Abstract
Background: Peripheral neuropathies are a group of diseases characterized by malfunctioning of peripheral nervous system. Neuropathic pain, one of the core manifestations of peripheral neuropathy remains as the most severe disabling condition affecting the social and daily routine life of patients suffering from peripheral neuropathy.
Method: The current review is aimed at unfolding the possible role of mitochondrial dysfunction in peripheral nerve damage and to discuss on the probable therapeutic strategies against neuronal mitotoxicity. The article also highlights the therapeutic significance of maintaining a healthy mitochondrial environment in neuronal cells via pharmacological management in context of peripheral neuropathies.
Results: Aberrant cellular signaling coupled with changes in neurotransmission, peripheral and central sensitization are found to be responsible for the pathogenesis of variant toxic neuropathies. Current research reports have indicated the possible involvement of mitochondria mediated redox imbalance as one of the principal causes of neuropathy aetiologies. In addition to imbalance in redox homeostasis, mitochondrial dysfunction is also responsible for alterations in physiological bioenergetic metabolism, apoptosis and autophagy pathways.
Conclusions: In spite of various etiological factors, mitochondrial dysfunction has been found to be a major pathomechanism underlying the neuronal dysfunction associated with peripheral neuropathies. Pharmacological modulation of mitochondria either directly or indirectly is expected to yield therapeutic relief from various primary and secondary mitochondrial diseases.
Keywords: AMPK, mitochondria, mitophagy, neuropathic pain, PGC-1α
1. INTRODUCTION
Peripheral neuropathies result when damage occurs to peripheral nerves due to a systemic disease, toxicant exposure or due to direct nerve injury [1]. Even though the severity and symptoms depend on the causative agent and the nerve affected, neuropathic pain develops when the damage occurs to somatosensory nervous system [2]. In fact, neuropathic pain is the most common clinical manifestation of peripheral neuropathy resulting from diabetes, chronic alcohol intake, certain cancers, vitamin B deficiency, infections, nerve-related diseases, toxins, and certain drugs. Symptoms of peripheral nerve damage include muscle wasting and pain in motor neuropathies and dysautonomia in case of autonomic neuropathies [3]. Another way of classifying peripheral neuropathies is based on their inheritance. Genetically inherited neuropathies consist of a broad variety of diseases associated with neuronal damage like Charcot Marie Tooth disease, familial dysautonomia, congenital neuropathies and other inherited motor, sensory, autonomic neuropathies etc [4]. The incidence of inherited neuropathies is genetically determined, acquired neuropathies occur in majority of cases due to a disease condition, infection, spinal cord injury or due to drug therapy. Recently it has been experimentally proved that most of these inherited and acquired neuropathies are found to be mediated through dysfunctioning of mitochondria and other important signaling pathways [5-7]. Innumerable number of genes associated with mitochondrial genome were found to be mutated in several inherited neuropathies [8]. Acquired neuropathies such as diabetic peripheral neuropathy, chemotherapy induced peripheral neuropathy and trauma/ injury induced neuropathies occur as a result of enhanced oxidative stress and inflammation in peripheral neurons.
Mitochondria have been identified to have vital regulatory role in cellular physiology and pathology, thus attracted focus of many researchers. Indeed it has been transformed from a status of extraneous prokaryotic organelle to the organelle of potential therapeutic importance. Being a source for fundamental cellular reactive oxygen species (ROS), mitochondria are expected to have regulation over cellular oxidative stress and consequent inflammatory signaling. Besides that, mitochondria also plays a critical role in regulating apoptosis and bioenergetic metabolism which were known to be deregulated in various toxic neuropathies. On the whole, the complex interwoven relation among these pathways culminates in mitochondrial dysfunction in peripheral neurons and result in nerve dysfunction.
The current review is aimed at identifying the role of mitochondrial dysfunction in peripheral neurotoxic changes associated with various acquired neuropathies. Efforts put forth by eminent neurologists in the field of mitochondrial biology could lead to the identification of several therapeutic ways of modulating mitochondrial functioning and dynamics in various neurodegenerative disorders [9]. Those therapeutic options amenable to alter the peripheral neuropathies are discussed herein with their possible therapeutic potential. The article also emphasizes the necessity of maintaining a healthy mitochondrial phenotype via pharmacological modulation to produce neuroprotection against various depicts associated with peripheral neuropathy.
1.1. Diabetic Peripheral Neuropathy
Diabetes is one of the most debilitating conditions affecting majority of people in both developed and developing countries. The micro vascular complications of diabetes are the end result of chronic hyperglycemia [10]. One of the important vascular complications of diabetes is peripheral symmetrical polyneuropathy, which affects the nerves in the limbs. Diabetic neuropathy (DN) is known to have a prevalence of 50-60% in people with enduring diabetes [11]. By 2035, patient toll with diabetes is expected to rise to a number of 592 million (International Diabetes Federation). With DN, patient experiences progressive increase in unpleasant sensory symptoms including tingling, burning, shooting, lancinating, contact pain, abnormal sensation to heat and cold, persistent aching in the limbs and cramp like sensations in the feet [12].
Though the etiology of neuropathy may differ depending on types of diabetes, pathophysiology may underlie a unifying mechanism i.e. hyperglycemic injury to peripheral neurons [13]. Hyperglycemic neuronal damage is predominantly mediated through the enhanced generation of ROS, lack of proper bioenergetic supply, increased activation of pro-inflammatory markers, inefficient removal of damaged proteins and organelles [14, 15]. Furthermore, lack of insulinotropic support to neurons makes them deprived of the growth signals [16]. It has been also identified that glucose induced generation of advanced glycation end products activates the nuclear factor kappa light chain enhancer of B cells (NF-κB) and leads to production of several proinflammatory mediators resulting in neuroinflammation, a predecessor to peripheral myelin damage [17]. All these events occur individually or in combination with others, mediate peripheral nerve damage and eventually leads to neuropathy (Fig. 1). In most of the cases chronic DN results in amputations or limb loss which is known to affect major proportion of diabetic patients in US and other developed countries [18].
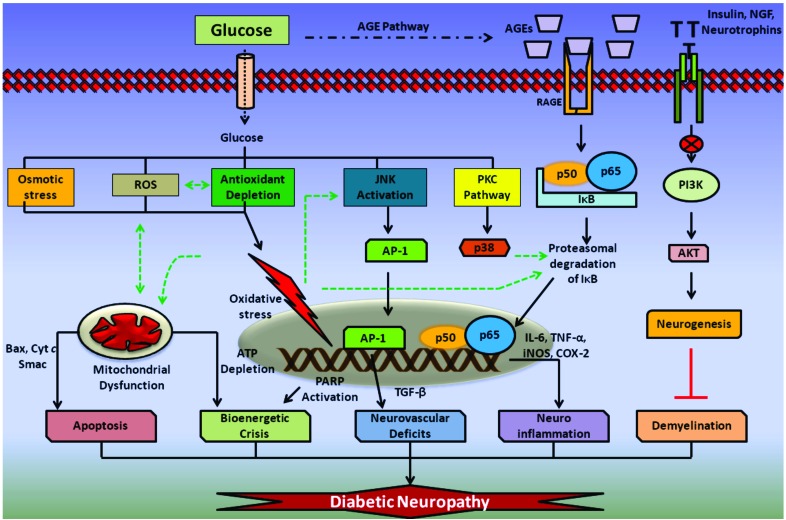
Fig. (1).
Pathophysiology of Diabetic neuropathy: Glucose induced osmotic stress, reactive oxygen species (ROS) and antioxidant depletion results in the formation of intense oxidative stress inside neuronal cells. Glucose is also found to activate JNK and protein kinase pathway (PKC). JNK activation leads to the facilitation of activator protein-1 (AP-1) directed expression of tumor growth factor-β (TGF-β) and other cytokines, which reduces vascular supply to the nervous tissue. Oxidative damage to mitochondria results in release of pro-apoptotic mediators from the mitochondria and also causes damage to electron transport chain components (ETC). This eventually results in apoptosis and bioenergetic dysfunction associated with DN. Glucose induced advanced glycation end products (AGE) and its further association with receptors for AGE (RAGE) also involved in activation of NF-κB heterodimer and thus releases proinflammatory mediators such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (COX-2) release and thus produces neuroinflammation. Further, lack of insulin, neurotrophic signaling leads to reduced PI3K/AKT mediated neurogeneis and thus may promote demyelination observed in chronic neuropathies.
Several treatment options that are available for DN could relieve only symptoms of neuropathy, by virtue of their effect on neurotransmission. The therapeutics includes modulators of adrenergic, GABAergic and tryptaminergic transmission. Examples include sodium valproate, duloxetine, paroxetine, carbamazepine, pregabalin and gabapentin [19]. Despite their efficacy to relieve symptoms of neuropathic pain, they were found to be ineffective in complete eradication of neuronal dysfunction associated with hyperglycemia. Significant work done in last two decades to understand the molecular pathology to a considerable extent, has helped and provided directions to discover new therapeutic strategies for the treatment of neuropathy. However, translational failures have resulted in pursuit for an ideal therapeutic intervention for management of DN [20].
Several lines of evidence have clearly shown that hyperglycemia impairs mitochondrial structure, function and dynamics [6, 8]. In fact, the hyperglycemic overload on neuronal cells and activation of various metabolic pathways co-integrate through mitochondrial generation of reactive oxygen species (ROS) [21].
Mitochondria have been proven experimentally to be a principle mediator of apoptosis, redox and inflammatory pathways which play major role in the pathophysiology of DN [22, 23]. In addition to the dysfunction, impaired mitochondrial fusion, excessive fission and aberrant axonal transport of mitochondria are also being suspected in contribution of damage in peripheral nerves of diabetic patients resulting in production of small, fragmented and non functional mitochondria which precipitate neurodegeneration [24]. Defective autophagy/mitophagy signaling is known to occur in metabolic conditions like insulin resistance, diabetes and is expected to be impaired in DN, a metabolic disease of peripheral nervous system [25]. Hence, identification of drugs which sustain the mitochondrial function and health could aid the search of finding a better therapeutic strategy, which might open new vistas in the treatment of DN.
1.2. Chemotherapy Induced Peripheral Neuropathy (CIPN)
Peripheral neuropathy is a set of symptoms caused by the damage of the peripheral nerves and spinal cord. CIPN is caused by neuronal derangement due to chemotherapeutic agents and most of the time stands as a severe dose limiting and disabling side effects. Some of the common agents which causes CIPN includes taxanes, vinca alkaloids, platinum compounds and also newer effective anticancer agents like bortezomib [26]. It is the most prevalent neurological complication of chronic cancer treatment and affects almost 30-40% of patients receiving chemotherapy. The increased incidence of CIPN in patients is a cause of concern and is also influenced by factors like patient’s age, dose intensity, cumulative dose, therapy duration and pre-existing conditions like alcoholism, diabetes etc [27]. However, 60% of incidence has been reported with the usage of taxanes, vinca alkaloids, platinum compounds and bortezomib. Oxaliplatin, a third generation platinum compound reported to cause peripheral neuropathy in 29-67%, whereas bortezomib indicated for multiple myeloma, incidences up to 64% have recently been reported [27].
Symptoms of CIPN include a blend of sensory, sensorimotor and autonomic nerve dysfunction symptoms resulting in aberrant somatosensory processing of peripheral and central nervous system. Peripheral neuropathy seriously affects both small fiber axons that cause shooting pain, tingling sensation, numbness, cold sensitivity etc., and large fiber sensory axons that affect proprioception and strength of muscles. The common clinical symptoms begin with paresthesias and dysesthesias in feet that spread proximally to affect the lower and upper extremities in a characteristic glove and stocking distribution [28]. CIPN can persist from months to years, reversible or irreversible beyond the chemotherapy completion, extremely painful and disabling that impairs the functional capacity and compromising the quality of life. Therapeutic management of CIPN still remained as a difficult task in cancer therapeutics as it ruins the oncological prognosis and quality of life.
Pathomechanisms underlying the CIPN differ with the inducing agent. Taxanes, vinca alkaloids, platinum compounds and bortezomib are the major group of chemotherapeutic drugs that adversely affect the peripheral nervous system through dissimilar mechanisms as shown in Fig. 2. All these mechanisms culminate in damage to the sensory neurons such as Aδ and C fibers, leading to neuropathic pain characterized by hyperalgesia and allodynia. However, oxidative stress and mitochondrial dysfunction have been suggested to be major contributors in the pathophysiology of CIPN.
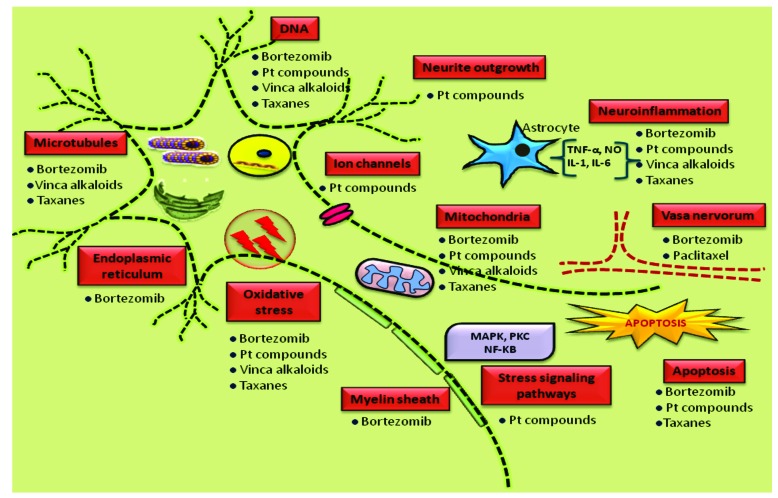
Fig. (2).
