A single dose of zoledronic acid administered at antiretroviral therapy (ART) initiation prevented ART-induced bone loss through the first 48 weeks of therapy, the period when ART-induced bone loss is most pronounced.
Keywords: antiretroviral therapy–induced bone loss, zoledronic acid, human immunodeficiency virus
Abstract
Background. Human immunodeficiency virus (HIV) infection and antiretroviral therapy (ART) are associated with bone loss leading to increased fracture rate among HIV-infected individuals. ART-induced bone loss is most intense within the first 48 weeks of therapy, providing a window for prophylaxis with long-acting antiresorptives.
Methods. In a phase 2, double-blind, placebo-controlled trial, we randomized 63 nonosteoporotic, ART-naive adults with HIV initiating ART with atazanavir/ritonavir + tenofovir/emtricitabine to a single zoledronic acid (ZOL) infusion (5 mg) vs placebo to determine the efficacy of ZOL in mitigating ART-induced bone loss. Plasma bone turnover markers and bone mineral density (BMD) were performed at weeks 0, 12, 24, and 48 weeks. Primary outcome was change in C-terminal telopeptide of collagen at 24 weeks. Repeated-measures analyses using mixed linear models were used to estimate and compare study endpoints.
Results. The ZOL arm had a 65% reduction in bone resorption relative to the placebo arm at 24 weeks (0.117 ng/mL vs 0.338 ng/mL; P < .001). This effect of ZOL occurred as early as 12 weeks (73% reduction; P < .001) and persisted through week 48 (57% reduction; P < .001). The ZOL arm had an 8% higher lumbar spine BMD at 12 weeks relative to the placebo arm (P = .003), and remained 11% higher at 24 and 48 weeks. Similar trends were observed in the hip and femoral neck.
Conclusions. A single dose of ZOL administered at ART initiation prevented ART-induced bone loss through the first 48 weeks of ART, the period when ART-induced bone loss is most pronounced. Validation of these results in larger multicenter randomized clinical trials is warranted.
Clinical Trials Registration. NCT01228318.
Increasing longevity of the human immunodeficiency virus (HIV) population has renewed interest in the long-term complications of HIV infection [1]. Among the metabolic complications of chronic HIV infection is skeletal deterioration [2]. Osteopenia prevalence in HIV-infected cohorts ranges from 22% to 71%, with rates of osteoporosis varying from 3% to 33% [3]. An intriguing aspect of this phenomenon is that antiretroviral therapy (ART) exacerbates rather than ameliorates bone loss [4]. The skeletal effects of ART, although varied in magnitude, appear to be universal to all ART types including tenofovir alafenamide (TAF)–containing and tenofovir disoproxil fumarate (TDF)–sparing regimens [5–10]. Losses of up to 6% in bone mineral density (BMD) were observed with earlier regimens within 1–2 years of ART initiation [2]. With TAF, an average loss in BMD of 0.66% at the hip and 1.30% at the lumbar spine was observed after 48 weeks in ART-naive, HIV-infected patients. Of note, although reductions in BMD cannot be quantitatively correlated to fracture incidence, a 2- to 9-fold higher fracture prevalence is reported with HIV infection relative to the general population [11, 12]. There is also a growing concern that the bone loss induced by ART on a background of a preexisting virally induced weakened skeleton will synergize with the natural age-related bone loss to cause an epidemic of fragility fracture [13].
To better understand the mechanism underlying this phenomenon, we previously examined bone turnover in HIV-infected patients initiating ART, and observed a surge in bone resorption, starting as early as 2 weeks and lasting through 24 weeks [14]. Because T-cell recovery with ART reaches a significant magnitude by 12 weeks [15], the time point at which we observed a peak in bone resorption, we speculated that there was a link between immune reconstitution and ART-induced bone loss. Using an animal model of immune reconstitution created by syngeneic adoptive transfer of T cells into T-cell knockout mice, we observed that immune reconstitution resulted in a profound loss in BMD [16]. Importantly, in this animal model, bone loss was prevented by zoledronic acid (ZOL), a potent, long-acting antiresorptive, administered before T-cell transfer [16].
In the current report, we tested the efficacy of ZOL in preventing ART-induced bone loss in treatment-naive HIV-infected patients initiating ART. We hypothesized that the preponderance of bone loss in this setting would occur during early period of therapy when T-cell recovery is most pronounced, providing an exploitable window for preemptive intervention to mitigate ART-induced bone resorption and preserve natural bone in this population.
METHODS
Trial Design
This phase IIb clinical trial was conducted at the Grady Infectious Diseases Program Clinic in Atlanta, Georgia, between January 2010 and January 2015. All subjects provided written informed consent, and the study was approved by the Institutional Review Board of Emory University. Investigational new drug approval was obtained from the US Food and Drug Administration for off-label use of ZOL. The study was registered at ClinicalTrials.gov (identifier NCT01228318).
