Abstract
In recent years there has been a growing body of clinical and laboratory evidence demonstrating the neuroprotective effects of estrogen and progesterone after traumatic brain injury (TBI) and spinal cord injury (SCI). In humans, women have been shown to have a lower incidence of morbidity and mortality after TBI compared with age-matched men. Similarly, numerous laboratory studies have demonstrated that estrogen and progesterone administration is associated with a mortality reduction, improvement in neurological outcomes, and a reduction in neuronal apoptosis after TBI and SCI. Here, we review the evidence that supports hormone-related neuroprotection and discuss possible underlying mechanisms. Estrogen and progesterone-mediated neuroprotection are thought to be related to their effects on hormone receptors, signaling systems, direct antioxidant effects, effects on astrocytes and microglia, modulation of the inflammatory response, effects on cerebral blood flow and metabolism, and effects on mediating glutamate excitotoxicity. Future laboratory research is needed to better determine the mechanisms underlying the hormones’ neuroprotective effects, which will allow for more clinical studies. Furthermore, large randomized clinical control trials are needed to better assess their role in human neurodegenerative conditions.
Keywords: Estrogen, neuroprotection, progesterone, spinal cord injury, traumatic brain injury
INTRODUCTION
Although estrogen and progesterone are gonadal steroid hormones, their actions are not restricted to reproductive organs and functions. Estrogen and progesterone exert effects on various organs systems including the central and peripheral nervous systems, and they play a significant role in growth and development [1, 2]. In the past three decades, there has been increasing evidence that estrogen and progesterone are neuroactive hormones. In recent years there has been a growing body of clinical evidence that has demonstrated neuroprotective effects of estrogen and progesterone. Studies have further proposed that estrogen and progesterone serve as general neurotrophic molecules that stabilize neuronal function, support neuronal viability, and, under certain conditions, prevent neuronal death [2-4]. Interestingly, epidemiological studies have observed gender differences in the incidence of a wide range of unrelated neurological and psychiatric disorders, and it has been suggested that estrogen and progesterone among other factors contribute to this difference [5]. The mechanisms of these neuroprotective effects, however, are poorly understood. The purpose here is to review both the preclinical and clinical literature that demonstrates the neuroprotective
effects of sex hormones on the central nervous system, and to describe possible mechanisms for these effects.
NEUROPROTECTIVE EFFECTS OF ESTROGEN
Estrogen’s neuroprotective effects have been shown in numerous experimental studies, including animal models of acute global and focal cerebral ischemia, spinal cord injury (SCI), traumatic brain injury (TBI), and experimental autoimmune encephalitis.
Traumatic Brain Injury
Preclinical Animal Model Studies
Clinical reports are supported by experimental preclinical evidence of the neuroprotective effects of estrogen in animal models. These laboratory studies suggest estrogen-mediated benefits in mortality, functional outcomes, and histological improvement after TBI. Using a model of impact-acceleration closed head injury, two studies have demonstrated a 100% survival in female rats compared with a 75% survival in male rats [6, 7] (Table 1). Furthermore, selective estrogen receptor (ER) agonists were implicated as a mediator of neuroprotection after acoustic TBI in mice [8].
Table 1.
Neuroprotective effects of estrogen after traumatic brain injury (animal models).
| Study | Animal Model | Animal Species | Estrogen Treatment Protocol | Primary End Points | Conclusions |
|---|---|---|---|---|---|
| Neese, 2010 [6] | Fluid percussion injury | Rats | Z-Bisdehydrodoisynolic acid (300μg/0.1cc/100g body weight, sc) two hours after FPI for 48 hours | 1. Behavioral testing: Coordination of limb movement, memory task 2. Lesion size |
Z-BDDA improved behavioral testing. There were no changes in cortical lesion size or cell death. |
| Meltser, 2008 [8] | Acoustic trauma | Mice | ER alpha-selective agonist – propyl (1H) pyrazole-1,3,5-triyl-trisphenol (PTT) | Auditory function | PPT pre-treatment partially protected from hearing loss after trauma. |
| Emerson, 1993 [9] | Fluid percussion injury | Rats | 17 beta-estradiol (144 micrograms/kg intraperitoneally), 4 hours prior to injury |
1. Motor function 1w after injury 2. Mortality |
Male rats had significant improvement of motor function and low mortality compared with female rats. |
| Zlotnik, 2012 [10] | Traumatic brain injury (weight-drop) |
Rats | Premarin treatment (99 ± 36 μM/l) after TBI | 1. Blood glutamate levels 2. Neurological outcome |
Premarin treatment followed TBI decreased blood glutamate levels and demonstrated better neurologic recovery. |
| Soustiel, 2005 [11] | Parietal cortex contusion by dynamic cortical deformation | Rats | Estrogen treatment for 3 days after brain injury | Histological analysis of cortical lesion | Estrogen-treated animals had a significant reduction in apoptosis compared with control animals. |
| Hu, 2012 [22] | Spinal cord injury using a weight-drop injury approach | Rats | 17β-estradiol administration at 15 mins and 24 hrs post injury | 1. Functional recovery 2. Cell death |
Estrogen treatment prevented spinal cord injury-induced apoptotic cell death and enhanced functional recovery after spinal cord injury. |
| Naderi, 2015 [14] | Traumatic brain injury (weight-drop) | Rats | Estrogen (E2) treatment (33.3 µg/Kg) injected 30 min after TBI | 1. Brain edema 2. BBB disruption |
E2 reduced brain edema and blood brain barrier disruption after TBI. |
| Kim, 2015 [17] | Lateral fluid percussion (LFP) at 24 h after craniectomy | Rats | Estrogen sulfate (E2-SO4) (1 mg/kg BW in 1 mL/kg BW) was intravenously administered at 1 h after TBI | 1. Intracranial pressure (ICP), cerebral perfusion pressure (CPP), cerebral oxygenation (pbtO2), cerebral glycolysis. 2. Brain edema and lesion size |
E2-SO4 significantly decreased ICP, and increased CPP and pbtO2. Brain edema in the treatment group was reduced compared with controls. Cerebral glycolysis in the injured brain region was increased. |
| Schaible, 2014 [15] | Traumatic brain injury (weight-drop) |
Mice | Intraperitoneal injection of 2-methoxyestradiol (2ME2) 30 min after TBI. | Histological analysis of brain damage | Early 2ME2 administration reduced secondary brain damage, likely mediated by ubiquitin proteasomes. |
| Day, 2013 [16] | Lateral fluid percussion (LFP | Rats | 17β-estradiol (E2) treatment | Histological analysis of brain damage | E2 significantly increased neuronal survival in the ipsilateral CA 2/3 region of the hippocampus, and decreased neuronal degeneration and apoptotic cell death in both the ipsilateral cortex and CA 2/3 region of the hippocampus in a dose-dependent manner. |
Emerson and colleagues reported that estrogen administration before TBI improved neurological outcome in male rats, but paradoxically worsened neurological outcomes in female rats [9]. Zlotnik and colleagues further demonstrated that injection of premarin (mixture of estrogens) in male rats after TBI resulted in a significant decrease in blood glutamate concentrations and improved neurological outcomes [10]. Furthermore, Neese and colleagues demonstrated the pretreatment with Z-BDDA (estrogenic seco-steroid) resulted in a significant improvement in learning and memory tests after moderate TBI [6].
Although Neese and colleagues failed to demonstrate a reduction in cell death in the cortical lesion zone [6], other studies have demonstrated that a significant reduction in neuronal apoptosis in rats pretreated with estrogen before TBI [11]. Recent studies have supported the neuroprotective role of 17-beta estradiol in decreasing cell death in the pericontusional zone after TBI, as well as the potential neuroprotective effects of 17-alpha estradiol in an in vivo model of early TBI to the immature brain of male and female rats [12, 13]. Recently, several laboratory studies demonstrated a reduction in brain edema, blood-brain barrier (BBB) disruption, and an improvement in neuronal survival when estrogen was administered after TBI [14-16] (Table 1). Furthermore, Kim et al. [17] showed significant reduction in intracranial pressure (ICP) with an elevation in cerebral perfusion pressure (CPP), partial pressure of oxygen in the brain, and rate of cerebral glycolysis in the injured region of the brain.
Human Studies
Clinical reports have suggested that severe TBI is associated with some degree of hypopituitarism [18]. In one study, 116 adult patients with severe TBI were followed for 6 months after TBI [18]. In the first seven days after TBI, there is a significant decline in blood hormone concentrations of estradiol, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) [18]. This reduction in hormone levels was followed by a reduction in the acute hormone stress response and a depression of cognitive function [18]. These findings correlated with previously published observational clinical reports that female humans had both lower mortality rates and better functional outcomes after TBI compared with men [1, 19-21] (Table 3). A clinical review of more than 72,000 patients in the 5-year cohort of the National Trauma Database demonstrated that women older than 45 year and postmenopausal women (older than 55 years) had significantly lower mortality and morbidity after moderate to severe TBI compared with age-matched men [20].
Table 3.