Pathogenesis of chemotherapy induced peripheral neuropathy: Multiple sites of peripheral neurons get attacked by various chemotherapeutic agents such as taxane derivatives, vinca alkaloids, Platinum compounds and bortezomib. They adversely affect diverse components at cellular and sub cellular level like ion channels, myelin sheath, DNA, microtubules, mitochondria, endoplasmic reticulum etc. Though these agents cause neurotoxicity by different mechanisms, several mechanisms shared by them in common like oxidative stress, neuroinflammation and mitochondrial dysfunction.
Vinca alkaloids like vincristine and vinblastine are widely used chemotherapeutics for the treatment of non Hodgkin’s lymphoma, Hodgkin’s lymphoma and acute lymphoblastic leukemia are reported to cause symmetric distal sensory neuropathy in patients [29]. The features of vincristine induced neuropathy are found to be mediated through the actions of a second messenger, nitric oxide (NO) and the other inflammatory mediators [30]. Recent studies indicated the impairment of several genes concerned with mitochondrial function in vincristine induced peripheral neuropathy, which rendering the possible involvement of mitochondrial dysfunction and corresponding oxidative stress generation in the disease pathology [31]. The axonopathy and axon transport failure resultant of vinca alkaloid chemotherapy may be due to mitochondrial dysfunction and bioenergetic failure apart from the microtubule dysfunction [29, 32]. The vincristine induced neurotoxicity is produced due to the peroxynitrite induced DNA damage and the poly ADP ribose polymerase (PARP) enzyme overactivation which directly activates the mitochondrial mediated apoptosis and neurodegeneration through bioenergetic failure [33, 34].
Platinum compounds like Cisplatin and Oxaliplatin were reported to cause sensory neuropathy [35]. Cisplatin is mainly used to treat solid malignancies like small cell lung cancer and ovarian cancer. Oxaliplatin is widely used in combination with fluorouracil for the treatment of colorectal cancer under the trade name of FOLFOX [36]. These platinum compounds accumulate in the neurons; generate ROS and damage DNA by forming Pt-DNA adducts with nDNA (nuclear DNA) and mt DNA (mitochondrial DNA) that induces neuronal apoptosis [37-39]. This cumulative DNA damage also activates PARP enzyme which causes bioenergetic depletion and drives the cell towards death [40]. The mt-DNA damage results in the production of defective ETC components which further increases the accumulation of ROS and causes mitochondrial dysfunction [41, 42]. Several in vitro and in vivo studies proved the involvement of nitro oxidative stress and loss of antioxidant defense with the platinum compounds induced peripheral neuropathy [43, 44]. Platinum antineoplastic agents have been shown to reduce plasma antioxidant levels, due to oxidative stress in human studies [45]. Recent insights in molecular studies have proved the involvement of mitochondrial dysfunction and reduced mitochondrial membrane potential in the disease pathogenesis [42]. Several other intervention studies conducted using antioxidants attenuated the neuropathic pain symptoms, thereby confirming the hypothesis that the platinum compounds induced neuronal damage is through the increased oxidative, nitrosative stress [46, 47]. Neurotoxicity induced by these compounds has been explained as channelopathy as they alter the function of voltage gated ion channels. Especially oxaliplatin induced neurotoxicity have long been attributed to oxalate, its metabolite which could disrupt Ca2+ channels by chelating Ca2+ and also inhibit nerve growth factor (NGF) mediated neuronal growth [48]. Oxaliplatin induced channelo- pathy can be correlated to the mitochondrial dysfunction attributed bioenergetic failure [49, 50].
The taxane derivatives like paclitaxel and docetaxel have been found to cause sensorimotor axonal polyneuropathy [51]. Paclitaxel is widely used to treat Kaposi’s sarcoma whereas docetaxel is used for the treatment of breast and prostate cancers. Being microtubule stabilizers, they were thought to mediate the neuronal damage through interfering with microtubule function and axonal transport [37, 51]. Recent experimental studies showed that the neuronal injury in PNS leads to the formation of peroxynitrite followed by the activation of nitric oxide synthase (NOS), NADPH oxidase and inactivation of MnSOD at spinal level through the release of cytokines on glial cell activation contributes to taxane induced neuropathic pain [52, 53]. Some recent studies also exemplified the involvement of peroxynitrite mediated damage to the glial derived proteins known to be involved in glutaminergic transmission in paclitaxel induced peripheral neuropathy [54]. Recent reports suggest that the possible link between oxidative stress and mitochondrial dysfunction; associated with swollen and vacuolated mitochondria in peripheral neurons of paclitaxel treated rats that further fortify the oxidative stress induced mitotoxicity hypothesis in the pathogenesis of taxane induced neuropathic pain [42, 55].
Bortezomib, a proteasome inhibitor widely used to treat multiple myeloma has also been known to induce peripheral neuropathy in 30% of patients [56]. Bortezomib induced neuronal dysfunction is characterized by interference with transcription, nuclear processing, transport and cytoplasmic translation of mRNA’s in the DRG neurons which might be due to the accumulation of aggresomes resulted through the proteasome inhibition [57]. These aggresomes also induce endoplasmic reticulum and mitochondrial stress, which is evident from the presence of vacuolated and swollen organelles in the neurons of animals with bortezomib induced peripheral neuropathy (BIPN). This ultimately results in mitochondrial dysfunction and accumulation of free radicals [58-60]. Some patients develop severe neuropathy even at conventional dose. As a result, genetic studies have been performed to identify the markers of increased susceptibility to BIPN, which revealed that there is a loss of MnSOD function in bortezomib treated patients which make neurons more susceptible to the oxidative stress [61]. This might be due to the posttranslational nitration of MnSOD by nitro oxidative stress and experimental studies also proved the decreased incidence of BIPN with peroxynitrite scavengers [62].
On a whole, oxidative stress and mitochondrial impairment are underpinning the pathobiology of CIPN. Although the symptoms of CIPN are similar to the diabetic neuropathy, its treatment may not necessarily be helpful for preventing or treating CIPN. Current treatment for CIPN is based on two pillars; one is prevention/ prophylactic treatment where antioxidants, nutraceuticals, vitamins, minerals, chelating agents and neurotrophic factors which are administered to achieve the neuroprotective effect and the other option is symptomatic treatment for the existing CIPN where analgesic therapeutics like steroids, tricyclic antidepressants, anticonvulsants, opioids etc., are advised which relieve the unremitting pain of CIPN [63].
Till date the treatment options and therapeutics used for CIPN have been giving only symptomatic relief, but the effective treatment to prevent or cure the CIPN has to be found. This is due to the existence of unmet clinical needs like questionable safety and efficacy of present drugs, spontaneous improvement of symptoms in many cases and partial success which may be completely reversible [64]. Finding an effective treatment to prevent or cure CIPN is challenging today, because of the limited research on the pathobiology of CIPN, ambiguity in understanding the pathomechanisms, lack of standard diagnostic methods to detect CIPN etc. As the role of oxidative stress and mitochondrial dysfunction has recently surfaced in the CIPN pathogenesis, modulating the mitochondrial pathways using pharmacological interventions could be beneficial in the prevention or cure of the CIPN.
1.3. Trauma/Nerve Injury Induced Peripheral Neuropathy
Neuropathic pain, a consequence of nerve injury of peripheral nervous tissue with allodynia and hyperalgesia as characteristic features, is one of the neurodegenerative diseases that reduces quality of life, decreases ability to participate in daily routines and compromises patients social life [2]. It is one of the challenges for clinicians, as it is serious and treatment resistant type of chronic pain.
The major nerve injury induced neuropathic pain has been identified to be a result of trauma or surgery induced peripheral neuropathy. The exact incidence of this type of neuropathy is difficult to define because of the heterogeneity and quality of studies. A latest study uncovered that peripheral nerve injuries were 87% of trauma and 12% owing to surgery. Nerve injuries occurred 81% of the instance in the upper extremities and 11% in the lower extremities, with the balance in other areas [65].
Clinical symptoms of neuropathic pain are characterized by abnormalities in the pain sensation which may be stimulus independent sensations like shooting pain, burning, tingling and numbness whereas, a stimulus evoked pain perceptions including thermal & mechanical hyperalgesia, tactile allodynia and sometimes negative symptoms like hypoalgesia [66].
Traumatic nerve injury to the peripheral nervous system frequently produces persistent debilitating pain states. It has been well demonstrated that next to the peripheral nerve injury the neuroinflammatory cascade of events and structural and functional alterations affects the peripheral nervous system, including nerve endings, afferent fibers, DRG and also central afferent terminals in the spinal cord. This results in events like nociceptor sensitization, ectopic discharges, collateral sprouting, central sensitization and disinhibition [67]. Peripheral nerve injury follows spontaneous discharge of inflammatory mediators and other modulators like proteinases, prostaglandins (PG’s), ATP, serotonin, substance P, bradykinin, nerve growth factor (NGF) etc., at the site of injury [68]. Then, inflammatory mediators induce hyperexcitability in the neurons resulting in short term peripheral sensitization (Fig. 3).
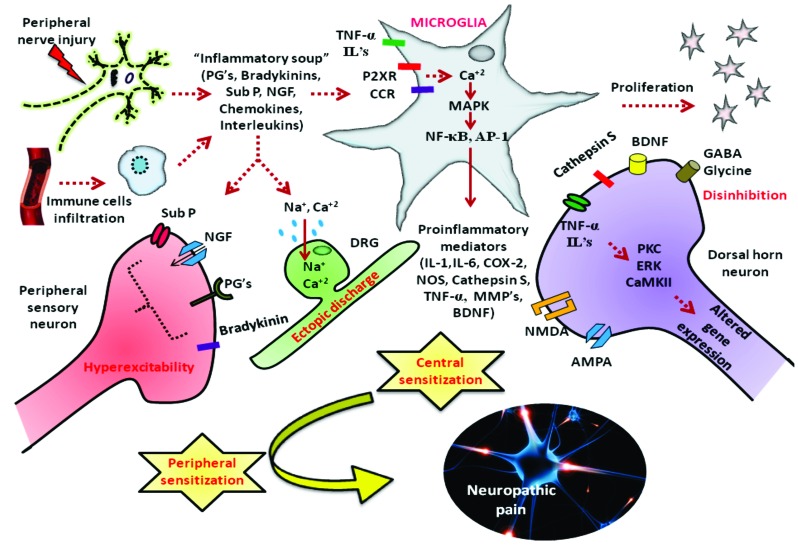
Fig. (3).
Pathogenesis of nerve injury induced neuropathic pain: Nerve injury can cause persistent neuropathic pain through pathological cascade of events like release of pain mediators, hyperexcitability in sensory neurons, ectopic discharges, central sensitization and disinhibition. Activation of glial cells in the peripheral nervous system through purinergic and chemokine receptors release inflammatory mediators like (IL-1, IL-6, COX-2, NOS, Cathepsin S, TNF-α, MMP’s, BDNF) through activation of transcription factors like NF-κB, AP-1. These act on central afferent fibers causing long term central sensitization and disinhibition phenomenon. This peripheral and central sensitization occurs as a result of oxidative stress and neuroinflammation generated as a consequence of nerve injury induced glial cell activation.
Nerve injury also affects the voltage gated ion channels along with the length of the sensory axons and DRG which can cause ectopic discharges [67]. These changes in the peripheral nervous system activate stress signaling pathways like PKC, ERK, CaMKII etc., in central afferent fibers resulting in altered gene expression of receptors like NMDA, AMPA, neurokinin-1 (NK-1), Tyrosine Kinase B etc [69]. These changes may also jointly contribute to long term sensitization due to an increase in excitatory synaptic transmission central sensitization. In addition to these changes, there occurs a phenomenon called disinhibition i.e. inhibition of inhibitory neurotransmitters (GABA and glycine) which further provoke the sensitization process [70]. Therefore, injury induced neuropathic pain can be said to be a consequence of peripheral and central sensitization but the mounting evidence proposed that the oxidative stress induced organelle damage and neuroinflammation are also the major contributors to the pathogenesis of neuropathic pain [71, 72].
It has been recently revealed that next to the nerve injury the inflammatory mediators not only produced at the site of injury through immune cells and Schwann cells but also due to glial cell activation [73]. The activated and proliferated glial cells upregulate several signaling pathways like ERK, NF-κB, JNK etc., which are the key mediators of oxidative stress, neuroinflammation and apoptosis [74, 75]. Research evidences suggest that MAPK and NGF starvation leads to apoptosis in the nerves confirmed by TUNEL assay [76]. Cell death after injury is associated with alteration in the ratio of Bcl-2 to Bax leading to mitochondrial mediated apoptosis. NF-κB induced nitric oxide (NO) synthesis and release of TNF-α, IL-1, and IL-6, causes oxidant induced damage to the cell organelles like endoplasmic reticulum and mitochondria [77]. Recent studies also examined that decrease in the expression of mitochondrial ATP synthase after the sciatic nerve injury causes bioenergetic deficits [71]. Thus, protection of mitochondria from inflammatory mediators induced damage through oxidative stress is essential to maintain cellular quality and nervous tissue function [77].
