletes GSH levels in human leukemia cells [64].
3.4. Aloe Emodin
Aloe emodin is one of the biologically active compounds isolated from leaves of A.vera. It has shown anticancer activity in multiple types of cancers [65, 66]. It suppressed the proliferation of U87 cells in time and dose dependent manner and altered mitochondrial membrane potential leading to mitochondria mediated apoptosis [67]. Genomic analysis carried out in Aloe emodin treated U87 cells revealed the upregulation of the important genes involved in mitochondrial apoptotic pathway and mitochondrial dynamics. Such genes of potential relevance include SHANK-associated RH domain interactor (SHARPIN) and mitochondrial fission 1 protein (FIS1) [68].
3.5. Dioscin
Dioscin, a natural steroidal saponin is isolated from medicinal plants of Dioscorea species. Research in past decades has suggested its wider range of pharmacological properties such as hepatoprotective, anticancer, anti-inflammatory activities [69-71]. Dioscin has shown promising anticancer activity in c6 rat glioma in both in vitro and in vivo models by inducing oxidative stress. Treatment with dioscin induced ROS generation, Ca2+ stress which leads to mitochondrial dysfunction. ROS accumulation further resulted in mitochondrial injury including alterations in mitochondrial structure such as decomposition of mitochondrial double membrane, swelling of mitochondria and mitochondrial permeability transition and change in mitochondrial membrane potential. Dioscin induced apoptosis through mitochondrial dependent pathway by release of cytochrome c, Programmed cell death 5 (PDCD-5), down regulated expression of anti- apoptotic factors such as Bcl-2, Bcl-xl, up regulated expression of pro-apoptotic factors such as Bax, Bak, Bid and increased activities of caspase-3. In vivo, Dioscin treatment significantly decreased tumor volume and extended survival time of rats in rat allograft model of glioma [72].
3.6. α-bisabolol
α-bisabolol, a sesquiterpene alcohol present in essential oil posses potent antitumor properties in human glioma U87 cells. α-bisabolol induced time and dose dependent cytotoxic effect through intrinsic apoptotic pathway as evident by dissipation of mitochondrial membrane potential, release of cytochrome c and PARP cleavage in U87 cells [73].
3.7. Dantron
Dantron (formerly known as danthron) is a natural anthraquinone derivative obtained from chinese medicine Rhubarb. It was withdrawn from the market as laxative by US FDA in 2009 due to its carcinogenic activity. Dantron induced cell death in c6 rat glioma cells through mitochondria dependent pathway by inducing ROS generation, collapse of mitochondrial transmembrane potential and release of cytochrome c, AIF, Endo G and increase in caspase 3, 9 levels [74].
3.8. Flavopiridol
Flavopiridol, a potent cyclin dependent kinase inhibitor is a semisynthetic flavone, originally isolated from Dysoxylum binectariferum, a plant used in Indian traditional medicine. Flavopiridol has shown efficacy in many solid and hematological malignancies with multiple mechanisms of action such as cell cycle arrest and apoptosis and regulation of genetic expression [75]. Ew Newcomb et al., demonstrated that flavopiridol inhibits growth of murine glioma GL261 cells both in vitro and in vivo by altering expression of CDK4, cyclin D and p21and induces apoptosis by mitochondria mediated apoptotic pathway. Flavopiridol caused mitochondrial damage such as swelling of mitochondria with displaced and distorted cristae and induced release of cytochrome c and nuclear translocation of AIF [76, 77].
3.9. Xanthohumol
Xanthohumol is a major prenylated flavanoid constituent of flowers of Hop plant (Humulus lupus) which is used as raw material in beer to preserve and to give its distinct flavor. Recent experimental findings have identified its numerous biological effects including chemopreventive and chemotherapeutic effects in wide variety of cancers [78]. In T98G human glioblastoma cell line, xanthohumol decreased cell viability by inducing apoptosis through mitochondrial dependent pathway by oxidative stress. Xanthohumol depolarized mitochondria and triggered mitochondrial permeability transition as evident by release of cytochrome c and decrease of mitochondria associated anti-apoptotic protein Bcl-2 thus leading to caspase-9 mediated apoptosis. It was found that xanthohumol induced ROS generation subsequently resulted in its effects on mitochondrial mediated apoptotic events [79].
3.10. Shikonin
Shikonin is a natural naphthoquinone pigment originated from Lithospermum erythrorhizon, a plant which is frequently used in traditional Chinese medicine for the treatment of various inflammatory conditions. Recent pharmacological reports suggest that shikonin exhibits strong cytotoxic action in numerous types of malignancies. Selective accumulation of Shikonin in mitochondria and its disruption of mitochondrial membrane potential were one of the major mechanisms of shikonin induced cell death in U937 leukemia cell line [80]. Study report by CH Chen et al., indicated that a similar mitochondria dependent mechanism has been involved in antitumor activity of shikonin in glioma cell lines. Shikonin treatment induced ROS generation which eventually leads to disruption of mitochondrial membrane potential. Shikonin triggered disruption of complex II of mitochondrial electron transport chain that has led to generation of mitochondrial originated superoxide radicals. ROS generation also resulted in depletion of GSH, down regulation of catalase along with up regulation of SOD1, modulation of Bcl-2 family apoptosis related proteins [81, 82].
3.11. Resveratrol
Resveratrol, chemically trans-3,4,5-trihydroxystilbene is a dietary phytoalexin present in abundant amounts in grapes, peanuts, red wine etc. Accumulating evidence suggest that resveratrol exhibits multiple pharmacological effects such as antioxidant, anti-inflammatory, anti-aging, chemopreventive and chemotherapeutic effects [83]. Resveratrol induced cell death through triggering apoptosis in u251 glioma cell lines by mitochondrial dependent pathway along with other mechanisms such as cell cycle arrest. Exposure to resveratrol has triggered release of cytochrome c, activated caspase 9 along with up regulation and translocation of pro-apoptotic family member Bax to mitochondria [84].
3.12. Quercetin
Quercetin is a dietary flavanoid that is present in large amounts in various fruits, vegetables, nuts and seeds. Pharmacological studies revealed the potential of quercetin as anticancer agent in many tumor models by multiple mechanisms [85]. Quercetin has exhibited potent antitumor action in the U373 glioma cells by inhibition of proliferation and induction of apoptosis after 48, 72 hr of treatment. The apoptosis has been mediated through mitochondria as evident by increase in activities of caspase 9 and caspase 3 and increase in cleavage of poly ADP ribose polymerase (PARP) protein. Quercetin treatment induced loss of mitochondrial membrane potential along with up regulation and translocation of p53 to mitochondria which further results in the release of cytochrome c from mitochondria. Experimental findings suggested that quercetin at initial stages promoted cytoprotective autophagy as combination with autophagy inhibitor chloroquine further enhanced its apoptosis effects through both intrinsic and extrinsic pathways [86]. However, in a recent study by LL Zamin et al., reported that quercetin treatment at 50mg/kg/day has promoted tumor growth in one rat glioma model. The contrasting effects between in vitro and in vivo models could be due to several reasons such as low concentration of quercetin in brain after treatment (0.53 µM in brain tissue), modulating effects on immune system, low stability and formation of conjugates of quercetin in vivo [87].
3.13. Hydroxygenkwanin
Hydroxygenkwanin is one of the bioactive flavanoids isolated from the flower buds of Daphne genkwa, a plant that is frequently used in traditional Chinese medicine for variety of inflammatory ailments [88]. Hydoxygenkwonin alone, or its combination with apigenin, another flavanoid has displayed antiglioma effects in vitro by mitochondria modulating effects. In combination treatment and alone, they exerted anti-proliferative, apoptotic effects on rat c6 glioma cell lines by loss of mitochondrial membrane potential, mitochondrial damage such as swelling of mitochondria and disappearance of cristae. At molecular level, over expression of Bid, Bak along with down regulation of Bcl-xl proteins were observed in this treatment approach [89].
3.14. Alantolactone
Alantolactone is a sesquiterpene lactone compound, isolated from many medicinal plant species such as Inula helinium, Inula japonica, Aucklandia lappa, Radix Inulae etc. Alantolactone has shown wide spectrum of pharmacological effects such as antimicrobial, antifungal, anti inflammatory and anticancer effects [90]. In a recent study, M Khan et al., reported that alantolactone has exhibited growth inhibitory effects in vitro in U87, U373, LN229 glioma cell lines by induction of oxidative stress and mitochondrial dysfunction. In U87 cell line, alantolactone has triggered apoptotic cell death by generation of ROS that in turn leading to depletion of GSH, dissipation of mitochondrial membrane potential, oxidation of cardiolipin all of which culminates in cell death. At molecular level, alantolactone treatment caused up regulation of p53, Bax and down regulation of Bcl2 along with release of cytochrome c. Furthermore, alantolactone has increased activities of caspase 9, 3 indicating mitochondria mediated apoptosis. In vivo studies proved that alantolactone crosses blood brain barrier and didn’t exert any hepatotoxic, nephrotoxic effects indicating its promise as effective chemotherapeutic agent in treatment of gliomas [91].
3.15. Kaempferol
Kaempferol, a phytoestrogen flavanoid present in fruits and vegetables has been reported to possess wide variety of biological effects including antioxidant, anti-inflammatory, chemopreventive and chemotherapeutic effects [92]. Kaempferol has been found to induce apoptosis in U87MG, LN229, T98G glioma cells. The anticancer effects of kaempferol in glioma might be due to elevation of intracellular oxidative stress, suppression of oxidant scavengers super oxide dismutase (SOD-1), thioredoxin (TRX-I) and loss of mitochondrial membrane potential, decreased expression of Bcl2. In addition to that, Kaempferol inhibited migration of glioma cells through ROS modulation effect. Furthermore, Kaempferol augmented doxorubicin cytotoxic effect through enhancement of ROS levels and decreasing cellular efflux of doxorubicin [93].
