As predicted by the Red Queen hypothesis, pneumococcal serotype diversity initially increased and subsequently decreased after introduction of the 7- and 13-valent pneumococcal conjugate vaccines. This finding was supported using clinical data from studies conducted in the United States and Europe.
Keywords: serotype replacement, vaccination, evolution, children
Abstract
Background. The Red Queen hypothesis is an evolutionary theory that describes the reciprocal coevolution of competing species. We sought to study whether introduction of the 7- and 13-valent pneumococcal conjugate vaccines (PCV7 and PCV13, respectively) altered pneumococcal serotype dynamics among children with invasive pneumococcal disease (IPD) as predicted by the Red Queen hypothesis.
Methods. This study examined pneumococcal isolates (n = 641) obtained from children <18 years of age hospitalized with IPD from 1997 to 2014 in Utah. A review of the literature also identified several additional studies conducted in the United States and Europe that were used to test the external generalizability of our Utah findings. Simpson's index was used to quantify pneumococcal serotype diversity.
Results. In Utah, the introduction of PCV7 and PCV13 was associated with rapid increases in serotype diversity (P < .001). Serotypes rarely present before vaccine introduction emerged as common causes of IPD. Diversity then decreased (P < .001) as competition selected for the fittest serotypes and new evolutionary equilibriums were established. This pattern was also observed more broadly in the United States, the United Kingdom, Norway, and Spain.
Conclusions. This vaccine-driven example of human/bacterial coevolution appears to confirm the Red Queen hypothesis, which reveals a limitation of serotype-specific vaccines and offers insights that may facilitate alternative strategies for the elimination of IPD.
To achieve and maintain dominance, pathogenic bacterial strains evolve at both the individual and population levels to cope with changes in human immunity. Vaccines alter bacterial strain dynamics by exerting selective pressure through the host immune system that may drive the emergence of new dominant strains. Protein conjugate vaccines targeting 7 and 13 of more than 90 specific Streptococcus pneumoniae serotypes were licensed for use in the United States in 2000 and 2010, respectively (PCV7 and PCV13, Wyeth/Pfizer). These vaccines were recommended for the universal immunization of children aged <2 years, with catch-up immunization for children <5 years. After the introduction of PCV7, disease attributable to vaccine serotypes declined dramatically [1]. Simultaneously, reports emerged of more frequent disease caused by serotypes not targeted by the vaccine (nonvaccine serotypes) [2, 3].
Recently, Jefferies et al proposed that serotype-specific pneumococcal vaccination strategies may lead to the reciprocal coevolution of human hosts and pneumococci [4]. This model of coevolution is analogous to the arms race between pathogenic bacteria and antimicrobials, in which resistance develops as an adaptive mutation in response to antimicrobial exposure [5]. Jefferies et al postulated that the coevolutionary cycle between pneumococci and their human hosts resembles Van Valen's “Red Queen dynamics” [6], in which continuing adaptation is required for a species to maintain its relative fitness among the other species with which it is coevolving [4].
Cobey and Lipsitch developed a dynamic individual-based mathematical model that demonstrated that immunization resulted in rapid replacement of vaccine serotypes, which in many of the modeled scenarios was associated with a brief increase in serotype diversity [7]. Moreover, their model predicted that a short-term increase in serotype diversity would occur following PCV7 introduction, which would then be followed by a sustained decrease in diversity.
We hypothesized that pneumococcal conjugate vaccine (PCV) administration would exert a strong selective pressure that would alter the distribution and diversity of pneumococcal serotypes, particularly in young children targeted to receive the vaccine. We sought to examine this hypothesis in a real-world clinical setting through investigation of the dynamics of pneumococcal serotype evolution among Utah children with invasive pneumococcal disease (IPD) from 1997 to 2014, which included the introduction of 2 different pneumococcal vaccines into the childhood immunization schedule. We then sought to externally validate our hypothesis using published data from other geographic regions by applying ecological measures of diversity.
METHODS
Ethics Statement
Approval for this study was obtained and a waiver of informed consent was granted from the institutional review boards of the University of Utah and Primary Children's Hospital (PCH).
Setting and Study Population
PCH is a 282-bed children's hospital that serves as the community hospital for Salt Lake County, Utah, and a tertiary referral center for neighboring states. All isolates obtained from Utah resident children aged <18 years with IPD at PCH were included. Data were analyzed for all children, as well as subgroups including children aged <2 years, the target population for PCV; children aged 2–4 years who were included in PCV catch-up recommendations; and children aged ≥5 years who were not recommended for PCV immunization.