Pharmacological treatment for neuropathic pain include tricyclic antidepressants (Amitriptyline), serotonin noradrenaline reuptake inhibitors (Venlafaxine, Duloxetine), anticonvulsants (Pregabalin, Gabapentin, Carbamazepine), opioids analgesics (Morphine, Oxycodeine) and miscellaneous analgesics like capsaicin, lidocaine patches, NMDA antagonist, Botulinum toxin, cannabinoids. These pharmacological agents provide symptomatic relief and acts by virtue of their effects on neurotransmission [64]. But recent lines of evidence suggest that nerve injury induced neuropathic pain is not merely a symptom of disease, but also a consequence of the aberrant functioning of peripheral nervous system induced by oxidative stress directed neuronal organelle dysfunction and microglia activated neuroinflammatory pathways. Therefore, we need to focus on preventing the oxidative stress and neuroinflammation induced neurodegeneration due to the organelle dysfunction.
Treatment for neuropathic pain today is an uphill battle for clinicians as the pathogenic mechanism underlying the pain is poorly known and also due to the existing difficulty in distinguishing between nociceptive and neuropathic pain. Currently existing remedies for neuropathic pain are not effective and associated with severe adverse effects [78]. The major unmet clinical needs include, their effectiveness is variable among the patients and sometimes relief from the symptoms is only partial [2, 64].
2. Mitochondrial dysfunction: a critical factor contributing to peripheral neuropathy
Peripheral nervous system (PNS) is vulnerable to a plethora of pathophysiological insults ranging from metabolic, inflammatory damage to trauma/injuries. This vulnerability of PNS is attributed to its structural and functional aspects. Lack of efficient blood nerve barrier system and lymph drainage, directly expose neurons to neurotoxins thus making them susceptible to damage [79]. Since, peripheral neurons require high energy for their functioning and mitochondria are the principal source of cellular energy, most of these neurotoxins known to act by a common mechanism of mitochondrial injury i.e. mitotoxicity [80].
Sensory neurons are more susceptible to the hyper- glycemic, chemotherapeutic insults as they contain long mitochondria rich axons that directly access blood supply [81]. Mitochondria are crucial cell organelles involved majorly in energy production, calcium homeostasis, maintenance of membrane potential, release and uptake of neurotransmitters at synapses [80]. Mitochondrial dynamic functions are required for axonal transport and for facilitating exocytosis mediated neurotransmitter release which helps in preserving neuronal plasticity [82].
Growing pool of evidence suggests that peripheral neuropathies are resultant of dysfunctional mitochondrial metabolism, functioning and dynamics [7, 71]. The mechanism of mitotoxicity and underlying neurodegeneration has been well characterized in neurodegenerative disorders but, less explained in the case of peripheral neuropathies. The following discussion briefly summarizes the role of mitochondria and associated pathways in the pathophysiology of various peripheral neuropathies.
Hyperglycaemia and chemotherapy are known to generate oxidative stress in the neuronal cells through their effect on mitochondria. The higher metabolic flux through ETC is the cause for ROS in the case of hyperglycemia whereas the mitochondrial DNA (mtDNA) damage is the basis for chemotherapeutic insult [83, 84]. Oxidative stress generated in this regard leads to structural and functional damage to mitochondria. Structural damage to the mitochondria is manifested in the form of reduced membrane integrity, causing cytochrome c translocation, mtDNA damage resulting in accumulation of swollen, vacuolated mitochondria [42, 85]. Functional disturbances associated with damaged mitochondria includes, reduced ATP supply through ETC chain, loss of membrane potential, release of pro apoptotic mediators from mitochondria to cytosol through the opening of mitochondrial permeability transition pore (mPTP) and calcium dyshomeostasis [86]. In addition to the role of neuronal mitochondria, mitochondria of Schwann cells are also found to be essential for maintaining high metabolic activity in neurons, which is crucial for neurotransmission [87]. Hyperglycemic insult may lead to altered proteome of schwann cells, even in absence of oxidative stress [88]. Oxidative damage induced production of cellular byproducts like 4-hydroxy nonenal (4-HNE), malondialdehyde (MDA) alters mitochondrial physiology and functioning by forming mitochondrial protein adducts [89]. Nitrosative stress is also known to profoundly affect mitochondrial function through peroxynitrite focused ETC damage and inhibiting the import of specific nuclear proteins into mitochondria [90]. A burst release of ROS/RNS is also responsible for depletion of several mitochondrial antioxidant enzymes such as MnSOD, GSH which further aggravates the mitochondrial damage [86]. Reduced ATP/ADP ratio as a consequence of failed coupling efficiency of ETC chain results in bioenergetic crisis causes necrotic death of neurons [91]. Prolonged ROS production destabilizes the mtDNA and cause transcriptional repression by preventing the attachment of transcription factors [92].
The dynamics of mitochondria i.e. fission and fusion are two important, highly regulated cellular functions which enable mitochondria to combine and distribute their components to meet cellular energy requirements and to maintain a healthy mitochondrial phenotype [80]. The regulation of these events is known to go awry in certain pathologies especially in diseases associated with neurodegeneration. Mitochondrial fission helps to enhance the number of mitochondria, which can be efficiently distributed to each corner of neuronal cells and thus helps them to maintain their energy demands. Mitochondrial fission is highly essential during the periods of energy starvation to produce new, efficient mitochondrial energy generating systems. However, enhanced fission associated with bioenergetic crisis causes BAX foci formation on mitochondrial membrane and thus causes mitochondrial outer membrane permeabilization (MOMP), releasing cytochrome c and other pro apoptotic mediators into cytosol, results in apoptosis [93]. Impairment in the mitochondrial dynamics has also been observed in case of inflammatory neuropathies and oxaliplatin induced neuropathy [94]. Excessive nitric oxide is known to cause s-nitrosylation of dynamin related protein-1 (Drp-1), and increases the mitochondrial fission [95, 96]. Tumor necrosis factor-α (TNF-α) reported to inhibit the kinensin 1 protein, and thus impairs trafficking by halting mitochondrial movement along axons [97]. In addition to impaired dynamics, aggregates of abnormal shaped, damaged mitochondria are responsible for aberrant mitochondrial trafficking, which contributes to axonal degeneration observed in various peripheral neuropathies [81].
Autophagy is the discerning cellular catabolic process responsible for recycling the damaged proteins/ organelles in the cells [98]. Mitophagy is a selective autophagic process involved in recycling of damaged mitochondria and helps in supplying the constituents for mitochondrial biogenesis [99]. Excessive accumulation and impaired clearance of dysfunctional mitochondria are known to be observed in various disorders associated with oxidative stress [100]. Oxidative damage to Atg 4, a key component involved in mitophagy causes impaired autophagosome formation and clearance of damaged mitochondria [101]. Loss in the function of molecular chaperons and associated accumulation of damaged proteins are known to be involved in various peripheral neuropathies including trauma induced neuropathy [102, 103]. A model of demyelinating neuropathy corresponds to the accumulation of improperly folded myelin protein PMP-22 is also being observed recently [104, 105].
Mitochondrial dysfunction and associated disturbances are well connected to neuroinflammatory changes that occur in various neurodegenerative diseases [106]. Dysfunctional mitochondria are also implicated in several pathologies such as cardiovascular and neurodegenerative diseases. Several mitochondrial toxins have been found to inhibit the respiration in microglial cells and also inhibit IL-4 induced alternative anti inflammatory response and thus potentiates neuroinflammation [107]. Mitochondrial ROS are well identified to be involved in several inflammatory pathways such as NF-κB, MAPK activation [108]. Similarly, the pro inflammatory mediators released as a result of an inflammatory episode found to be interfere with the functioning of the mitochondrial electron transport chain and thus compromise ATP production [109]. TNF-α is known to inhibit the complex I, IV of ETC and decreases energy production. Nitric oxide (NO) is a potent inhibitor of cytochrome c oxidase (complex IV) and similarly IL-6 is also known to enhance mitochondrial generation of superoxide [110]. Mitochondrial dysfunction initiates inflammation by increased formation of complexes of damaged mitochondrial parts and cytoplasmic pattern recognition receptors (PRR’s). The resulting inflammasome directed activation of interleukin-1β production, which starts an immune response and leads to chronic inflammation [111]. Mitochondrial DNA and N-formyl peptides released from mitochondria during oxidative damage can serve as an effective damage associated molecular patterns (DAMPs), which acts on several receptors to stimulate neutrophil chemotaxis and cytokine secretion [112]. These DAMPs can also activate NLRP3 (NLR family, pyrin domain-containing 3) inflammasome, the inflammatory responses of which have been implicated in microbial infections, tissue damage and metabolic disturbances [113]. In fact, traumatic injury and corresponding inflammation are considered to occur as a result of an increased burst of mitochondria at the injured site [112].
All together an imbalance in the ultra structure, physiology and dynamics of mitochondria results in lack of cell to cell communication, calcium buffering capacity, failure in axonal transport, neuronal and synaptic plasticity (Fig. 4). These events subsequently manifest in the form of distal axonal neurodegeneration, a major feature of several peripheral neuropathies. Neurodegeneration in these types of neuropathies is associated with mitochondrial dysfunction without any clinically proved gene mutation as seen with inherited neuropathies and diseases like Parkinson’s, Alzheimer’s, Huntington’s etc., but the expression of mitochondrial proteins were altered as discussed [4, 9].
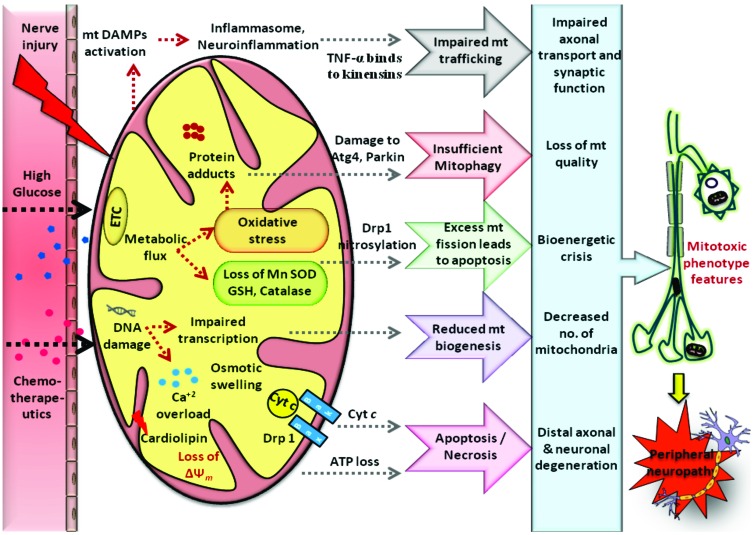
Fig. (4).
Mitotoxicity in peripheral neuropathies: Various pathophysiological insults like hyperglycemic, chemotherapeutic and traumatic injury to the peripheral nerves results in mitochondrial dysfunction through enhanced generation of ROS induced biomolecular damage and bioenergetic crisis. Following the nerve injury accumulation of mitochondria occurs resulting in the release of mtDNA & formyl peptides into circulation which acts as Death associated molecular patterns (DAMP’s). These are recognized by immune cells as foreign bodies and can elicit a local immune/inflammatory response. Interaction between inflammatory mediators and structural proteins involved in mitochondrial trafficking will cause impairment in mitochondrial motility. Oxidative stress induced damage to the mt proteins like Atg4, Parkin etc cause insufficient mitophagy. Excess nitrosative stress also results in excessive mt fission associated with apoptosis. In addition, mtDNA damage impairs its transcription and reduces mitochondrial biogenesis. Ca2+ dyshomeostasis, loss in mitochondrial potential and bioenergetic crisis cause neuronal death via apoptosis/necrosis. All these modifications cause defects in ultra structure, physiology and trafficking of mitochondria resulting in loss of neuronal function producing peripheral neuropathy.
3. Future strategies against mitochondrial dysfunction in peripheral nerve damage
Dysfunctional mitochondria associated with neuro- degeneration results in enhanced oxidative stress, accumulation of damaged proteins/ organelles, glial inflammation and neurovascular impairment [114]. Several novel therapeutic strategies of targeting mitochondria have been identified. These strategies include a broad range of pharmacological modulators with potential effects on the function and dynamics of mitochondria and associated components. Even another way of altering the mitochondrial function through genetic manipulation has been identified.
Mitochondrial permeability transition pore (mPTP) is found to be responsible for the release of certain pro apoptotic mediators from the mitochondria to cytosol and found to mediate apoptosis [115]. Several in vitro studies have clearly indicated the possible involvement of apoptotic neuronal death as a pathophysiological damage involved in neurodegeneration [116]. Hence, the therapeutic strategies directed at preventing neuronal apoptosis through modulation of mPTP would be one of the therapeutic options. The mPTP is a protein complex present in mitochondrial membrane made of different protein subunits. Alteration in protein components can change the dynamics of mPTP; therefore such drugs can be used to target mPTP [117]. VDAC (Voltage dependent anion channel), hexokinase (HK), cyclophilin D binding site of mPTP can be pharmacologically modulated through several natural and synthetic modulators [118]. As increased intracellular calcium is responsible for opening mPTP, interventions targeted at preventing the Ca2+ accumulation in mitochondria can also prevent mPTP opening [118].