3.16. Honokiol
Honokiol, a bioactive constituent isolated from genus Mangolia has been reported to exhibit multiple pharmacological actions including anti-angiogenesis, anti-inflammatory, anti-proliferative effects. In a study in DBTRG-05MG glioblastoma cells, honokiol has found to induce apoptosis through mitochondria mediated apoptotic pathway. Honokiol treatment induced ROS generation, enhanced intracellular Ca++ release, reduced mitochondrial membrane potential, released cytochrome c into cytosol and increased activities of caspase 9,3 [94]. In another study, J J Jeong et al., has shown that honokiol also modulated Bcl2 apoptotic family proteins in the induction of apoptosis. Furthermore, honokiol is proved to have the ability to cross the blood brain barrier (BBB), and blood- cerebrospinal fluid (BCSFB) suggesting its strong potential in the therapy of malignant brain tumors [95].
3.17. Parthenolide
Parthenolide, a sesquiterpene lactone is the principal bioactive constituent from plant Tanacetum parthenium (Feverfew) [96]. It inhibits nuclear factor light chain kappa beta (NF-kB) by inhibiting inhibitory kappa beta (IkB) kinase as well as by direct binding to p65 in the NF-kB complex. Parthenolide has displayed strong anti-invasion, anti-angiogenesis and anti-proliferative effects in glioblastoma cells in vitro. The involvement of mitochondrial mediated signaling in apoptosis induction effect by parthenolide has been evident by the decrease in mitochondrial membrane potential, release of cytochrome c along with activation of caspases 9 and 3. The over expression of Bax, Bak, including reducing expression of Bcl2 also indicate the role of mitochondria in the cytotoxic effects of parthenolide. Furthermore, in vivo intracerebral glioblastoma xenograft mouse model, parthenolide effectively reduced tumor growth, angiogenesis processes indicating its possible use in glioblastoma therapy [97, 98].
3.18. Phloretin
Phloretin, a dihydrochalcone flavanoid present abundantly in apples has known to possess many interesting biological properties such as anti-atherosclerotic, antioxidant, antimicrobial activities [99, 100]. Phloretin is a well known BK channel (functional big potassium channels) activator and has been reported to cause cytotoxic effect in U251, T9 glioma cells. Phloretin induced cellular swelling and vacuolization at initial hours of treatment along with mitochondrial and endoplasmic reticulum swelling due to osmotic imbalance created by opening of BK channels. This damage on mitochondria leads to loss of ATP, over expression of heat shock proteins Hsp 60, 70, 90 which progressed to cytotoxic effect in glioma cells and release of danger signals such as High mobility group box-1 (HMGB-1) protein which increases tumor immunity [101, 102].
3.19. Gossypol
Gossypol, a natural polyphenolic compound obtained from cotton seeds is reported as BH3 mimetic with Pan Bcl-2 inhibitory action. Bcl2 family proteins suppress both apoptosis and autophagy. Pharmacological investigations have revealed that gossypol induces autophagic cell death along with inducing mitochondrial dysfunction in U87, U373, MZ-54 glioblastoma cell lines. Gossypol treatment caused decrease in mitochondrial membrane potential along with release of cytochrome c [103]. In addition to that, gossypol augmented the cytotoxic action of Temozolimide in vivo glioma DBTRG-05MG models and also showed anti angiogenesis and anti invasive properties alone and in combination in vitro [104]. Gossypol was tested in clinical trials in patients of glial tumors and proved to be well tolerated at the dose of 10mg bid PO. This treatment displayed low, measurable response in already pretreated patients with recurrent tumors that are associated with poor prognosis [105]. Moreover, gossypol was also tested in other 2 early phase clinical trials I) as single agent in phase 2 trial in patients of progressive / recurrent GBM (NCT00540722), II) in combination with Temozolimide (TMZ) with/ without radiation therapy in Phase 1 trial in patients of newly diagnosed GBM (NCT0I0390403). Although these clinical trials were completed, so far results were not accessible [104].
3.20. Berberine
Berberine is a natural isoquinoline alkaloid isolated from various medicinal herbs Berberis vulgaris, Berberis aquifolium, Berberis aristata, Hydrastis canadensis and Tinospora cordifolia. It displayed wide variety of pharmacological effects including antioxidant, antimicrobial, anthelmintic and antitumor effects [106]. Berberine has shown remarkable anti glioma effects in various in vitro and in vivo models by multiple mechanisms such as mitochondria mediated apoptosis, G1 cell cycle arrest and induction of senescence and endoplasmic reticulum stress. In T98G cells, berberine has reduced cell viability through enhanced ROS generation and increase in intracellular Ca2+ levels. Significant activation of mitochondria dependent apoptotic pathway has also been observed in berberine treated T98G cells as evident by loss of mitochondrial membrane potential, enhanced Bax/Bcl-2 ratio and increase in activities of caspase 9,3. Furthermore, berberine treatment also showed anti-invasive and anti-metastasis properties and potently inhibited growth of tumor xenografts [107-109]. In a recent study, mitochondriotropic derivatives of berberine, synthesized by addition of moderate length alkyl chains have shown efficacy in inhibition of glioma cell proliferation at low micromolar range along with potent suppression of invasion and metastasis. These derivatives have enhanced lipophilicity and localized into mitochondria and triggered ROS generation [110].
Growing literature suggests the prooxidant property of the phytoconstituents i.e., the ability to oxidize the cellular system depend upon numerous factors such as concentration, presence of metal ions and pH [111].Usually at higher concentrations and in the presence of metal ions, phytochemicals exhibit this property generating enhanced amounts of ROS in in vitro biological systems. For instance, curcumin at 25-100 µM significantly increased cellular ROS levels in human hepatoma Hep3B cells in time dependent manner [112]. This prooxidant property is recently attributed as one of major mechanisms for the antitumor properties of plant polyphenols [113]. Moreover, the selective cytotoxicity of many phytoconstituents might be mediated through prooxidant action of these compounds as cancer cells are more vulnerable to ROS mediated cytotoxicity than their normal counterparts [114].
4. NATURAL COMPOUNDS FOR THE TREATMENT OF GLIOMAS: TRANSLATIONAL HURDLES
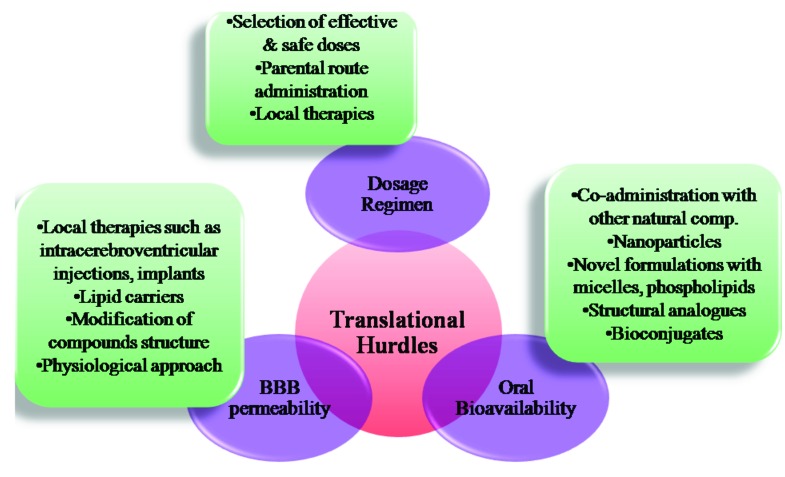
Hitherto, most of these natural compounds except gossypol are under preclinical evaluation for their efficacy for anti-glioma activity, safety and their effects on metabolism. Translation of these possible chemotherapeutic agents from bench to clinic for the treatment of gliomas offers many challenges such as bioavailability, BBB permeability, selection of optimal dosage regimen etc. According to the new stringent regulations of US patent and trademark office (USPTO) for biological patents, complete dosage regimen for treatment of a disease should be established for a natural compound which is both expensive and time consuming to make them patentable. Hence battery of preclinical tests on various aspects such as pharmacokinetics, pharmacodynamics, toxicity effects both in vitro and in vivo including genotoxicity, mutagenicity and safety pharmacology are needed to be performed to get approved by food and drug administration (FDA) and to enter into clinical markets [115]. Growing body of evidence suggest that many of these aforementioned natural products are well tolerated without significant organ toxicities in both animal and human studies at pharmacological doses [116, 114]. However, dosage schedule and route of administration are also crucial to ascertain bioavailability, efficacy and patient compliance in clinical trials without significant toxicities and thus need to be carefully determined.
Poor oral bioavailability is a major obstacle for a number of natural products for their clinical utility [117]. Solubility, GI membrane permeability, metabolism and chemical stability are contributed as important factors for their low bioavailability. For instance, Results from both human and animal studies demonstrate the lower plasma concentrations of curcumin after its oral intake. Poor aqueous solubility, rapid metabolism and rapid systemic elimination are prime factors contributing for less bioavailability of curcumin [118]. Many researchers have made several attempts to enhance the bioavailability of the natural compounds by various approaches such as co-administration with other natural compounds like piperine, nanoparticle administration, novel formulations with use of micelles, phospholipid complexes and development of structural analogues and bioconjugates [119]. Parenteral route of administration eliminates the need for the proper aqueous solubility and permeability through gastrointestinal membrane hence can be useful in the cases where natural compounds have poor oral absorption. However, in parental routes of administration such as i.p and i.v routes, half-lives of compounds in the plasma are affected by the rate of metabolism and excretion and their tissue distribution patterns. In many cases, these polyphenolic natural compounds undergo phase II metabolism and these conjugates have reasonable half lives in plasma and can hydrolyse to parent compounds thus ensuring clinical efficacy [117]. The clinical application of these aforementioned approaches and alternative route of administrations might enhance the bioavailable fraction of the administered compound and improve their biological efficacy in the clinical studies.