Objectives
The primary objective was to evaluate whether ZOL ameliorates ART-induced bone resorption in the study population. Secondary objectives included ZOL's impact on BMD outcomes at key fracture-prone anatomical sites, and safety and clinical measures.
Participants
Viremic (HIV type 1 [HIV-1] RNA > 1000 copies/mL), treatment-naive, HIV-infected patients aged 30–50 years who were planning ART initiation, had no history of bone or active immunological disease other than HIV infection, and were in generally good health, were eligible for the study if they had serum vitamin D3 level ≥12 ng/mL and serum calcium level ≥8 mg/dL within 60 days before enrollment. Sexually active women of reproductive age were required to agree to use at least one reliable method of contraception during the study. Patients who had osteoporosis (t score < −2.5), prior or current use of antiresorptives, recent (within 6 months) or planned invasive dental procedures, active peptic ulcer disease or recent history of gastrointestinal bleed, serious systemic illness, or were pregnant or breastfeeding were excluded.
Randomization
Treatment assignments were stratified according to screening HIV-1 RNA (<100 000 or ≥100 000 copies/mL), age (30–39 or 40–49 years), and sex, and were generated using a pseudo-random-number generator with permuted blocks for each of the 8 levels of stratification. The unblinded study pharmacist maintained 8 color-coded sets of sealed, sequenced, opaque envelopes containing the treatment assignment. Each envelope uniquely identified each stratum and the sequence number. All other individuals involved in the study were blinded to the randomization, with the exception of the data coordinating center biostatisticians.
Interventions
At entry, participants initiated ART per standard of care with standard doses of atazanavir/ritonavir + TDF/emtricitabine (FTC) [17]. ART change was allowed after study entry in the case of drug intolerance or virologic failure. On the same day of ART initiation, participants also received a single intravenous infusion of ZOL (5 mg per 100 mL ready-to-infuse solution), if assigned to the ZOL arm, or a single infusion of placebo (220 mg mannitol and 24 mg sodium citrate per 100 mL ready-to-infuse solution), if assigned to the placebo arm.
Follow-up
Study outcomes were assessed at baseline and at study weeks 12, 24, 36, and 48. The study was unblinded when the last enrolled participant completed the 24-week visit. Clinical and safety laboratory tests were performed at week 2, week 12, and every 3 months thereafter.
Outcome Measures
Blood samples for biomarkers of bone turnover were processed within 60 minutes of collection, and plasma was separated by centrifugation and frozen at −80°C until analysis. Commercial enzyme-linked immunosorbent assays from Immunodiagnostic Systems, (Scottsdale, Arizona) were used according to the manufacturer's instructions [18, 19] to quantify plasma C-terminal telopeptide of collagen (CTx) and osteocalcin, sensitive and specific markers of bone resorption and bone formation, respectively. BMD was assessed using a Lunar prodigy scanner (GE Lunar, Madison, Wisconsin) dual-energy X-ray absorptiometry (DXA) machine and Encore Software, version 2010 13.31, at Emory University Hospital. Osteopenia was defined as t scores between −1.0 and −2.5, and osteoporosis as t scores < −2.5 per World Health Organization criteria [20]. Clinical and safety laboratory tests were performed at a Clinical Laboratory Improvement Amendments–adherent laboratory. Safety reports were generated by the data coordinating center every year and reviewed by an independent medical safety monitor.
Sample Size and Power Considerations
Pilot data from a study of treatment-naive HIV-infected patients on therapy for 24 weeks with lopinavir/ritonavir + TDF/FTC [14] form the basis for estimating sample size. Assuming an increase on average of 1.2 µg/L for CTx in the active placebo arm and on average no change in the ZOL arm and an estimated standard deviation in each group of 1.4 µg/L, a sample size of 30 patients per treatment arm achieves 90% power to detect a treatment difference of 1.2 µg/L in CTx between active placebo and ZOL at 24 weeks if the true difference between treatments is 1.2 µg/L (2-sided, 2-sample equal-variance t test and α = .05).