Neuroprotective effects of estrogen and progesterone in Traumatic Brain Injury (clinical studies).
| Study | Study Design | Sample Size | Treatment Protocol | Primary End Points | Conclusions |
|---|---|---|---|---|---|
| Berry, 2009 [20] | Retrospective review | 72,294 patients with moderate to severe TBI, | None | 1. Complications after TBI 2. Mortality |
Female gender was independently associated with reduced mortality and decreased complications after TBI. |
| Garringer, 2013 [21] | Prospective study | 100 patients with severe TBI compared to healthy volunteers | None. | 1. Glasgow Outcome Scale (GOS) scores 6 months after TBI 2. Mortality 3. CSF estrogen/testosterone E2/T ratios. |
TBI subjects had lower CSF estradiol over time compared with controls. CSF testosterone was initially high, but declined over time. E2/T ratios were initially low compared with controls, but increased over time. A higher mean E2/T ratio was associated with a lower mortality and better GOS scores after 6 months. |
| Wright, 2007 [46] | Phase II, randomized, double-blind, placebo-controlled trial | 100 patients with Glasgow Coma Scale (GCS) between 4 and 12 | Progesterone treatment | 1. Neurological outcomes 2. Mortality |
Patients randomized to progesterone had a lower 30-day mortality rate. Moderate TBI survivors who received progesterone were more likely to have a moderate to good outcome than placebo controls. |
| Xiao, 2008 [47] | A prospective, randomized, placebo-controlled trial | 159 patients with GCS ≤ 8 | Progesterone treatment | 1. Neurological outcomes 2. Mortality |
Progesterone treatment improved neurologic outcomes for up to 6 months. The mortality rate in the progesterone group was significantly lower than in the placebo group after 6 months. |
| Aminmansour, 2012 [48] | Prospective, randomized controlled trial | 60 patients with severe TBI, GCS < 8 | Progesterone vs. progesterone-vitamin D treatment | 1. Recovery rates 3 months after TBI 2. Mortality |
Patients who received progesterone and vitamin D together had significantly higher recovery rates than patients who received progesterone only. |
| Shakeri, 2013 [49] | Prospective, randomized controlled trial | 76 patients with severe TBI an DAI | Progesterone treatment (1mg/kg per 12h for 5d) | Neurologic outcomes | Progesterone significantly improved neurologic outcomes in patients with severe TBI up to 3 months after injury, especially those with GCS between 5 and 8. |
| Skolnick, 2014 [52] | Multinational placebo-controlled trial | 1195 patients, 16 to 70 years of age, with severe TBI (GCS ≤8) | Progesterone treatment initiated within 8 hours after injury and continued for 120h. | 1. GOS score at 6 months after the injury. 2. Mortality |
Primary and secondary efficacy analyses showed no clinical benefit of progesterone in patients with severe TBI. |
| Santarsieri, 2014 | Prospective study | 130 adults with severe TBI. | None | Cerebrospinal fluid (CSF) progesterone and cortisol levels after TBI | CSF cortisol levels were significantly and persistently elevated during the first week after TBI, and high CSF cortisol levels were associated with poor outcome. Serum and CSF levels for both cortisol and progesterone were strongly correlated after TBI relative to controls, possibly because of blood-brain barrier disruption. |
| Wright, 2014 [51] | Double-blinded, multicenter clinical trial | 882 patients with moderate-to-severe, or moderate acute TBI (GCS score between 4 and 12) |
Progesterone treatment | 1. Neurological outcome 2. Mortality |
There were no significant differences between the progesterone group and the placebo group with regards to favorable outcomes. |
Spinal Cord Injury
There is evidence to suggest that estrogen has neuroprotective effects in the context of SCI. Estrogen treatment in SCI was shown to prevent apoptosis of neurogenic cells and improve its functional recovery in rats [22]. Similarly, estrogen treatment prevented SCI-induced apoptotic cell death and enhanced functional recovery after spinal cord injury [23]. Furthermore, pretreatment with an ER agonist attenuated TNF alpha-induced apoptosis in spinal motoneurons [8, 24]. To date, no clinical studies have investigated the neuroprotective effects of estrogen after SCI.
NEUROPROTECTIVE EFFECTS OF PROGERSTERONE
In contrast to estrogen, the neuroprotective effects of progesterone have been more extensively studied in models of TBI and SCI than in models of ischemia. Moreover, the neuroprotective role of progesterone has also been studied in laboratory models of neurodegenerative CNS diseases, including Alzheimer’s disease.
Traumatic Brain Injury
Preclinical Animal Model Studies
Similar to estrogen, experimental laboratory data suggests that progesterone also has neuroprotective effects in the context of TBI [7, 25, 26] (Table 2). In three independent studies, progesterone administration consistently and significantly decreased brain edema 24-48 hours post-TBI in both male and female rats compared to vehicle-treatment animals [27-29]. When progesterone treatment was initiated
Table 2.
Neuroprotective effects of progesterone after traumatic brain injury (animal models).
| Study | Animal Model | Animal Species | Progesterone Treatment Protocol | Primary End Points | Conclusions |
|---|---|---|---|---|---|
| Roof, 1996 [27] | Medial frontal cortex contusion by dynamic cortical deformation | Rats | Progesterone treatment 1h after injury | Brain edema | Progesterone effectively reduced brain edema when treatment was delayed until 24h after injury. |
| Wright, 2001 [29] | Bilateral medial frontal cortex injury | Rats | Progesterone (4 mg/kg), intraperitoneally at 1, 6, and 24 h post injury. | Brain edema | Progesterone significantly decreased cerebral edema after TBI in adult male rats. |
| Thomas, 1999 [56] | Spinal cord injury (Laminectomy with contusion) | Rats | Progesterone treatment | 1. Functional status 2. Histologic analysis |
Progesterone improved clinical and histologic outcomes compared with the control groups. |
| Allitt, 2015 [33] | Cortical impact acceleration-induced diffuse TBI. | Rats | Progesterone (P4) treatment | Short-term (4 days post-TBI) and long-term (8 weeks post-TBI) functional outcomes | Short-term: neural responses in supragranular layers were suppressed, and TBI-induced suppression in the granular and infragranular layers was reversed. Long-term: There were inconsistent effects of P4 on the TBI-induced hyperexcitation in supragranular layers. |
| Lopez-Rodriguez, 2015 [34] | Traumatic brain injury (weight-drop) | Mice | None | Correlation between levels of neuroactive steroids in the brain and plasma at 24h, 72h and 2w after injury and clinical outcomes. | Brain levels of progesterone, tetrahydro- progesterone, isopregnanolone and 17β-estradiol were decreased at 24h, 72h and 2w after TBI. Brain levels of progesterone and dehydroepiandrosterone showed a positive correlation with neurological recovery. |
| Peterson, 2015 [35] | Cortical contusion injury after craniotomy | Rats | Combination treatment of nicotinamide (NAM) and progesterone | 1. Behavioral testing 2. Histological analysis of brain lesion |
NAM and progesterone treatment resulted in significant improvements in recovery of function, and reduced lesion cavitation, degenerating neurons, and reactive astrocytes 24h after injury. |
| Nudi, 2015 [40] | Controlled cortical impact (medial frontal cortex) | Rats | 10-mg/kg progesterone or vehicle injections 4h after injury and every 12h for 72 h after injury, followed by embryonic neural stem cells (eNSC) transplantation | Behavioral testing | Multimodal therapeutic approach after TBI improved functional recovery to a greater magnitude than either method alone. |
| Si, 2014 [42] | Traumatic brain injury (modified Feeney's weight-drop) | Rats | Progesterone treatment | 1. Neurological outcomes 2. Histological analysis of the brain lesion |
Progesterone treatment significantly reduced the post-injury inflammatory response, brain edema, and Evans blue dye extravasation, and improved neurological scores compared with control animals. |
| Xu, 2014 [43] | Surgical brain injury (SBI) | Rats | Low and high doses of progesterone vs. dexamethasone treatment | Histological analysis of the brain lesion | Progesterone reduced astrocyte and microglia responses, and attenuated brain edema with preservation of the blood brain barrier. Progesterone was as effective as dexamethasone in reducing brain edema and inflammation. |
| Geddes, 2014 [41] | Controlled cortical impact (CCI) model, pediatric model | Rats | 4,8 and 16 mg/kg doses of progesterone treatment 7d after injury | Behavioral testing | Progesterone ameliorated the injury-induced neurological deficits. |
| Pascual, 2013 [44] | Traumatic brain injury after craniotomy | Mice | Progesterone treatment 16 mg/kg intraperitoneally | 1. Neurological outcomes 2. Histological analysis of the brain lesion |
Progesterone treatment reduced the size of the pericontusional lesion and blood brain barrier macromolecular leakage after TBI, and improved neurological outcomes. |
as late as 24h after TBI, it was effective in decreasing brain edema [30]. Brain edema reduction strongly correlated with plasma progesterone concentrations [29]. However, while these studies examined the extent of brain edema as a measure of neuroprotection, neither neurological outcome nor mortality rates were assessed.
It is important to emphasize that while the traumatic lesions in the frontal cortex were similar in size, female rats had less ventricular enlargement and brain edema [31]. The extent of brain edema is clinically significant because of its serious consequences, including impaired cerebral blood flow and neuronal loss, and subsequent secondary neuronal death [32-35]. Moreover, brain edema to a significant extent is associated with an increase in extracellular brain glutamate. The excessive glutamate release after TBI may be an important laboratory marker of severity of brain injury [36]. Interestingly, previously published studies showed that blood concentrations of glutamate and progesterone are inversely correlated [37, 38].
Roof and colleagues demonstrated in male rats that treatment with progesterone 1h after TBI improved spatial navigation and reduced thalamic neuronal loss after 21 days [28]. Moreover, there is evidence that progesterone administration results in a decrease in TBI-induced diffuse axonal injury [39]. Nudi et al. [40] demonstrated better functional recovery after TBI where progesterone treatment was followed with embryonic neural stem cells (eNSC) transplantation. Progesterone treatment after TBI significantly reduced post-injury astrocyte and microglia inflammatory responses, and reduced the pericontusional lesion and BBB macromolecular leakage [41-44] (Table 2).