Another important factor for successful efficacious therapy in gliomas is the drug’s ability to cross BBB - physiological barrier formed by tight junctions between capillary endothelial cells. Only small molecules (usually 400- 500 Daltons) with appropriate lipophilic nature and charge diffuse through BBB while large compounds with >1 Kilodaltons are unable to permeate through BBB [120]. Several delivery approaches were investigated to enhance the delivery of the compounds through BBB. Among them, invasive approach includes various methods such as physically breaching the BBB and injecting or implanting the drug into the brain by intra cerebroventricular injection, convention enhanced delivery and use of intracerebral implants. Moreover, modifications of compound’s structure to enhance its lipophilicity, usage of lipid carriers were also successfully employed to bypass BBB. An alternative approach; physiological approach takes advantage of the receptors expressed in BBB to facilitate receptor mediated transcytosis. Tagging with endogenous ligands or antibodies targeting to those receptors such as transferrin receptor, insulin receptor, LDL receptor, nicotinic acetylcholine receptors were evaluated to augment the brain delivery of therapeutic compounds [121, 122].
Local delivery of the drugs into gliomas by methods such as either direct bolus injection or continuous infusion and controlled release methods by using carriers allow direct delivery of drug into tumors achieving maximum efficacy [123]. This method offers advantages such as lower systemic toxicity due to minimal dose requirement and allows entry of the hydrophilic large molecular drugs to tumors that are having lower BBB diffusion properties. Few chemotherapeutic drugs and proteins like TNF-α were injected into gliomas directly in clinical trials. However none of the aforementioned phytoconstituents were directly administered into gliomas [124]. The disadvantages of this administration include lower patient compliance, possible chances of intracranial hemorrhage, central nervous system toxicity and infection. These limitations can be possibly avoided by selecting optimal dosage schedule and utility of the best surgical practices and patient care and the use of advanced methods including drug impregnated microchips, local gene therapy [125]. Thus the testing of novel compounds in in vitro and in vivo models to check their ability to cross BBB and implementation of strategies such as local delivery with novel methods to enhance their delivery into brain is necessary to achieve the desirable therapeutic outcome in the treatment of brain tumors.
The aforestated clinical challenges if addressed properly by conducting large scale controlled clinical trials with these natural compounds paves a way for them to clinic. All of these phytoconstituents exhibited potent anti-glioma action in preclinical studies through multitude of biological effects such as decrease in cell viability, apoptosis induction, inhibition of invasion and migration. Moreover, they also enhance the anticancer activity in the gliomas when given in combination with other standard drugs such as temozolimide and TRAIL. Interestingly functional expression of P-glycoprotein pump (P-gp), an efflux pump that extrudes the drugs has been observed in brain endothelial cells in BBB [126]. Further, enhancement of the CNS effects of drugs such as loperamide, methadone (P-gp substrates) was observed when they are co-administered with the P-gp inhibitors like quinidine, verapamil [127, 128]. As numerous phytoconstituents such as quercetin, kaempferol were reported to inhibit P-gp, possible consideration of combination strategies of chemotherapeutic agents that are P-gp substrates along with these phytochemicals might be beneficial in increasing the permeability of the anticancer therapeutics thus may exhibit synergistic anticancer effects [129]. In the view of these attributes, these phytocostituents can be directly used as drug or adjuvant therapy in combination with other standard chemotherapeutic agents to achieve desirable therapeutic outcome.
5. FUTURE DIRECTIONS
Recent studies have focused on the discovery and synthesis of compounds that are capable of selectively accessing mitochondria - mitochondriotropics- with aim of either inducing or preventing cellular death [130]. Mitocans- one class of such mitochondriotropics- are the anticancer agents that selectively target the mitochondria, destabilize them and thus exhibit cytotoxicity [131]. Many natural products target mitochondria at multiple nodes such as direct interaction with key molecules in mitochondria or indirect alteration of metabolic consequences arise as a result of mitochondrial dysfunction. Further selective targeting of such natural compounds to enhance their efficacy as antitumor agents has been achieved by tagging them with membrane –permeant cations such as triphenyl phosphonium (TPP) which selectively localizes into regions of negative potential. Recently, such mitochondria targeted analogues of natural compounds like resveratrol, quercetin, vitamin E were prepared and reported to have enhanced selective cytotoxic action owing to their enhanced ROS generation and specific targeting of mitochondrial components in variety of malignancies [132-134]. Future investigations of such mitocans are highly warranted in both in vitro and in vivo models of gliomas. Clinical success of anticancer agents also depends upon an important factor “therapeutic index” i.e., the selectivity between targeting cancer and normal cells. Hence identification and exploitation of differences between cancer cells and normal cells such as elevated ROS, abnormal antioxidant system balance, deregulated mitochondria mediated apoptotic signaling in cancer cells when compared to normal cells are necessary for the safe and efficacious therapy in targeting mitochondrial dysfunction.
6. CONCLUDING REMARKS
Mitochondria are attractive pharmacological targets owing to their notable roles in cellular metabolism, regulation of redox signaling, ion homeostasis and induction of apoptotic cell death mechanism. Gliomas frequently harbor mitochondrial alterations such as shift from oxidative phosphorylation to glycolysis (Warburg effect), enhanced oxidative stress, abnormalities in mitochondrial membrane potential and apoptotic machinery, mitochondrial genome mutations along with somatic mutations in tricarboxylic acid cycle gene IDH. Plant derived phytochemicals target mitochondria by either directly binding to mitochondrial components or indirectly affecting metabolic alterations as a result of mitochondrial dysfunction. Targeting and disruption of mitochondrial electron transport chain by selective inhibition of various electron transport chain complexes, enhancement of ROS levels, disruption of cellular antioxidant system, targeting of Bcl2 anti-apoptotic family proteins and ion channels in the membrane of mitochondria are some identified mechanisms of action of mitochondria targeted natural compounds. These natural compounds have shown promise in treatment of gliomas in in vitro and in vivo models by their mitochondria specific effects and thus warrant further preclinical and clinical development in the treatment of gliomas. Critical observation of pharmacokinetics, bioavailability and tissue distribution patterns of each compound and implementation of innovative methods to enhance their bioavailability are highly warranted for their clinical success.
Fig. (3).
Traslational challenges and possible solutions for the natural compounds to enter clinic for the management of gliomas (BBB- Blood Brain Barrier).
