Changes in Veterans Aging Cohort Study (VACS) Index scores correspond to changes in neurocognitive function among human immunodeficiency virus-infected persons, with higher VACS Index scores conferring a notable increased risk for neurocognitive decline and incident impairment.
Keywords: HIV, biomarkers, cognitive impairment
Abstract
Background. The Veterans Aging Cohort Study (VACS) Index, a composite marker of disease severity among human immunodeficiency virus (HIV)–infected persons, has been associated with concurrent risk for neurocognitive impairment (NCI). The present study examined whether the VACS Index predicts longitudinal neurocognitive change.
Methods. Participants included 655 HIV-infected persons followed for up to 6 years in cohort studies at the University of California, San Diego, HIV Neurobehavioral Research Program (mean age at baseline, 42.5 years; 83% male; 60% white; AIDS in 67%; median current CD4+ T-cell count, 346/μL; 61% receiving antiretroviral therapy). The VACS Index was calculated through standard methods. Participants completed a comprehensive neurocognitive battery. Neurocognitive status was plotted over time using demographically and practice-adjusted global and domain T scores. NCI was defined by global deficit scores derived from T scores.
Results. Baseline VACS Index scores were not predictive of changes in global T scores during the follow-up period (P = .14). However, in time-dependent analyses adjusting for covariates, higher VACS Index scores were significantly associated with worse global and domain neurocognitive performance (Ps < .01), as well as increased risk for developing NCI in a subgroup of persons who were neurocognitively normal at baseline (hazard ratio [HR], 1.17; P < .001). We categorized VACS Index scores by quartiles and found that the upper-quartile group was significantly more likely to develop NCI than the lower quartile (HR, 2.16; P < .01) and middle groups (HR, 1.76; P < .01).
Conclusions. Changes in VACS Index scores correspond to changes in neurocognitive function. HIV-infected persons with high VACS Index scores are at increased risk for decline and incident NCI. The VACS Index shows promise as a tool for identifying HIV-infected persons at risk for NCI.
The advent of combination antiretroviral therapy (ART) has transformed human immunodeficiency virus (HIV) infection and its effects on the central nervous system (CNS). HIV infection is now a chronic disease with multiple interacting causes of morbidity [1]. Although neurocognitive impairment (NCI) remains common in some HIV cohorts (35%–50% prevalence), neurocognitive deficits now tend to be milder than in the pre-ART era [2, 3]. Identifying persons with milder NCI is more difficult than identifying frank dementia and tends to require comprehensive and costly assessments. Even the milder forms of NCI may be associated with problems with everyday functioning (eg, impaired driving and medication nonadherence) [4, 5]. This highlights the importance of identifying, assessing and intervening in HIV-infected persons at risk for impaired and/or worsening neurocognitive function. Ascertaining biomarkers of HIV-associated NCI is one promising approach to detecting those at risk for NCI, particularly if these biomarkers are obtained as part of routine clinical care. Clinical investigation of biomarkers is also relevant to improve understanding of the biomedical mechanisms underlying NCI in the ART era.
The Veterans Aging Cohort Study (VACS) Index was developed as a composite marker of disease severity among HIV-infected persons based on routine clinical blood tests. It integrates age, “traditional” biomarkers of HIV disease (ie, plasma RNA and current CD4+ T-cell counts) and “nontraditional” biomarkers, including markers of renal and liver function, anemia, and hepatitis C virus (HCV) coinfection [6, 7]. The VACS Index has been consistently associated with increased risk of death in HIV-infected persons [6, 8, 9]. It has also been linked to poor health outcomes, including increased risk for hospitalizations and medical intensive care unit admissions [10, 11], fragility fractures [12], frailty [13, 14], and concurrent extremity strength [15]. Prior work by our group found the cross-sectional association of the VACS Index with NCI to be significant but small [16], and particularly weak among Hispanics [17].
The overall goal of the present study was to extend prior cross-sectional findings to examine the ability of the VACS Index to predict neurocognitive change and incident NCI in a large and well-characterized cohort of HIV-infected persons. We did so by examining (1) the association between baseline VACS Index scores and subsequent neurocognitive change; (2) whether longitudinal changes in the VACS Index corresponded to changes in neurocognitive function; and (3) whether VACS Index scores predicted time to incident NCI in a subgroup of participants who were neurocognitively normal at baseline.
METHODS
Participants
Participants included 655 HIV-infected individuals followed for up to 6 years in National Institutes of Health–funded cohort studies at the University of California, San Diego, HIV Neurobehavioral Research Program from 14 April 1999 to 11 May 2012 [18–20]. Studies were approved by the university's institutional review board. All participants provided informed consent for participation in these cohort studies and agreed for their data to be used for future studies assessing the impact of HIV on the nervous system. Exclusion criteria included histories of neurological (eg, seizure disorders, closed head injuries, and cerebrovascular accidents) or severe psychiatric (eg, schizophrenia) conditions. Inclusion criteria were being HIV infected (based on enzyme-linked immunosorbent assay with Western blot confirmation), having ≥2 study visits with valid global neurocognitive scores, having laboratory data available to compute the VACS Index within 3 months of neurocognitive data, being primarily English speaking, providing informed consent, and being free of sensory or physical problems that would interfere with neurocognitive testing. Forty-five percent of the sample had previously undergone neurocognitive testing.