Statistical Analysis
The primary analyses of the data were performed on an intention-to-treat basis, and data from all randomized participants were included in the final analysis. Time to viral suppression was analyzed using Kaplan–Meier curves and compared between treatment arms using log-rank tests. Repeated-measures analyses of CTx, osteocalcin, and BMD (lumbar spine, mean of right and left measurements for hip and femoral neck) were performed with a means model using SAS version 9 (Proc Mixed, mixed linear models) providing separate estimates of the means by time on study (baseline and weeks 12 [range, 6–16], 24 [20–28], and 48 [44–52]) and treatment arm. Clinical visits for CD4 T-cell count included baseline and 4, 16, 24, 36, and 48 weeks. The same model was used to analyze percentage change from baseline for CTx, osteocalcin, and BMD. Each model included 3 predictors (treatment arm, time on study, and the statistical interaction between treatment arm and time on study). A compound-symmetric variance-covariance form in repeated measurements was assumed for each outcome, and robust estimates of the standard errors of parameters were used to perform statistical tests and construct 95% confidence intervals (CIs) [21]. The model-based means are unbiased with unbalanced and missing data, so long as the missing data are noninformative (missing at random), and t tests were used to compare the differences between the model-based treatment means (least-squares means) at each time point and to compare differences over time within each treatment arm. Specific statistical tests were done within the framework of the mixed-effects linear model. All statistical tests were 2-sided and unadjusted for multiple comparisons. A value of P < .05 indicated statistical significance. The study was designed as a fixed-sample size study and no formal interim analyses were performed for safety and efficacy. Statistical stopping boundaries were not established as an aide to early stopping of the study. Monthly data reports were generated to summarize the timeliness and completeness of expected study case report forms. Monthly data quality checks and data queries were generated when creating analytic data sets used to generate monthly safety reports and monthly summary statistics. Each of 36 solicited adverse events was counted only once per patient as the most severe level reported across the 4 study visits during the 48-week follow-up period and compared between treatment arms with a χ2 or Fisher exact test. Statistical analyses were limited to the 20 most commonly reported adverse effects (AEs) after excluding those symptoms reported as mild.
RESULTS
Demographic and Clinical Characteristics
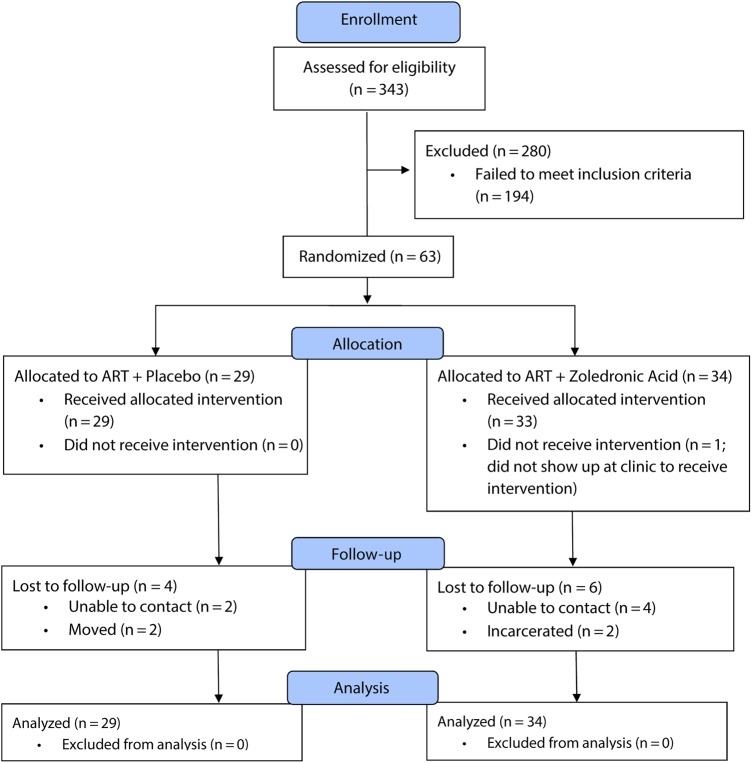
A total of 343 patients were assessed for eligibility (Figure 1), and 63 were randomized to receive either ZOL (n = 34) or active placebo (n = 29). Demographic and clinical characteristics were comparable between the 2 study arms (Table 1).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram. The progress through the phases (enrollment, intervention allocation, follow-up, and data analysis) of a double-blind, randomized controlled trial in nonosteoporotic, viremic, antiretroviral therapy (ART)–naive, human immunodeficiency virus-infected adults comparing a single zoledronic acid (5 mg) infusion at the time of ART initiation with active placebo infusion is shown.
Table 1.