ACKNOWLEDGEMENTs
Authors would like to thank Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India for financial support for Research scholars Lalita Guntuku and Veera Ganesh Yerra.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.GLOBOCAN 2012 v1.0. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. International Agency for Research on Cancer. 2013 [Google Scholar]
- 2.Ostrom Q.T., Gittleman H., Liao P., Rouse C., Chen Y., Dowling J., Wolinsky Y., Kruchko C., Barnholtz-Sloan J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro-oncol. 2014;16(Suppl. 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [http://dx.doi.org/10.1093/ neuonc/nou223]. [PMID: 25304271]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edick M.J., Cheng C., Yang W., Cheok M., Wilkinson M.R., Pei D., Evans W.E., Kun L.E., Pui C.H., Relling M.V. Lymphoid gene expression as a predictor of risk of secondary brain tumors. Genes Chromosomes Cancer. 2005;42(2):107–116. doi: 10.1002/gcc.20121. [http://dx.doi.org/10.1002/gcc.20121]. [PMID: 15543619]. [DOI] [PubMed] [Google Scholar]
- 4.Ostrom Q.T., Gittleman H., Stetson L., Virk S.M., Barnholtz-Sloan J.S. Current Understanding and Treatment of Gliomas. Springer; 2015. Epidemiology of gliomas. pp. 1–14. [DOI] [PubMed] [Google Scholar]
- 5.Ohka F., Natsume A., Wakabayashi T. Current trends in targeted therapies for glioblastoma multiforme. 2012. [DOI] [PMC free article] [PubMed]
- 6.Brennan C., Momota H., Hambardzumyan D., Ozawa T., Tandon A., Pedraza A., Holland E. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One. 2009;4(11):e7752. doi: 10.1371/journal.pone.0007752. [http://dx.doi.org/10.1371/journal.pone.0007752]. [PMID: 19915670]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhaak R.G., Hoadley K.A., Purdom E., Wang V., Qi Y., Wilkerson M.D., Miller C.R., Ding L., Golub T., Mesirov J.P., Alexe G., Lawrence M., O’Kelly M., Tamayo P., Weir B.A., Gabriel S., Winckler W., Gupta S., Jakkula L., Feiler H.S., Hodgson J.G., James C.D., Sarkaria J.N., Brennan C., Kahn A., Spellman P.T., Wilson R.K., Speed T.P., Gray J.W., Meyerson M., Getz G., Perou C.M., Hayes D.N. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [http://dx.doi.org/10.1016/ j.ccr.2009.12.020]. [PMID: 20129251]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conroy S., Kruyt F.A., Joseph J.V., Balasubramaniyan V., Bhat K.P., Wagemakers M., Enting R.H., Walenkamp A.M., den Dunnen W.F. Subclassification of newly diagnosed glioblastomas through an immunohistochemical approach. PLoS One. 2014;9(12):e115687. doi: 10.1371/journal.pone.0115687. [http://dx.doi.org/10.1371/journal.pone.0115687]. [PMID: 25546404]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motomura K., Natsume A., Watanabe R., Ito I., Kato Y., Momota H., Nishikawa R., Mishima K., Nakasu Y., Abe T., Namba H., Nakazato Y., Tashiro H., Takeuchi I., Mori T., Wakabayashi T. Immunohistochemical analysis-based proteomic subclassification of newly diagnosed glioblastomas. Cancer Sci. 2012;103(10):1871–1879. doi: 10.1111/j.1349-7006.2012.02377.x. [http://dx.doi.org/10.1111/j.1349-7006. 2012.02377.x]. [PMID: 22747609]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranjit M., Motomura K., Ohka F., Wakabayashi T., Natsume A. Applicable advances in the molecular pathology of glioblastoma. Brain Tumor Pathol. 2015;32(3):153–162. doi: 10.1007/s10014-015-0224-6. [http://dx.doi.org/ 10.1007/s10014-015-0224-6]. [PMID: 26078107]. [DOI] [PubMed] [Google Scholar]
- 11.McLendon R., Friedman A., Bigner D., Van Meir E.G., Brat D.J., Mastrogianakis G.M., Olson J.J., Mikkelsen T., Lehman N., Aldape K. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [http://dx.doi.org/10.1038/nature07385]. [PMID: 18772890]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Groot J.F., Gilbert M.R. New molecular targets in malignant gliomas. Curr. Opin. Neurol. 2007;20(6):712–718. doi: 10.1097/WCO.0b013e3282f15650. [http://dx.doi. org/10.1097/WCO.0b013e3282f15650]. [PMID: 17992095]. [DOI] [PubMed] [Google Scholar]
- 13.Brown G.C., Cooper C.E., Nicholls D.G. Mitochondria and cell death. Portland. 1999;7(1):134–135. [Google Scholar]
- 14.Barbosa I.A., Machado N.G., Skildum A.J., Scott P.M., Oliveira P.J. Mitochondrial remodeling in cancer metabolism and survival: potential for new therapies. Biochimica et Biophysica Acta (BBA)-. Rev. Can. 2012;1826(1):238–254. doi: 10.1016/j.bbcan.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg S.E., Chandel N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015;11(1):9–15. doi: 10.1038/nchembio.1712. [http://dx.doi.org/10.1038/nchembio.1712]. [PMID: 25517383]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsetos C.D., Anni H., Dráber P. Mitochondrial dysfunction in gliomas. Semin. Pediatr. Neurol. 2013;20(3):216–227. doi: 10.1016/j.spen.2013.09.003. [http://dx.doi.org/10.1016/j.spen.2013.09.003]. [PMID: 24331363]. [DOI] [PubMed] [Google Scholar]
- 17.Arismendi-Morillo G. Electron microscopy morphology of the mitochondrial network in gliomas and their vascular micro- environment. Biochimica et Biophysica Acta (BBA)-. Bioenergetics. 2011;1807(6):602–608. doi: 10.1016/j.bbabio.2010.11.001. [http://dx.doi.org/10.1016/j.bbabio.2010. 11.001]. [DOI] [PubMed] [Google Scholar]
- 18.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [http://dx. doi.org/10.1126/science.1160809]. [PMID: 19460998]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seyfried T.N., Sanderson T.M., El-Abbadi M.M., McGowan R., Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br. J. Cancer. 2003;89(7):1375–1382. doi: 10.1038/sj.bjc.6601269. [http://dx.doi.org/10.1038/sj.bjc.6601269]. [PMID: 14520474]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nebeling L.C., Miraldi F., Shurin S.B., Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J. Am. Coll. Nutr. 1995;14(2):202–208. doi: 10.1080/07315724.1995.10718495. [http://dx.doi.org/10.1080/07315724.1995. 10718495]. [PMID: 7790697]. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee P., Abate L.E., Seyfried T.N. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin. Cancer Res. 2004;10(16):5622–5629. doi: 10.1158/1078-0432.CCR-04-0308. [http://dx.doi.org/10.1158/1078-0432.CCR-04-0308]. [PMID: 15328205]. [DOI] [PubMed] [Google Scholar]
- 22.Jelluma N., Yang X., Stokoe D., Evan G.I., Dansen T.B., Haas-Kogan D.A. Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol. Cancer Res. 2006;4(5):319–330. doi: 10.1158/1541-7786.MCR-05-0061. [http://dx.doi.org/10.1158/1541-7786.MCR-05-0061]. [PMID: 16687487]. [DOI] [PubMed] [Google Scholar]
- 23.Leonardi R., Subramanian C., Jackowski S., Rock C.O. Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. J. Biol. Chem. 2012;287(18):14615–14620. doi: 10.1074/jbc.C112.353946. [http://dx.doi.org/10.1074/jbc.C112.353946]. [PMID: 22442146]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balss J., Meyer J., Mueller W., Korshunov A., Hartmann C., von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116(6):597–602. doi: 10.1007/s00401-008-0455-2. [http://dx.doi.org/10.1007/s00401-008-0455-2]. [PMID: 18985363]. [DOI] [PubMed] [Google Scholar]
- 25.Ward P.S., Patel J., Wise D.R., Abdel-Wahab O., Bennett B.D., Coller H.A., Cross J.R., Fantin V.R., Hedvat C.V., Perl A.E., Rabinowitz J.D., Carroll M., Su S.M., Sharp K.A., Levine R.L., Thompson C.B. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17(3):225–234. doi: 10.1016/j.ccr.2010.01.020. [http://dx.doi.org/10.1016/j.ccr.2010.01.020]. [PMID: 20171147]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krell D., Assoku M., Galloway M., Mulholland P., Tomlinson I., Bardella C. Screen for IDH1, IDH2, IDH3, D2HGDH and L2HGDH mutations in glioblastoma. PLoS One. 2011;6(5):e19868. doi: 10.1371/journal.pone.0019868. [http://dx.doi.org/10.1371/journal.pone.0019868]. [PMID: 21625441]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evan G.I., Vousden K.H. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342–348. doi: 10.1038/35077213. [http://dx.doi.org/10. 1038/35077213]. [PMID: 11357141]. [DOI] [PubMed] [Google Scholar]
- 28.Johnstone R.W., Ruefli A.A., Lowe S.W. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108(2):153–164. doi: 10.1016/s0092-8674(02)00625-6. [http://dx.doi.org/10.1016/S0092-8674(02)00625-6]. [PMID: 11832206]. [DOI] [PubMed] [Google Scholar]