Human Studies
The promising laboratory data has resulted in the performance of randomized clinical trials, in which progesterone treatment after moderate TBI in humans was studied [45-47] (Table 3). Wright et al. [46] treated 77 patients with intravenous progesterone during first 72 hours after moderate to severe TBI (Glasgow Coma Scale [GCS] between 4 and 12), and showed a reduction in mortality rate at 30 days and improved Glasgow Outcome Scale score compared with the placebo group. Furthermore, there were no any adverse side effects during the treatment protocol. Xiao and colleagues observed decreased mortality rates and improved neurological outcomes after a six-month follow up of 159 acute severe TBI patients (GCS < 8) after intramuscular progesterone injections (1 mg/kg every 12 hours for 5 days after admission to a trauma center) [47]. Aminmansour and colleagues [48] used a similar progesterone treatment protocol [47], and administered either a combination of progesterone and vitamin D, progesterone only, or placebo (n=20 in each group). The authors demonstrated an improvement in the recovery of patients with severe TBI (GCS < 8) after 3 months [48]. Shakeri and colleagues [49] observed a significant improvement in neurological outcomes up to 3 months after early treatment with progesterone (1mg/kg every 12h for 5 days intramuscularly) in patients with severe head trauma with diffuse axonal injury (5-8). Santarsieri et al. [50] found that serum and
cerebrospinal (CSF) levels of both cortisol and progesterone were strongly associated with clinical outcomes in 130 adults after severe TBI. An elevation in CSF cortisol levels was associated with poor clinical outcomes, which was attributed to blood-brain barrier disruption. As a precursor to cortisol, progesterone was thought to mediate these effects [50] (Table 3). Recently two large, randomized, placebo-controlled and double-blinded clinical investigations by Skolnick et al. [51, 52] and Wright et al. [51, 52] investigated the neuroprotective effects of progesterone treatment in severe and moderate-to-severe TBI. In 1195 patients (age 16 to 70) with severe TBI, Skolnick et al. administered intravenous progesterone within 8 hours after injury (0.71 mg/kg for the first hour and 0.5 mg/kg for the next 119 hours). Patients were followed for 6 months after injury, and no clinical benefits (improvement in Glasgow Outcome Scale and decrease mortality rate) were observed with progesterone treatment. Wright et al. used the same progesterone treatment protocol [51, 52], initiated within 4 hours after injury and continued during first 96 hours for severe TBI patients (GCS 4 to 12). Similar, no clinical benefit after 6 months clinical follow up was demonstrated.
Currently, several additional randomized control trials are investigating the effects of progesterone treatment in various populations with TBI. A phase II, multicenter clinical trial (NCT01336413) is studying the effects of pregnenalone, a precursor of progesterone, in treating mild TBI. This study is currently recruiting patients.
Despite the growing data of evidence in the literature of effectiveness of estrogen and progesterone in animal models of TBI, the human clinical data has not demonstrated a clinical benefit. To this end, two recent meta-analyses failed to demonstrate a reduction in mortality or clinical benefit after progesterone treatment in acute TBI compared to placebo [53, 54]. There are several possible reasons for the negative findings. First, most of the progesterone treatment protocols in the preclinical animal model studies administered progesterone in the very early time period after injury. Second, the dosing regimens in preclinical studies were different than those used in the human studies. Third, the animal models used a controlled and isolated injury, which differed from the heterogeneous and diffuse injuries often observed in humans. Finally, secondary injury associated with CNS hypoxia and organ failure is rare in healthy young animals but common in human patients with TBI [55].
Spinal Cord Injury
Progesterone may have neuroprotective effects in the context of SCI as well. Thomas and colleagues found that progesterone treatment significantly improved the neurological deficits in rats after traumatic SCI, with an associated improved histological recovery [56]. De Nicola and colleagues further demonstrated that progesterone treatment restores proliferation and differentiation of oligodendrocytes, and prevents motor neuron degeneration after SCI in mice [57]. These results may be in part explained by a progesterone-mediated up-regulation of mRNA and protein 25-Dx in the dorsal horn of the injured spinal cord in rats [58]. There have been no clinical trials that have evaluated the effects of progesterone treatment in humans with SCI.
PROPOSED MECHANISMS OF ESTROGEN NEUROPROTECTION
Effects on Estrogen Receptors
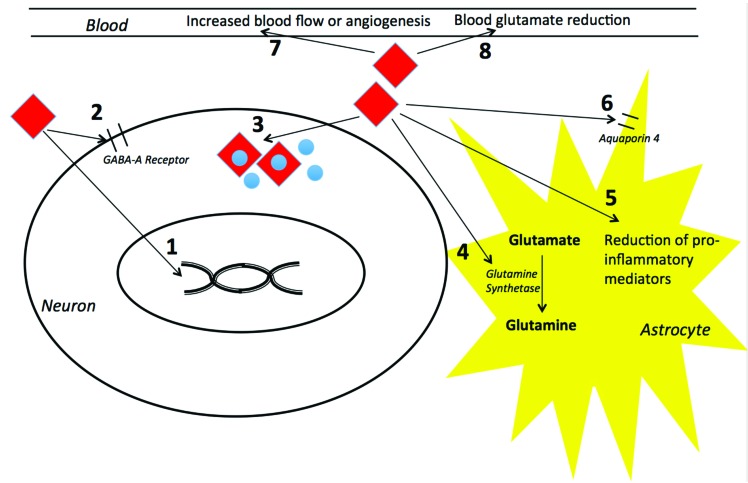
Estrogen receptors (ERs) are expressed in a wide range of tissues, including the brain (Fig. 1). The majority of ERs are present in the olfactory lobe, cortex, and cerebellum. Although ER-α and ER-ß are both expressed in the neuronal cell membrane of hypothalamus, the hippocampus in contrast appears to primarily express ER-ß [59]. Progesterone receptors (PRs) are also highly expressed in the adult brain. There are two classical isoforms of the PR present in brain tissue: PR-B and PR-A [3, 4].
Fig. (1).
Proposed mechanisms of neuroprotective effects of estrogen and progesterone on neurons, astrocytes, and the blood. Estrogen and progesterone exert its neuroprotective effects via complex and integrated mechanisms. Some of the mechanisms proposed include: 1) Estrogen and progesterone enter neurons by passive diffusion where they regulate gene transcription; 2) Estrogen and progesterone acts on GABA-A receptors after brain injury and increase cellular survival; 3) Estrogen and progesterone have antioxidant activity via intrinsic properties of scavenging oxygen free radicals; 4) Estrogen enhances the activity of the astroglial enzyme glutamine synthetase, which increases glutamate metabolism and cycling; 5) Estrogen may have effects on microglial proinflammatory mediators; 6) Progesterone modulates the expression of Aquaporin-4 channels after brain injury, resulting in decreased brain edema; 7) Estrogen may increase cerebral blood flow or promote angiogenesis; and 8) Estrogen and progesterone may scavenge blood glutamate and increase the brain to blood glutamate efflux. See text for more details.
Recently, estrogen and progesterone receptors were found on neurons and glial elements [4]. Studies have demonstrated a wide expression of classic nuclear ERs and progesterone receptors (PRs), as well as extranuclear sites present in the hypothalamus, neocortex, and other brain regions [2, 3, 60]. As estrogen and progesterone are both highly lipid-soluble, they pass the blood-brain barrier and enter the cell by passive diffusion and bind to both the nuclear and extranuclear sites. The important physiologic functions of gonadal hormones in the brain may be characterized not only by their neuroendocrine regulation, but also by their control of neuronal differentiation, cell migration and death, and synaptic organization of neurons [60, 61]. Hormonal binding on both the nuclear and extranuclear sites induces the promotion region of genes by regulating gene transcription, resulting in the formation of multi-protein units. However, they may also be integrated with other effectors and linked to different steroid signaling pathways in the cell [4, 60].
Estrogen has a plethora of cellular and extracellular effects that are mediated by ER-α and ER-ß. Estrogen classically exerts its effects by a nuclear ER-related mechanism. In this model, the steroid enters the cell by passive diffusion and binds to the nuclear ER [4]. Following a series of activation steps, the ER complex associates with an estrogen responsive element and functions as an enhancer for estrogen -containing genes. Estrogen induction of these genes may contribute to its neuroprotective effects.
The activation of ERs in the mammalian brain induces long-term genomic effects on elements such as the development and modulation of function in certain nerve cells [4, 62]. These effects of estrogen are termed “delayed”. They are dependent on genomic alterations induced by estrogen, and may include the delayed electrophysiological effects of estrogen in the hypothalamus [59], on seizure activity, on hippocampal long- term potentiation as well as general hippocampal physiology [63]. Furthermore, the delayed effects of estrogen on neuronal connectivity include estrogen-induced synaptic remodeling in the hippocampus [3, 4, 59]. Indeed, with respect to neuroprotection, estrogen promotes neurite outgrowth, sprouting of neurons, synaptogenesis, an increase in the level of the neurotransmitter acetylcholine, increased density of N-methyl-D-aspartate (NMDA) receptors, and higher expression of neurotropic factors including nerve growth factor [4, 59, 63]. These effects require a long activation time, and do not explain the rapid neuroprotective effects observed when estrogen is administered immediately prior to brain injury or immediately after inducing injury. However, these delayed effects may be important in posttraumatic brain rehabilitation. Thus, previously published studies [12, 62, 64-66] found an estrogen -mediated up regulation of the anti-apoptotic gene, bcl-2, the anti-apoptotic pro-survival factor, surviving and the neuroprotective factor, and brain derived neurotrophic factor (BDNF) following brain injury, which were associated with decreased apoptotic cell death.
Several studies have investigated the neuroprotective role of 17-beta estradiol after traumatic cerebral contusion [11, 12, 67]. They found that 17-beta estradiol protects from apoptosis in the cortical precontusional zone via the enhancement of ER-alpha. Furthermore, there was a decrease in caspase-3 activity via the activation and upregulation of nuclear and extranuclear ER-ά mRNA induction and protein expression (bcl-2), as well as inhibited caspase-3 activation in the pericontusional zone of the brain [12]. Moreover, there is evidence that a G protein-coupled ER agonist G-1 may mediate the neuroprotective effects of estrogen against SCI, and prevent SCI-induced apoptotic cell death after estrogen pretreatment [22].