Materials and Procedures
Participants completed comprehensive neuromedical, neurocognitive, psychiatric, and substance use evaluations, similar to prior research by our group on the VACS Index and NCI [16, 17], every 6 months to 1 year.
Neuromedical Evaluation
Routine clinical chemistry panels, complete blood cell counts, rapid plasma reagin, HCV antibody, and CD4+ T-cell counts (flow cytometry) were performed at a Clinical Laboratory Improvement Amendments–certified, or equivalent, laboratory. HIV RNA levels in plasma were measured by means of reverse-transcription polymerase chain reaction (Roche Amplicor, version 1.5; lower limit of quantitation, 50 copies/mL). CNS penetration effectiveness was ascertained as described elsewhere [21, 22]. Self-reported data were gathered on duration of HIV infection, nadir CD4+ T-cell count, history of ART, and duration of current ART. HCV status was based on HCV antibody testing (as described above) and/or self-report. The VACS Index was computed as described elsewhere, with higher scores indicating worse disease status [10].
Neurocognitive Evaluation
The neurocognitive battery comprised 15 measures covering 7 neurocognitive domains (see Cysique et al [23] for a list of tests by domain). Raw test scores were transformed into scaled scores adjusted for repeated testing [23], which were then converted into T scores adjusted for demographics (age, education, sex, and race) [24, 25]. The adjusted T scores for each test were then averaged to derive global and domain T scores, which were used for analyses in the overall sample. To determine whether the VACS Index predicted time to incident NCI, we converted the adjusted T scores for each test into deficit scores, ranging from 0 (T score, >39; no impairment) to 5 (T score, <20; severe impairment) and averaged these scores to derive global deficit scores [18]. Consistent with previous studies, NCI was defined as a global deficit score of ≥0.50 [16].
Psychiatric and Substance Use Characteristics
We assessed current mood symptoms via the Beck Depression Inventory (BDI) and used published cutoff scores to determine severity of depression symptoms (ie, minimal, mild, moderate, or severe) [26, 27]. Current (last 30 days) and lifetime history of major depressive and substance use disorders were obtained using structured diagnostic interviews [28–30] that follow criteria of the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) [31]. Presence of a “substance use disorder” was defined as meeting criteria for abuse or dependence for alcohol, cannabis, and any of the following substances: opioids, methamphetamine, cocaine, sedatives, and hallucinogens. Data on lifetime intravenous drug use were ascertained by self-report.
Statistical Methods
Three analytic approaches were used. First, to evaluate effects of baseline VACS Index scores on changes in neurocognitive function over time, we used a mixed effects linear regression with subject-specific random intercepts and slopes, which assumes that participants have different baseline T scores (intercepts) and varying trajectories of change in T scores over time (slopes). The model regressed mean global T scores on time (in years). Individual slopes were obtained from the model and used as outcomes in a linear regression with baseline VACS Index as predictor. The slopes estimated the average changes in global T scores with every year passed.
A second set of analyses was used to evaluate the association of the VACS Index as a time-dependent predictor (ie, measures from each visit, not just baseline, were used) of longitudinal cognitive status based on adjusted T scores. Mixed effects linear regressions with subject-specific random intercepts and slopes were used. Because the model on global T scores was significant, we investigated the association of the VACS Index as a time-dependent predictor with changes in domain T scores using a similar approach and evaluated the impact of potential covariates. Covariates examined included baseline (lifetime major depressive and substance use disorder [cannabis, alcohol, and other substances], and nadir CD4+ T-cell count) and time-dependent characteristics (current major depressive and substance use disorder [cannabis, alcohol, and other substances], BDI, and ART status). Covariates were included into multivariable models, if univariable analyses showed that they were associated with the outcome variable at the .10 significance level. Backward model selection process was applied and the final models kept only those covariates that had P values <.05. For reported models, effects of predictors are described by regression coefficients (estimates) and their standard errors (SEs).
A third set of analyses was performed in the subgroup of participants who did not show NCI at baseline. We used Cox proportional hazard models to assess time to NCI. Two models were run: (1) a baseline model, which used values of the VACS Index and baseline covariates; and (2) a time-dependent model, which included time-dependent measures of the VACS Index and covariates. Covariate selection followed the same process as described above. Hazard ratio (HR) was used as a measure of effect size for these analyses. Given that the VACS Index was initially developed in HIV-infected persons initiating therapy, we investigated interactions between the VACS Index and ART status (on or off) for all 3 types of analyses.
Based on prior cross-sectional findings showing that HIV-infected persons with particularly elevated VACS Index scores might be at highest risk for NCI [16], we categorized VACS Index scores at each time point based on cutoff scores. These cutoff scores were based on the distribution of the VACS Index at baseline of the current sample (low VACS Index was defined as the lower quartile, VACS Index <13; high VACS Index, the upper quartile, VACS Index ≥38; and middle VACS Index, between the lower and upper quartiles). We then performed again the main analyses described above using these categories of the VACS Index (ie, low, middle and high). P values for pairwise comparisons of the groups were adjusted for multiple testing using Tukey and false discovery rate methods, and Cohen's d was computed to measure effect sizes of group differences.