- 29.Villunger A., Michalak E.M., Coultas L., Müllauer F., Böck G., Ausserlechner M.J., Adams J.M., Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302(5647):1036–1038. doi: 10.1126/science.1090072. [http://dx.doi. org/10.1126/science.1090072]. [PMID: 14500851]. [DOI] [PubMed] [Google Scholar]
- 30.Reifenberger J., Ring G.U., Gies U., Cobbers L., Oberstrass J., An H-X., Niederacher D., Wechsler W., Reifenberger G. Analysis of p53 mutation and epidermal growth factor receptor amplification in recurrent gliomas with malignant progression. J. Neuropathol. Exp. Neurol. 1996;55(7):822–831. doi: 10.1097/00005072-199607000-00007. [http://dx.doi. org/10.1097/00005072-199607000-00007]. [PMID: 8965097]. [DOI] [PubMed] [Google Scholar]
- 31.Krajewski S., Krajewska M., Ehrmann J., Sikorska M., Lach B., Chatten J., Reed J.C. Immunohistochemical analysis of Bcl-2, Bcl-X, Mcl-1, and Bax in tumors of central and peripheral nervous system origin. Am. J. Pathol. 1997;150(3):805–814. [PMID: 9060818]. [PMC free article] [PubMed] [Google Scholar]
- 32.Nakasu S., Nakasu Y., Nioka H., Nakajima M., Handa J. bcl-2 protein expression in tumors of the central nervous system. Acta Neuropathol. 1994;88(6):520–526. doi: 10.1007/BF00296488. [http://dx.doi.org/10.1007/ BF00296488]. [PMID: 7879598]. [DOI] [PubMed] [Google Scholar]
- 33.Libermann T.A., Nusbaum H.R., Razon N., Kris R., Lax I., Soreq H., Whittle N., Waterfield M.D., Ullrich A., Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313(5998):144–147. doi: 10.1038/313144a0. [http://dx.doi.org/10. 1038/313144a0]. [PMID: 2981413]. [DOI] [PubMed] [Google Scholar]
- 34.Ordys B.B., Launay S., Deighton R.F., McCulloch J., Whittle I.R. The role of mitochondria in glioma pathophysiology. Mol. Neurobiol. 2010;42(1):64–75. doi: 10.1007/s12035-010-8133-5. [http://dx.doi.org/10.1007/s12035-010-8133-5]. [PMID: 20414816]. [DOI] [PubMed] [Google Scholar]
- 35.Schlame M., Rua D., Greenberg M.L. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000;39(3):257–288. doi: 10.1016/s0163-7827(00)00005-9. [http://dx.doi.org/10.1016/S0163-7827(00)00005-9]. [PMID: 10799718]. [DOI] [PubMed] [Google Scholar]
- 36.Fry M., Green D.E. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J. Biol. Chem. 1981;256(4):1874–1880. [PMID: 6257690]. [PubMed] [Google Scholar]
- 37.Kiebish M.A., Han X., Cheng H., Chuang J.H., Seyfried T.N. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J. Lipid Res. 2008;49(12):2545–2556. doi: 10.1194/jlr.M800319-JLR200. [http://dx.doi.org/10.1194/jlr.M800319-JLR200]. [PMID: 18703489]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carré M., André N., Carles G., Borghi H., Brichese L., Briand C., Braguer D. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J. Biol. Chem. 2002;277(37):33664–33669. doi: 10.1074/jbc.M203834200. [http://dx. doi.org/10.1074/jbc.M203834200]. [PMID: 12087096]. [DOI] [PubMed] [Google Scholar]
- 39.Sheldon K.L., Maldonado E.N., Lemasters J.J., Rostovtseva T.K., Bezrukov S.M. Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PLoS One. 2011;6(10):e25539. doi: 10.1371/journal.pone.0025539. [http://dx.doi.org/10.1371/ journal.pone.0025539]. [PMID: 22022409]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mariani M., Shahabi S., Sieber S., Scambia G., Ferlini C. Class III β-tubulin (TUBB3): more than a biomarker in solid tumors? Curr. Mol. Med. 2011;11(9):726–731. doi: 10.2174/156652411798062368. [http://dx.doi.org/10.2174/ 156652411798062368]. [PMID: 21999149]. [DOI] [PubMed] [Google Scholar]
- 41.Maldonado E.N., Patnaik J., Mullins M.R., Lemasters J.J. Free tubulin modulates mitochondrial membrane potential in cancer cells. Cancer Res. 2010;70(24):10192–10201. doi: 10.1158/0008-5472.CAN-10-2429. [http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2429]. [PMID: 21159641]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedman J.R., Webster B.M., Mastronarde D.N., Verhey K.J., Voeltz G.K. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J. Cell Biol. 2010;190(3):363–375. doi: 10.1083/jcb.200911024. [http://dx.doi.org/10.1083/jcb.200911024]. [PMID: 20696706]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levine B. Cell biology: autophagy and cancer. Nature. 2007;446(7137):745–747. doi: 10.1038/446745a. [http://dx.doi.org/10.1038/446745a]. [PMID: 17429391]. [DOI] [PubMed] [Google Scholar]
- 44.Kim E.H., Choi K.S. A critical role of superoxide anion in selenite-induced mitophagic cell death. Autophagy. 2008;4(1):76–78. doi: 10.4161/auto.5119. [http://dx.doi.org/10.4161/auto.5119]. [PMID: 17952022]. [DOI] [PubMed] [Google Scholar]
- 45.Gong A., Ye S., Xiong E., Guo W., Zhang Y., Peng W., Shao G., Jin J., Zhang Z., Yang J., Gao J. Autophagy contributes to ING4-induced glioma cell death. Exp. Cell Res. 2013;319(12):1714–1723. doi: 10.1016/j.yexcr.2013.05.004. [http://dx.doi.org/10.1016/j.yexcr.2013.05.004]. [PMID: 23684856]. [DOI] [PubMed] [Google Scholar]
- 46.Menon V.P., Sudheer A.R. The molecular targets and therapeutic uses of curcumin in health and disease. Springer; 2007. Antioxidant and anti-inflammatory properties of curcumin. pp. 105–125. [http://dx.doi.org/10.1007/978-0-387-46401-5_3] [DOI] [PubMed] [Google Scholar]
- 47.Youns M., Fathy G.M. Upregulation of extrinsic apoptotic pathway in curcumin-mediated antiproliferative effect on human pancreatic carcinogenesis. J. Cell. Biochem. 2013;114(12):2654–2665. doi: 10.1002/jcb.24612. [http://dx.doi.org/10.1002/jcb.24612]. [PMID: 23794119]. [DOI] [PubMed] [Google Scholar]
- 48.Karmakar S., Banik N.L., Ray S.K. Curcumin suppressed anti-apoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells. Neurochem. Res. 2007;32(12):2103–2113. doi: 10.1007/s11064-007-9376-z. [http://dx.doi.org/10.1007/s11064-007-9376-z]. [PMID: 17562168]. [DOI] [PubMed] [Google Scholar]
- 49.Gao X., Deeb D., Jiang H., Liu Y.B., Dulchavsky S.A., Gautam S.C. Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of procaspases and release of cytochrome c from mitochondria. J. Exp. Ther. Oncol. 2005;5(1):39–48. [PMID: 16416600]. [PubMed] [Google Scholar]
- 50.Karmakar S., Banik N.L., Patel S.J., Ray S.K. Curcumin activated both receptor-mediated and mitochondria-mediated proteolytic pathways for apoptosis in human glioblastoma T98G cells. Neurosci. Lett. 2006;407(1):53–58. doi: 10.1016/j.neulet.2006.08.013. [http://dx.doi.org/10. 1016/j.neulet.2006.08.013]. [PMID: 16949208]. [DOI] [PubMed] [Google Scholar]
- 51.Morin D., Barthélémy S., Zini R., Labidalle S., Tillement J-P. Curcumin induces the mitochondrial permeability transition pore mediated by membrane protein thiol oxidation. FEBS Lett. 2001;495(1-2):131–136. doi: 10.1016/s0014-5793(01)02376-6. [http://dx.doi.org/10.1016/S0014-5793(01) 02376-6]. [PMID: 11322961]. [DOI] [PubMed] [Google Scholar]
- 52.Sinha S., Pal B.C., Jagadeesh S., Banerjee P.P., Bandyopadhaya A., Bhattacharya S. Mahanine inhibits growth and induces apoptosis in prostate cancer cells through the deactivation of Akt and activation of caspases. Prostate. 2006;66(12):1257–1265. doi: 10.1002/pros.20415. [http://dx.doi.org/10.1002/pros.20415]. [PMID: 16683271]. [DOI] [PubMed] [Google Scholar]
- 53.Roy M.K., Thalang V.N., Trakoontivakorn G., Nakahara K. Mahanine, a carbazole alkaloid from Micromelum minutum, inhibits cell growth and induces apoptosis in U937 cells through a mitochondrial dependent pathway. Br. J. Pharmacol. 2005;145(2):145–155. doi: 10.1038/sj.bjp.0706137. [http://dx.doi.org/10.1038/sj.bjp.0706137]. [PMID: 15753952]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhattacharya K., Bag A.K., Tripathi R., Samanta S.K., Pal B.C., Shaha C., Mandal C. Mahanine, a novel mitochondrial complex-III inhibitor induces G0/G1 arrest through redox alteration-mediated DNA damage response and regresses glioblastoma multiforme. Am. J. Cancer Res. 2014;4(6):629–647. [PMID: 25520856]. [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y-R., Han J., Kori R., Kong A-N., Tan T-H. Phenylethyl isothiocyanate induces apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J. Biol. Chem. 2002;277(42):39334–39342. doi: 10.1074/jbc.M202070200. [http://dx.doi.org/10.1074/ jbc.M202070200]. [PMID: 12171915]. [DOI] [PubMed] [Google Scholar]