Not all the neuromodulator effects of estrogen follow the “classical” hormone receptor pathway via nuclear receptor occupation and activation. The rapid effects of estrogen include the modulation of excitability, which was observed in various brain regions including the hypothalamus, amygdala, striatum, cerebellum, neocortex, and hippocampus [59]. The modulation of ligand-gated ion channels or G-protein-coupled receptors by these neuroactive steroids may also directly affect nerve cell survival. Estrogen shares such modulatory interactions with neuronal membranes, which are independent of the presence and/or activation of ERs.
Effects on Signaling Systems and Neurotransmitters
McClean and colleagues showed that 17- alpha estradiol acts via the GABA-A receptor and induces a reduction in hippocampal volume and impaired hippocampal-dependent performance after water percussion brain injury model [13]. Another study in male rats demonstrated that pretreatment with 17-beta estradiol 4 h prior to fluid percussion injury stabilized free extracellular magnesium concentrations within 4 hours after injury, and improved motor function 1 week after TBI [6].
Antioxidant Effects
Recently, estrogen was shown to have intrinsic oxygen free radical scavenging antioxidant activity. Estrogens such as 17ß-estradiol and its derivatives ethinyl estradiol and hydroxyl estradiol equally protected cultured neurons against oxidative cell death induced by amyloid- ß protein (Aß), glutamate, superoxide anions and hydrogen peroxide [5, 25, 59, 68]. In brain injury, estrogen may exert its effects by blocking the formation of oxygen free radicals and preventing cellular membrane damage induced by lipid peroxidation [30].
Effects on Astrocytes and Microglia
The indirect effects of estrogen on astrocytes and microglia may significantly contribute to the neuroprotective effects observed in many neurodegenerative disorders [4]. In organotypic cortical explants and neuronal/astrocyte co-cultures, there is evidence that the neuroprotective effects of estrogen at physiological concentrations are astrocyte-mediated. Furthermore, estrogen is known to enhance the activity of glutamine synthetase, an astrocytic enzyme that produces glutamine from glutamate. The glutamine from astrocytes is transported to neighboring neurons, where it can replenish the neuronal glutamate supply [69, 70].
Modulation of Inflammatory Response
After neuronal injury, microglia secrete pro-inflammatory mediators that may result in further secondary neuronal damage. Several studies have postulated that estrogen-mediated neuroprotection may result from its effects on the microglia’s pro-inflammatory mediators, including a reduction in kappa-B phosphorylation, NF-kappa-B activation, and overexpression of inducible nitric oxide synthase (iNOS) [69, 71].
Effects on Cerebral Blood Flow and Metabolism
ERs are widely distributed in the cerebral vasculature [5]. It has been postulated that estrogen-related neuroprotection may be related to an increase in cerebral blood flow (CBF) or angiogenesis, and the formation of new collateral blood flow to ischemic regions after brain insults. This theory has been studied extensively with conflicting results. Estrogen treatment has been shown to increase CBF after middle cerebral artery occlusion (MCAO) [72] and TBI [25]. In general, the direct effects of estrogen on the wall of blood vessels may play a significant role in neuroprotection. Several mechanisms by which estrogen may induce a rapid vasomotor effect were postulated. These mechanisms include estrogen-mediated release of endothelium-derived relaxing factor, antagonism of endothelin-related vasoconstriction, direct hyperpolarizing of vascular smooth muscle, and antagonism of calcium channels in the vascular smooth muscle [25]. Taken together, the effects of estrogen on the cerebral vasculature have a predominant role in improving microcirculation, with an inconsistent effect on CBF. The improvement of microcirculation is critical to maintaining the brain to blood glutamate efflux by increasing the effective surface of glutamate exchange from the brain to the blood [73].
Effects on Glutamate, Glutamate Receptors, and Glutamate Transporters
Many acute and chronic neurological disorders, such as TBI, stroke, intracerebral hemorrhage, meningitis, brain hypoxia, and glioma are associated with pathologically elevated glutamate levels in the extracellular fluid (ECF) of the brain [74-81]. These pathologically elevated glutamate levels have been shown in both animal and human studies [82].
Teichberg and colleagues demonstrated that pathologi- cally increased brain ECF glutamate is shifted to the plasma by glutamate transporters [73, 83]. Compared with the brain ECF, the concentration of glutamate is normally much higher in the blood. Therefore, the transport of excess glutamate from the brain ECF to the blood must work against a large concentration gradient. Reducing the blood glutamate concentration is thought to increase this gradient and facilitate the brain to blood glutamate efflux. This proposed neuroprotective mechanism reflects an exaggerated brain to blood glutamate efflux via mechanisms of blood glutamate reduction [73, 83].
The effect of plasma estrogen and progesterone levels on glutamate was evaluated along the course of female menstrual cycle [37]. There are predictable changes in plasma levels of estrogen and progesterone during the course of the menstrual cycle [84-90]. At the start of the menstrual cycle, plasma estrogen and progesterone concentrations are low. Plasma estrogen concentrations begin to rise on the fifth or sixth day of the cycle, with maximum values observed a day or two prior to ovulation on day 14. There is a sharp drop after 3 days at maximal levels, followed by a secondary rise in plasma estrogen between days 18 and 24, decreasing again to its original level at the end of the cycle [84-90].
In another study, the physiological rise of estrogen and progesterone was accompanied with a significant reduction in blood glutamate levels [38]. Moreover, Stover and Kempsky found that women have lower glutamate levels in the blood compared with men during craniotomies [83]. In animal studies, injecting premarin (estrogens mixture) in naïve and head-injured rats led to sustained decreases in blood glutamate levels, and was associated with a significant neuroprotective effect.
It is important to note that studies have demonstrated that estrogen increased the expression of the astrocytic glutamate uptake transporters GLAST (glutamate and aspartate transporters) and GLT-1 (glutamate transporter) [69, 91]. This effect may be an important mediator of neuroprotection by reducing the excitotoxic ECF concentrations of glutamate, which may attenuate glutaminergic receptor activation [92].
The removal of excess glutamate in the ECF of the brain is thought to attenuate the glutamate-mediated excitotoxicity that significantly impacts neurological outcomes [36]. The neuroprotective effects of 17-beta-estrodial were investigated in a rat model of glutamate neurotoxicity in which glutamate was infused in the cortex through a microdialysis probe. Prior to increasing brain glutamate, the rats were administered intravenous 17- beta-estradiol. The study demonstrated that pretreatment with beta-estradiol resulted in a significant reduction in the size of the glutamate-mediated lesion [93].
PROPOSED MECHANISMS OF PROGESTROGEN NEUROPROTECTION
The neuroprotective effects that characterize progesterone may be similar that of estrogen [94], and are also thought to be mediated by several mechanisms [95].
Effects on Progesterone and Other Receptors
The effects of progesterone on neurotrophin brain derived neurotrophic factor (BDNF) expression may be related to transcriptional activation through the progesterone receptor (PR)-A and PR-B receptor-signaling pathway. Some authors have argued that progesterone acts by activating specific neuroprotective signaling pathways, and by increasing the expression of anti-apoptotic proteins [96]. In contrast, others suggest that the neuroprotective effects of progesterone may be related to its regulation of cellular events via progesterone’s interaction with the PR or GABA receptor. To this end, metabolites of progesterone bind to the GABA-receptor complex, increase the effects of GABA on its receptor, and subsequently increase the survival of the cell [25, 95].
Effects on AQP4 Channel Expression
Aquaporin-4 (AQP4) is a water-permeable channel that may play a significant role in regulating cerebral edema. After TBI, it was demonstrated that progesterone decreased cerebral edema by modulating the expression AQP4 72 hours after brain injury in a rat model [97].
Antioxidant Effects
Like estrogen, the antioxidant effects of progesterone may contribute to increasing neuronal survival after injury [25, 27]. Progesterone might reduce the inflammatory response after an acute insult, and progesterone pretreatment has been shown to reduce levels of inflammatory mediators such as glial fibrillary acidic protein (GFAP), complement factor C3, and nuclear factor kappa beta (NFκB) after cortical brain injury [98]. As with estrogen, progesterone treatment after brain injury resulted in reduced lipid peroxidation in male rats [25].
Effects on Glutamate, Glutamate Receptors, and Glutamate Transporters
As discussed in detail above in the context of estrogen, the plasma levels of progesterone fluctuate during the female menstrual cycle and are inversely correlated with blood glutamate levels [84, 90, 99]. Tsesis and colleagues investigated whether blood glutamate levels are influenced by changes in estrogen and progesterone levels observed during normal pregnancy in humans [38]. Estrogen and progesterone levels are known to increase exponentially during pregnancy. Elevated female hormones lead to a significant decrease in blood glutamate concentrations in the second and third trimesters of pregnancy compared to the first trimester. Despite higher levels of estrogen and progesterone during the third trimester compared with the second trimester, blood glutamate was not reduced additionally in the third trimester [38]. This seems to suggest that once the maximum glutamate-lowering capacity of estrogen and progesterone are reached, increasing estrogen and progesterone levels further do not result in more glutamate reduction.
Additionally, in-vitro studies have demonstrated that progesterone reduces the neuronal response to excitatory amino acids and prevents glutamate neurotoxicity [25]. The activation of ionotropic glutamate receptors after TBI directly results in depolarizing currents, unbalanced calcium influx through the L-type calcium channels, resulting in neuronal apoptosis [100]. Luoma and colleagues demonstrated that progesterone might block calcium entry through L-type calcium channels and prevent subsequent calcium-induced neurotoxicity [101]. Collectively these data provide evidence that progesterone, with or without estrogen, promotes neuronal survival. The observed progesterone-induced neuroprotection seems to be mediated by several complex and integrated mechanisms.