Baseline Demographic and Clinical Characteristics by Treatment
| Characteristic | ART + PL (n = 29) | ART + ZOL (n = 34) |
|---|---|---|
| Age, y, mean (SD) | 39.4 (6.9) | 39.7 (6.6) |
| Sex, No. (%) | ||
| Male | 23 (79.3) | 27 (79.4) |
| Female | 6 (20.7) | 7 (20.6) |
| Race | ||
| White | 3 (10.3) | 7 (20.6) |
| Black | 26 (89.7) | 27 (79.4) |
| History of smoking | ||
| Yes | 26 (89.7) | 24 (70.6) |
| No | 3 (10.3) | 10 (29.4) |
| Current smoking | ||
| Yes | 23 (79.3) | 19 (55.9) |
| No | 6 (20.7) | 15 (44.1) |
| Cigarettes smoked per day (in patients with history of smoking) | 7.6 (4.5) | 7.5 (6.0) |
| Years of cigarette smoking (in patients with history of smoking) | 13.4 (8.1) | 13.9 (9.6) |
| Alcohol use in past 30 d | ||
| Daily | 1 (3.4) | 2 (5.9) |
| 5–6 times/wk | 0 (0) | 1 (2.9) |
| 3–4 times/wk | 1 (3.4) | 4 (11.8) |
| 1–2 times/wk | 8 (27.6) | 3 (8.8) |
| 2–3 times/mo | 2 (6.9) | 2 (5.9) |
| Once/mo | 3 (10.3) | 8 (23.5) |
| Never | 14 (48.3) | 14 (41.2) |
| Baseline osteopenia in any areaa | ||
| Yes | 10 (34.5) | 7 (21.9) |
| No | 19 (65.6) | 25 (78.1) |
| Baseline lumbar spine BMD, g/cm3 | 1.23 (0.14) | 1.29 (0.14) |
| Baseline lumbar spine BMD t score | 0.16 (1.16) | 0.67 (1.22) |
| History of bone fracture | ||
| Yes | 5 (17.2) | 10 (29.4) |
| No | 24 (82.8) | 24 (70.6) |
| HIV-1 RNA, log10 copies/mL (SD) | 4.81 (0.96) | 5.26 (0.44) |
| CD4+ count, cells/uL (SD) | 155 (145) | 102 (69) |
| Serum calcium, mg/dL (SD) | 9.3 (0.4) | 9.1 (0.4) |
| Serum vitamin D, ng/mL (SD) | 27.8 (10.0) | 28.1 (11.7) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; BMD, bone mineral density; HIV, human immunodeficiency virus; PL, active placebo; SD, standard deviation; ZOL, zoledronic acid.
a Osteoporotic patients not enrolled in the study. Baseline dual-energy X-ray absorptiometry measurements for 2 patients were performed with a different machine and were not included in the analyses.
ZOL Blunted ART-Induced Bone Resorption
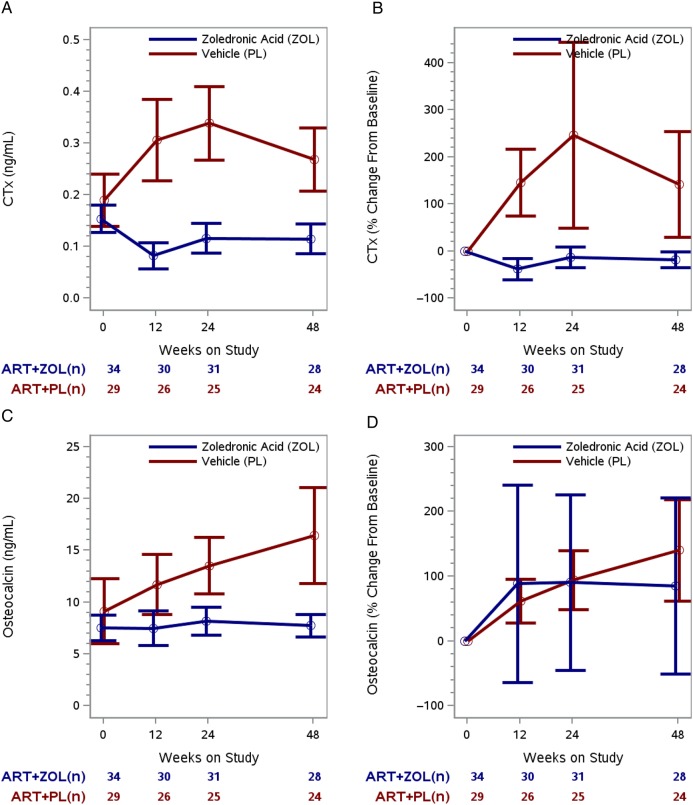
CTx in the treatment arms changed in significantly different ways (ie, different temporal patterns over time) during the 48 weeks of follow-up (P < .001, test for interaction between time on study and treatment arm). Mean CTx was similar in both treatment arms at randomization (0.154 ng/mL vs 0.190 ng/mL for ZOL vs placebo, respectively; P = .22) but became significantly lower in the ZOL arm at 12 weeks (0.083 ng/mL vs 0.305 ng/mL; P < .001), 24 weeks (0.117 ng/mL vs 0.338 ng/mL; P < .001), and 48 weeks (0.116 ng/mL vs 0.269 ng/mL; P < .001; Figure 2A). Treatment with ZOL led to a 73% reduction in bone resorption relative to placebo at 12 weeks (CTx mean difference = −0.222 ng/mL [95% CI, −.306 to −.139]), with a 65% and 57% relative reduction at 24 weeks (mean difference, −0.221 ng/mL [95% CI, −.300 to −.145]) and 48 weeks (mean difference, −0.153 ng/mL [95% CI, −.221 to −.085]). The CTx mean percentage increase from baseline to 12, 24, and 48 weeks was 145%, 244%, and 140%, respectively, in the placebo arm. The CTx mean percentage decrease from baseline to 12, 24, and 48 weeks was 39%, 13%, and 18%, respectively, in the ZOL arm (Figure 2B; Table 2).
Figure 2.