All analyses were carried with the statistical software R (version 3.1.1) [32] and used 2-sided tests and a significance level of .05, unless stated otherwise. Sample sizes vary by model, because complete data were not available for some of the covariates (Table 1).
Table 1.
Baseline Characteristics of the Full Study Cohort and a Subset of Participants With No Neurocognitive Impairment at Baseline
| Variable | Full Study Cohort (N = 655) | No Baseline NCI (n = 392) |
|---|---|---|
| Demographic characteristics | ||
| Age, mean (SD), y | 42.5 (8.9) | 42 (8.5) |
| Male sex, % | 83 | 82 |
| Race, % | ||
| White | 60 | 58 |
| Black | 22 | 29 |
| Hispanic | 13 | 9 |
| Other | 5 | 4 |
| Educational level, mean (SD), y | 13.1 (2.6) | 13.1 (2.5) |
| Calendar year of baseline assessments, median (IQR) | 2001 (2000–2003) | 2001 (2000–2003) |
| Duration of follow-up, median (IQR), y | 4.0 (2.1–5.9) | 5.0 (2.5–6.0) |
| HIV disease characteristics | ||
| CD4+ T-cell count, median (IQR), cells/μL | ||
| Current | 346 (179–544) | 369 (196–575) |
| Nadir | 120 (20–277) | 157 (26–300) |
| AIDS, % | 67 | 62 |
| Estimated duration of HIV infection, median (IQR), y | 9 (5–14) (n = 572)a | 9 (5–13) (n = 350)a |
| Receiving ART, % | 61 | 57 |
| Detectable plasma HIV viral load, %b | 49 | 50 |
| Duration of current ART regimen, median (IQR), mob | 12 (4–27) | 12 (4–25) |
| CPEb | 7 (5–9) | 7 (5–8) |
| Psychiatric and substance use characteristics | ||
| Depression symptoms (BDI), %c | ||
| Minimal | 46 | 51 |
| Mild | 30 | 30 |
| Moderate | 18 | 15 |
| Severe | 7 | 4 |
| Current major depressive disorder, % | 21 (n = 437)a | 17 (n = 228)a |
| Lifetime major depressive disorder, % | 58 (n = 437)a | 54 (n = 228)a |
| Current substance use disorder, % | ||
| Any | 17 (n = 437)a | 14 (n = 228)a |
| Alcohol | 6 | 6 |
| Cannabis | 7 | 5 |
| Otherd | 9 | 8 |
| Lifetime substance use disorder, % | ||
| Any | 76 (n = 437)a | 76 (n = 228)a |
| Alcohol | 54 | 54 |
| Cannabis | 37 | 40 |
| Otherd | 59 | 59 |
| Lifetime history of intravenous drug use, % | 27 (n = 233)a | 24 (n = 148)a |
| NCI, % | 40 | 0 |
| VACS Index, median (IQR) | 22 (12–37) | 21 (11–33) |
Abbreviations: ART, antiretroviral therapy; BDI, Beck Depression Inventory; CPE, central nervous system penetration-effectiveness; HIV, human immunodeficiency virus; IQR, interquartile range; NCI, neurocognitive impairment; SD, standard deviation; VACS, Veterans Aging Cohort Study.
a n represents total number of participants with available data on the corresponding cell.
b Among those receiving ART.
c BDI cutoff scores were defined based on the version administered (original BDI version [26] or BDI-II [27]).
d Other substance use disorder considered opioids, methamphetamine, cocaine, sedatives, and hallucinogens.
RESULTS
Cohort Participants
Participants' baseline characteristics are listed in Table 1. The cohort consisted of mostly white men aged 18–70 years. The majority were receiving ART and had AIDS; about half had detectable plasma viral loads.
Association Between Baseline VACS Index Scores and Neurocognitive Change
A univariable model predicting changes in global T scores from baseline VACS Index scores was not significant (estimate, −0.01; SE, 0.01; P = .14). A multivariable model showed no significant interaction between the VACS Index and ART status (P = .85).
Time-Dependent Association Between VACS Index and Neurocognitive Decline
Table 2 shows results of time-dependent multivariable models predicting changes in global and domain T scores from VACS Index scores. Higher values of the VACS Index were significantly associated with worse global and domain neurocognitive performance in both unadjusted and adjusted analyses (Ps < .01). In general, the decreases in neurocognitive T scores per every 10 points of the VACS Index were <1 point (range, −0.34 to −0.74).
Table 2.