Oxidative stress and related ROS generation are identified to be responsible for etiopathogenesis of many diseases. In fact, it is the most common mechanism of cellular/tissue damage in several disease pathologies [119]. A wide variety of cellular sources of ROS have been identified, ranging from xanthine oxidase enzyme system to NADPH oxidase system. Most of these enzymes mediate ROS generation and are known to be involved in the physiological redox signaling and phagocytosis [120]. However, excessive oxidative stress may lead to an intense oxidative burst followed by inflammation. Mitochondrial electron transport chain (ETC) is known to be a potential source of cellular ROS mainly superoxide (O2-.). This superoxide generation occurs as a result of the partial electron transfer to oxygen during their movement through redox centers in the inner mitochondrial membrane [121]. Electron leakage or uncoupling of electron transfer through ETC mainly occurs at complexes I and III of ETC, the resultant uncoupling of ETC reduces ATP generation. An imbalanced mitochondrial ETC functioning, oxidative stress and bioenergetic dysfunction are known to exert their pathological foot print in various peripheral neuropathies [42, 122]. Mainly hyperglycemic and chemotherapeutic neuronal damage are associated with mitochondrial dysfunction and corresponding oxidative stress. Several therapeutic drugs directed at these mitochondrial abnormalities have been reported to benefit in various metabolic and neuro- degenerative diseases [123, 124]. These mitochondrial targeted drugs encompass a broad range of antioxidant molecules conjugated to a lipophilic or peptide moiety. Many of the available antioxidants are conjugated to tri phenyl phosphonium (TPP+), a cationic, lipophilic molecule and successfully delivered to the mitochondria. These TPP+ conjugated molecules can easily cross the inner mitochondrial membrane because of its large negative charge inside the mitochondrial matrix [125]. Similarly, antioxidant nutraceuticals have been successfully targeted to mitochondria using szeto-schiller (SS) peptides, which are made up of a backbone consisting of alternating four basic amino acids [124]. In addition to these antioxidants, mitochondrial dysfunction and associated energy depletion can be rescued by using redox active agents with mild redox potential. These mild redox agents, regulates the electron flow through the ETC chain, prevents the electron leakage and thus maintain enough ATP production, which is essential for neuronal function [126].
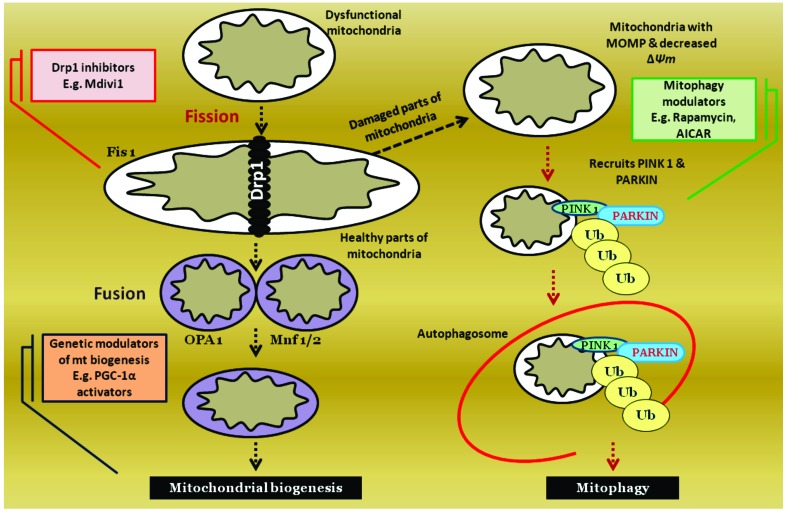
Mitochondria are not static but, instead they are dynamic in nature. The dynamic functions of mitochondria are especially important for functioning of the neurons due to their high requirement for mitochondrial ATP [127]. Some neurons have very long axons and hence maintaining the ATP production far distant from cell body is highly essential to maintain vital neurotransmission and impulse conduction [128]. In this regard, mitochondria travel from the axons to the nerve terminals. Several genes involved in mitochondrial transport are found to be mutated in inherited neuropathies [8]. In addition to it, fission and fusion of mitochondria are the two important functions which keep the mitochondrial quantity at a constant number per cell. Imbalance in mitochondrial homeostatic mechanisms is the possible culprit in various acquired peripheral neuropathies [80]. Hence modulation of these pathways by pharmacological tuning could mitigate the disease condition by facilitating mitochondrial function [8]. Accumulation of shortened mitochondria was observed in the neurons of diabetic animals resulting in an impaired axonal transport and therefore, therapeutics aimed at inhibiting excessive mitochondrial fission (e.g. Dynamin related protein 1 (Drp1) inhibitors such as Mdivi, Dynasore etc.) may be useful in the treatment of DN [129].
Mitophagy is the selective removal of damaged mitochondria from the cellular system by self digestion. Mitophagy and autophagy are self driven cellular catabolic pathways during the periods of bioenergetic crisis [99]. Accumulation of damaged mitochondria due to oxidative stress is well identified in several neuropathies and neurodegenerative diseases. Hence, efficient removal of these damaged organelles with production of new healthy mitochondria is essential to maintain neuronal health and metabolism [130, 131]. The protein machinery of mitophagic pathway can be pharmacologically modulated by several means [132] (Fig. 5). For example, enhancement of mitophagy by AMPK activation (e.g. Metformin) or HDAC inhibition (e.g. Valproic acid, SAHA) augments the survival conditions of neurons in neuropathies and thus can be explored [133].
Fig. (5).
Regulation of mitochondrial quantity & quality: Mitochondria are dynamic cellular organelles, the quantity and quality of which are maintained through regulated processes of fission, fusion and mitophagy. Fission generally precedes mitophagy. Dysfunctional mitochondria undergoes fission using the help of GTPase proteins such as Fis1, dynamin related protein (Drp1). Damaged parts of mitochondria will further undergo recycling using a catabolic mitophagy process. Such damaged mitochondria undergoes PINK1, PARKIN recruitment mediated ubiquitination and then accumulation in autophagosomes. Healthy parts of mitochondria in turn undergo fusion process using the help of proteins such as OPA1, Mnf 1/2. All of these stages of mitochondrial regulation can be pharmacologically modulated pertaining to the catering needs of cells. Excessive mitochondrial fission can be inhibited by using Drp1 inhibitor (e.g. Mdivi1). Mitophagy can be enhanced by various cellular transcription modulators (e.g. mTOR inhibitors).
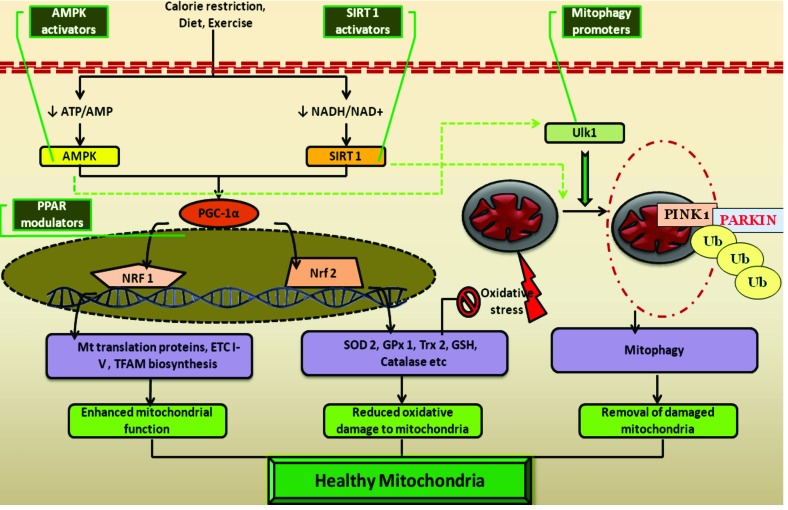
In addition to the above mentioned means of targeting mitochondria, its function can also be modulated through transcriptional regulation of various nuclear genes [134] (Fig. 6). Peroxisome proliferator activated receptor-γ coactivator-1 (PGC-1α) is an important transcription factor responsible for mitochondrial function and maintenance [135]. It enhances the production of several mitochondrial enzymes such as enzymes of the tri carboxylic acid cycle (TCA) and fatty acid oxidation through nuclear respiratory factor-1 (NRF-1) expression. PGC-1α also plays a key role in the mitochondrial protein synthesis by increasing the production of mitochondrial transcription factor A (Tfam) [136]. More convincing evidence of PGC-1α upregulation is known to enhance the nuclear erythroid factor (NEF)-2 related factor (Nrf-2) and thereby facilitating the production of several antioxidants such as glutathione (GSH), superoxide dismutase (SOD), glutathione-s-transferase (GST), Hemeoxygenase-1 (HO-1), NADPH quinone oxidase reductase-1 (NQOR-1) [137, 138]. Although a physical interaction between PGC-1α and Nrf2 protein has not been discovered, it has been identified that activation of one transcription factor induces the other during the period of redox imbalance [139]. Elevated Nrf2 expression effectively inhibits the cellular oxidative damage; therefore the PGC-1α expression is not only associated with enhanced mitochondrial function but also with reduced oxidative mitochondrial damage and thus is important for maintaining a healthy mitochondrial phenotype [140]. Several drugs are known to act as PGC-1α activators and proved to have therapeutic potential in many neurodegenerative disorders [138]. Examples of these drugs include PPARγ agonists (e.g. Rosiglitazone, Pioglitazone). It has been recently observed that PPARγ agonists can effectively prevent the glucose induced neurotoxicity and various deficits in streptozotocin (STZ) induced experimental DN [141].
Fig. (6).
Cellular manipulators of mitochondria and their pharmacological modulation: Cellular energetic sensors such as adenosine monophosphate kinase (AMPK), silent information regulator of transcription homologue type 1 (SIRT1) activated in response to nutrient starvation and at the periods of high metabolic demand. These energetic sensors activate PPAR-γ coactivator-1α (PGC-1α) by direct phosphorylation. The activated PGC-1α in turn regulates mitochondrial biosynthesis through transcriptional facilitation of nuclear respiratory factor-1 (NRF-1) and reduces cellular oxidative damage by enhancing the transcription of antioxidant response element (ARE) of genome through activation of nuclear erythroid factor-1(NEF-1) related facor-2 (Nrf-2). Further AMPK and SIRT1 are also found to promote mitophagy either directly activating Unc-51 like kinase 1 (Ulk1) or indirectly through mTOR inhibition. All these resulting events facilitate the mitochondrial function, reduce damage to it and recycle the constituents if damage occurs, the characteristic features of a healthy mitochondrial phenotype. The mitochondrial transcription facilitator PGC-1α can be promoted by using peroxisome proliferator activated receptor modulators (e.g. Glitazones). Similarly, PGC-1α mediated mitochondrial functions can also be indirectly activated through specific AMPK and SIRT1 activators. Direct pharmacological promoters of mitophagy can also be useful in maintaining a healthy population of mitochondria (e.g. mTOR inhibitors).
Recently it has been identified that PGC1α is inactivated in the dorsal root ganglion of STZ induced rats. PGC-1α null diabetic mice have shown typical features of exacerbated protein damage and nerve degeneration, which indicates the critical importance of PGC-1α mediated mitoprotection [142]. Indeed a reduced Tfam levels in the DRG of diabetic rats and corresponding reduction in mt biogenesis has been identified to play an important role in the pathogenesis of DN. Tfam upregulation was found to ameliorate nerve conduction and improve the epidermal nerve fiber density in experimental DN [143].
Being a mitochondrial sensor, PGC-1α function and expression is regulated by several cellular signaling proteins and transcription factors. These cellular proteins can be therapeutically altered to enhance PGC-1α function [144]. Few of important upstream activators of PGC-1α include adenosine monophosphate kinase (AMPK) and silent information regulators of transcription (SIRT-1) [145]. AMPK and SIRT1 act as cellular metabolic sensors which respond to the changes in level of ATP and NADH respectively. AMPK is activated in response to reduced ATP/ADP ratio, and further activates several key metabolic pathways and inhibits anabolic pathways. Similarly, SIRT-1 is activated when a reduction in cellular NADH /NAD+ pool occurs [146]. SIRT-1 deacetylates several genes involved in energy utilization pathways which result in transcriptional modulation of these genes [147]. In addition to direct action on metabolic pathways, AMPK and SIRT-1 are known to activate nuclear PGC-1α through direct phosphorylation and deacetylation respectively. Activation of PGC-1α by AMPK and SIRT-1 accelerates the mitochondrial TCA cycle and free fatty acid oxidation and thus enhances bioenergetic supply [145]. AMPK and SIRT-1 are well-known to activate cellular Nrf2 signaling through PGC-1α expression and found to inhibit oxidative damage by ROS [148, 149]. Further, activation of AMPK and SIRT-1 facilitates the cellular self digestion process, autophagy by activating its protein machinery [150, 151]. Particularly, AMPK is associated with activation of mitophagy (an autophagic process involving the removal of damaged mitochondria) [152]. The dual effect of AMPK on mitophagy and PGC-1α directed transcription facilitates the replacement of cellular damaged mitochondria with new functional mitochondria [150]. Similarly, SIRT-1 is also known to activate mitophagy by indirectly regulating the NAD+/NADH ratio [153]. In addition to the above mentioned protective roles, activation of AMPK and SIRT1 intensifies bioenergetic supply and supports the metabolism of malnourished neurons in neurodegenerative conditions [144]. On the whole, activation of AMPK/SIRT1/PGC-1α axis helps in maintaining a healthy mitochondrial pool by improving the function and by reducing oxidative damage [145]. Thus, pharmacological activators of this pathway are expected to produce a potentially beneficial therapeutic effect in various secondary mitochondrial diseases. The therapeutic potential of AMPK and SIRT1 is explored in certain neurodegenerative, metabolic diseases and their effect needs to be explored in various peripheral neuropathies [154]. With an overwhelming evidence of mitochondrial dysfunction and oxidative stress in the pathogenesis of various peripheral neuropathies, one can clearly state that pharmacological modulation of AMPK and SIRT1 offers a potential foreseeable therapeutic choice for the treatment of neuropathies.