Study Periods
The 18-year Utah study included 4 years (1997–2000) before the licensure of PCV7 (pre-PCV7). The PCV7 period included 10 years (2001–2010), which we divided into the early PCV7 period (2001–2004) during which PCV7 immunization coverage increased from 0% to 70% and the late PCV7 period (2005–2010) when vaccine coverage was >80% for Utah children. Finally, we analyzed data following the licensure of PCV13 (2011–2014). In this period, PCV13 largely replaced PCV7 in Utah and during this period the 3-dose and 4-dose series PCV immunization rates among Utah children were 91.5% and 80.1%, respectively.
Microbiologic Selection Criteria and Pneumococcal Serotyping
Culture-confirmed IPD was defined as an illness with S. pneumoniae isolated from a normally sterile site (eg, blood, cerebrospinal fluid, pleural fluid, joint fluid, peritoneal fluid, or abscess). As described previously, all pneumococcal isolates were serotyped (E. O. M.) using the capsular swelling technique [3]. There were no changes in the definition or methods used to identify or serotype IPD during the study period.
Measurement of Diversity
We evaluated pneumococcal serotype diversity using Simpson's index of diversity (D), which is a quantitative measure originally developed for ecological evaluations of species diversity [8]. The measurement calculates the probability that 2 individuals (in this study, pneumococcal isolates) selected at random, with replacement, within a given time period will belong to different groups (serotypes). D values range from 0 to 1, with larger values indicating greater diversity (Supplementary Materials).
Supplemental Data Sources
As the epidemiology of pediatric IPD in Utah has been recognized to be distinct from that of the majority of the United States [9], we sought to explore the generalizability of our findings by calculating Simpson's D for published studies that were identified by the following criteria: pneumococcal isolates obtained from a sterile site matching our case definition of IPD, the population included children aged <18 years, the most frequent isolates were reported in a line list with the frequency noted per year or by study period (pre-PCV7, early PCV7, late PCV7, early PCV13), and ≥50 isolates had to be serotyped and reported. Nontypeable isolates were excluded from all analyses. When studies included serotype distributions for multiple age groups, we included results for all pediatric subpopulations (eg, <2 years, <5 years). As previously reported by Cobey and Lipsitch [7], we permitted inconsistent data collection methods to retain statistical power.
Statistical Analyses
The purpose of this study was to assess whether the distribution of pneumococcal serotypes was altered following the introduction of PCV. Segmented regression analyses were performed to compare the proportion of IPD attributable to PCV7 and PCV13 serotypes before and after each vaccine's introduction. The Durbin-Watson statistic was calculated to test for serial autocorrelation of the error terms in the regression models. All models had a Durbin-Watson statistic value close to the preferred value of 2 and therefore the models were not adjusted to account for autocorrelation. Differences in diversity were assessed using Simpson's D, as described above. We used a nonparametric estimator to derive 95% confidence intervals for Simpson's D values using methods developed by Zhang and Zhou [10]. To test whether estimates of Simpson's D were significantly different between the pre- and post-PCV periods, P values were calculated using the Welch t test [11]. All comparisons were performed 2-sided with P < .05 set as the statistical significance threshold using R software version 3.2.3. (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Serotype Distribution in Utah