FUTURE INVESTIGATIONS AND CONCLUSIONS
Over the past three decades, there has been much evidence that supports the neuroprotective role of estrogen and progesterone. Although several mechanisms have been suggested, the precise underling mechanisms hormone-related neuroprotection is still largely undetermined. Laboratory investigations that aim to elucidate the underlying mechanisms may result in novel, targeted treatments. Furthermore, very few small clinical studies regarding progesterone neuroprotection have been published in recent years, and none have studied estrogen treatment in the context of TBI. The optimal hormone dose and length of therapy in humans is currently unknown. As estrogen and progesterone treatment are generally considered safe, large clinical multicenter, prospective, randomized control studies should be done to better assess their clinical role in human brain neuroprotection after an acute traumatic insult.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Bazarian J.J., Blyth B., Mookerjee S., He H., McDermott M.P. Sex differences in outcome after mild traumatic brain injury. J. Neurotrauma. 2010;27(3):527–539. doi: 10.1089/neu.2009.1068. [http://dx.doi.org/10.1089/ neu.2009.1068]. [PMID: 19938945]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wise P.M., Smith M.J., Dubal D.B., Wilson M.E., Rau S.W., Cashion A.B., Böttner M., Rosewell K.L. Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog. Horm. Res. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [http://dx.doi.org/10.1210/ rp.57.1.235]. [PMID: 12017546]. [DOI] [PubMed] [Google Scholar]
- 3.Beyer C. Estrogen and the developing mammalian brain. 1999 doi: 10.1007/s004290050236. [DOI] [PubMed] [Google Scholar]
- 4.Johann S., Beyer C. Neuroprotection by gonadal steroid hormones in acute brain damage requires cooperation with astroglia and microglia. J. Steroid Biochem. Mol. Biol. 2013;137:71–81. doi: 10.1016/j.jsbmb.2012.11.006. [http:// dx.doi.org/10.1016/j.jsbmb.2012.11.006]. [PMID: 23196064]. [DOI] [PubMed] [Google Scholar]
- 5.Behl C. Oestrogen as a neuroprotective hormone. Nat. Rev. Neurosci. 2002;3(6):433–442. doi: 10.1038/nrn846. [PMID: 12042878]. [DOI] [PubMed] [Google Scholar]
- 6.Neese S.L., Clough R.W., Banz W.J., Smith D.C. Z-Bisdehydrodoisynolic acid (Z-BDDA): an estrogenic seco-steroid that enhances behavioral recovery following moderate fluid percussion brain injury in male rats. Brain Res. 2010;1362:93–101. doi: 10.1016/j.brainres.2010.09.055. [http:// dx.doi.org/10.1016/j.brainres.2010.09.055]. [PMID: 20869954]. [DOI] [PubMed] [Google Scholar]
- 7.Roof R.L., Hall E.D. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma. 2000;17(5):367–388. doi: 10.1089/neu.2000.17.367. [http://dx.doi.org/10.1089/ neu.2000.17.367]. [PMID: 10833057]. [DOI] [PubMed] [Google Scholar]
- 8.Meltser I., Tahera Y., Simpson E., Hultcrantz M., Charitidi K., Gustafsson J.A., Canlon B. Estrogen receptor beta protects against acoustic trauma in mice. J. Clin. Invest. 2008;118(4):1563–1570. doi: 10.1172/JCI32796. [http://dx.doi.org/10.1172/JCI32796]. [PMID: 18317592]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emerson C.S., Headrick J.P., Vink R. Estrogen improves biochemical and neurologic outcome following traumatic brain injury in male rats, but not in females. Brain Res. 1993;608(1):95–100. doi: 10.1016/0006-8993(93)90778-l. [http://dx.doi.org/10.1016/0006-8993(93)90778-L]. [PMID: 8495351]. [DOI] [PubMed] [Google Scholar]
- 10.Zlotnik A., Leibowitz A., Gurevich B., Ohayon S., Boyko M., Klein M., Knyazer B., Shapira Y., Teichberg V.I. Effect of estrogens on blood glutamate levels in relation to neurological outcome after TBI in male rats. Intensive Care Med. 2012;38(1):137–144. doi: 10.1007/s00134-011-2401-3. [http://dx.doi.org/10.1007/s00134-011-2401-3]. [PMID: 22124768]. [DOI] [PubMed] [Google Scholar]
- 11.Soustiel J.F., Palzur E., Nevo O., Thaler I., Vlodavsky E. Neuroprotective anti-apoptosis effect of estrogens in traumatic brain injury. J. Neurotrauma. 2005;22(3):345–352. doi: 10.1089/neu.2005.22.345. [http://dx.doi.org/10.1089/neu.2005.22.345]. [PMID: 15785230]. [DOI] [PubMed] [Google Scholar]
- 12.Bao Y.J., Li L.Z., Li X.G., Wang Y.J. 17Beta-estradiol differentially protects cortical pericontusional zone from programmed cell death after traumatic cerebral contusion at distinct stages via non-genomic and genomic pathways. Mol. Cell. Neurosci. 2011;48(3):185–194. doi: 10.1016/j.mcn.2011.07.004. [http://dx.doi.org/10.1016/ j.mcn.2011.07.004]. [PMID: 21803156]. [DOI] [PubMed] [Google Scholar]
- 13.McClean J., Nuñez J.L. 17alpha-Estradiol is neuroprotective in male and female rats in a model of early brain injury. Exp. Neurol. 2008;210(1):41–50. doi: 10.1016/j.expneurol.2007.09.027. [http://dx.doi.org/10.1016/j.expneurol.2007. 09.027]. [PMID: 17997403]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naderi V., Khaksari M., Abbasi R., Maghool F. Estrogen provides neuroprotection against brain edema and blood brain barrier disruption through both estrogen receptors α and β following traumatic brain injury. Iran. J. Basic Med. Sci. 2015;18(2):138–144. [PMID: 25810887]. [PMC free article] [PubMed] [Google Scholar]
- 15.Schaible E.V., Windschügl J., Bobkiewicz W., Kaburov Y., Dangel L., Krämer T., Huang C., Sebastiani A., Luh C., Werner C., Engelhard K., Thal S.C., Schäfer M.K. 2-Methoxyestradiol confers neuroprotection and inhibits a maladaptive HIF-1α response after traumatic brain injury in mice. J. Neurochem. 2014;129(6):940–954. doi: 10.1111/jnc.12708. [http://dx.doi.org/10.1111/ jnc.12708]. [PMID: 24606183]. [DOI] [PubMed] [Google Scholar]
- 16.Day N.L., Floyd C.L., D’Alessandro T.L., Hubbard W.J., Chaudry I.H. 17β-estradiol confers protection after traumatic brain injury in the rat and involves activation of G protein-coupled estrogen receptor 1. J. Neurotrauma. 2013;30(17):1531–1541. doi: 10.1089/neu.2013.2854. [http://dx.doi.org/10.1089/neu.2013.2854]. [PMID: 23659385]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H., Cam-Etoz B., Zhai G., Hubbard W.J., Zinn K.R., Chaudry I.H. Salutary Effects of Estrogen Sulfate for Traumatic Brain Injury. J. Neurotrauma. 2015;32(16):1210–1216. doi: 10.1089/neu.2014.3771. [http://dx.doi.org/10.1089/neu.2014.3771]. [PMID: 25646701]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner A.K., McCullough E.H., Niyonkuru C., Ozawa H., Loucks T.L., Dobos J.A., Brett C.A., Santarsieri M., Dixon C.E., Berga S.L., Fabio A. Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. J. Neurotrauma. 2011;28(6):871–888. doi: 10.1089/neu.2010.1586. [http://dx.doi.org/10.1089/ neu.2010.1586]. [PMID: 21488721]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groswasser Z., Cohen M., Keren O. Female TBI patients recover better than males. Brain Inj. 1998;12(9):805–808. doi: 10.1080/026990598122197. [http://dx.doi.org/10.1080/026990598122197]. [PMID: 9755371]. [DOI] [PubMed] [Google Scholar]
- 20.Berry C., Ley E.J., Tillou A., Cryer G., Margulies D.R., Salim A. The effect of gender on patients with moderate to severe head injuries. J. Trauma. 2009;67(5):950–953. doi: 10.1097/TA.0b013e3181ba3354. [http://dx.doi.org/ 10.1097/TA.0b013e3181ba3354]. [PMID: 19901653]. [DOI] [PubMed] [Google Scholar]
- 21.Garringer J.A., Niyonkuru C., McCullough E.H., Loucks T., Dixon C.E., Conley Y.P., Berga S., Wagner A.K. Impact of aromatase genetic variation on hormone levels and global outcome after severe TBI. J. Neurotrauma. 2013;30(16):1415–1425. doi: 10.1089/neu.2012.2565. [http://dx.doi.org/10.1089/neu.2012.2565]. [PMID: 23540392]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu R., Sun H., Zhang Q., Chen J., Wu N., Meng H., Cui G., Hu S., Li F., Lin J., Wan Q., Feng H. G-protein coupled estrogen receptor 1 mediated estrogenic neuroprotection against spinal cord injury. Crit. Care Med. 2012;40(12):3230–3237. doi: 10.1097/CCM.0b013e3182657560. [http://dx.doi.org/10.1097/CCM.0b013e3182657560]. [PMID: 22975889]. [DOI] [PubMed] [Google Scholar]