Results of Time-dependent Multivariable Mixed-Effect Linear Models Assessing the Association of the Veterans Aging Cohort Study Index With Global and Domain T Scores at any Time Point During the Follow-up Period
| Predictor | Regression Coefficient (SE) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Global Cognition | Verbal Fluency | SIP | Learning | Memory | Executive Function | Working Memory | Complex Motor Skills | |
| Unadjusteda analyses | n = 655 | n = 653 | n = 648 | n = 654 | n = 654 | n = 643 | n = 650 | n = 641 |
| VACS Indexb | −0.51 (.05)c | −0.52 (.08)c | −0.62 (.07)c | −0.50 (.08)c | −0.37 (.09)c | −0.51 (.08)c | −0.51 (.07)c | −0.74 (.09)c |
| Time in study, y | 0.15 (.04)c | 0.04 (.06) | 0.11 (.06)d | −0.19 (.06)c | −0.17 (.06)c | 0.75 (.06)c | 0.32 (.06)c | −0.17 (.08)d |
| Adjusted analyses | n = 402 | n = 400 | n = 398 | n = 401 | n = 414 | n = 396 | n = 399 | n = 392 |
| VACS Indexb | −0.41 (.06)c | −0.62 (.10)c | −0.53 (.09)c | −0.34 (.10)c | −0.59 (.17)c | −0.37 (.12)c | −0.48 (.10)c | −0.63 (.12)c |
| Time, y | 0.19 (.05)c | 0.17 (.08)d | 0.03 (.08) | −0.02 (.09) | −0.02 (.09) | 0.72 (.10)c | 0.42 (.08)c | −0.34 (.11)c |
| Current MDD | −0.83 (.24)c | … | −0.83 (.36)d | … | −1.09 (.43)d | … | −1.08 (.37)c | … |
| Lifetime MDD | … | 1.85 (.84)d | … | … | … | … | … | … |
| Depression | ||||||||
| Minimal | Reference | Reference | Reference | Reference | … | Reference | … | Reference |
| Mild | −0.74 (.21)c | −0.71 (.35)d | −0.89 (.31)c | −0.44 (.37) | … | −1.25 (.41)c | … | −1.45 (.43)c |
| Moderate | −0.99 (.30)c | −1.53 (.47)c | −1.02 (.44)d | −1.57 (.49)c | … | −2.25 (.56)c | … | −1.08 (.58) |
| Severe | −1.13 (.44)d | −1.08 (.72) | −2.33 (.67)c | −0.71 (.74) | … | −3.15 (.85)c | … | −1.99 (.90)d |
| Current CUD | 1.10 (.44)d | … | … | 2.46 (.77)c | … | … | 2.15 (.71)c | … |
| Lifetime CUD | … | … | … | … | 1.98 (.83)d | … | … | … |
| Current OSUD | … | … | … | … | … | … | … | −1.57 (.67)d |
| Nadir CD4+ T-cell counte | 0.49 (.17)c | … | … | 0.50 (.20)d | 0.51 (.20)d | 0.75 (.21)c | … | 0.50 (.24)d |
| On ART | … | … | … | −1.34 (.41)c | −2.46 (.70)c | … | … | … |
| VACS × On ART | … | … | … | … | 0.39 (.18)d | … | … | … |
Values represent regression coefficient (standard error).
Abbreviations: ART, antiretroviral therapy; CUD, cannabis use disorder; MDD, major depressive disorder; OSUD, other substance use disorder (opioids, methamphetamine, cocaine, sedatives, and hallucinogens); SE, standard error; SIP, speed of information processing; VACS, Veterans Aging Cohort Study.
a Models were adjusted for time (years), but not for covariates.
b Effect is shown as change in T scores per 10-point increase in VACS.
c P < .01.
d P < .05.
e Effect is shown as change in T scores per increase in baseline nadir CD4+ T-cell count of 100/μL.
A significant interaction between the VACS Index and ART (P = .03) was observed in the memory domain, indicating that higher VACS Index scores were associated with lower memory among participants off ART (estimate, −0.58; SE, 0.17; P < .001) but not among those receiving ART (estimate, −0.19; SE, 0.12; P = .11). No other significant interactions were observed.
Association Between VACS Index and Risk of Developing NCI
Sixty percent of the cohort (n = 392) showed no NCI at baseline (Table 1). Results of Cox proportional hazard models (Table 3) showed that higher baseline VACS Index values were associated with increased hazards of impairment (HR, 1.17, 95% confidence interval [CI], 1.06–1.29; P < .01) in unadjusted analyses. After controlling for significant covariates (ie, nadir CD4+ T-cell count and baseline BDI), this association was no longer statistically significant (P = .07). In time-dependent analyses, higher VACS Index scores were associated with a significant increased risk of incident NCI (Table 3). There were no significant interactions between the VACS Index and ART status.
Table 3.
Results of Multivariable Cox Proportional Hazards Models Assessing the Association of the Veterans Aging Cohort Study Index With Probability of Neurocognitive Impairment
| Predictor | Baseline Modela |
Time-Dependent Modelb |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| VACS Indexc | 1.11 (.99–1.24) | .07 | 1.17 (1.09–1.25) | <.001 |
| Nadir CD4+ T-cell countd | 0.87 (.77–.98) | .03 | 0.90 (.82–.99) | .02 |
| Depression | ||||
| Minimal | Reference | … | Reference | … |
| Mild | 1.09 (.69–1.73) | .70 | 1.23 (.86–1.77) | .26 |
| Moderate | 1.97 (1.17–3.33) | .01 | 2.14 (1.35–3.40) | .001 |
| Severe | 2.59 (1.07–6.27) | .04 | 1.80 (.81–3.99) | .15 |
| Lifetime MDD | … | … | 0.46 (.33–.64) | <.001 |
| Lifetime AUD | … | … | 1.83 (1.31–2.54) | <.001 |
Abbreviations: AUD, alcohol use disorder; CI, confidence interval; HR, hazard ratio; MDD, major depressive disorder; VACS, Veterans Aging Cohort Study.
a VACS Index and depression severity are measured at baseline (n = 365).
b VACS Index and depression severity are measured at multiple visits (n = 193).
c The HR for impairment is associated with a 10-point increase in the VACS Index.
d The HR for impairment is associated with a increase in the baseline nadir CD4+ T-cell count of 100/μL.