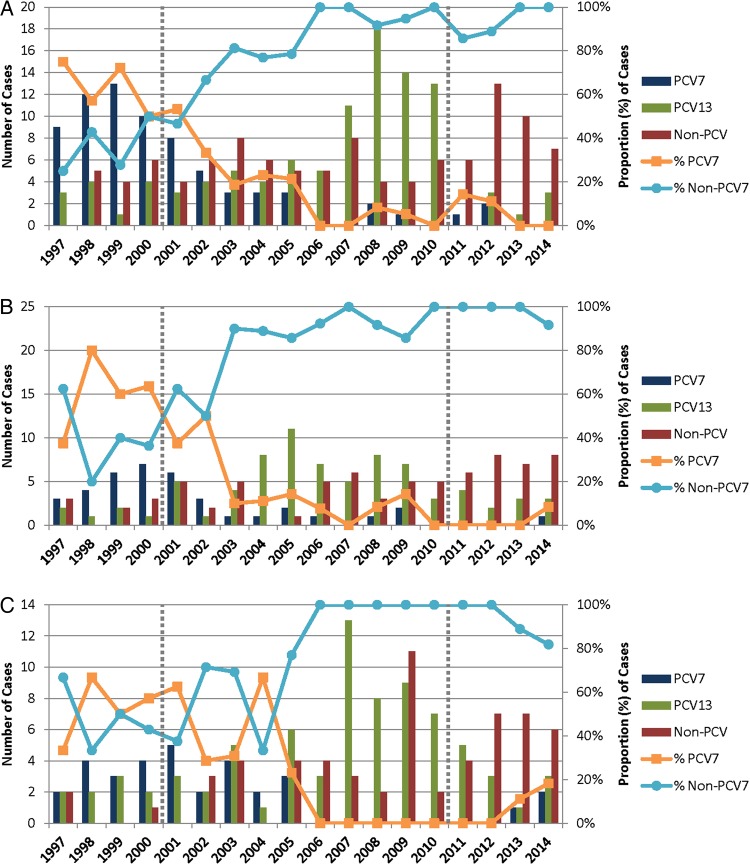
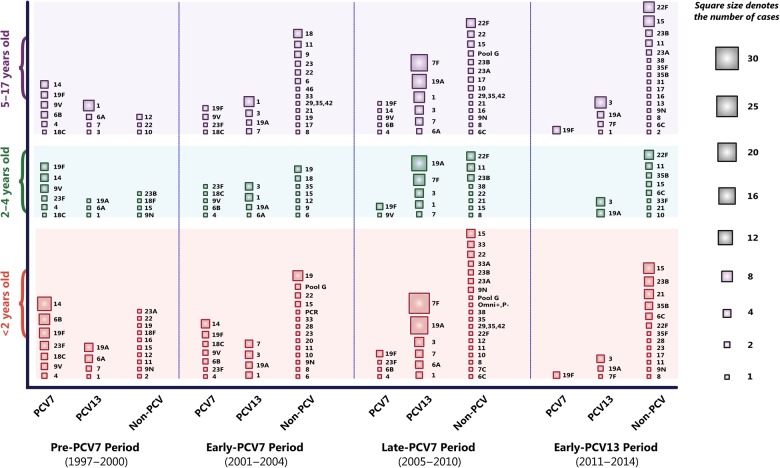
From 1997 to 2014, 641 pneumococcal isolates were identified and serotyped from Utah children with IPD. The proportion of pediatric IPD attributable to PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) declined from 59% in the pre-PCV7 (1997–2000) period to 6% by the late PCV7 period (2005–2010) (P < .001) (Figure 1 and Supplementary Table 1). A similar pattern was observed following the introduction of PCV13 in 2010. In the late PCV7 period, serotypes exclusive to PCV13 (not included in PCV7; 1, 3, 5, 6A, 7F, and 19A) caused 61% of pediatric IPD. In the 4 years following PCV13 licensure and inclusion in the childhood vaccine schedule, the proportion of PCV13-exclusive serotypes in pediatric IPD declined to 24% (P < .001) (Supplementary Table 2). Serotype data for all periods are featured in Figure 2.
Figure 1.
Pneumococcal serotypes associated with children hospitalized with invasive pneumococcal disease, according to 7- and 13-valent pneumococcal conjugate vaccine (PCV7 and PCV13, respectively) serotype status and year of isolation. PCV7 licensure in 2000 and PCV13 licensure in 2010 are denoted by dotted vertical gray lines. Graphs are presented by age group: <2 years (A), 2–4 years (B), and 5–17 years (C).
Figure 2.
Streptococcus pneumoniae serotype distribution by age group among children hospitalized with invasive pneumococcal disease prior to the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) (1997–2000), during a period of increasing PCV7 coverage (2001–2004), in the late PCV7 period (2005–2010) when vaccine coverage exceeded 80%, and in the early 13-valent pneumococcal conjugate vaccine (PCV13) period (2011–2014). The area of the squares represents the number of cases attributable to each serotype.
Serotype Diversity
Following the introduction of PCV7, the number of serotypes identified among IPD cases increased rapidly. A total of 23 different serotypes were detected in the pre-PCV7 period (mean, 14.3 serotypes detected per year). In the early PCV7 period, 35 different serotypes were detected (mean of 17.5 serotypes detected per year). There was also an increase in pneumococcal serotype diversity following the introduction of PCV7, which was reflected by a rise in Simpson's D from 0.920 to 0.949 (P < .001) [8]. This pattern was most pronounced among children aged <2 years, the target population for PCV7 immunization, with an increase from 0.910 to 0.952 (P < .001).
In the late PCV7 period, 32 different serotypes caused IPD (a mean of 13.2 serotypes per year). During this period, pneumococcal diversity decreased from 0.949 to 0.867 (P < .001). This resulted in lower levels of serotype diversity in the late PCV7 period compared with measurements from the pre-PCV7 period (P < .001). Both the increase and decrease in Simpson's D were most pronounced among children aged <2 years.
Within months after the introduction of PCV13, there was a decline in the 6 additional serotypes contained in PCV13, and several new non-PCV13 serotypes emerged. In this period, pneumococcal serotype diversity again increased from 0.867 to 0.921 (P < .001). As was seen following the introduction of PCV7, the largest numerical increase in Simpson's D was observed among young children.