CONCLUSION
The role of mitochondria in various physiological functions has been well characterized and realized, but the role of aberrant mitochondrial functioning and associated cellular signaling in the pathogenesis of various metabolic, neurodegenerative diseases has been observed recently. In spite of various etiological factors, mitochondrial pathology has been found to be a major pathomechanism underlying the neuronal dysfunction associated with peripheral neuropathies. Pharmacological modulation of mitochondria either directly or indirectly through transcriptional facilitation is expected to yield therapeutic relief from various primary and secondary mitochondrial diseases. Crosstalk among mitochondria and associated oxidative stress, energy supply, autophagy and apoptotic pathways can be better targeted for maintaining healthy mitochondrial population. In addition to the above mentioned strategies, identification of inter organellar association between mitochondria and other organelles such as nucleus, endoplasmic reticulum could lead to provide further molecular insight into the neuropathology and can be appreciated therapeutically.
ACKNOWLEDGEMENTS
Authors would like to acknowledge Department of Pharmaceuticals, Ministry of Chemical and fertilizers, Government of India for their support. Authors would also like to thank support from NIPER-Hyderabad for the preparation of this manuscript.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Ganesh Yerra V., Negi G., Sharma S.S., Kumar A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013;1:394–397. doi: 10.1016/j.redox.2013.07.005. [http://dx.doi.org/10.1016/j.redox.2013.07.005]. [PMID: 24024177]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron R., Binder A., Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–819. doi: 10.1016/S1474-4422(10)70143-5. [http://dx.doi.org/10.1016/S1474-4422(10) 70143-5]. [PMID: 20650402]. [DOI] [PubMed] [Google Scholar]
- 3.Weisberg L.A., Garcia C., Strub R.L. Essentials of clinical neurology. St Louis: Mosby; 1996. Diseases of the peripheral nerves and motor neurons. pp. 458–494. [Google Scholar]
- 4.Saporta M.A., Shy M.E. Inherited peripheral neuropathies. Neurol. Clin. 2013;31(2):597–619. doi: 10.1016/j.ncl.2013.01.009. [http://dx.doi.org/10.1016/ j.ncl.2013.01.009]. [PMID: 23642725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carelli V., Ross-Cisneros F.N., Sadun A.A. Mitochondrial dysfunction as a cause of optic neuropathies. Prog. Retin. Eye Res. 2004;23(1):53–89. doi: 10.1016/j.preteyeres.2003.10.003. [http://dx.doi.org/10.1016/j.preteyeres.2003.10. 003]. [PMID: 14766317]. [DOI] [PubMed] [Google Scholar]
- 6.Fernyhough P., Huang T.J., Verkhratsky A. Mechanism of mitochondrial dysfunction in diabetic sensory neuropathy. J. Peripher. Nerv. Syst. 2003;8(4):227–235. doi: 10.1111/j.1085-9489.2003.03028.x. [http://dx.doi.org/10. 1111/j.1085-9489.2003.03028.x]. [PMID: 14641647]. [DOI] [PubMed] [Google Scholar]
- 7.Han Y., Smith M.T. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front. Pharmacol. 2013;4:156. doi: 10.3389/fphar.2013.00156. [http://dx.doi.org/10.3389/fphar.2013.00156]. [PMID: 24385965]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sajic M. Mitochondrial dynamics in peripheral neuropathies. Antioxid. Redox Signal. 2014;21(4):601–620. doi: 10.1089/ars.2013.5822. [http://dx. doi.org/10.1089/ars.2013.5822]. [DOI] [PubMed] [Google Scholar]
- 9.Moreira P.I., Zhu X., Wang X., Lee H-g., Nunomura A., Petersen R.B., Perry G., Smith M.A. Mitochondria: a therapeutic target in neurodegeneration. Biochimica et Biophysica Acta (BBA)-. Molecular Basis of Disease. 2010;1802(1):212–220. doi: 10.1016/j.bbadis.2009.10.007. [http://dx.doi.org/10.1016/j.bbadis.2009.10.007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [http://dx.doi.org/10.1152/ physrev.00045.2011]. [PMID: 23303908]. [DOI] [PubMed] [Google Scholar]
- 11.Negi G., Kumar A., Joshi R.P., Sharma S.S. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem. Biophys. Res. Commun. 2011;408(1):1–5. doi: 10.1016/j.bbrc.2011.03.087. [http://dx.doi.org/10.1016/j.bbrc.2011.03.087]. [PMID: 21439933]. [DOI] [PubMed] [Google Scholar]
- 12.Sandireddy R., Yerra V.G., Areti A., Komirishetty P., Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int. J. Endocrinol. 2014;2014:674987. doi: 10.1155/2014/674987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Said G. Diabetic neuropathy--a review. Nat. Clin. Pract. Neurol. 2007;3(6):331–340. doi: 10.1038/ncpneuro0504. [http://dx.doi.org/10.1038/ncpneuro0504]. [PMID: 17549059]. [DOI] [PubMed] [Google Scholar]
- 14.Vincent A.M., Brownlee M., Russell J.W. Oxidative stress and programmed cell death in diabetic neuropathy. Ann. N. Y. Acad. Sci. 2002;959(1):368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x. [http://dx.doi.org/10.1111/j.1749-6632.2002.tb02108.x]. [PMID: 11976211]. [DOI] [PubMed] [Google Scholar]
- 15.Schmeichel A.M., Schmelzer J.D., Low P.A. Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes. 2003;52(1):165–171. doi: 10.2337/diabetes.52.1.165. [http://dx.doi.org/10.2337/diabetes.52.1.165]. [PMID: 12502508]. [DOI] [PubMed] [Google Scholar]
- 16.Brussee V., Cunningham F.A., Zochodne D.W. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes. 2004;53(7):1824–1830. doi: 10.2337/diabetes.53.7.1824. [http://dx.doi.org/10.2337/diabetes.53.7.1824]. [PMID: 15220207]. [DOI] [PubMed] [Google Scholar]
- 17.Cameron N.E., Cotter M.A. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr. Drug Targets. 2008;9(1):60–67. doi: 10.2174/138945008783431718. [http://dx.doi.org/10.2174/ 138945008783431718]. [PMID: 18220713]. [DOI] [PubMed] [Google Scholar]
- 18.Clayton W., Elasy T.A. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin. Diabetes. 2009;27(2):52–58. [http://dx.doi.org/10.2337/diaclin. 27.2.52]. [Google Scholar]
- 19.Várkonyi T., Kempler P. Diabetic neuropathy: new strategies for treatment. Diabetes Obes. Metab. 2008;10(2):99–108. doi: 10.1111/j.1463-1326.2007.00741.x. [PMID: 17593238]. [DOI] [PubMed] [Google Scholar]
- 20.Jensen T.S., Backonja M-M., Hernández Jiménez S., Tesfaye S., Valensi P., Ziegler D. New perspectives on the management of diabetic peripheral neuropathic pain. Diab. Vasc. Dis. Res. 2006;3(2):108–119. doi: 10.3132/dvdr.2006.013. [http://dx.doi.org/10.3132/dvdr.2006.013]. [PMID: 17058631]. [DOI] [PubMed] [Google Scholar]
- 21.Leinninger G.M., Edwards J.L., Lipshaw M.J., Feldman E.L. Mechanisms of disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nat. Clin. Pract. Neurol. 2006;2(11):620–628. doi: 10.1038/ncpneuro0320. [http://dx.doi.org/10.1038/ncpneuro0320]. [PMID: 17057749]. [DOI] [PubMed] [Google Scholar]
- 22.Sandireddy R., Yerra V.G., Komirishetti P., Areti A., Kumar A. Fisetin Imparts Neuroprotection in Experimental Diabetic Neuropathy by Modulating Nrf2 and NF-κB Pathways. Cell. Mol. Neurobiol. 2015:1–10. doi: 10.1007/s10571-015-0272-9. [PMID: 26399251]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akude E., Zherebitskaya E., Roy Chowdhury S.K., Girling K., Fernyhough P. 4-Hydroxy-2-nonenal induces mitochondrial dysfunction and aberrant axonal outgrowth in adult sensory neurons that mimics features of diabetic neuropathy. Neurotox. Res. 2010;17(1):28–38. doi: 10.1007/s12640-009-9074-5. [http://dx.doi.org/10.1007/s12640-009-9074-5]. [PMID: 19557324]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards J.L., Quattrini A., Lentz S.I., Figueroa-Romero C., Cerri F., Backus C., Hong Y., Feldman E.L. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia. 2010;53(1):160–169. doi: 10.1007/s00125-009-1553-y. [http://dx.doi.org/10.1007/ s00125-009-1553-y]. [PMID: 19847394]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung H.S., Lee M-S. Role of autophagy in diabetes and mitochondria. Ann. N. Y. Acad. Sci. 2010;1201(1):79–83. doi: 10.1111/j.1749-6632.2010.05614.x. [http:// dx.doi.org/10.1111/j.1749-6632.2010.05614.x]. [PMID: 20649543]. [DOI] [PubMed] [Google Scholar]
- 26.Balayssac D., Ferrier J., Descoeur J., Ling B., Pezet D., Eschalier A., Authier N. Chemotherapy-induced peripheral neuropathies: from clinical relevance to preclinical evidence. Expert Opin. Drug Saf. 2011;10(3):407–417. doi: 10.1517/14740338.2011.543417. [http://dx.doi.org/10.1517/ 14740338.2011.543417]. [PMID: 21210753]. [DOI] [PubMed] [Google Scholar]
- 27.Argyriou A.A., Bruna J., Marmiroli P., Cavaletti G. Chemo- therapy-induced peripheral neurotoxicity (CIPN): an update. Crit. Rev. Oncol. Hematol. 2012;82(1):51–77. doi: 10.1016/j.critrevonc.2011.04.012. [http://dx.doi.org/10. 1016/j.critrevonc.2011.04.012]. [PMID: 21908200]. [DOI] [PubMed] [Google Scholar]
- 28.Boland B.A., Sherry V., Polomano R.C. Chemotherapy-induced peripheral neuropathy in cancer survivors. Oncol. Nurse Edn. 2010;24:33–38. [Google Scholar]
- 29.Lobert S., Vulevic B., Correia J.J. Interaction of vinca alkaloids with tubulin: a comparison of vinblastine, vincristine, and vinorelbine. Biochemistry. 1996;35(21):6806–6814. doi: 10.1021/bi953037i. [http://dx. doi.org/10.1021/bi953037i]. [PMID: 8639632]. [DOI] [PubMed] [Google Scholar]
- 30.Aley K.O., Levine J.D. Different peripheral mechanisms mediate enhanced nociception in metabolic/toxic and traumatic painful peripheral neuropathies in the rat. Neuroscience. 2002;111(2):389–397. doi: 10.1016/s0306-4522(02)00009-x. [http://dx.doi.org/10.1016/S0306-4522(02)00009-X]. [PMID: 11983324]. [DOI] [PubMed] [Google Scholar]
- 31.Press C., Milbrandt J. Nmnat delays axonal degeneration caused by mitochondrial and oxidative stress. J. Neurosci. 2008;28(19):4861–4871. doi: 10.1523/JNEUROSCI.0525-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI.0525-08.2008]. [PMID: 18463239]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett G.J., Doyle T., Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat. Rev. Neurol. 2014;10(6):326–336. doi: 10.1038/nrneurol.2014.77. [http://dx.doi.org/10.1038/nrneurol.2014.77]. [PMID: 24840972]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brederson J.D., Joshi S.K., Browman K.E., Mikusa J., Zhong C., Gauvin D., Liu X., Shi Y., Penning T.D., Shoemaker A.R., Giranda V.L. PARP inhibitors attenuate chemotherapy-induced painful neuropathy. J. Peripher. Nerv. Syst. 2012;17(3):324–330. doi: 10.1111/j.1529-8027.2012.00413.x. [http://dx.doi.org/10.1111/j.1529-8027.2012.00413.x]. [PMID: 22971094]. [DOI] [PubMed] [Google Scholar]
- 34.Krantic S., Mechawar N., Reix S., Quirion R. Apoptosis-inducing factor: a matter of neuron life and death. Prog. Neurobiol. 2007;81(3):179–196. doi: 10.1016/j.pneurobio.2006.12.002. [http://dx.doi.org/10.1016/ j.pneurobio.2006.12.002]. [PMID: 17267093]. [DOI] [PubMed] [Google Scholar]
- 35.Krarup-Hansen A., Rietz B., Krarup C., Heydorn K., Rørth M., Schmalbruch H. Histology and platinum content of sensory ganglia and sural nerves in patients treated with cisplatin and carboplatin: an autopsy study. Neuropathol. Appl. Neurobiol. 1999;25(1):29–40. doi: 10.1046/j.1365-2990.1999.00160.x. [http://dx.doi.org/10.1046/j.1365-2990.1999. 00160.x]. [PMID: 10194773]. [DOI] [PubMed] [Google Scholar]
- 36.Argyriou A.A., Polychronopoulos P., Iconomou G., Chroni E., Kalofonos H.P. A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat. Rev. 2008;34(4):368–377. doi: 10.1016/j.ctrv.2008.01.003. [http://dx.doi. org/10.1016/j.ctrv.2008.01.003]. [PMID: 18281158]. [DOI] [PubMed] [Google Scholar]
- 37.Argyriou A.A., Bruna J., Marmiroli P., Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit. Rev. Oncol. Hematol. 2012;82(1):51–77. doi: 10.1016/j.critrevonc.2011.04.012. [http://dx.doi.org/10.1016/ j.critrevonc.2011.04.012]. [PMID: 21908200]. [DOI] [PubMed] [Google Scholar]