- 23.Samantaray S., Smith J.A., Das A., Matzelle D.D., Varma A.K., Ray S.K., Banik N.L. Low dose estrogen prevents neuronal degeneration and microglial reactivity in an acute model of spinal cord injury: effect of dosing, route of administration, and therapy delay. Neurochem. Res. 2011;36(10):1809–1816. doi: 10.1007/s11064-011-0498-y. [http://dx.doi. org/10.1007/s11064-011-0498-y]. [PMID: 21611834]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das A., Smith J.A., Gibson C., Varma A.K., Ray S.K., Banik N.L. Estrogen receptor agonists and estrogen attenuate TNF-α-induced apoptosis in VSC4.1 motoneurons. J. Endocrinol. 2011;208(2):171–182. doi: 10.1677/JOE-10-0338. [http://dx.doi.org/10.1677/JOE-10-0338]. [PMID: 21068071]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh M., Su C. Progesterone and neuroprotection. Horm. Behav. 2013;63(2):284–290. doi: 10.1016/j.yhbeh.2012.06.003. [http://dx.doi.org/10.1016/j.yhbeh.2012. 06.003]. [PMID: 22732134]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein D.G. A clinical/translational perspective: can a developmental hormone play a role in the treatment of traumatic brain injury? Horm. Behav. 2013;63(2):291–300. doi: 10.1016/j.yhbeh.2012.05.004. [http://dx.doi.org/10.1016/ j.yhbeh.2012.05.004]. [PMID: 22626570]. [DOI] [PubMed] [Google Scholar]
- 27.Roof R.L., Duvdevani R., Heyburn J.W., Stein D.G. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp. Neurol. 1996;138(2):246–251. doi: 10.1006/exnr.1996.0063. [http://dx.doi. org/10.1006/exnr.1996.0063]. [PMID: 8620923]. [DOI] [PubMed] [Google Scholar]
- 28.Roof R.L., Duvdevani R., Braswell L., Stein D.G. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp. Neurol. 1994;129(1):64–69. doi: 10.1006/exnr.1994.1147. [http://dx.doi.org/10.1006/exnr.1994.1147]. [PMID: 7925843]. [DOI] [PubMed] [Google Scholar]
- 29.Wright D.W., Bauer M.E., Hoffman S.W., Stein D.G. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J. Neurotrauma. 2001;18(9):901–909. doi: 10.1089/089771501750451820. [http://dx.doi.org/10.1089/089771501750451820]. [PMID: 11565602]. [DOI] [PubMed] [Google Scholar]
- 30.Green P.S., Yang S.H., Simpkins J.W. Neuroprotective effects of phenolic A ring oestrogens. Novartis Found. Symp. 2000;230:202–213. doi: 10.1002/0470870818.ch15. [http://dx.doi.org/10.1002/0470870818.ch15]. [PMID: 10965510]. [DOI] [PubMed] [Google Scholar]
- 31.Attella M.J., Nattinville A., Stein D.G. Hormonal state affects recovery from frontal cortex lesions in adult female rats. Behav. Neural Biol. 1987;48(3):352–367. doi: 10.1016/s0163-1047(87)90918-6. [http://dx.doi.org/10.1016/ S0163-1047(87)90918-6]. [PMID: 3689284]. [DOI] [PubMed] [Google Scholar]
- 32.Unterberg A.W., Stover J., Kress B., Kiening K.L. Edema and brain trauma. Neuroscience. 2004;129(4):1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [http://dx. doi.org/10.1016/j.neuroscience.2004.06.046]. [PMID: 15561417]. [DOI] [PubMed] [Google Scholar]
- 33.Allitt B.J., Johnstone V.P., Richards K., Yan E.B., Rajan R. Progesterone Exacerbates Short-Term Effects of Traumatic Brain Injury on Supragranular Responses in Sensory Cortex and Over-Excites Infragranular Responses in the Long Term. J. Neurotrauma. 2016;33(4):375–389. doi: 10.1089/neu.2015.3946. [http://dx.doi.org/10.1089/neu. 2015.3946]. [PMID: 26258958]. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Rodriguez A.B., Acaz-Fonseca E., Giatti S., Caruso D., Viveros M.P., Melcangi R.C., Garcia-Segura L.M. Correlation of brain levels of progesterone and dehydroepiandrosterone with neurological recovery after traumatic brain injury in female mice. Psychoneuroendocrinology. 2015;56:1–11. doi: 10.1016/j.psyneuen.2015.02.018. [http://dx.doi.org/ 10.1016/j.psyneuen.2015.02.018]. [PMID: 25770855]. [DOI] [PubMed] [Google Scholar]
- 35.Peterson T.C., Hoane M.R., McConomy K.S., Farin F.M., Bammler T.K., MacDonald J.W., Kantor E.D., Anderson G.D. A Combination Therapy of Nicotinamide and Progesterone Improves Functional Recovery following Traumatic Brain Injury. J. Neurotrauma. 2015;32(11):765–779. doi: 10.1089/neu.2014.3530. [http://dx.doi.org/10.1089/ neu.2014.3530]. [PMID: 25313690]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zlotnik A., Gurevich B., Tkachov S., Maoz I., Shapira Y., Teichberg V.I. Brain neuroprotection by scavenging blood glutamate. Exp. Neurol. 2007;203(1):213–220. doi: 10.1016/j.expneurol.2006.08.021. [http://dx.doi.org/ 10.1016/j.expneurol.2006.08.021]. [PMID: 17014847]. [DOI] [PubMed] [Google Scholar]
- 37.Zlotnik A., Gruenbaum B.F., Mohar B., Kuts R., Gruenbaum S.E., Ohayon S., Boyko M., Klin Y., Sheiner E., Shaked G., Shapira Y., Teichberg V.I. The effects of estrogen and progesterone on blood glutamate levels: evidence from changes of blood glutamate levels during the menstrual cycle in women. Biol. Reprod. 2011;84(3):581–586. doi: 10.1095/biolreprod.110.088120. [http://dx.doi.org/10.1095/ biolreprod.110.088120]. [PMID: 20980684]. [DOI] [PubMed] [Google Scholar]
- 38.Tsesis S., Gruenbaum B.F., Ohayon S., Boyko M., Gruenbaum S.E., Shapira Y., Weintraub A., Zlotnik A. The effects of estrogen and progesterone on blood glutamate levels during normal pregnancy in women. Gynecol. Endocrinol. 2013;29(10):912–916. doi: 10.3109/09513590.2013.813467. [http://dx.doi.org/10.3109/09513590.2013.813467]. [PMID: 23862584]. [DOI] [PubMed] [Google Scholar]
- 39.Vink R., Van Den Heuvel C. Recent advances in the development of multifactorial therapies for the treatment of traumatic brain injury. Expert Opin. Investig. Drugs. 2004;13(10):1263–1274. doi: 10.1517/13543784.13.10.1263. [http://dx.doi.org/10.1517/13543784.13.10.1263]. [PMID: 15461556]. [DOI] [PubMed] [Google Scholar]
- 40.Nudi E.T., Jacqmain J., Dubbs K., Geeck K., Salois G., Searles M.A., Smith J.S. Combining Enriched Environment, Progesterone, and Embryonic Neural Stem Cell Therapy Improves Recovery after Brain Injury. J. Neurotrauma. 2015;32(14):1117–1129. doi: 10.1089/neu.2014.3618. [http:// dx.doi.org/10.1089/neu.2014.3618]. [PMID: 25268854]. [DOI] [PubMed] [Google Scholar]
- 41.Geddes R.I., Sribnick E.A., Sayeed I., Stein D.G. Progesterone treatment shows benefit in a pediatric model of moderate to severe bilateral brain injury. PLoS One. 2014;9(1):e87252. doi: 10.1371/journal.pone.0087252. [http://dx. doi.org/10.1371/journal.pone.0087252]. [PMID: 24489882]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Si D., Li J., Liu J., Wang X., Wei Z., Tian Q., Wang H., Liu G. Progesterone protects blood-brain barrier function and improves neurological outcome following traumatic brain injury in rats. Exp. Ther. Med. 2014;8(3):1010–1014. doi: 10.3892/etm.2014.1840. [PMID: 25120639]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu F.F., Sun S., Ho A.S., Lee D., Kiang K.M., Zhang X.Q., Wang A.M., Wu E.X., Lui W.M., Liu B.Y., Leung G.K. Effects of progesterone vs. dexamethasone on brain oedema and inflammatory responses following experimental brain resection. Brain Inj. 2014;28(12):1594–1601. doi: 10.3109/02699052.2014.943289. [http://dx.doi.org/10.3109/ 02699052.2014.943289]. [PMID: 25093611]. [DOI] [PubMed] [Google Scholar]
- 44.Pascual J.L., Murcy M.A., Li S., Gong W., Eisenstadt R., Kumasaka K., Sims C., Smith D.H., Browne K., Allen S., Baren J. Neuroprotective effects of progesterone in traumatic brain injury: blunted in vivo neutrophil activation at the blood-brain barrier. Am. J. Surg. 2013;206(6):840–845. doi: 10.1016/j.amjsurg.2013.07.016. [http://dx.doi.org/ 10.1016/j.amjsurg.2013.07.016]. [PMID: 24112683]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein D.G. Progesterone exerts neuroprotective effects after brain injury. Brain Res. Brain Res. Rev. 2008;57(2):386–397. doi: 10.1016/j.brainresrev.2007.06.012. [http://dx. doi.org/10.1016/j.brainresrev.2007.06.012]. [PMID: 17826842]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright D.W., Kellermann A.L., Hertzberg V.S., Clark P.L., Frankel M., Goldstein F.C., Salomone J.P., Dent L.L., Harris O.A., Ander D.S., Lowery D.W., Patel M.M., Denson D.D., Gordon A.B., Wald M.M., Gupta S., Hoffman S.W., Stein D.G. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann. Emerg. Med. 2007;49(4):391–402. doi: 10.1016/j.annemergmed.2006.07.932. [http://dx.doi.org/10.1016/j.annemergmed.2006.07.932]. [DOI] [PubMed] [Google Scholar]