Longitudinal change in bone resorption outcomes by treatment arm. A, Model-based mean longitudinal changes in C-terminal telopeptide of collagen (CTx) by treatment arm and weeks on study. B, Model-based mean CTx percentage change from baseline by treatment arm and weeks on study. C, Model-based mean longitudinal changes in osteocalcin by treatment arm and weeks on study. D, Model-based mean osteocalcin percentage change from baseline by treatment arm and weeks on study. For each of the 4 panels, the vertical bars are the 95% confidence intervals and the numbers below the time points signify the number of subjects in each treatment group at each time interval. Abbreviations: ART, antiretroviral therapy; PL, active placebo; ZOL, zoledronic acid.
Table 2.
Baseline-Adjusted Means at 48 Weeks of Follow-up for Bone Resorption and Bone Mineral Density Outcomes by Treatment Arm
| Variables | Treatment | No. | Adjusted Mean (95% CI)a | Mean Difference (95% CI) | P Value |
|---|---|---|---|---|---|
| CTx, ng/mL | ART + ZOL | 28 | 0.126 (.021–.231) | −0.215 (−.374 to .056) | .0118 |
| ART + PL | 24 | 0.341 (.222–.460) | |||
| Osteocalcin, ng/mL | ART + ZOL | 28 | 8.752 (3.077–14.426) | −8.739 (−17.634 to .155) | .0536 |
| ART + PL | 24 | 17.491 (11.003–23.978) | |||
| Lumbar spine, g/cm2 | ART + ZOL | 26 | 1.268 (1.247–1.289) | 0.072 (.042 to .102) | <.0001 |
| ART + PL | 23 | 1.196 (1.175–1.217) | |||
| Lumbar spine t score | ART + ZOL | 26 | 0.471 (.298–.644) | 0.589 (.337 to .840) | <.0001 |
| ART + PL | 23 | −0.118 (−.295 to .059) | |||
| Lumbar spine z score | ART + ZOL | 26 | −0.284 (−.481 to −.088) | 0.449 (.165 to .733) | .0027 |
| ART + PL | 23 | −0.734 (−.934 to −.533) | |||
| Hip, g/cm2 | ART + ZOL | 26 | 1.066 (1.052–1.079) | 0.040 (.021 to .060) | .0001 |
| ART + PL | 23 | 1.025 (1.011–1.039) | |||
| Hip t score | ART + ZOL | 26 | −0.027 (−.151 to .097) | 0.311 (.133 to .489) | .0010 |
| ART + PL | 23 | −0.337 (−.465 to −.210) | |||
| Hip z score | ART + ZOL | 26 | −0.840 (−.965 to −.716) | 0.268 (.090 to .445) | .0041 |
| ART + PL | 23 | −1.108 (−1.235 to −.981) | |||
| Femoral neck, g/cm2 | ART + ZOL | 26 | 1.061 (1.044–1.077) | 0.036 (.013 to .060) | .0035 |
| ART + PL | 23 | 1.024 (1.008–1.041) | |||
| Femoral neck t score | ART + ZOL | 26 | 0.106 (−.034 to 0.245) | 0.318 (.119 to .517) | .0025 |
| ART + PL | 23 | −0.212 (−.354 to −.070) | |||
| Femoral neck z score | ART + ZOL | 26 | −0.641 (−.783 to −.499) | 0.216 (.014 to .419) | .0371 |
| ART + PL | 23 | −0.857 (−1.002 to −.712) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CTx, C-terminal telopeptide of collagen; PL, active placebo; ZOL, zoledronic acid.
a Adjusted mean defined as the predicted response value obtained by fitting the regression equation for each treatment arm at the mean baseline value for the 2 treatment arms, and estimated using analysis of covariance at 48 weeks for each outcome.
Bone Formation Was Not Affected by ZOL
Osteocalcin levels in the treatment arms were consistently different (P < .001), with the placebo arm having significantly higher serum osteocalcin levels than ZOL arm at each time point except baseline. Mean difference in osteocalcin between the arms pooled over the 48-week follow-up period was −4.8 ng/mL (95% CI, −7.4 to −2.2 ng/mL; Figure 2C). Mean difference at 12 weeks, 24 weeks, and 48 weeks was −4.2 ng/mL (95% CI, −7.6 to −.8 ng/mL), −5.3 ng/mL (95% CI, −8.4 to −2.3 ng/mL), and −8.1 ng/mL (95% CI, −12.9 to −3.3 ng/mL), respectively. The osteocalcin mean percentage increases from baseline to 12 and 48 weeks were 61% (95% CI, 27% to 95%) and 137% (95% CI, 58% to 216%), respectively, in the placebo arm (Figure 2D). Osteocalcin did not change from baseline to 48 weeks in the ZOL arm (P = .22).