Changes in Neurocognitive Function by Categorical Values of the VACS Index
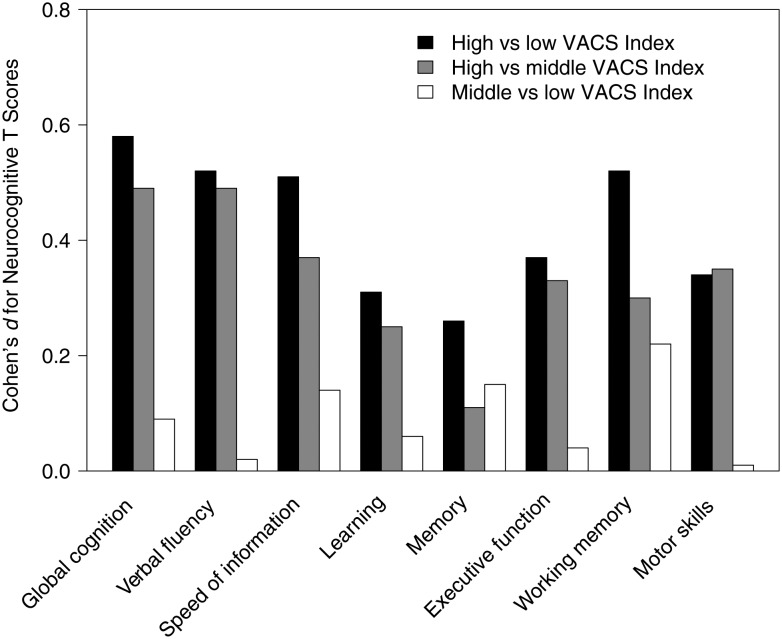
Results from analyses predicting neurocognitive change in the overall sample based on baseline VACS Index group (low, n = 171; middle, n = 321; and high, n = 163) showed no significant group differences (P = .23). In time-dependent analyses, being in the high VACS Index group was associated with worse global T score than being in the low and middle groups, with no significant differences between the low and middle VACS Index groups. This pattern was also observed in most cognitive domains (Figure 1).
Figure 1.
Cohen's d effect sizes for Veterans Aging Cohort Study (VACS) Index group comparisons on global and domain T scores. Results are based on time-dependent multivariable mixed-effect linear models assessing the association of VACS Index group with global and domain T scores at any time point during the follow-up period, adjusting for significant covariates. Covariates are similar to those presented in Table 2. High indicates VACS Index scores in the upper quartile; low, scores in the lower quartile; and middle, scores between the lower and upper quartiles.
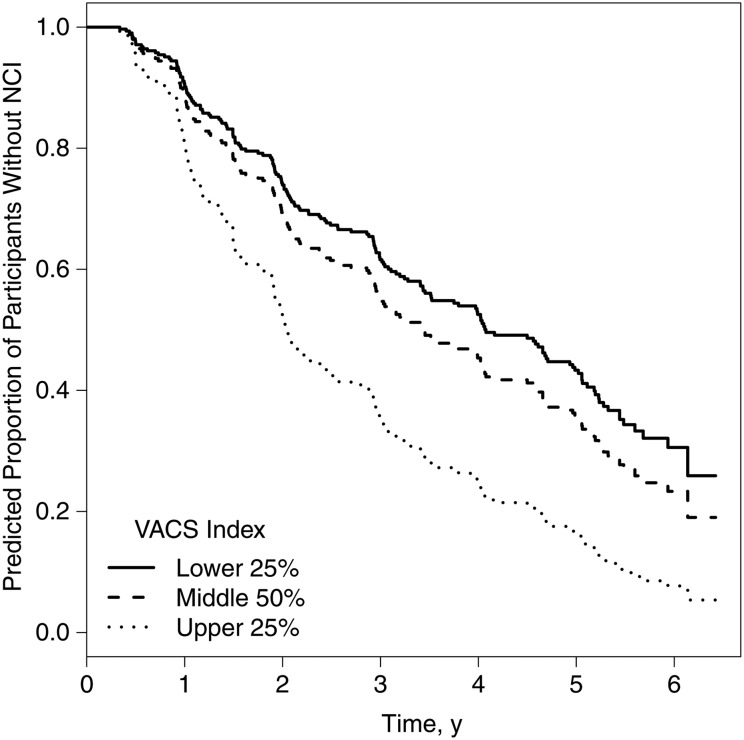
Models predicting incident NCI by baseline VACS Index group (low, middle, or high) showed no significant group differences (P = .24). Time-dependent analyses adjusting for significant covariates showed that the high VACS Index group was significantly more likely to develop NCI than both low and middle VACS Index groups (Figure 2).
Figure 2.