- 47.Xiao G., Wei J., Yan W., Wang W., Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit. Care. 2008;12(2):R61. doi: 10.1186/cc6887. [http://dx.doi.org/10.1186/cc6887]. [PMID: 18447940]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aminmansour B., Nikbakht H., Ghorbani A., Rezvani M., Rahmani P., Torkashvand M., Nourian M., Moradi M. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: A randomized clinical trial with placebo group. Adv. Biomed. Res. 2012;1:58. doi: 10.4103/2277-9175.100176. [http://dx.doi.org/10.4103/2277-9175.100176]. [PMID: 23326789]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shakeri M., Boustani M.R., Pak N., Panahi F., Salehpour F., Lotfinia I., Meshkini A., Daghighi S. vahedi, P.; Khani, M.; Taghiloo, D. Effect of progesterone administration on prognosis of patients with diffuse axonal injury due to severe head trauma. Clin. Neurol. Neurosurg. 2013;115(10):2019–2022. doi: 10.1016/j.clineuro.2013.06.013. [http://dx.doi.org/ 10.1016/j.clineuro.2013.06.013]. [PMID: 23871679]. [DOI] [PubMed] [Google Scholar]
- 50.Santarsieri M., Niyonkuru C., McCullough E.H., Dobos J.A., Dixon C.E., Berga S.L., Wagner A.K. Cerebrospinal fluid cortisol and progesterone profiles and outcomes prognostication after severe traumatic brain injury. J. Neurotrauma. 2014;31(8):699–712. doi: 10.1089/neu.2013.3177. [http://dx.doi.org/10.1089/neu.2013.3177]. [PMID: 24354775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright D.W., Yeatts S.D., Silbergleit R., Palesch Y.Y., Hertzberg V.S., Frankel M., Goldstein F.C., Caveney A.F., Howlett-Smith H., Bengelink E.M., Manley G.T., Merck L.H., Janis L.S., Barsan W.G., Investigators N. Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 2014;371(26):2457–2466. doi: 10.1056/NEJMoa1404304. [http://dx.doi.org/ 10.1056/NEJMoa1404304]. [PMID: 25493974]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skolnick B.E., Maas A.I., Narayan R.K., van der Hoop R.G., MacAllister T., Ward J.D., Nelson N.R., Stocchetti N., Investigators S.T. A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 2014;371(26):2467–2476. doi: 10.1056/NEJMoa1411090. [http://dx.doi.org/10.1056/NEJMoa1411090]. [PMID: 25493978]. [DOI] [PubMed] [Google Scholar]
- 53.Lin C., He H., Li Z., Liu Y., Chao H., Ji J., Liu N. Efficacy of progesterone for moderate to severe traumatic brain injury: a meta-analysis of randomized clinical trials. Sci. Rep. 2015;5:13442. doi: 10.1038/srep13442. [http://dx.doi.org/10.1038/srep13442]. [PMID: 26304556]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng Y., Zhang Y., Ma J., Xu J. Progesterone for Acute Traumatic Brain Injury: A Systematic Review of Randomized Controlled Trials. PLoS One. 2015;10(10):e0140624. doi: 10.1371/journal.pone.0140624. [http://dx. doi.org/10.1371/journal.pone.0140624]. [PMID: 26473361]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vink R., Nimmo A.J. Multifunctional drugs for head injury. Neurotherapeutics. 2009;6(1):28–42. doi: 10.1016/j.nurt.2008.10.036. [http://dx.doi.org/10.1016/ j.nurt.2008.10.036]. [PMID: 19110197]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas A.J., Nockels R.P., Pan H.Q., Shaffrey C.I., Chopp M. Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine. 1999;24(20):2134–2138. doi: 10.1097/00007632-199910150-00013. [http:// dx.doi.org/10.1097/00007632-199910150-00013]. [PMID: 10543012]. [DOI] [PubMed] [Google Scholar]
- 57.De Nicola A.F., Labombarda F., Gonzalez D.M., Gonzalez S.L., Garay L., Meyer M., Gargiulo G., Guennoun R., Schumacher M. Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Front. Neuroendocrinol. 2009;30(2):173–187. doi: 10.1016/j.yfrne.2009.03.001. [http://dx.doi.org/10.1016/j.yfrne.2009. 03.001]. [PMID: 19318112]. [DOI] [PubMed] [Google Scholar]
- 58.Guennoun R., Meffre D., Labombarda F., Gonzalez S.L., Gonzalez Deniselle M.C., Stein D.G., De Nicola A.F., Schumacher M. The membrane-associated progesterone-binding protein 25-Dx: expression, cellular localization and up-regulation after brain and spinal cord injuries. Brain Res. Brain Res. Rev. 2008;57(2):493–505. doi: 10.1016/j.brainresrev.2007.05.009. [http://dx.doi.org/10.1016/j.brainresrev. 2007.05.009]. [PMID: 17618691]. [DOI] [PubMed] [Google Scholar]
- 59.Behl C., Manthey D. Neuroprotective activities of estrogen: an update. J. Neurocytol. 2000;29(5-6):351–358. doi: 10.1023/a:1007109222673. [http://dx.doi.org/ 10.1023/A:1007109222673]. [PMID: 11424951]. [DOI] [PubMed] [Google Scholar]
- 60.Dluzen D.E. Unconventional effects of estrogen uncovered. Trends Pharmacol. Sci. 2005;26(10):485–487. doi: 10.1016/j.tips.2005.08.001. [http://dx.doi.org/ 10.1016/j.tips.2005.08.001]. [PMID: 16122814]. [DOI] [PubMed] [Google Scholar]
- 61.Griffin G.D., Flanagan-Cato L.M. Ovarian hormone action in the hypothalamic ventromedial nucleus: remodelling to regulate reproduction. J. Neuroendocrinol. 2011;23(6):465–471. doi: 10.1111/j.1365-2826.2011.02143.x. [http://dx.doi.org/10.1111/j.1365-2826.2011.02143.x]. [PMID: 21518031]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simpkins J.W., Singh M., Brock C., Etgen A.M. Neuroprotection and estrogen receptors. Neuroendocrinology. 2012;96(2):119–130. doi: 10.1159/000338409. [http://dx.doi.org/10.1159/000338409]. [PMID: 22538356]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woolley C.S., McEwen B.S. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 1993;336(2):293–306. doi: 10.1002/cne.903360210. [http://dx.doi.org/10.1002/cne.903360210]. [PMID: 8245220]. [DOI] [PubMed] [Google Scholar]
- 64.Dubal D.B., Shughrue P.J., Wilson M.E., Merchenthaler I., Wise P.M. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J. Neurosci. 1999;19(15):6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [PMID: 10414967]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Q.G., Wang R., Khan M., Mahesh V., Brann D.W. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J. Neurosci. 2008;28(34):8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI.2752-08.2008]. [PMID: 18716201]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang L.C., Zhang Q.G., Zhou C.F., Yang F., Zhang Y.D., Wang R.M., Brann D.W. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One. 2010;5(5):e9851. doi: 10.1371/journal.pone.0009851. [http://dx.doi.org/10.1371/journal. pone.0009851]. [PMID: 20479872]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li L.Z., Bao Y.J., Zhao M. 17beta-estradiol attenuates programmed cell death in cortical pericontusional zone following traumatic brain injury via upregulation of ERalpha and inhibition of caspase-3 activation. Neurochem. Int. 2011;58(1):126–133. doi: 10.1016/j.neuint.2010.11.006. [http://dx.doi.org/10.1016/j.neuint.2010.11.006]. [PMID: 21093516]. [DOI] [PubMed] [Google Scholar]
- 68.Moosmann B., Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl. Acad. Sci. USA. 1999;96(16):8867–8872. doi: 10.1073/pnas.96.16.8867. [http://dx.doi.org/10.1073/pnas.96.16.8867]. [PMID: 10430862]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brann D.W., Dhandapani K., Wakade C., Mahesh V.B., Khan M.M. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72(5):381–405. doi: 10.1016/j.steroids.2007.02.003. [http://dx.doi.org/10.1016/j.steroids.2007.02.003]. [PMID: 17379265]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arevalo M.A., Santos-Galindo M., Bellini M.J., Azcoitia I., Garcia-Segura L.M. Actions of estrogens on glial cells: Implications for neuroprotection. Biochim. Biophys. Acta. 2010;1800(10):1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [http://dx.doi.org/10.1016/j.bbagen.2009. 10.002]. [PMID: 19818384]. [DOI] [PubMed] [Google Scholar]
- 71.Bruce-Keller A.J., Keeling J.L., Keller J.N., Huang F.F., Camondola S., Mattson M.P. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141(10):3646–3656. doi: 10.1210/endo.141.10.7693. [PMID: 11014219]. [DOI] [PubMed] [Google Scholar]
- 72.Rusa R., Alkayed N.J., Crain B.J., Traystman R.J., Kimes A.S., London E.D., Klaus J.A., Hurn P.D. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30(8):1665–1670. doi: 10.1161/01.str.30.8.1665. [http://dx.doi.org/10.1161/01.STR.30.8.1665]. [PMID: 10436119]. [DOI] [PubMed] [Google Scholar]
- 73.Teichberg V.I., Cohen-Kashi-Malina K., Cooper I., Zlotnik A. Homeostasis of glutamate in brain fluids: an accelerated brain-to-blood efflux of excess glutamate is produced by blood glutamate scavenging and offers protection from neuropathologies. Neuroscience. 2009;158(1):301–308. doi: 10.1016/j.neuroscience.2008.02.075. [http://dx.doi.org/10.1016/j. neuroscience.2008.02.075]. [PMID: 18423998]. [DOI] [PubMed] [Google Scholar]