ZOL Prevented ART-Induced BMD Loss
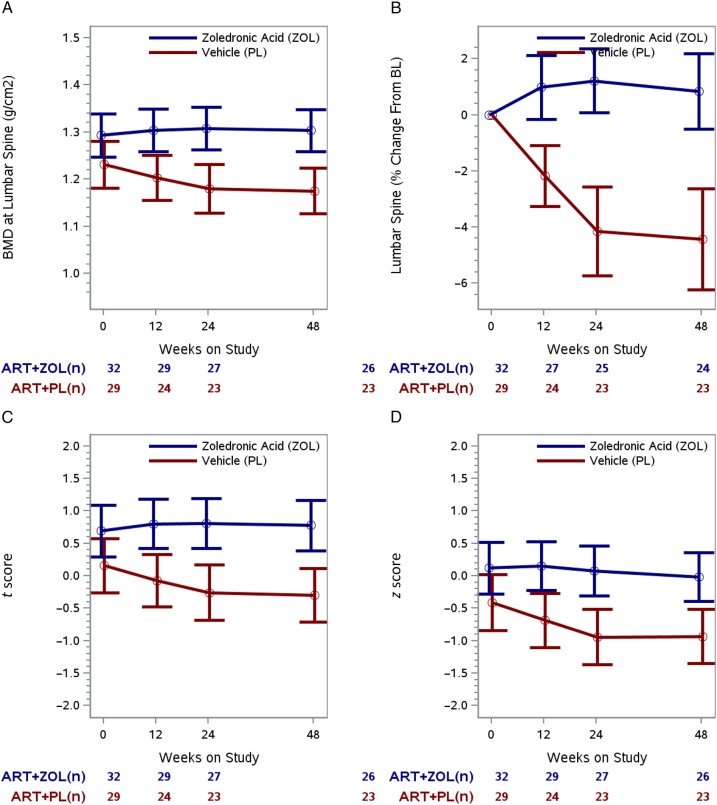
Lumbar spine BMD in the treatment arms changed in significantly different ways (ie, different temporal patterns over time) during the 48 weeks of follow-up (P < .001, test for interaction between time on study and treatment arm). Mean lumbar spine was similar in both treatment arms at randomization (P = .08) but became significantly higher in the ZOL arm at 12 weeks (1.304 g/cm2 vs 1.203 g/cm2; P = .003), 24 weeks (1.307 g/cm2 vs 1.179 g/cm2; P < .001), and 48 weeks (1.303 g/cm2 vs 1.175 g/cm2; P < .001; Figure 3A). ZOL led to an 8% increase in lumbar spine BMD at 12 weeks relative to placebo (mean difference, 0.101 g/cm2 [95% CI, .034 to .167 g/cm2]), with an 11% increase at 24 weeks (mean difference, 0.128 g/cm2 [95% CI, .059 to .197 g/cm2]). The mean difference at 48 weeks remained 0.128 g/cm2 (11% increase relative to placebo). BMD at the lumbar spine did not change from baseline to 48 weeks in the ZOL arm (mean percentage change, +0.9% [95% CI, −.47% to 2.19%]; P = .20), but decreased −4.4% (95% CI, −6.21% to −2.63%; P < .001) in the placebo arm (Figure 3B). Similar trends were observed for BMD in the hip and femoral neck (Supplementary Figure 1).
Figure 3.
Longitudinal change in lumbar spine bone mineral density (BMD) outcomes by treatment arm. A, Model-based mean longitudinal changes in BMD at the lumbar spine by treatment arm and weeks on study. B, Model-based mean BMD at the lumbar spine percentage change from baseline (BL) by treatment arm and weeks on study. C, Model-based mean longitudinal changes in lumbar spine t scores by treatment arm and weeks on study. D, Model-based mean longitudinal changes in lumbar spine z scores by treatment arm and weeks on study. For each of the 4 panels, the vertical bars are the 95% confidence intervals and the numbers below the time points signify the number of subjects in each treatment group at each time interval. Abbreviations: ART, antiretroviral therapy; PL, active placebo; ZOL, zoledronic acid.
ZOL Treatment Did Not Impact the Rate of Virologic Suppression or Immunologic Response
Supplementary Figure 2A summarizes the cumulative initial virologic suppression by treatment arm (P = .24, log-rank test). By 48 weeks, initial virologic suppression was 97% in the ZOL arm and 84% in the placebo arm. CD4 T-cell counts in the 2 treatment arms increased over time (P < .001). Neither the pattern of change (P = .63) nor the difference between treatment arms was significant (P = .25; Supplementary Figure 2B). The week 48 mean (±SEM) CD4 T-cell count was 270 ± 24 cells/µL and 311 ± 40 cells/µL for the ZOL and placebo arms, respectively (P = .38).