- 38.McDonald E.S., Windebank A.J. Cisplatin-induced apoptosis of DRG neurons involves bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol. Dis. 2002;9(2):220–233. doi: 10.1006/nbdi.2001.0468. [http://dx.doi.org/10.1006/nbdi.2001.0468]. [PMID: 11895373]. [DOI] [PubMed] [Google Scholar]
- 39.Ta L.E., Espeset L., Podratz J., Windebank A.J. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology. 2006;27(6):992–1002. doi: 10.1016/j.neuro.2006.04.010. [http://dx.doi.org/10.1016/j.neuro.2006.04.010]. [PMID: 16797073]. [DOI] [PubMed] [Google Scholar]
- 40.Ta L.E., Schmelzer J.D., Bieber A.J., Loprinzi C.L., Sieck G.C., Brederson J.D., Low P.A., Windebank A.J. A novel and selective poly (ADP-ribose) polymerase inhibitor ameliorates chemotherapy-induced painful neuropathy. PLoS One. 2013;8(1):e54161. doi: 10.1371/journal.pone.0054161. [http:// dx.doi.org/10.1371/journal.pone.0054161]. [PMID: 23326593]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Podratz J.L., Knight A.M., Ta L.E., Staff N.P., Gass J.M., Genelin K., Schlattau A., Lathroum L., Windebank A.J. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol. Dis. 2011;41(3):661–668. doi: 10.1016/j.nbd.2010.11.017. [http://dx.doi.org/ 10.1016/j.nbd.2010.11.017]. [PMID: 21145397]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng H., Xiao W.H., Bennett G.J. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp. Neurol. 2011;232(2):154–161. doi: 10.1016/j.expneurol.2011.08.016. [http://dx.doi.org/10.1016/j.expneurol.2011. 08.016]. [PMID: 21907196]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Cesare Mannelli L., Zanardelli M., Failli P., Ghelardini C. Oxaliplatin-induced neuropathy: oxidative stress as pathological mechanism. Protective effect of silibinin. J. Pain. 2012;13(3):276–284. doi: 10.1016/j.jpain.2011.11.009. [http://dx.doi.org/10.1016/j.jpain.2011.11.009]. [PMID: 22325298]. [DOI] [PubMed] [Google Scholar]
- 44.Di Cesare Mannelli L., Zanardelli M., Failli P., Ghelardini C. Oxaliplatin-induced oxidative stress in nervous system-derived cellular models: could it correlate with in vivo neuropathy? Free Radic. Biol. Med. 2013;61:143–150. doi: 10.1016/j.freeradbiomed.2013.03.019. [http://dx.doi.org/10.1016/j. freeradbiomed.2013.03.019]. [PMID: 23548635]. [DOI] [PubMed] [Google Scholar]
- 45.Pace A., Savarese A., Picardo M., Maresca V., Pacetti U., Del Monte G., Biroccio A., Leonetti C., Jandolo B., Cognetti F., Bove L. Neuroprotective effect of vitamin E supplementation in patients treated with cisplatin chemotherapy. J. Clin. Oncol. 2003;21(5):927–931. doi: 10.1200/JCO.2003.05.139. [http://dx.doi.org/10.1200/JCO.2003.05.139]. [PMID: 12610195]. [DOI] [PubMed] [Google Scholar]
- 46.Carozzi V.A., Marmiroli P., Cavaletti G. The role of oxidative stress and anti-oxidant treatment in platinum-induced peripheral neurotoxicity. Curr. Cancer Drug Targets. 2010;10(7):670–682. doi: 10.2174/156800910793605820. [http://dx.doi.org/10.2174/156800910793605820]. [PMID: 20578989]. [DOI] [PubMed] [Google Scholar]
- 47.Schloss J.M., Colosimo M., Airey C., Masci P.P., Linnane A.W., Vitetta L. Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review. Clin. Nutr. 2013;32(6):888–893. doi: 10.1016/j.clnu.2013.04.007. [http://dx.doi.org/10.1016/j.clnu.2013.04.007]. [PMID: 23647723]. [DOI] [PubMed] [Google Scholar]
- 48.Pasetto L.M., D’Andrea M.R., Rossi E., Monfardini S. Oxaliplatin-related neurotoxicity: how and why? Crit. Rev. Oncol. Hematol. 2006;59(2):159–168. doi: 10.1016/j.critrevonc.2006.01.001. [http://dx.doi.org/10.1016/ j.critrevonc.2006.01.001]. [PMID: 16806962]. [DOI] [PubMed] [Google Scholar]
- 49.Canta A., Pozzi E., Carozzi V.A. Mitochondrial Dysfunction in Chemotherapy-Induced Peripheral Neuropathy (CIPN). Toxics. 2015;3(2):198–223. doi: 10.3390/toxics3020198. [http://dx.doi.org/10.3390/toxics3020198]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zenker J., Ziegler D., Chrast R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 2013;36(8):439–449. doi: 10.1016/j.tins.2013.04.008. [http://dx.doi.org/10.1016/j.tins.2013.04.008]. [PMID: 23725712]. [DOI] [PubMed] [Google Scholar]
- 51.Hagiwara H., Sunada Y. Mechanism of taxane neurotoxicity. Breast Cancer. 2004;11(1):82–85. doi: 10.1007/BF02968008. [http://dx.doi.org/10.1007/ BF02968008]. [PMID: 14718798]. [DOI] [PubMed] [Google Scholar]
- 52.Peters C.M., Jimenez-Andrade J.M., Kuskowski M.A., Ghilardi J.R., Mantyh P.W. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res. 2007;1168:46–59. doi: 10.1016/j.brainres.2007.06.066. [http://dx.doi.org/10.1016/j.brainres.2007. 06.066]. [PMID: 17698044]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doyle T., Chen Z., Muscoli C., Bryant L., Esposito E., Cuzzocrea S., Dagostino C., Ryerse J., Rausaria S., Kamadulski A., Neumann W.L., Salvemini D. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J. Neurosci. 2012;32(18):6149–6160. doi: 10.1523/JNEUROSCI.6343-11.2012. [http:// dx.doi.org/10.1523/JNEUROSCI.6343-11.2012]. [PMID: 22553021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weng H-R., Aravindan N., Cata J.P., Chen J-H., Shaw A.D., Dougherty P.M. Spinal glial glutamate transporters downregulate in rats with taxol-induced hyperalgesia. Neurosci. Lett. 2005;386(1):18–22. doi: 10.1016/j.neulet.2005.05.049. [http://dx.doi.org/10.1016/j.neulet.2005.05.049]. [PMID: 15975716]. [DOI] [PubMed] [Google Scholar]
- 55.Flatters S.J., Bennett G.J. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122(3):245–257. doi: 10.1016/j.pain.2006.01.037. [http://dx.doi.org/10.1016/j.pain.2006.01.037]. [PMID: 16530964]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Argyriou A.A., Iconomou G., Kalofonos H.P. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood. 2008;112(5):1593–1599. doi: 10.1182/blood-2008-04-149385. [http://dx.doi.org/10.1182/blood-2008-04-149385]. [PMID: 18574024]. [DOI] [PubMed] [Google Scholar]
- 57.Casafont I., Berciano M.T., Lafarga M. Bortezomib induces the formation of nuclear poly(A) RNA granules enriched in Sam68 and PABPN1 in sensory ganglia neurons. Neurotox. Res. 2010;17(2):167–178. doi: 10.1007/s12640-009-9086-1. [http://dx.doi.org/10.1007/s12640-009-9086-1]. [PMID: 19609631]. [DOI] [PubMed] [Google Scholar]
- 58.Meregalli C., Canta A., Carozzi V.A., Chiorazzi A., Oggioni N., Gilardini A., Ceresa C., Avezza F., Crippa L., Marmiroli P., Cavaletti G. Bortezomib-induced painful neuropathy in rats: a behavioral, neurophysiological and pathological study in rats. Eur. J. Pain. 2010;14(4):343–350. doi: 10.1016/j.ejpain.2009.07.001. [http://dx.doi.org/10.1016/j.ejpain. 2009.07.001]. [PMID: 19695912]. [DOI] [PubMed] [Google Scholar]
- 59.Bruna J., Udina E., Alé A., Vilches J.J., Vynckier A., Monbaliu J., Silverman L., Navarro X. Neurophysiological, histological and immunohistochemical characterization of bortezomib-induced neuropathy in mice. Exp. Neurol. 2010;223(2):599–608. doi: 10.1016/j.expneurol.2010.02.006. [http:// dx.doi.org/10.1016/j.expneurol.2010.02.006]. [PMID: 20188093]. [DOI] [PubMed] [Google Scholar]
- 60.Zheng H., Xiao W.H., Bennett G.J. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp. Neurol. 2012;238(2):225–234. doi: 10.1016/j.expneurol.2012.08.023. [http://dx.doi.org/10.1016/j.expneurol.2012.08. 023]. [PMID: 22947198]. [DOI] [PubMed] [Google Scholar]
- 61.Broyl A., Corthals S.L., Jongen J.L., van der Holt B., Kuiper R., de Knegt Y., van Duin M., el Jarari L., Bertsch U., Lokhorst H.M., Durie B.G., Goldschmidt H., Sonneveld P. Mechanisms of peripheral neuropathy associated with bortezomib and vincristine in patients with newly diagnosed multiple myeloma: a prospective analysis of data from the HOVON-65/GMMG-HD4 trial. Lancet Oncol. 2010;11(11):1057–1065. doi: 10.1016/S1470-2045(10)70206-0. [http://dx.doi.org/10.1016/S1470-2045(10)70206-0]. [PMID: 20864405]. [DOI] [PubMed] [Google Scholar]
- 62.Janes K., Doyle T., Bryant L., Esposito E., Cuzzocrea S., Ryerse J., Bennett G.J., Salvemini D. Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain. 2013;154(11):2432–2440. doi: 10.1016/j.pain.2013.07.032. [http://dx.doi.org/10.1016/j.pain.2013. 07.032]. [PMID: 23891899]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piccolo J., Kolesar J.M. Prevention and treatment of chemotherapy-induced peripheral neuropathy. Am. J. Health Syst. Pharm. 2014;71(1):19–25. doi: 10.2146/ajhp130126. [http://dx.doi.org/10.2146/ajhp130126]. [PMID: 24352178]. [DOI] [PubMed] [Google Scholar]
- 64.Finnerup N.B., Sindrup S.H., Jensen T.S. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573–581. doi: 10.1016/j.pain.2010.06.019. [http://dx.doi.org/10.1016/j.pain.2010.06.019]. [PMID: 20705215]. [DOI] [PubMed] [Google Scholar]
- 65.Pfister B.J., Gordon T., Loverde J.R., Kochar A.S., Mackinnon S.E., Cullen D.K. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit. Rev. Biomed. Eng. 2011;39(2):81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [http://dx.doi.org/10.1615/CritRevBiomedEng.v39.i2.20]. [PMID: 21488817]. [DOI] [PubMed] [Google Scholar]
- 66.Woolf C.J., Mannion R.J. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353(9168):1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [http://dx.doi.org/10.1016/S0140-6736(99)01307-0]. [PMID: 10371588]. [DOI] [PubMed] [Google Scholar]
- 67.Freeman R. The treatment of neuropathic pain. CNS Spectr. 2005;10(9):698–706. doi: 10.1017/s1092852900019696. [PMID: 16142210]. [DOI] [PubMed] [Google Scholar]
- 68.Ji R-R., Suter M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain. 2007;3(33):33. doi: 10.1186/1744-8069-3-33. [http://dx.doi.org/ 10.1186/1744-8069-3-33]. [PMID: 17974036]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inoue K., Tsuda M., Koizumi S. ATP receptors in pain sensation: Involvement of spinal microglia and P2X(4) receptors. Purinergic Signal. 2005;1(2):95–100. doi: 10.1007/s11302-005-6210-4. [http://dx.doi.org/10.1007/s11302-005-6210-4]. [PMID: 18404495]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bridges D., Thompson S.W., Rice A.S. Mechanisms of neuropathic pain. Br. J. Anaesth. 2001;87(1):12–26. doi: 10.1093/bja/87.1.12. [http://dx.doi. org/10.1093/bja/87.1.12]. [PMID: 11460801]. [DOI] [PubMed] [Google Scholar]
- 71.Chen K-H., Lin C-R., Cheng J-T., Cheng J-K., Liao W-T., Yang C-H. Altered mitochondrial ATP synthase expression in the rat dorsal root ganglion after sciatic nerve injury and analgesic effects of intrathecal ATP. Cell. Mol. Neurobiol. 2014;34(1):51–59. doi: 10.1007/s10571-013-9986-8. [http://dx.doi.org/10.1007/s10571-013-9986-8]. [PMID: 24048632]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao Y-J., Ji R-R. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol. Ther. 2010;126(1):56–68. doi: 10.1016/j.pharmthera.2010.01.002. [http://dx.doi.org/10.1016/j.pharmthera.2010.01. 002]. [PMID: 20117131]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsuda M., Inoue K., Salter M.W. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28(2):101–107. doi: 10.1016/j.tins.2004.12.002. [http://dx.doi.org/10.1016/j.tins. 2004.12.002]. [PMID: 15667933]. [DOI] [PubMed] [Google Scholar]
- 74.Thacker M.A., Clark A.K., Marchand F., McMahon S.B. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth. Analg. 2007;105(3):838–847. doi: 10.1213/01.ane.0000275190.42912.37. [http://dx.doi. org/10.1213/01.ane.0000275190.42912.37]. [PMID: 17717248]. [DOI] [PubMed] [Google Scholar]