- 74.Castillo J., Dávalos A., Naveiro J., Noya M. Neuroexcitatory amino acids and their relation to infarct size and neurological deficit in ischemic stroke. Stroke. 1996;27(6):1060–1065. doi: 10.1161/01.str.27.6.1060. [http://dx.doi.org/10.1161/01.STR.27.6.1060]. [PMID: 8650715]. [DOI] [PubMed] [Google Scholar]
- 75.Zauner A., Bullock R., Kuta A.J., Woodward J., Young H.F. Glutamate release and cerebral blood flow after severe human head injury. Acta Neurochir. Suppl. (Wien) 1996;67:40–44. doi: 10.1007/978-3-7091-6894-3_9. [PMID: 8870800]. [DOI] [PubMed] [Google Scholar]
- 76.Castillo J., Dávalos A., Alvarez-Sabín J., Pumar J.M., Leira R., Silva Y., Montaner J., Kase C.S. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology. 2002;58(4):624–629. doi: 10.1212/wnl.58.4.624. [http://dx.doi.org/10.1212/WNL.58.4.624]. [PMID: 11865143]. [DOI] [PubMed] [Google Scholar]
- 77.Johnston M.V., Trescher W.H., Ishida A., Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr. Res. 2001;49(6):735–741. doi: 10.1203/00006450-200106000-00003. [http://dx.doi.org/10.1203/ 00006450-200106000-00003]. [PMID: 11385130]. [DOI] [PubMed] [Google Scholar]
- 78.Andreadou E., Kapaki E., Kokotis P., Paraskevas G.P., Katsaros N., Libitaki G., Petropoulou O., Zis V., Sfagos C., Vassilopoulos D. Plasma glutamate and glycine levels in patients with amyotrophic lateral sclerosis. In Vivo. 2008;22(1):137–141. [PMID: 18396796]. [PubMed] [Google Scholar]
- 79.Bunting H., Still R., Williams D.R., Gravenor M., Austin M.W. Evaluation of plasma glutamate levels in normal tension glaucoma. Ophthalmic Res. 2010;43(4):197–200. doi: 10.1159/000272024. [http://dx.doi.org/10.1159/ 000272024]. [PMID: 20068372]. [DOI] [PubMed] [Google Scholar]
- 80.Espey M.G., Basile A.S., Heaton R.K., Ellis R.J. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2002;58(9):1439. doi: 10.1212/wnl.58.9.1439. [http://dx.doi.org/10.1212/WNL.58. 9.1439]. [PMID: 12011305]. [DOI] [PubMed] [Google Scholar]
- 81.Takano T., Lin J.H., Arcuino G., Gao Q., Yang J., Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat. Med. 2001;7(9):1010–1015. doi: 10.1038/nm0901-1010. [http://dx.doi.org/10.1038/nm0901-1010]. [PMID: 11533703]. [DOI] [PubMed] [Google Scholar]
- 82.Zlotnik A., Ohayon S., Gruenbaum B.F., Gruenbaum S.E., Mohar B., Boyko M., Klin Y., Sheiner E., Shaked G., Shapira Y., Teichberg V.I. Determination of factors affecting glutamate concentrations in the whole blood of healthy human volunteers. J. Neurosurg. Anesthesiol. 2011;23(1):45–49. doi: 10.1097/ANA.0b013e3181f82a8f. [http://dx.doi.org/ 10.1097/ANA.0b013e3181f82a8f]. [PMID: 21248494]. [DOI] [PubMed] [Google Scholar]
- 83.Stover J.F., Kempski O.S. Anesthesia increases circulating glutamate in neurosurgical patients. Acta Neurochir. (Wien) 2005;147(8):847–853. doi: 10.1007/s00701-005-0562-y. [http://dx.doi.org/10.1007/s00701-005-0562-y]. [PMID: 15968470]. [DOI] [PubMed] [Google Scholar]
- 84.Fernholm R., Thoren M., Hoybye C., Anderstam B., Pernow Y., Saaf M., Hall K. Amino acid profiles in adults with growth hormone (GH) deficiency before and during GH replacement therapy. Growth Horm. IGF Res. 2009;19(3):206–211. doi: 10.1016/j.ghir.2008.09.001. [http://dx.doi.org/10.1016/j.ghir.2008.09.001]. [PMID: 18990596]. [DOI] [PubMed] [Google Scholar]
- 85.Abraham G.E., Odell W.D., Swerdloff R.S., Hopper K. Simultaneous radioimmunoassay of plasma FSH, LH, progesterone, 17-hydroxyprogesterone, and estradiol-17 beta during the menstrual cycle. J. Clin. Endocrinol. Metab. 1972;34(2):312–318. doi: 10.1210/jcem-34-2-312. [http://dx.doi.org/10.1210/jcem-34-2-312]. [PMID: 5059762]. [DOI] [PubMed] [Google Scholar]
- 86.Barile G., Sica G., Montemurro A., Iacobelli S., Corradini M. Levels of estrogen and progesterone receptor in human endometrium during the menstrual cycle. Eur. J. Obstet. Gynecol. Reprod. Biol. 1979;9(4):243–246. doi: 10.1016/0028-2243(79)90062-5. [http://dx.doi.org/10.1016/ 0028-2243(79)90062-5]. [PMID: 264091]. [DOI] [PubMed] [Google Scholar]
- 87.Goebelsmann U., Midgley A.R., Jr, Jaffe R.B. Regulation of human gonadotropins: VII. Daily individual urinary estrogens, pregnanediol and serum luteinizing and follicle stimulating hormones during the menstrual cycle. J. Clin. Endocrinol. Metab. 1969;29(9):1222–1230. doi: 10.1210/jcem-29-9-1222. [http://dx.doi.org/10.1210/jcem-29-9-1222]. [PMID: 5808527]. [DOI] [PubMed] [Google Scholar]
- 88.Roger M., Veinante A., Soldat M.C., Tardy J., Tribondeau E., Scholler R. Simultaneous study of plasma gonadotropins, estrogens, progesterone and 17-hydroxyprogesterone during the ovulatory cycle. Nouv. Presse Med. 1975;4(30):2173–2178. [Simultaneous study of plasma gonadotropins, estrogens, progesterone and 17-hydroxyprogesterone during the ovulatory cycle]. [PMID: 1178478]. [PubMed] [Google Scholar]
- 89.Sherman B.M., Korenman S.G. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J. Clin. Invest. 1975;55(4):699–706. doi: 10.1172/JCI107979. [http://dx.doi.org/10.1172/ JCI107979]. [PMID: 1120778]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sherman B.M., West J.H., Korenman S.G. The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J. Clin. Endocrinol. Metab. 1976;42(4):629–636. doi: 10.1210/jcem-42-4-629. [http://dx.doi.org/ 10.1210/jcem-42-4-629]. [PMID: 1262439]. [DOI] [PubMed] [Google Scholar]
- 91.Cimarosti H., O’Shea R.D., Jones N.M., Horn A.P., Simão F., Zamin L.L., Nassif M., Frozza R., Netto C.A., Beart P.M., Salbego C. The effects of estradiol on estrogen receptor and glutamate transporter expression in organotypic hippocampal cultures exposed to oxygen--glucose deprivation. Neurochem. Res. 2006;31(4):483–490. doi: 10.1007/s11064-006-9043-9. [http://dx.doi.org/10.1007/s11064-006-9043-9]. [PMID: 16758356]. [DOI] [PubMed] [Google Scholar]
- 92.Green P.S., Simpkins J.W. Neuroprotective effects of estrogens: potential mechanisms of action. Int. J. Dev. Neurosci. 2000;18(4-5):347–358. doi: 10.1016/s0736-5748(00)00017-4. [http://dx.doi.org/10.1016/S0736-5748(00)00017-4]. [PMID: 10817919]. [DOI] [PubMed] [Google Scholar]
- 93.Chen J., Lei T., Ritz M.F., Mendelowitsch A. Effect of 17 beta-estradiol on the brain damage and metabolic changes in rats. J. Tongji Med. Univ. 2001;21(1):62–64, 74. doi: 10.1007/BF02888040. [http://dx.doi.org/10.1007/BF02888040]. [PMID: 11523252]. [DOI] [PubMed] [Google Scholar]
- 94.Stein D.G., Wright D.W., Kellermann A.L. Does progesterone have neuroprotective properties? Ann. Emerg. Med. 2008;51(2):164–172. doi: 10.1016/j.annemergmed.2007.05.001. [http://dx.doi.org/10.1016/j.annemergmed.2007.05.001]. [PMID: 17588708]. [DOI] [PubMed] [Google Scholar]
- 95.Singh M. Progesterone-induced neuroprotection. Endocrine. 2006;29(2):271–274. doi: 10.1385/ENDO:29:2:271. [http://dx.doi.org/10.1385/ENDO:29:2:271]. [PMID: 16785602]. [DOI] [PubMed] [Google Scholar]
- 96.Djebaili M., Guo Q., Pettus E.H., Hoffman S.W., Stein D.G. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J. Neurotrauma. 2005;22(1):106–118. doi: 10.1089/neu.2005.22.106. [http://dx.doi.org/ 10.1089/neu.2005.22.106]. [PMID: 15665606]. [DOI] [PubMed] [Google Scholar]
- 97.Guo Q., Sayeed I., Baronne L.M., Hoffman S.W., Guennoun R., Stein D.G. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp. Neurol. 2006;198(2):469–478. doi: 10.1016/j.expneurol.2005.12.013. [http://dx.doi.org/10.1016/j. expneurol.2005.12.013]. [PMID: 16445913]. [DOI] [PubMed] [Google Scholar]
- 98.Pettus E.H., Wright D.W., Stein D.G., Hoffman S.W. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049(1):112–119. doi: 10.1016/j.brainres.2005.05.004. [http://dx.doi.org/10.1016/j.brainres.2005.05.004]. [PMID: 15932748]. [DOI] [PubMed] [Google Scholar]
- 99.Punnonen R., Nummi S., Ylikorkala O., Alapiessa U., Karvonen P., Vinikka L. A composite picture of the normal menstrual cycle. Acta Obstet. Gynecol. Scand. Suppl. 1976;51:63–70. [PMID: 1065183]. [PubMed] [Google Scholar]
- 100.Szydlowska K., Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47(2):122–129. doi: 10.1016/j.ceca.2010.01.003. [http://dx.doi. org/10.1016/j.ceca.2010.01.003]. [PMID: 20167368]. [DOI] [PubMed] [Google Scholar]
- 101.Luoma J.I., Kelley B.G., Mermelstein P.G. Progesterone inhibition of voltage-gated calcium channels is a potential neuroprotective mechanism against excitotoxicity. Steroids. 2011;76(9):845–855. doi: 10.1016/j.steroids.2011.02.013. [PMID: 21371490]. [DOI] [PMC free article] [PubMed] [Google Scholar]