Serious Adverse Effects, Adverse Effects, and Laboratory Toxicities
No serious adverse effects (SAEs) was reported to be possibly or definitively related to ZOL treatment. SAEs were similar between the ZOL and the placebo arms. Three patients in each arm were hospitalized during the 48 weeks of follow-up. Supplementary Table 2 summarizes patient-reported AEs by treatment arm. There were no statistically significant differences in the rates of moderate or severe diarrhea, weight loss, rash, insomnia, and myalgia between the ZOL and placebo arm. Moderate or severe dyspepsia was more frequent in the placebo arm (P = .04). There were no statistically significant differences between the 2 treatment arms for the other 14 self-reported AEs. Supplementary Table 3 summarizes laboratory toxicities. There were no statistically significant differences between treatment arms for the incidence of any grade 3 or higher laboratory toxicities during 48 weeks of follow-up.
DISCUSSION
ART initiation led to an early surge in bone resorption in patients randomized to the placebo arm. A compensatory increase in bone formation was noted that may have tempered the extent of bone loss in some patients. Nevertheless, the net effect of these changes in biomarkers of bone turnover was an expected significant loss of BMD in the placebo arm. Importantly, however, we demonstrated that ART-induced bone loss can be successfully prevented with an antiresorptive. Specifically, the heightened bone resorption following ART initiation was completely blunted by ZOL, resulting in durable BMD preservation at fracture-prone sites that lasted through 48 weeks. These findings corroborate our earlier animal studies in which ZOL prophylaxis completely ablated immune reconstitution–induced bone loss following T-cell adoptive transfer in immunocompromised T-cell knockout mice [16].
These findings are relevant for several reasons. First, the magnitude of bone loss observed with almost all ART regimens during the relatively short study period approaches, if not exceeds, that seen in the first years of the archetypal fragility bone disease of postmenopausal osteoporosis [22]. Second, this significant bone loss is occurring in a population with compromised skeletal reserve, likely contributing to the increased fracture rates observed in the aging HIV population. Finally, homeostasis between bone formation and resorption is very short lived. Shortly after peak BMD is achieved in early adulthood (20–30 years of age), resorption begins to outpace formation, leading to a steady decline in BMD with age. Once lost after early adulthood, bone is seldom recovered without pharmacological intervention. Thus, preserving natural modeled/remodeled bone, as was done in this study, is essential for optimal skeletal health over the long term.
The above-enumerated considerations underscore the significance of current efforts to preemptively preserve naturally accreted bone in the HIV population. The overall 60% lower bone resorption in the ZOL arm relative to the placebo arm and the corresponding 11% relative higher BMD at the lumbar spine observed with ZOL at 48 weeks are the largest reported effect sizes for any prophylactic intervention directed at ameliorating ART-induced bone loss. At 48 weeks, supplementation with vitamin D and calcium carbonate in treatment-naive, HIV-infected patients in a recent study resulted in a relative treatment difference in total hip BMD of 1.86% [23], while in the A5303 study, the use of a TDF-sparing ART regimen was associated with relative treatment differences in total hip and lumbar spine BMD of 0.89% and 1.47%, respectively [9].
Although modern-era ART regimens induce less bone loss, they are, however, not completely innocuous to the skeleton [5–10]. As an example, in the report by Sax et al [10], 16% and 27% of patients treated with TAF-containing regimens sustained >3% loss in BMD at the hip and lumbar spine, respectively, within 48 weeks of ART therapy. These findings suggest that there are subsets of HIV-infected patients for whom bone loss prophylaxis will be beneficial following ART initiation, regardless of the regimen.
Furthermore, ZOL had no suppressive effect on bone formation and resulted in negligible overall change in BMD in our study. This relative lack of effect on formation with a single dose of ZOL is important, as remodeled bone that occurs with the prolonged use of bisphosphonates is paradoxically susceptible to microcracks [24, 25]. ZOL at a single dose was safe and well tolerated, and resulted in comparable rate of virologic suppression and similar magnitude of CD4 T-cell reconstitution.
Our study was a proof-of-concept phase IIb study with a small sample size, conducted at a single site, and thus is subject to several limitations. The study population was relatively homogenous, reflecting the demography of our clinic population with a predominance of African American men, thereby limiting the generalizability of our findings. Furthermore, the 48-week study duration could not evaluate the impact of our intervention on long-term bone outcomes.
These limitations notwithstanding, a single infusion of ZOL at the time of ART initiation mitigated ART-induced bone resorption and prevented bone loss in nonosteoporotic, HIV-infected patients. These effects were observed as early as 12 weeks, and persisted through 48 weeks, the period when ART-induced bone loss is most intense. These data define an optimal window for a preemptive intervention to forestall ART-induced bone loss and provide robust information needed to guide the design and implementation of larger confirmatory phase 3, multicenter randomized clinical trials.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The authors are grateful to all the patients who participated in this study. We also thank Allison Ross Eckard in the Departments of Pediatrics and Medicine, Division of Infectious Diseases, at the Medical University of South Carolina, for service as the medical safety monitor.
Author contributions. I. O. and M. N. W. conceived the original idea with contribution from J. L. L.; I. O. wrote the first draft with contribution from M. N. W. and K. A. E.; K. T. and A. V. performed all laboratory assays and contributed to data analysis; K. A. E., L. W., and A. K. were responsible for data management and statistical analysis; J. L. L., C. D. L., A. F., S. E. S., and A. N. S. were part of the investigating team and were involved with outcomes and safety monitoring and manuscript development. All authors read the current version of the manuscript.