- 75.Mika J., Zychowska M., Popiolek-Barczyk K., Rojewska E., Przewlocka B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013;716(1-3):106–119. doi: 10.1016/j.ejphar.2013.01.072. [http://dx.doi.org/ 10.1016/j.ejphar.2013.01.072]. [PMID: 23500198]. [DOI] [PubMed] [Google Scholar]
- 76.Zimmermann M. Pathobiology of neuropathic pain. Eur. J. Pharmacol. 2001;429(1-3):23–37. doi: 10.1016/s0014-2999(01)01303-6. [http://dx.doi.org/10.1016/ S0014-2999(01)01303-6]. [PMID: 11698024]. [DOI] [PubMed] [Google Scholar]
- 77.Piantadosi C.A., Suliman H.B. Transcriptional control of mitochondrial biogenesis and its interface with inflammatory processes. Biochimica et Biophysica Acta (BBA)-. General Subjects. 2012;1820(4):532–541. doi: 10.1016/j.bbagen.2012.01.003. [http://dx.doi.org/10.1016/ j.bbagen.2012.01.003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dworkin R.H., Backonja M., Rowbotham M.C., Allen R.R., Argoff C.R., Bennett G.J., Bushnell M.C., Farrar J.T., Galer B.S., Haythornthwaite J.A., Hewitt D.J., Loeser J.D., Max M.B., Saltarelli M., Schmader K.E., Stein C., Thompson D., Turk D.C., Wallace M.S., Watkins L.R., Weinstein S.M. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch. Neurol. 2003;60(11):1524–1534. doi: 10.1001/archneur.60.11.1524. [http://dx.doi.org/10.1001/archneur.60.11.1524]. [PMID: 14623723]. [DOI] [PubMed] [Google Scholar]
- 79.Kk J., editor. Drug-induced neurological disorders. Seattle: Hogrefe & Huber; 2001. J. Drug-induced peripheral neuropathies. pp. 263–294. [Google Scholar]
- 80.Knott A.B., Perkins G., Schwarzenbacher R., Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008;9(7):505–518. doi: 10.1038/nrn2417. [http://dx.doi.org/10.1038/nrn2417]. [PMID: 18568013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Court F.A., Coleman M.P. Mitochondria as a central sensor for axonal degenerative stimuli. Trends Neurosci. 2012;35(6):364–372. doi: 10.1016/j.tins.2012.04.001. [http://dx.doi.org/10.1016/j.tins.2012.04.001]. [PMID: 22578891]. [DOI] [PubMed] [Google Scholar]
- 82.Chen H., Chan D.C. Critical dependence of neurons on mitochondrial dynamics. Curr. Opin. Cell Biol. 2006;18(4):453–459. doi: 10.1016/j.ceb.2006.06.004. [http://dx.doi.org/10.1016/j.ceb.2006.06.004]. [PMID: 16781135]. [DOI] [PubMed] [Google Scholar]
- 83.Jaggi A.S., Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291(1-3):1–9. doi: 10.1016/j.tox.2011.10.019. [http://dx.doi.org/10.1016/j.tox.2011.10.019]. [PMID: 22079234]. [DOI] [PubMed] [Google Scholar]
- 84.Russell J.W., Golovoy D., Vincent A.M., Mahendru P., Olzmann J.A., Mentzer A., Feldman E.L. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16(13):1738–1748. doi: 10.1096/fj.01-1027com. [http://dx.doi.org/10.1096/fj.01-1027com]. [PMID: 12409316]. [DOI] [PubMed] [Google Scholar]
- 85.Fernyhough P., Roy Chowdhury S.K., Schmidt R.E. Mitochondrial stress and the pathogenesis of diabetic neuropathy. Expert Rev. Endocrinol. Metab. 2010;5(1):39–49. doi: 10.1586/eem.09.55. [http://dx.doi. org/10.1586/eem.09.55]. [PMID: 20729997]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Areti A., Yerra V.G., Naidu V., Kumar A. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuro- pathy. Redox Biol. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006. [http://dx.doi.org/10.1016/ j.redox.2014.01.006]. [PMID: 24494204]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viader A., Golden J.P., Baloh R.H., Schmidt R.E., Hunter D.A., Milbrandt J. Schwann cell mitochondrial metabolism supports long-term axonal survival and peripheral nerve function. J. Neurosci. 2011;31(28):10128–10140. doi: 10.1523/JNEUROSCI.0884-11.2011. [http://dx.doi.org/10.1523/ JNEUROSCI.0884-11.2011]. [PMID: 21752989]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L., Yu C., Vasquez F.E., Galeva N., Onyango I., Swerdlow R.H., Dobrowsky R.T. Hyperglycemia alters the schwann cell mitochondrial proteome and decreases coupled respiration in the absence of superoxide production. J. Proteome Res. 2010;9(1):458–471. doi: 10.1021/pr900818g. [http://dx.doi.org/10.1021/pr900818g]. [PMID: 19905032]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akude E.L., Zherebitskaya E., Chowdhury S.K., Girling K., Fernyhough P. 4-Hydroxy-2-nonenal Impairs Mitochondrial Physiology and Induces Aberrant Axonal Outgrowth in Adult Sensory Neurons that Mimics Features of Diabetic Neuropathy. Neurotox. Res. 2010;17(1):28–38. doi: 10.1007/s12640-009-9074-5. [http://dx.doi.org/10.1007/ s12640-009-9074-5]. [PMID: 19557324]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pop-Busui R., Sima A., Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab. Res. Rev. 2006;22(4):257–273. doi: 10.1002/dmrr.625. [http://dx.doi.org/10.1002/dmrr.625]. [PMID: 16506271]. [DOI] [PubMed] [Google Scholar]
- 91.Hinder L.M., Vincent A.M., Burant C.F., Pennathur S., Feldman E.L. Bioenergetics in diabetic neuropathy: what we need to know. J. Peripher. Nerv. Syst. 2012;17(s2) Suppl. 2:10–14. doi: 10.1111/j.1529-8027.2012.00389.x. [http://dx.doi.org/10.1111/j.1529-8027.2012.00389.x]. [PMID: 22548617]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ryu H., Lee J., Impey S., Ratan R.R., Ferrante R.J. Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. Proc. Natl. Acad. Sci. USA. 2005;102(39):13915–13920. doi: 10.1073/pnas.0502878102. [http://dx.doi.org/10.1073/pnas.0502878102]. [PMID: 16169904]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuan H., Gerencser A.A., Liot G., Lipton S.A., Ellisman M., Perkins G.A., Bossy-Wetzel E. Mitochondrial fission is an upstream and required event for bax foci formation in response to nitric oxide in cortical neurons. Cell Death Differ. 2007;14(3):462–471. doi: 10.1038/sj.cdd.4402046. [http://dx.doi.org/10.1038/sj.cdd.4402046]. [PMID: 17053808]. [DOI] [PubMed] [Google Scholar]
- 94.Ferrari L.F., Chum A., Bogen O., Reichling D.B., Levine J.D. Role of Drp1, a key mitochondrial fission protein, in neuropathic pain. J. Neurosci. 2011;31(31):11404–11410. doi: 10.1523/JNEUROSCI.2223-11.2011. [http://dx.doi.org/ 10.1523/JNEUROSCI.2223-11.2011]. [PMID: 21813700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leinninger G.M., Backus C., Sastry A.M., Yi Y-B., Wang C-W., Feldman E.L. Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol. Dis. 2006;23(1):11–22. doi: 10.1016/j.nbd.2006.01.017. [http://dx.doi. org/10.1016/j.nbd.2006.01.017]. [PMID: 16684605]. [DOI] [PubMed] [Google Scholar]
- 96.Nakamura T., Cieplak P., Cho D-H., Godzik A., Lipton S.A. S-nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion. 2010;10(5):573–578. doi: 10.1016/j.mito.2010.04.007. [http://dx.doi.org/10.1016/j.mito.2010.04.007]. [PMID: 20447471]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Vos K., Severin F., Van Herreweghe F., Vancompernolle K., Goossens V., Hyman A., Grooten J. Tumor necrosis factor induces hyperphosphorylation of kinesin light chain and inhibits kinesin-mediated transport of mitochondria. J. Cell Biol. 2000;149(6):1207–1214. doi: 10.1083/jcb.149.6.1207. [http://dx.doi.org/10.1083/jcb.149.6.1207]. [PMID: 10851018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chhangani D., Mishra A. Protein quality control system in neurodegeneration: a healing company hard to beat but failure is fatal. Mol. Neurobiol. 2013;48(1):141–156. doi: 10.1007/s12035-013-8411-0. [http://dx.doi.org/ 10.1007/s12035-013-8411-0]. [PMID: 23378031]. [DOI] [PubMed] [Google Scholar]
- 99.Gottlieb R.A., Carreira R.S., Gottlieb R.A., Carreira R.S. Autophagy in health and disease. 5. Mitophagy as a way of life. Am. J. Physiol. Cell Physiol. 2010;299(2):C203–C210. doi: 10.1152/ajpcell.00097.2010. [http://dx.doi.org/10.1152/ajpcell.00097.2010]. [PMID: 20357180]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Federico A., Cardaioli E., Da Pozzo P., Formichi P., Gallus G.N., Radi E. Mitochondria, oxidative stress and neuro- degeneration. J. Neurol. Sci. 2012;322(1-2):254–262. doi: 10.1016/j.jns.2012.05.030. [http://dx. doi.org/10.1016/j.jns.2012.05.030]. [PMID: 22669122]. [DOI] [PubMed] [Google Scholar]
- 101.Palacino J.J., Sagi D., Goldberg M.S., Krauss S., Motz C., Wacker M., Klose J., Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 2004;279(18):18614–18622. doi: 10.1074/jbc.M401135200. [http://dx.doi.org/10.1074/jbc.M401135200]. [PMID: 14985362]. [DOI] [PubMed] [Google Scholar]
- 102.Urban M.J., Pan P., Farmer K.L., Zhao H., Blagg B.S., Dobrowsky R.T. Modulating molecular chaperones improves sensory fiber recovery and mitochondrial function in diabetic peripheral neuropathy. Exp. Neurol. 2012;235(1):388–396. doi: 10.1016/j.expneurol.2012.03.005. [http:// dx.doi.org/10.1016/j.expneurol.2012.03.005]. [PMID: 22465570]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marinelli S., Nazio F., Tinari A., Ciarlo L., D’Amelio M., Pieroni L., Vacca V., Urbani A., Cecconi F., Malorni W., Pavone F. Schwann cell autophagy counteracts the onset and chronification of neuropathic pain. Pain. 2014;155(1):93–107. doi: 10.1016/j.pain.2013.09.013. [http://dx.doi.org/10.1016/j.pain.2013.09.013]. [PMID: 24041962]. [DOI] [PubMed] [Google Scholar]
- 104.Fortun J., Verrier J.D., Go J.C., Madorsky I., Dunn W.A., Notterpek L. The formation of peripheral myelin protein 22 aggregates is hindered by the enhancement of autophagy and expression of cytoplasmic chaperones. Neurobiol. Dis. 2007;25(2):252–265. doi: 10.1016/j.nbd.2006.09.018. [http://dx.doi.org/10.1016/j.nbd.2006.09.018]. [PMID: 17174099]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Watanabe T., Nagase K., Chosa M., Tobinai K. Schwann cell autophagy induced by SAHA, 17-AAG, or clonazepam can reduce bortezomib-induced peripheral neuropathy. Br. J. Cancer. 2010;103(10):1580–1587. doi: 10.1038/sj.bjc.6605954. [http://dx.doi.org/10.1038/sj.bjc.6605954]. [PMID: 20959823]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Witte M.E., Geurts J.J., de Vries H.E., van der Valk P., van Horssen J. Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration? Mitochondrion. 2010;10(5):411–418. doi: 10.1016/j.mito.2010.05.014. [http://dx.doi.org/10.1016/j.mito.2010.05.014]. [PMID: 20573557]. [DOI] [PubMed] [Google Scholar]
- 107.Ferger A.I., Campanelli L., Reimer V., Muth K.N., Merdian I., Ludolph A.C., Witting A. Effects of mitochondrial dysfunction on the immunological properties of microglia. J. Neuroinflammation. 2010;7(45):45. doi: 10.1186/1742-2094-7-45. [http://dx.doi.org/10.1186/1742-2094-7-45]. [PMID: 20701773]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.López-Armada M.J., Riveiro-Naveira R.R., Vaamonde-García C., Valcárcel-Ares M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13(2):106–118. doi: 10.1016/j.mito.2013.01.003. [http://dx.doi.org/10.1016/j.mito.2013.01.003]. [PMID: 23333405]. [DOI] [PubMed] [Google Scholar]
- 109.Brown G.C., Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol. Neurobiol. 2003;27(3):325–355. doi: 10.1385/MN:27:3:325. [http://dx.doi.org/10.1385/ MN:27:3:325]. [PMID: 12845153]. [DOI] [PubMed] [Google Scholar]
- 110.Pieczenik S.R., Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007;83(1):84–92. doi: 10.1016/j.yexmp.2006.09.008. [http://dx.doi.org/10.1016/j.yexmp.2006.09.008]. [PMID: 17239370]. [DOI] [PubMed] [Google Scholar]
- 111.Tait S.W., Green D.R. Mitochondria and cell signalling. J. Cell Sci. 2012;125(Pt 4):807–815. doi: 10.1242/jcs.099234. [http://dx.doi.org/10.1242/jcs.099234]. [PMID: 22448037]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [http://dx.doi.org/10.1038/nature08780]. [PMID: 20203610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tschopp J. Mitochondria: Sovereign of inflammation? Eur. J. Immunol. 2011;41(5):1196–1202. doi: 10.1002/eji.201141436. [http://dx.doi.org/10.1002/eji. 201141436]. [PMID: 21469137]. [DOI] [PubMed] [Google Scholar]