- 56.Moon Y.J., Brazeau D.A., Morris M.E. Dietary phenethyl isothiocyanate alters gene expression in human breast cancer cells. Evid. Based Complement. Alternat. Med. 2010 doi: 10.1155/2011/462525. [PMID: 20953429]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y., Wei S., Wang J., Fang Q., Chai Q. Phenethyl isothiocyanate inhibits growth of human chronic myeloid leukemia K562 cells via reactive oxygen species generation and caspases. Mol. Med. Rep. 2014;10(1):543–549. doi: 10.3892/mmr.2014.2167. [PMID: 24788892]. [DOI] [PubMed] [Google Scholar]
- 58.Cheung K.L., Kong A-N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12(1):87–97. doi: 10.1208/s12248-009-9162-8. [http://dx.doi.org/10.1208/s12248-009-9162-8]. [PMID: 20013083]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trachootham D., Zhou Y., Zhang H., Demizu Y., Chen Z., Pelicano H., Chiao P.J., Achanta G., Arlinghaus R.B., Liu J., Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell. 2006;10(3):241–252. doi: 10.1016/j.ccr.2006.08.009. [http://dx.doi.org/10.1016/ j.ccr.2006.08.009]. [PMID: 16959615]. [DOI] [PubMed] [Google Scholar]
- 60.Xu K., Thornalley P.J. Involvement of glutathione metabolism in the cytotoxicity of the phenethyl isothiocyanate and its cysteine conjugate to human leukaemia cells in vitro. Biochem. Pharmacol. 2001;61(2):165–177. doi: 10.1016/s0006-2952(00)00526-8. [http://dx.doi.org/10.1016/S0006-2952(00) 00526-8]. [PMID: 11163331]. [DOI] [PubMed] [Google Scholar]
- 61.Chou Y-C., Chang M-Y., Wang M-J., Harnod T., Hung C-H., Lee H-T., Shen C-C., Chung J-G. PEITC induces apoptosis of Human Brain Glioblastoma GBM8401 Cells through the extrinsic- and intrinsic -signaling pathways. Neurochem. Int. 2015;81:32–40. doi: 10.1016/j.neuint.2015.01.001. [http://dx.doi.org/10.1016/j.neuint.2015.01.001]. [PMID: 25582659]. [DOI] [PubMed] [Google Scholar]
- 62.Lee D-H., Kim D-W., Lee H-C., Lee J-H., Lee T-H. Phenethyl isothiocyanate sensitizes glioma cells to TRAIL-induced apoptosis. Biochem. Biophys. Res. Commun. 2014;446(4):815–821. doi: 10.1016/j.bbrc.2014.01.112. [http://dx.doi.org/10.1016/j.bbrc.2014.01.112]. [PMID: 24491546]. [DOI] [PubMed] [Google Scholar]
- 63.Su J-C., Lin K., Wang Y., Sui S-H., Gao Z-Y., Wang Z-G. In vitro studies of phenethyl isothiocyanate against the growth of LN229 human glioma cells. Int. J. Clin. Exp. Pathol. 2015;8(4):4269–4276. [PMID: 26097624]. [PMC free article] [PubMed] [Google Scholar]
- 64.Chen G., Chen Z., Hu Y., Huang P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by β-phenethyl isothiocyanate: mechanisms for anti-leukemia activity. Antioxid. Redox Signal. 2011;15(12):2911–2921. doi: 10.1089/ars.2011.4170. [http://dx.doi. org/10.1089/ars.2011.4170]. [PMID: 21827296]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pecere T., Gazzola M.V., Mucignat C., Parolin C., Vecchia F.D., Cavaggioni A., Basso G., Diaspro A., Salvato B., Carli M., Palù G. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000;60(11):2800–2804. [PMID: 10850417]. [PubMed] [Google Scholar]
- 66.Guo J.M., Xiao B.X., Liu Q., Zhang S., Liu D.H., Gong Z.H. Anticancer effect of aloe-emodin on cervical cancer cells involves G2/M arrest and induction of differentiation. Acta Pharmacol. Sin. 2007;28(12):1991–1995. doi: 10.1111/j.1745-7254.2007.00707.x. [http://dx.doi.org/10.1111/j.1745-7254. 2007.00707.x]. [PMID: 18031614]. [DOI] [PubMed] [Google Scholar]
- 67.Ismail S., Haris K., Abdul Ghani A.R., Abdullah J.M., Johan M.F., Mohamed Yusoff A.A. Enhanced induction of cell cycle arrest and apoptosis via the mitochondrial membrane potential disruption in human U87 malignant glioma cells by aloe emodin. J. Asian Nat. Prod. Res. 2013;15(9):1003–1012. doi: 10.1080/10286020.2013.818982. [http://dx.doi.org/ 10.1080/10286020.2013.818982]. [PMID: 23869465]. [DOI] [PubMed] [Google Scholar]
- 68.Haris K., Ismail S., Idris Z., Abdullah J.M., Yusoff A.A. Expression profile of genes modulated by Aloe emodin in human U87 glioblastoma cells. Asian Pac. J. Cancer Prev. 2014;15(11):4499–4505. doi: 10.7314/apjcp.2014.15.11.4499. [http://dx.doi.org/10.7314/APJCP.2014.15.11.4499]. [PMID: 24969876]. [DOI] [PubMed] [Google Scholar]
- 69.Lu B., Yin L., Xu L., Peng J. Application of proteomic and bioinformatic techniques for studying the hepatoprotective effect of dioscin against CCl4-induced liver damage in mice. Planta Med. 2011;77(5):407–415. doi: 10.1055/s-0030-1250461. [http://dx.doi.org/10.1055/s-0030-1250461]. [PMID: 20979020]. [DOI] [PubMed] [Google Scholar]
- 70.Wei Y., Xu Y., Han X., Qi Y., Xu L., Xu Y., Yin L., Sun H., Liu K., Peng J. Anti-cancer effects of dioscin on three kinds of human lung cancer cell lines through inducing DNA damage and activating mitochondrial signal pathway. Food Chem. Toxicol. 2013;59:118–128. doi: 10.1016/j.fct.2013.05.054. [http://dx.doi.org/10.1016/j.fct.2013.05.054]. [PMID: 23764357]. [DOI] [PubMed] [Google Scholar]
- 71.Wu S., Xu H., Peng J., Wang C., Jin Y., Liu K., Sun H., Qin J. Potent anti-inflammatory effect of dioscin mediated by suppression of TNF-α-induced VCAM-1, ICAM-1and EL expression via the NF-κB pathway. Biochimie. 2015;110:62–72. doi: 10.1016/j.biochi.2014.12.022. [http://dx.doi.org/10.1016/j.biochi.2014.12.022]. [PMID: 25577996]. [DOI] [PubMed] [Google Scholar]
- 72.Lv L., Zheng L., Dong D., Xu L., Yin L., Xu Y., Qi Y., Han X., Peng J. Dioscin, a natural steroid saponin, induces apoptosis and DNA damage through reactive oxygen species: a potential new drug for treatment of glioblastoma multiforme. Food Chem. Toxicol. 2013;59:657–669. doi: 10.1016/j.fct.2013.07.012. [http://dx.doi.org/10.1016/j.fct.2013. 07.012]. [PMID: 23871826]. [DOI] [PubMed] [Google Scholar]
- 73.Cavalieri E., Mariotto S., Fabrizi C., de Prati A.C., Gottardo R., Leone S., Berra L.V., Lauro G.M., Ciampa A.R., Suzuki H. α-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem. Biophys. Res. Commun. 2004;315(3):589–594. doi: 10.1016/j.bbrc.2004.01.088. [http://dx.doi.org/10.1016/j.bbrc.2004.01.088]. [PMID: 14975741]. [DOI] [PubMed] [Google Scholar]
- 74.Chiou S-M., Chiu C-H., Yang S-T., Yang J-S., Huang H-Y., Kuo C-L., Chen P-Y., Chung J-G. Danthron triggers ROS and mitochondria-mediated apoptotic death in C6 rat glioma cells through caspase cascades, apoptosis-inducing factor and endonuclease G multiple signaling. Neurochem. Res. 2012;37(8):1790–1800. doi: 10.1007/s11064-012-0792-3. [http://dx.doi.org/10.1007/s11064-012-0792-3]. [PMID: 22592642]. [DOI] [PubMed] [Google Scholar]
- 75.Kwak M-S., Yu S.J., Yoon J-H., Lee S-H., Lee S-M., Lee J-H., Kim Y.J., Lee H-S., Kim C.Y. Synergistic anti-tumor efficacy of doxorubicin and flavopiridol in an in vivo hepatocellular carcinoma model. J. Cancer Res. Clin. Oncol. 2015;141(11):2037–2045. doi: 10.1007/s00432-015-1990-6. [http://dx.doi.org/10.1007/s00432-015-1990-6]. [PMID: 25989942]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Newcomb E.W., Tamasdan C., Entzminger Y., Alonso J., Friedlander D., Crisan D., Miller D.C., Zagzag D. Flavopiridol induces mitochondrial-mediated apoptosis in murine glioma GL261 cells via release of cytochrome c and apoptosis inducing factor. Cell Cycle. 2003;2(3):243–250. [http://dx.doi.org/10.4161/cc. 2.3.357]. [PMID: 12734434]. [PubMed] [Google Scholar]
- 77.Newcomb E.W., Tamasdan C., Entzminger Y., Arena E., Schnee T., Kim M., Crisan D., Lukyanov Y., Miller D.C., Zagzag D. Flavopiridol inhibits the growth of GL261 gliomas in vivo: implications for malignant glioma therapy. Cell Cycle. 2004;3(2):230–234. [http://dx.doi.org/10.4161/cc.3.2.667]. [PMID: 14712094]. [PubMed] [Google Scholar]
- 78.Liu M., Hansen P.E., Wang G., Qiu L., Dong J., Yin H., Qian Z., Yang M., Miao J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules. 2015;20(1):754–779. doi: 10.3390/molecules20010754. [http://dx.doi.org/10.3390/molecules20010754]. [PMID: 25574819]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Festa M., Capasso A., D’Acunto C.W., Masullo M., Rossi A.G., Pizza C., Piacente S. Xanthohumol induces apoptosis in human malignant glioblastoma cells by increasing reactive oxygen species and activating MAPK pathways. J. Nat. Prod. 2011;74(12):2505–2513. doi: 10.1021/np200390x. [http://dx.doi.org/10.1021/np200390x]. [PMID: 22111577]. [DOI] [PubMed] [Google Scholar]