Incident neurocognitive impairment (NCI). Graph shows predicted proportion of participants who are neurocognitively normal (ie, do not show neurocognitive impairment in standardized testing) by Veterans Aging Cohort Study (VACS) Index group, with adjustment for significant covariates, namely, Beck Depression Inventory, lifetime major depressive, lifetime alcohol use disorder, and nadir CD4+ T-cell count (n = 193; VACS Index group pairwise comparisons with false discovery rate adjustment: hazard ratio for high (upper 25%) vs low (lower 25%), 2.16 [95% confidence interval, 1.27–3.69; P < .01]; high vs middle, 1.76 [1.19–2.62; P < .01]; and middle vs low, 1.23 [.82–1.83; P = .31]).
Follow-up Analyses
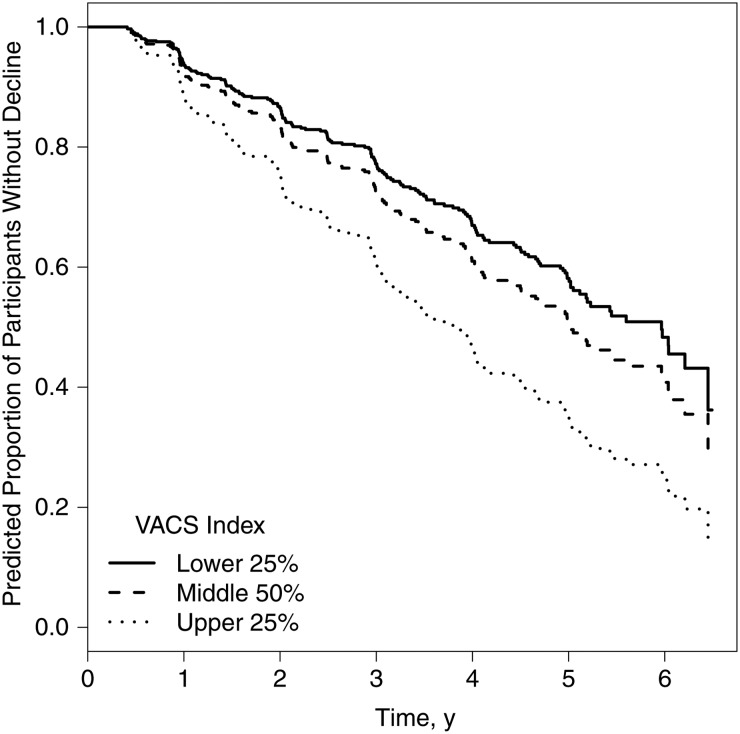
For a subset of participants (n = 340), the baseline visit for current analyses was their first time to complete neurocognitive assessments. This allowed us to investigate neurocognitive change based on published summary regression-based change scores [23] (which identify change relative to first neurocognitive assessment) in this subset of participants. Following methods outlined elsewhere [33], we determined overall neurocognitive change (ie, decline/no decline) and performed again core analyses presented above. Results were comparable to previous findings; that is, the VACS Index (as both a continuous and a categorical variable; Figure 3) was significantly associated with neurocognitive decline in time-dependent models after adjustment for significant covariates.
Figure 3.
Overall neurocognitive decline. Graph shows predicted proportion of participants who do not show neurocognitive decline by Veterans Aging Cohort Study (VACS) Index group. Results are based on a time-dependent mixed effects model predicting overall neurocognitive decline (based on regression based norms for change), with adjustment for significant covariates, namely, current major depressive disorder, lifetime other substance use disorder, baseline nadir CD4+ T-cell count, and antiretroviral therapy status (n = 241; VACS Index group pairwise comparisons with false discovery rate adjustment: hazard ratio for high (upper 25%) vs low (lower 25%), 1.93 [95% confidence interval, 1.13–3.29; P < .01]; high vs middle, 1.57 [1.08–2.28; P = .02]; middle vs low, 1.23 [.83–1.83; P = .30]).
We also investigated whether lifetime history of intravenous drug use affected the association of the VACS Index with neurocognitive change in the subset of participants for whom data were available, as well as whether CNS penetration effectiveness affected this association among persons on ART. Findings from all core models showed that the association between the VACS Index and neurocognitive change was substantially similar to that of prior analyses.
DISCUSSION
The present longitudinal study adds to the evolving scientific literature on the VACS Index and NCI by showing that changes in VACS Index scores correspond to changes in neurocognitive function over time in a large, well-characterized HIV-infected cohort. Baseline VACS Index scores were not significantly associated with change in global neurocognitive function during the overall follow-up period of up to 6 years (average follow-up time, 4 years). This suggests that the VACS Index might not be a good predictor of subsequent neurocognitive change over the span of a number of years. However, we found statistically significant associations in time-dependent analyses, indicating that changes in the VACS Index correspond to concurrent changes in global neurocognitive function. These significant associations, albeit relatively small, were found across multiple neurocognitive domains, and remained significant after adjusting for the effect of potential covariates, suggesting an independent effect of the VACS Index.
Analyses of a subset of participants who were neurocognitively normal at baseline yielded a similar pattern of results. Baseline VACS Index scores were not significantly associated with incident NCI in adjusted analyses. However, time-dependent analyses showed that increases in VACS Index scores (indicative of worsening health status) significantly increased the probability of becoming neurocognitively impaired.