- 114.Lee J., Giordano S., Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 2012;441(2):523–540. doi: 10.1042/BJ20111451. [http://dx.doi.org/10.1042/BJ20111451]. [PMID: 22187934]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Friberg H., Wieloch T. Mitochondrial permeability transition in acute neurodegeneration. Biochimie. 2002;84(2-3):241–250. doi: 10.1016/s0300-9084(02)01381-0. [http:// dx.doi.org/10.1016/S0300-9084(02)01381-0]. [PMID: 12022955]. [DOI] [PubMed] [Google Scholar]
- 116.Okouchi M., Ekshyyan O., Maracine M., Aw T.Y. Neuronal apoptosis in neurodegeneration. Antioxid. Redox Signal. 2007;9(8):1059–1096. doi: 10.1089/ars.2007.1511. [http://dx.doi.org/10.1089/ars.2007.1511]. [DOI] [PubMed] [Google Scholar]
- 117.Rao V.K., Carlson E.A., Yan S.S. Mitochondrial permeability transition pore is a potential drug target for neurodegeneration. Biochimica et Biophysica Acta (BBA)-. Molecular Basis of Disease. 2013;1842(8):1267–1272. doi: 10.1016/j.bbadis.2013.09.003. [http://dx.doi.org/10.1016/ j.bbadis.2013.09.003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Javadov S., Karmazyn M., Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J. Pharmacol. Exp. Ther. 2009;330(3):670–678. doi: 10.1124/jpet.109.153213. [http://dx.doi.org/10.1124/jpet.109.153213]. [PMID: 19509316]. [DOI] [PubMed] [Google Scholar]
- 119.Auten R.L., Davis J.M. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr. Res. 2009;66(2):121–127. doi: 10.1203/PDR.0b013e3181a9eafb. [http://dx.doi.org/10.1203/PDR.0b013e3181a9eafb]. [PMID: 19390491]. [DOI] [PubMed] [Google Scholar]
- 120.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [http://dx.doi.org/10.1038/35041687]. [PMID: 11089981]. [DOI] [PubMed] [Google Scholar]
- 121.Naoi M., Maruyama W., Shamoto-Nagai M., Yi H., Akao Y., Tanaka M. Oxidative stress in mitochondria: decision to survival and death of neurons in neurodegenerative disorders. Mol. Neurobiol. 2005;31(1-3):81–93. doi: 10.1385/MN:31:1-3:081. [http://dx.doi.org/10.1385/MN: 31:1-3:081]. [PMID: 15953813]. [DOI] [PubMed] [Google Scholar]
- 122.Chowdhury S.K., Smith D.R., Fernyhough P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol. Dis. 2013;51:56–65. doi: 10.1016/j.nbd.2012.03.016. [http://dx.doi.org/10.1016/j.nbd. 2012.03.016]. [PMID: 22446165]. [DOI] [PubMed] [Google Scholar]
- 123.Sheu S-S., Nauduri D., Anders M.W. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochimica et Biophysica Acta (BBA)-. Molecular Basis of Disease. 2006;1762(2):256–265. doi: 10.1016/j.bbadis.2005.10.007. [http://dx.doi.org/10.1016/j.bbadis.2005.10.007]. [DOI] [PubMed] [Google Scholar]
- 124.Szeto H.H. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J. 2006;8(3):E521–E531. doi: 10.1208/aapsj080362. [http://dx.doi.org/10.1208/aapsj080362]. [PMID: 17025271]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith R.A., Murphy M.P. Mitochondria-targeted antioxidants as therapies. Discov. Med. 2011;11(57):106–114. [PMID: 21356165]. [PubMed] [Google Scholar]
- 126.Atamna H., Mackey J., Dhahbi J.M. Mitochondrial pharmacology: electron transport chain bypass as strategies to treat mitochondrial dysfunction. Biofactors. 2012;38(2):158–166. doi: 10.1002/biof.197. [http://dx.doi.org/10.1002/biof.197]. [PMID: 22419586]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schwarz T.L. Mitochondrial trafficking in neurons. Cold Spring Harb. Perspect. Biol. 2013;5(6):a011304. doi: 10.1101/cshperspect.a011304. [http://dx.doi.org/10. 1101/cshperspect.a011304]. [PMID: 23732472]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cai Q., Davis M.L., Sheng Z-H. Regulation of axonal mitochondrial transport and its impact on synaptic transmission. Neurosci. Res. 2011;70(1):9–15. doi: 10.1016/j.neures.2011.02.005. [http://dx.doi.org/10.1016/ j.neures.2011.02.005]. [PMID: 21352858]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reddy P.H. Inhibitors of mitochondrial fission as a therapeutic strategy for diseases with oxidative stress and mitochondrial dysfunction. J. Alzheimers Dis. 2014;40(2):245–256. doi: 10.3233/JAD-132060. [PMID: 24413616]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.García-Arencibia M., Hochfeld W.E., Toh P.P., Rubinsztein D.C. Autophagy, a guardian against neurodegeneration. Semin. Cell Dev. Biol. 2010;21(7):691–698. doi: 10.1016/j.semcdb.2010.02.008. [http://dx.doi.org/10.1016/ j.semcdb.2010.02.008]. [PMID: 20188203]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giordano S., Darley-Usmar V., Zhang J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2014;2:82–90. doi: 10.1016/j.redox.2013.12.013. [http://dx.doi.org/10.1016/j.redox. 2013.12.013]. [PMID: 24494187]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rubinsztein D.C., Codogno P., Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012;11(9):709–730. doi: 10.1038/nrd3802. [http://dx.doi.org/10.1038/nrd3802]. [PMID: 22935804]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheng Y., Ren X., Hait W.N., Yang J-M. Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol. Rev. 2013;65(4):1162–1197. doi: 10.1124/pr.112.007120. [http://dx.doi.org/10.1124/pr.112. 007120]. [PMID: 23943849]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith R.A., Hartley R.C., Cochemé H.M., Murphy M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012;33(6):341–352. doi: 10.1016/j.tips.2012.03.010. [http://dx.doi.org/10.1016/j.tips.2012.03.010]. [PMID: 22521106]. [DOI] [PubMed] [Google Scholar]
- 135.Scarpulla R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochimica et Biophysica Acta (BBA)-. Molecular Cell Research. 2011;1813(7):1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Austin S., St-Pierre J. PGC1α and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012;125(Pt 21):4963–4971. doi: 10.1242/jcs.113662. [http://dx.doi. org/10.1242/jcs.113662]. [PMID: 23277535]. [DOI] [PubMed] [Google Scholar]
- 137.Valle I., Alvarez-Barrientos A., Arza E., Lamas S., Monsalve M. PGC-1α regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005;66(3):562–573. doi: 10.1016/j.cardiores.2005.01.026. [http://dx.doi.org/10.1016/j.cardiores.2005.01.026]. [PMID: 15914121]. [DOI] [PubMed] [Google Scholar]
- 138.Negi G., Kumar A., Sharma S.S. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-κB and Nrf2 cascades. J. Pineal Res. 2011;50(2):124–131. doi: 10.1111/j.1600-079X.2010.00821.x. [PMID: 21062351]. [DOI] [PubMed] [Google Scholar]
- 139.Baldelli S., Aquilano K., Ciriolo M.R. Punctum on two different transcription factors regulated by PGC-1α: Nuclear factor erythroid-derived 2-like 2 and nuclear respiratory factor 2. Biochimica et Biophysica Acta (BBA)-. General Subjects. 2013;1830(8):4137–4146. doi: 10.1016/j.bbagen.2013.04.006. [http://dx.doi.org/10.1016/j.bbagen.2013. 04.006]. [DOI] [PubMed] [Google Scholar]
- 140.Lee S., Notterpek L. Dietary restriction supports peripheral nerve health by enhancing endogenous protein quality control mechanisms. Exp. Gerontol. 2013;48(10):1085–1090. doi: 10.1016/j.exger.2012.12.008. [http://dx.doi.org/10. 1016/j.exger.2012.12.008]. [PMID: 23267845]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wiggin T.D., Kretzler M., Pennathur S., Sullivan K.A., Brosius F.C., Feldman E.L. Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology. 2008;149(10):4928–4937. doi: 10.1210/en.2008-0869. [http://dx.doi.org/10.1210/en.2008-0869]. [PMID: 18583417]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi J., Chandrasekaran K., Inoue T., Muragundla A., Russell J.W. PGC-1α regulation of mitochondrial degeneration in experimental diabetic neuropathy. Neurobiol. Dis. 2014;64:118–130. doi: 10.1016/j.nbd.2014.01.001. [http://dx.doi.org/10.1016/j.nbd.2014.01.001]. [PMID: 24423644]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chandrasekaran K., Muragundla A., Inoue T., Choi J., Chen C., Ide T., Russell J.W. Mitochondrial transcription factor a regulation of mitochondrial degeneration in experimental diabetic neuropathy. American Journal of Physiology-Endocrinology and Metabolism. 2015 doi: 10.1152/ajpendo.00620.2014. 00620.02014. [http://dx.doi.org/10.1152/ajpendo.00620.2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tsunemi T., La Spada A.R. PGC-1α at the intersection of bioenergetics regulation and neuron function: from Huntington’s disease to Parkinson’s disease and beyond. Prog. Neurobiol. 2012;97(2):142–151. doi: 10.1016/j.pneurobio.2011.10.004. [http://dx.doi.org/10.1016/j.pneurobio.2011.10.004]. [PMID: 22100502]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cantó C., Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009;20(2):98–105. doi: 10.1097/MOL.0b013e328328d0a4. [http://dx.doi.org/10.1097/MOL. 0b013e328328d0a4]. [PMID: 19276888]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ruderman N.B., Xu X.J., Nelson L., Cacicedo J.M., Saha A.K., Lan F., Ido Y. AMPK and SIRT1: a long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010;298(4):E751–E760. doi: 10.1152/ajpendo.00745.2009. [http://dx.doi.org/10.1152/ajpendo.00745.2009]. [PMID: 20103737]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cantó C., Auwerx J. Targeting sirtuin 1 to improve metabolism: all you need is NAD. Pharmacol. Rev. 2012;64(1):166–187. doi: 10.1124/pr.110.003905. [http://dx.doi.org/10.1124/pr.110.003905]. [PMID: 22106091]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salminen A., Kaarniranta K., Kauppinen A. Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int. J. Mol. Sci. 2013;14(2):3834–3859. doi: 10.3390/ijms14023834. [http://dx.doi.org/10.3390/ ijms14023834]. [PMID: 23434668]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mo C., Wang L., Zhang J., Numazawa S., Tang H., Tang X., Han X., Li J., Yang M., Wang Z. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxinshocked mice. Antioxid. Redox Signal. 2013;20(4):574–588. doi: 10.1089/ars.2012.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13(9):1016–1023. doi: 10.1038/ncb2329. [http://dx.doi.org/10.1038/ncb2329]. [PMID: 21892142]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paraíso A.F., Mendes K.L., Santos S.H. Brain activation of SIRT1: role in neuropathology. Mol. Neurobiol. 2013;48(3):681–689. doi: 10.1007/s12035-013-8459-x. [http://dx.doi.org/10.1007/s12035-013-8459-x]. [PMID: 23615921]. [DOI] [PubMed] [Google Scholar]
- 152.Egan D.F., Shackelford D.B., Mihaylova M.M., Gelino S., Kohnz R.A., Mair W., Vasquez D.S., Joshi A., Gwinn D.M., Taylor R., Asara J.M., Fitzpatrick J., Dillin A., Viollet B., Kundu M., Hansen M., Shaw R.J. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331(6016):456–461. doi: 10.1126/science.1196371. [http://dx.doi.org/10.1126/science.1196371]. [PMID: 21205641]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jang S.Y., Kang H.T., Hwang E.S. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J. Biol. Chem. 2012;287(23):19304–19314. doi: 10.1074/jbc.M112.363747. [http://dx.doi.org/10.1074/jbc.M112.363747]. [PMID: 22493485]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Price N.L., Gomes A.P., Ling A.J., Duarte F.V., Martin-Montalvo A., North B.J., Agarwal B., Ye L., Ramadori G., Teodoro J.S., Hubbard B.P., Varela A.T., Davis J.G., Varamini B., Hafner A., Moaddel R., Rolo A.P., Coppari R., Palmeira C.M., de Cabo R., Baur J.A., Sinclair D.A. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–690. doi: 10.1016/j.cmet.2012.04.003. [http://dx.doi.org/10.1016/j.cmet.2012.04.003]. [PMID: 22560220]. [DOI] [PMC free article] [PubMed] [Google Scholar]