Disclaimer. The National Institutes of Health (NIH) and Novartis did not play any additional role in the study other than financial support.
Financial support. The study was sponsored by the NIH, and zoledronic acid and its active placebo were provided by Novartis. Research reported in this publication was supported by the National Institute on Aging (award number R01AG040013) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (award numbers R01AR059364 and R01AR068157 to M. N. W. and I. O.). M. N. W. is also supported by a grant from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development (5I01BX000105) and by NIAMS (award number R01AR056090). M. N. W. is also supported by NIAMS (award number R01AR053898). C. D. L. is also supported by National Center for Advancing Translational Sciences of the NIH (award numbers UL1TR000454 and KL2TR000455). A. N. S. is also supported by the National Institute for Allergy and Infectious Diseases (NIAID) (award number 1K23AI114407). The authors gratefully acknowledge services provided by the Emory Center for AIDS Research funded though the NIAID (award number P30AI050409) and the Atlanta Clinical and Translational Science Institute, funded though the National Center for Advancing Translational Sciences (award number UL1TR000454).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown TT, Hoy J, Borderi M et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis 2015; 60:1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Carpio-Cano FE, Dela Cadena RA, Sawaya BE. HIV and bone disease: a perspective of the role of microRNAs in bone biology upon HIV infection. J Osteoporos 2013; 2013:571418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallant JE, Staszewski S, Pozniak AL et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292:191–201. [DOI] [PubMed] [Google Scholar]
- 5.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr 2009; 51:554–61. [DOI] [PubMed] [Google Scholar]
- 6.Duvivier C, Kolta S, Assoumou L et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 2009; 23:817–24. [DOI] [PubMed] [Google Scholar]
- 7.Brown TT, Moser C, Currier JS et al. Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis 2015; 212:1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tebas P, Kumar P, Hicks C et al. Greater change in bone turnover markers for efavirenz/emtricitabine/tenofovir disoproxil fumarate versus dolutegravir + abacavir/lamivudine in antiretroviral therapy-naive adults over 144 weeks. AIDS 2015; 29:2459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taiwo BO, Chan ES, Fichtenbaum CJ et al. Less bone loss with maraviroc- versus tenofovir-containing antiretroviral therapy in the AIDS Clinical Trials Group A5303 Study. Clin Infect Dis 2015; 61:1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sax PE, Wohl D, Yin MT et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015; 385:2606–15. [DOI] [PubMed] [Google Scholar]
- 11.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 2008; 93:3499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prieto-Alhambra D, Guerri-Fernandez R, De Vries F et al. HIV infection and its association with an excess risk of clinical fractures: a nationwide case-control study. J Acquir Immune Defic Syndr 2014; 66:90–5. [DOI] [PubMed] [Google Scholar]
- 13.Amorosa V, Tebas P. Bone disease and HIV infection. Clin Infect Dis 2006; 42:108–14. [DOI] [PubMed] [Google Scholar]
- 14.Ofotokun I, Titanji K, Vunnava A et al. Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS 2016; 30:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco JM, Rubio A, Martinez-Moya M et al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood 2002; 99:3702–6. [DOI] [PubMed] [Google Scholar]
- 16.Ofotokun I, Titanji K, Vikulina T et al. Role of T-cell reconstitution in HIV-1 antiretroviral therapy-induced bone loss. Nat Commun 2015; 6:8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lennox JL, Landovitz RJ, Ribaudo HJ et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med 2014; 161:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Immunodiagnostic Systems Inc. C-terminal telopeptide of Collagen Crosslaps ELISA. Serum CrossLaps ELISA - Oxford Biosystems. http://www.idsplc.com/products/serum-crosslaps-ctx-i-elisa-2/. Accessed 20 October 2015.
- 19.Immunodiagnostic Systems Inc. Fountain Hills, AZ: Osteocalcin N-mid ELISA. http://www.antibodies-online.com/kit/368354/N-MID+Osteocalcin+ELISA/. Accessed 31 May 2016.
- 20.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002; 359:1929–36. [DOI] [PubMed] [Google Scholar]
- 21.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford, UK: Clarendon Press, 1994:68–77. [Google Scholar]
- 22.Riggs BL, Khosla S, Melton LJ 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 2002; 23:279–302. [DOI] [PubMed] [Google Scholar]
- 23.Overton ET, Chan ES, Brown TT et al. Vitamin D and calcium attenuate bone loss with antiretroviral therapy initiation: a randomized trial. Ann Intern Med 2015; 162:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med 2008; 358:1304–6. [DOI] [PubMed] [Google Scholar]
- 25.Shane E. Evolving data about subtrochanteric fractures and bisphosphonates. N Engl J Med 2010; 362:1825–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.