Because the VACS Index was initially developed in samples of HIV-infected persons initiating ART, we investigated whether ART status modified the association between the VACS Index and neurocognitive change. We found that this was the case for the memory domain in time-dependent analyses, where there was a significant association between VACS Index scores and memory decline among untreated participants but not among participants receiving ART. Other biomarkers not included in the VACS Index might be important for memory decline in participants on ART. In line with this, effect sizes of analyses by VACS Index group tended to be smaller for learning and memory. Memory problems are the hallmark of neurocognitive conditions of aging, such as Alzheimer's disease. Considering biomarkers of these other age-related conditions might assist in developing an index that fully encompasses the neurocognitive changes observed among persons aging with HIV infection. We have also found that the association of learning and memory to NCI was not significant among Hispanics [17]. Unfortunately, we did not have enough Hispanic participants with longitudinal data to examine ethnic/racial differences in the current study.
Prior findings had indicated that it is valuable to categorize the VACS Index to better understand its association with NCI [16]. In the current study, we found that participants who scored high on the VACS Index (upper quartile) were significantly more likely to experience global neurocognitive decline, with effect sizes indicating a relatively strong effect. Among participants who were neurocognitively normal at baseline, those who scored within the high VACS Index range were twice as likely to develop incident NCI as those in the low VACS Index group, and 76% more likely than those in the middle group. Overall, results from these analyses on categorized VACS Index scores support the notion that those with particularly higher VACS Index values are indeed at risk for worse neurocognitive outcomes.
Our study had several limitations. Our group of participants was relatively young. Including older participants with higher rates of medical comorbid conditions might strengthen the association between the VACS Index and neurocognitive change. Most baseline assessments in the present study were completed in the early 2000s and the median duration of HIV infection was 9 years. Thus, many of the participants were diagnosed with HIV infection in the era before combination ART era, and findings might differ in more recently infected cohorts. A relatively small proportion of participants in our sample were of Hispanic origin. Given recent findings [17], future studies including more Hispanic subjects would better address whether longitudinal associations between the VACS Index and neurocognitive change differ by ethnic group.
It has also yet to be established whether other biomarkers not included in the VACS Index might increase its predictive utility for detecting NCI. Some covariates examined in present analyses were unexpectedly associated with neurocognitive change; for example, cannabis use and being off ART were associated with better neurocognitive performance in some models. Although these potentially interesting associations warrant further investigation, our models were not constructed to investigate their impact on neurocognitive change, and thus caution is warranted in interpreting these findings. Most relevant for the purpose of the current study, the VACS Index was significantly associated with neurocognitive change, even after adjustment for multiple covariates. Another future direction is to investigate whether the combination of the VACS Index and NCI might help improve predictive power for mortality risk among HIV-infected persons. Some of the strengths of the study include the use of a comprehensive neurocognitive battery that has been validated in persons with HIV infection and enabled the examination of domains based on composite scores.
Overall, baseline VACS Index scores may not be a good predictor of neurocognitive change in the longer term. Changes in VACS Index scores, however, correspond to changes in neurocognition. Although the strength of this association is relatively weak in analyses using continuous scores of the VACS Index, results from categorized VACS Index scores indicate that having very high VACS Index scores might indicate a notable increased risk for neurocognitive decline and for incident NCI. These findings support the VACS Index as a simple tool for identifying HIV-infected patients who are at high risk for NCI and might warrant further neurocognitive follow-up.
Notes
Acknowledgments. The San Diego Human Immunodeficiency Virus Neurobehavioral Research Center group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes the following: Director: R. K. H., PhD, Codirector: I. G., MD; Associate Directors: J. Hampton Atkinson, MD, R. J. E., MD, PhD, and S. L. L., MD; Center Manager: Thomas D. Marcotte, PhD; Jennifer Marquie-Beck, MPH; Melanie Sherman; Neuromedical Component: R. J. E., MD, PhD (principal investigator [PI]), S. L. L., MD, J. Allen McCutchan, MD, Brookie Best, PharmD, Rachel Schrier, PhD, Debra Rosario, MPH; Neurobehavioral Component: R. K. H., PhD (PI), J. Hampton Atkinson, MD, Steven Paul Woods, PsyD, Thomas D. Marcotte, PhD, Mariana Cherner, PhD, D. J. M., PhD, Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, PhD (PI), Monte S. Buchsbaum, MD, John Hesselink, MD, Sarah L. Archibald, MA, Gregory Brown, PhD, Richard Buxton, PhD, Anders Dale, PhD, Thomas Liu, PhD; Neurobiology Component: Eliezer Masliah, MD (PI), Cristian Achim, MD, PhD; Neurovirology Component: David M. Smith, MD (PI), Douglas Richman, MD; International Component: J. Allen McCutchan, MD, (PI), Mariana Cherner, PhD; Developmental Component: Cristian Achim, MD, PhD; (PI), Stuart Lipton, MD, PhD; Participant Accrual and Retention Unit: J. Hampton Atkinson, MD (PI), Jennifer Marquie-Beck, MPH; Data Management and Information Systems Unit: Anthony C. Gamst, PhD (PI), Clint Cushman; Statistics Unit: Ian Abramson, PhD (PI), Florin Vaida, PhD (Co-PI), Reena Deutsch, PhD, A. U., MS.
Disclaimer. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States government.