- 80.Wiench B., Eichhorn T., Paulsen M., Efferth T. Shikonin directly targets mitochondria and causes mitochondrial dysfunction in cancer cells. Evid. Based Complement. Alternat. Med. 2012 doi: 10.1155/2012/726025. 726025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen C-H., Lin M-L., Ong P-L., Yang J-T. Novel multiple apoptotic mechanism of shikonin in human glioma cells. Ann. Surg. Oncol. 2012;19(9):3097–3106. doi: 10.1245/s10434-012-2324-4. [http://dx.doi.org/10.1245/s10434-012-2324-4]. [PMID: 22446899]. [DOI] [PubMed] [Google Scholar]
- 82.Yang J-T., Li Z-L., Wu J-Y., Lu F-J., Chen C-H. An oxidative stress mechanism of shikonin in human glioma cells. PLoS One. 2014;9(4):e94180. doi: 10.1371/journal.pone.0094180. [http://dx.doi.org/10.1371/journal.pone. 0094180]. [PMID: 24714453]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Venugopal R., Liu R.H. Phytochemicals in diets for breast cancer prevention: The importance of resveratrol and ursolic acid. Food Science and Human Wellness. 2012;1(1):1–13. [http://dx.doi.org/ 10.1016/j.fshw.2012.12.001]. [Google Scholar]
- 84.Jiang H., Zhang L., Kuo J., Kuo K., Gautam S.C., Groc L., Rodriguez A.I., Koubi D., Hunter T.J., Corcoran G.B., Seidman M.D., Levine R.A. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol. Cancer Ther. 2005;4(4):554–561. doi: 10.1158/1535-7163.MCT-04-0056. [http:// dx.doi.org/10.1158/1535-7163.MCT-04-0056]. [PMID: 15827328]. [DOI] [PubMed] [Google Scholar]
- 85.Batra P., Sharma A.K. Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech. 2013;3(6):439–459. doi: 10.1007/s13205-013-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim H., Moon J.Y., Ahn K.S., Cho S.K. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/596496. 596496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zamin L.L., Filippi-Chiela E.C., Vargas J., Demartini D.R., Meurer L., Souza A.P., Bonorino C., Salbego C., Lenz G. Quercetin promotes glioma growth in a rat model. Food Chem. Toxicol. 2014;63:205–211. doi: 10.1016/j.fct.2013.11.002. [http://dx.doi.org/10.1016/j.fct.2013. 11.002]. [PMID: 24252772]. [DOI] [PubMed] [Google Scholar]
- 88.Li S., Chou G., Hseu Y., Yang H., Kwan H., Yu Z. Isolation of anticancer constituents from flos genkwa (Daphne genkwa Sieb.et Zucc.) through bioassay-guided procedures. Chem. Cent. J. 2013;7(1):159. doi: 10.1186/1752-153X-7-159. [http://dx.doi.org/10.1186/1752-153X-7-159]. [PMID: 24059652]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y., Xu Y.S., Yin L.H., Xu L.N., Peng J.Y., Zhou H., Kang W. Synergistic anti-glioma effect of Hydroxygenkwanin and Apigenin in vitro. Chem. Biol. Interact. 2013;206(2):346–355. doi: 10.1016/j.cbi.2013.10.009. [http://dx.doi.org/10.1016/j.cbi.2013.10.009]. [PMID: 24144774]. [DOI] [PubMed] [Google Scholar]
- 90.Rasul A., Khan M., Ali M., Li J., Li X. Targeting apoptosis pathways in cancer with alantolactone and isoalantolactone. Scientific World Journal. 2013;2013 doi: 10.1155/2013/248532. 248532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khan M., Yi F., Rasul A., Li T., Wang N., Gao H., Gao R., Ma T. Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life. 2012;64(9):783–794. doi: 10.1002/iub.1068. [http://dx.doi.org/10.1002/iub. 1068]. [PMID: 22837216]. [DOI] [PubMed] [Google Scholar]
- 92.Kim S-H., Choi K-C. Anti-cancer effect and underlying mechanism (s) of Kaempferol, a Phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol. Res. 2013;29(4):229–234. doi: 10.5487/TR.2013.29.4.229. [http://dx.doi.org/10.5487/TR.2013.29.4.229]. [PMID: 24578792]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharma V., Joseph C., Ghosh S., Agarwal A., Mishra M.K., Sen E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol. Cancer Ther. 2007;6(9):2544–2553. doi: 10.1158/1535-7163.MCT-06-0788. [http:// dx.doi.org/10.1158/1535-7163.MCT-06-0788]. [PMID: 17876051]. [DOI] [PubMed] [Google Scholar]
- 94.Liang W-Z., Chou C-T., Chang H-T., Cheng J-S., Kuo D-H., Ko K-C., Chiang N-N., Wu R-F., Shieh P., Jan C-R. The mechanism of honokiol-induced intracellular Ca(2+) rises and apoptosis in human glioblastoma cells. Chem. Biol. Interact. 2014;221:13–23. doi: 10.1016/j.cbi.2014.07.012. [http://dx.doi.org/10.1016/j.cbi.2014.07.012]. [PMID: 25106108]. [DOI] [PubMed] [Google Scholar]
- 95.Jeong J.J., Lee J.H., Chang K.C., Kim H.J. Honokiol exerts an anticancer effect in T98G human glioblastoma cells through the induction of apoptosis and the regulation of adhesion molecules. Int. J. Oncol. 2012;41(4):1358–1364. doi: 10.3892/ijo.2012.1582. [PMID: 22895699]. [DOI] [PubMed] [Google Scholar]
- 96.Sohma I., Fujiwara Y., Sugita Y., Yoshioka A., Shirakawa M., Moon J-H., Takiguchi S., Miyata H., Yamasaki M., Mori M., Doki Y. Parthenolide, an NF-κB inhibitor, suppresses tumor growth and enhances response to chemotherapy in gastric cancer. Cancer Genomics Proteomics. 2011;8(1):39–47. [PMID: 21289336]. [PubMed] [Google Scholar]
- 97.Nakabayashi H., Shimizu K. Involvement of Akt/NF-κB pathway in antitumor effects of parthenolide on glioblastoma cells in vitro and in vivo. BMC Cancer. 2012;12(1):453. doi: 10.1186/1471-2407-12-453. [http://dx.doi.org/ 10.1186/1471-2407-12-453]. [PMID: 23039130]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zanotto-Filho A., Braganhol E., Schröder R., de Souza L.H., Dalmolin R.J., Pasquali M.A., Gelain D.P., Battastini A.M., Moreira J.C. κB inhibitors induce cell death in glioblastomas. Biochem. Pharmacol. 2011;81(3):412–424. doi: 10.1016/j.bcp.2010.10.014. [http://dx.doi.org/ 10.1016/j.bcp.2010.10.014]. [PMID: 21040711]. [DOI] [PubMed] [Google Scholar]
- 99.Stangl V., Lorenz M., Ludwig A., Grimbo N., Guether C., Sanad W., Ziemer S., Martus P., Baumann G., Stangl K. The flavonoid phloretin suppresses stimulated expression of endothelial adhesion molecules and reduces activation of human platelets. J. Nutr. 2005;135(2):172–178. doi: 10.1093/jn/135.2.172. [PMID: 15671209]. [DOI] [PubMed] [Google Scholar]
- 100.Barreca D., Bellocco E., Laganà G., Ginestra G., Bisignano C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014;160:292–297. doi: 10.1016/j.foodchem.2014.03.118. [http://dx.doi.org/10.1016/j.foodchem. 2014.03.118]. [PMID: 24799241]. [DOI] [PubMed] [Google Scholar]
- 101.Hoa N.T., Zhang J.G., Delgado C.L., Myers M.P., Callahan L.L., Vandeusen G., Schiltz P.M., Wepsic H.T., Jadus M.R. Human monocytes kill M-CSF-expressing glioma cells by BK channel activation. Lab. Invest. 2007;87(2):115–129. doi: 10.1038/labinvest.3700506. [http://dx.doi.org/10.1038/labinvest.3700506]. [PMID: 17318194]. [DOI] [PubMed] [Google Scholar]
- 102.Hoa N., Myers M.P., Douglass T.G., Zhang J.G., Delgado C., Driggers L., Callahan L.L., VanDeusen G., Pham J.T., Bhakta N., Ge L., Jadus M.R. Molecular mechanisms of paraptosis induction: implications for a non-genetically modified tumor vaccine. PLoS One. 2009;4(2):e4631–e4631. doi: 10.1371/journal.pone.0004631. [http://dx.doi.org/ 10.1371/journal.pone.0004631]. [PMID: 19247476]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Voss V., Senft C., Lang V., Ronellenfitsch M.W., Steinbach J.P., Seifert V., Kögel D. The pan-Bcl-2 inhibitor (-)-gossypol triggers autophagic cell death in malignant glioma. Mol. Cancer Res. 2010;8(7):1002–1016. doi: 10.1158/1541-7786.MCR-09-0562. [http://dx.doi.org/10.1158/1541-7786.MCR-09-0562]. [PMID: 20587533]. [DOI] [PubMed] [Google Scholar]
- 104.Jarzabek M.A., Amberger-Murphy V., Callanan J.J., Gao C., Zagozdzon A.M., Shiels L., Wang J., Ligon K.L., Rich B.E., Dicker P., Gallagher W.M., Prehn J.H., Byrne A.T. Interrogation of gossypol therapy in glioblastoma implementing cell line and patient-derived tumour models. Br. J. Cancer. 2014;111(12):2275–2286. doi: 10.1038/bjc.2014.529. [http://dx.doi.org/10.1038/bjc.2014.529]. [PMID: 25375271]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bushunow P., Reidenberg M.M., Wasenko J., Winfield J., Lorenzo B., Lemke S., Himpler B., Corona R., Coyle T. Gossypol treatment of recurrent adult malignant gliomas. J. Neurooncol. 1999;43(1):79–86. doi: 10.1023/a:1006267902186. [http://dx.doi.org/10.1023/A: 1006267902186]. [PMID: 10448875]. [DOI] [PubMed] [Google Scholar]
- 106.Sun Y., Xun K., Wang Y., Chen X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anticancer Drugs. 2009;20(9):757–769. doi: 10.1097/CAD.0b013e328330d95b. [http://dx.doi.org/ 10.1097/CAD.0b013e328330d95b]. [PMID: 19704371]. [DOI] [PubMed] [Google Scholar]