Financial support. This work was supported by the National Institutes of Health (grants P30MH062512, U01MH083506, R24MH59745, P01DA12065, K99AG048762, T32MH019934, and K23MH105297).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the HIV Neurobehavioral Research Program Group, Robert K. Heaton, Igor Grant, J. Hampton Atkinson, Ronald J. Ellis, Scott Letendre, Thomas D. Marcotte, Jennifer Marquie-Beck, Melanie Sherman, Ronald J. Ellis, Scott Letendre, J. Allen McCutchan, Brookie Best, Rachel Schrier, Debra Rosario, Robert K. Heaton, J. Hampton Atkinson, Steven Paul Woods, Thomas D. Marcotte, Mariana Cherner, David J. Moore, Matthew Dawson, Christine Fennema-Notestine, Monte S. Buchsbaum, John Hesselink, Sarah L. Archibald, Gregory Brown, Richard Buxton, Anders Dale, Thomas Liu, Eliezer Masliah, Cristian Achim, David M. Smith, Douglas Richman, J. Allen McCutchan, Mariana Cherner, Cristian Achim, Stuart Lipton, J. Hampton Atkinson, Jennifer Marquie-Beck, Anthony C. Gamst, Clint Cushman, Ian Abramson, Florin Vaida, Reena Deutsch, and Anya Umlauf
References
- 1.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. Br Med J 2009; 338:a3172. [DOI] [PubMed] [Google Scholar]
- 2.Heaton RK, Clifford DB, Franklin DR et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton RK, Franklin DR, Ellis RJ et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J NeuroVirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaton RK, Marcotte TD, Mindt MR et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Inter Neuropsychol Soc 2004; 10:317–31. [DOI] [PubMed] [Google Scholar]
- 5.Andrade ASA, Deutsch R, Celano SA et al. Relationships among neurocognitive status, medication adherence measured by pharmacy refill records, and virologic suppression in HIV-infected persons. J Acqui Immune Defic Syndr 2013; 62:282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Justice AC, Freiberg MS, Tracy R et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis 2012; 54:984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Justice AC, McGinnis KA, Skanderson M et al. Towards a combined prognostic index for survival in HIV infection: the role of ‘non-HIV’ biomarkers. HIV Med 2010; 11:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justice AC, Modur SP, Tate JP et al. Predictive accuracy of the Veterans Aging Cohort Study Index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Def Syndr 2013; 62:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tate JP, Justice AC, Hughes MD et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS 2013; 27:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akgun KM, Gordon K, Pisani M et al. Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV-infected Veterans. J Acquir Immune Def Syndr 2013; 62:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akgun KM, Tate JP, Crothers K et al. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Def Syndr 2014; 67:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Womack JA, Goulet JL, Gibert C et al. Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis 2013; 56:1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escota GV, Patel P, Brooks JT et al. Short communication: The Veterans Aging Cohort Study Index is an effective tool to assess baseline frailty status in a contemporary cohort of HIV-infected persons. AIDS Res Hum Retroviruses 2015; 31:313–7. [DOI] [PubMed] [Google Scholar]
- 14.Erlandson KM, Allshouse AA, Jankowski CM et al. Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials 2012; 13:324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oursler KK, Tate JP, Gill TM et al. Association of the Veterans Aging Cohort Study Index with exercise capacity in HIV-infected adults. AIDS Res Hum Retroviruses 2013; 29:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marquine MJ, Umlauf A, Rooney AS et al. The Veterans Aging Cohort Study Index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Def Syndr 2014; 65:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marquine MJ, Sakamoto M, Dufour CA et al. The impact of ethnicity/race on the association between the Veterans Aging Cohort Study (VACS) index and neurocognitive function among HIV-infected persons. J Neurovirol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant I, Heaton RK, Atkinson JH;. HNRC Group. Neurocognitive disorders in HIV-1 infection. Curr Top Microbiol Immunol 1995; 202:11–32. [PubMed] [Google Scholar]
- 19.Rippeth JD, Heaton RK, Carey CL et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Intern Neuropsychol Soc 2004; 10:1–14. [DOI] [PubMed] [Google Scholar]
- 20.Woods SP, Rippeth JD, Frol AB et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 2004; 26:759–78. [DOI] [PubMed] [Google Scholar]
- 21.Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top Antivir Med 2011; 19:137–42. [PMC free article] [PubMed] [Google Scholar]
- 22.Letendre S, Marquie-Beck J, Capparelli E et al. CHARTER group Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008; 65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cysique LA, Franklin D, Abramson I et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol 2011; 33:505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: demographically adjusted neuropsychological norms for African American and Caucasian adults scoring program. Odessa, FL: Psychological Assessment Resources, 2004. [Google Scholar]
- 25.Norman MA, Moore DJ, Taylor M et al. ; HNRC Group. and Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol 2011; 33:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–71. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess 1996; 67:588–97. [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders. Washington, DC: American Psychiatric Press, 1996. [Google Scholar]
- 29.Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry 1996; 153:1195–201. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. Composite International Diagnostic Interview. Geneva, Switzerland: World Health Organization, 1997. [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
- 32.R Core Team. The R project for statistical computing. Available at: http://www.R-project.org/ Accessed 15 May 2015.
- 33.Heaton RK, Franklin DR, Deutsch R et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER Study. Clin Infect Dis 2015; 60:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]