- 107.Eom K.S., Kim H-J., So H-S., Park R., Kim T.Y. Berberine-induced apoptosis in human glioblastoma T98G cells is mediated by endoplasmic reticulum stress accompanying reactive oxygen species and mitochondrial dysfunction. Biol. Pharm. Bull. 2010;33(10):1644–1649. doi: 10.1248/bpb.33.1644. [http://dx.doi.org/10.1248/bpb.33.1644]. [PMID: 20930370]. [DOI] [PubMed] [Google Scholar]
- 108.Liu Q., Xu X., Zhao M., Wei Z., Li X., Zhang X., Liu Z., Gong Y., Shao C. Berberine induces senescence of human glioblastoma cells by downregulating the EGFR-MEK-ERK signaling pathway. Mol. Cancer Ther. 2015;14(2):355–363. doi: 10.1158/1535-7163.MCT-14-0634. [http://dx.doi.org/10.1158/1535-7163.MCT-14-0634]. [PMID: 25504754]. [DOI] [PubMed] [Google Scholar]
- 109.Eom K-S., Hong J-M., Youn M-J., So H-S., Park R., Kim J-M., Kim T-Y. Berberine induces G1 arrest and apoptosis in human glioblastoma T98G cells through mitochondrial/caspases pathway. Biol. Pharm. Bull. 2008;31(4):558–562. doi: 10.1248/bpb.31.558. [http://dx.doi.org/ 10.1248/bpb.31.558]. [PMID: 18379040]. [DOI] [PubMed] [Google Scholar]
- 110.Fu S., Xie Y., Tuo J., Wang Y., Zhu W., Wu S., Yan G., Hu H. Discovery of mitochondria-targeting berberine derivatives as the inhibitors of proliferation, invasion and migration against rat C6 and human U87 glioma cells. MedChemComm. 2015;6(1):164–173. [http://dx.doi.org/10.1039/C4MD00264D]. [PMID: 26811742]. [Google Scholar]
- 111.Procházková D., Boušová I., Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82(4):513–523. doi: 10.1016/j.fitote.2011.01.018. [http://dx.doi.org/10.1016/j.fitote.2011.01.018]. [PMID: 21277359]. [DOI] [PubMed] [Google Scholar]
- 112.Kang J., Chen J., Shi Y., Jia J., Zhang Y. Curcumin-induced histone hypoacetylation: the role of reactive oxygen species. Biochem. Pharmacol. 2005;69(8):1205–1213. doi: 10.1016/j.bcp.2005.01.014. [http://dx.doi.org/ 10.1016/j.bcp.2005.01.014]. [PMID: 15794941]. [DOI] [PubMed] [Google Scholar]
- 113.de la Lastra C.A., Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem. Soc. Trans. 2007;35(Pt 5):1156–1160. doi: 10.1042/BST0351156. [http://dx.doi.org/10.1042/ BST0351156]. [PMID: 17956300]. [DOI] [PubMed] [Google Scholar]
- 114.López-Lázaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008;52(S1) Suppl. 1:S103–S127. doi: 10.1002/mnfr.200700238. [PMID: 18496811]. [DOI] [PubMed] [Google Scholar]
- 115.DeGeorge J.J., Ahn C-H., Andrews P.A., Brower M.E., Giorgio D.W., Goheer M.A., Lee-Ham D.Y., McGuinn W.D., Schmidt W., Sun C.J., Tripathi S.C. Regulatory considerations for preclinical development of anticancer drugs. Cancer Chemother. Pharmacol. 1998;41(3):173–185. doi: 10.1007/s002800050726. [http://dx.doi.org/10.1007/ s002800050726]. [PMID: 9443633]. [DOI] [PubMed] [Google Scholar]
- 116.Gescher A., Steward W.P., Brown K. Resveratrol in the management of human cancer: how strong is the clinical evidence? Ann. N. Y. Acad. Sci. 2013;1290(1):12–20. doi: 10.1111/nyas.12205. [http://dx.doi.org/10. 1111/nyas.12205]. [PMID: 23855461]. [DOI] [PubMed] [Google Scholar]
- 117.Oral bioavailability challenges of natural products used in cancer chemoprevention. 化学进. 2013;25(9) [Google Scholar]
- 118.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [http://dx.doi.org/10.1021/mp700113r]. [PMID: 17999464]. [DOI] [PubMed] [Google Scholar]
- 119.Prasad S., Tyagi A.K., Aggarwal B.B. 2014.
- 120.Pardridge W.M. Transport of small molecules through the blood-brain barrier: biology and methodology. Adv. Drug Deliv. Rev. 1995;15(1):5–36. [http://dx.doi.org/10.1016/0169-409X(95) 00003-P]. [PubMed] [Google Scholar]
- 121.Gabathuler R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010;37(1):48–57. doi: 10.1016/j.nbd.2009.07.028. [http://dx.doi.org/10.1016/j.nbd.2009.07.028]. [PMID: 19664710]. [DOI] [PubMed] [Google Scholar]
- 122.Wei X., Chen X., Ying M., Lu W. Brain tumor-targeted drug delivery strategies. Acta Pharm. Sin. B. 2014;4(3):193–201. doi: 10.1016/j.apsb.2014.03.001. [http://dx.doi.org/10.1016/j.apsb.2014.03.001]. [PMID: 26579383]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chaichana K.L., Pinheiro L., Brem H. Delivery of local therapeutics to the brain: working toward advancing treatment for malignant gliomas. 2015;6(3):353–369. doi: 10.4155/tde.14.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oshiro S., Tsugu H., Komatsu F., Ohnishi H., Ueno Y., Sakamoto S., Fukushima T., Soma G. Evaluation of intratumoral administration of tumor necrosis factor-alpha in patients with malignant glioma. Anticancer Res. 2006;26(6A):4027–4032. [PMID: 17195453]. [PubMed] [Google Scholar]
- 125.Sawyer A.J., Piepmeier J.M., Saltzman W.M. New methods for direct delivery of chemotherapy for treating brain tumors. Yale J. Biol. Med. 2006;79(3-4):141–152. [PMID: 17940624]. [PMC free article] [PubMed] [Google Scholar]
- 126.Doran A., Obach R.S., Smith B.J., Hosea N.A., Becker S., Callegari E., Chen C., Chen X., Choo E., Cianfrogna J., Cox L.M., Gibbs J.P., Gibbs M.A., Hatch H., Hop C.E., Kasman I.N., Laperle J., Liu J., Liu X., Logman M., Maclin D., Nedza F.M., Nelson F., Olson E., Rahematpura S., Raunig D., Rogers S., Schmidt K., Spracklin D.K., Szewc M., Troutman M., Tseng E., Tu M., Van Deusen J.W., Venkatakrishnan K., Walens G., Wang E.Q., Wong D., Yasgar A.S., Zhang C. The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: evaluation using the MDR1A/1B knockout mouse model. Drug Metab. Dispos. 2005;33(1):165–174. doi: 10.1124/dmd.104.001230. [http://dx.doi.org/10.1124/dmd.104.001230]. [PMID: 15502009]. [DOI] [PubMed] [Google Scholar]
- 127.Kharasch E.D., Hoffer C., Whittington D. The effect of quinidine, used as a probe for the involvement of P-glycoprotein, on the intestinal absorption and pharmacodynamics of methadone. Br. J. Clin. Pharmacol. 2004;57(5):600–610. doi: 10.1111/j.1365-2125.2003.02053.x. [http://dx.doi.org/ 10.1111/j.1365-2125.2003.02053.x]. [PMID: 15089813]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sadeque A.J., Wandel C., He H., Shah S., Wood A.J. Increased drug delivery to the brain by P-glycoprotein inhibition. Clin. Pharmacol. Ther. 2000;68(3):231–237. doi: 10.1067/mcp.2000.109156. [http://dx.doi.org/10. 1067/mcp.2000.109156]. [PMID: 11014404]. [DOI] [PubMed] [Google Scholar]
- 129.Kitagawa S. Inhibitory effects of polyphenols on p-glycoprotein-mediated transport. Biol. Pharm. Bull. 2006;29(1):1–6. doi: 10.1248/bpb.29.1. [http://dx. doi.org/10.1248/bpb.29.1]. [PMID: 16394499]. [DOI] [PubMed] [Google Scholar]
- 130.Horobin R.W., Trapp S., Weissig V. Mitochondriotropics: a review of their mode of action, and their applications for drug and DNA delivery to mammalian mitochondria. J. Control. Release. 2007;121(3):125–136. doi: 10.1016/j.jconrel.2007.05.040. [http://dx.doi.org/10.1016/j.jconrel.2007. 05.040]. [PMID: 17658192]. [DOI] [PubMed] [Google Scholar]
- 131.Neuzil J., Dong L-F., Rohlena J., Truksa J., Ralph S.J. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion. 2013;13(3):199–208. doi: 10.1016/j.mito.2012.07.112. [http://dx.doi.org/10.1016/ j.mito.2012.07.112]. [PMID: 22846431]. [DOI] [PubMed] [Google Scholar]
- 132.Neuzil J., Tomasetti M., Zhao Y., Dong L-F., Birringer M., Wang X-F., Low P., Wu K., Salvatore B.A., Ralph S.J. Vitamin E analogs, a novel group of “mitocans,” as anticancer agents: the importance of being redox-silent. Mol. Pharmacol. 2007;71(5):1185–1199. doi: 10.1124/mol.106.030122. [http://dx.doi.org/10.1124/mol.106.030122]. [PMID: 17220355]. [DOI] [PubMed] [Google Scholar]
- 133.Sassi N., Mattarei A., Azzolini M., Bernardi P., Szabo’ I., Paradisi C., Zoratti M., Biasutto L. Mitochondria-targeted resveratrol derivatives act as cytotoxic pro-oxidants. Curr. Pharm. Des. 2014;20(2):172–179. doi: 10.2174/13816128113199990034. [http://dx.doi.org/10.2174/ 13816128113199990034]. [PMID: 23701548]. [DOI] [PubMed] [Google Scholar]
- 134.Mattarei A., Biasutto L., Marotta E., De Marchi U., Sassi N., Garbisa S., Zoratti M., Paradisi C. A mitochondriotropic derivative of quercetin: a strategy to increase the effectiveness of polyphenols. ChemBioChem. 2008;9(16):2633–2642. doi: 10.1002/cbic.200800162. [http://dx. doi.org/10.1002/cbic.200800162]. [PMID: 18837061]. [DOI] [PubMed] [Google Scholar]