Global Changes in the Distribution of Pneumococcal Serotypes
Recently, Moore et al reported a US Centers for Disease Control and Prevention (CDC)–sponsored study that assessed the impact of PCV13 on the incidence of IPD in the United States [12]. Using data derived from this national cohort study, we measured significant increases in serotype diversity from the late PCV7 period following the introduction of PCV13 among children aged <5 years (Simpson's D = 0.686–0.883; P < .001). We found similar results when we calculated Simpson's D for published reports from the United Kingdom, Norway, and Spain following the introduction of PCV7 and PCV13 (Table 1) [12–24].
Table 1.
Global Changes in Pneumococcal Serotype Diversity Among Children Colonized and Infected With Streptococcus pneumoniae Before, During, and After the Introduction of the 7- and 13-Valent Pneumococcal Conjugate Vaccines
| Study, First Author (Year) | Country | Study Population | Age Group | Sample Size | Simpson's D (95% CI) |
|||
|---|---|---|---|---|---|---|---|---|
| Pre-PCV7 Period | Early PCV7 Period | Late PCV7 Period | Post–PCV13 Introduction | |||||
| Black et al (2007) [13] | United States | IPD | <5 y | 519 | 0.864 (.847–.881) | 0.947 (.935–.958) | NR | NR |
| Lacapa et al (2008) [14] | United States | IPD | <5 y | 59 | 0.928 (.919–.937) | 0.940 (.896–.984) | NR | NR |
| Guevara et al (2009) [15] | Spain | IPD | <5 y | 78 | NR | 0.916 (.900–.932) | 0.857 (.789–.925) | NR |
| All ages | 318 | NR | 0.923 (.909–.937) | 0.882 (.855–.909) | NR | |||
| Perez-Trallero et al (2009) [16] | Spain | IPD | <5 y | 91 | 0.901 (.878–.924) | 0.927 (.902–.953) | 0.879 (.804–.954) | NR |
| Kaplan et al (2010) [17] | United States | IPD | <18 y | 1030 | NR | 0.841 (.808–.873) | 0.667 (.618–.716) | NR |
| Wenger et al (2010) [18] | United States | IPD | <5 y | 384 | 0.852 (.822–.883) | 0.917 (.880–.954) | 0.808 (.754–.862) | NR |
| Vestrheim et al (2010) [19] | Norway | Carriage | <5 y | 204 | 0.830 (.790–.871) | 0.940 (.922–.958) | NR | NR |
| Flasche et al (2011) [20] | United Kingdom | Carriage | <5 y | 372 | 0.891 (.878–.904) | 0.960 (.949–.971) | NR | NR |
| Richter et al (2013) [21] | United States | IPD | <5 y | 243 | 0.851 (.823–.879) | 0.900 (.843–.957) | 0.857 (.779–.934) | 0.606 (.452–.760) |
| All ages | 1431 | 0.897 (.885–.908) | 0.906 (.886–.927) | 0.841 (.810–.873) | 0.680 (.630–.729) | |||
| Guevara et al (2014) [22] | Spain | IPD | <5 y | 207 | NR | 0.893 (.869–.917) | 0.871 (.834–.907) | 0.915 (.854–.977) |
| All ages | 688 | NR | 0.930 (.919–.941) | 0.921 (.911–.932) | 0.925 (.909–.942) | |||
| Moore et al (2014)a [23] | United Kingdom | IPD | <2 y | 455 | 0.868 (.846–.891) | 0.927 (.906–.949) | 0.946 (.923–.968) | |
| All ages | 4850 | 0.927 (.923–.931) | 0.941 (.936–.947) | 0.927 (.921–.933) | ||||
| Richter et al (2014) [24] | United States | IPD and carriage | All ages | 3444 | NR | NR | 0.902 (.895–.908) | 0.917 (.911–.923) |
| Moore et al (2015) [12] | United States | IPD | <5 y | 2189 | NR | NR | 0.686 (.605–.768) | 0.883 (.865–.902) |
| 5–17 y | 797 | NR | NR | 0.843 (.802–.884) | 0.870 (.845–.896) | |||
| Stockmann et al (2016) [35] | United States | IPD | <2 y | 266 | 0.910 (.888–.931) | 0.952 (.948–.957) | 0.850 (.819–.881) | 0.913 (.905–.921) |
| 2–4 y | 180 | 0.928 (.921–.933) | 0.935 (.921–.950) | 0.877 (.856–.898) | 0.908 (.903–.913) | |||
| 5–17 y | 168 | 0.920 (.900–.939) | 0.959 (.874–1.000) | 0.872 (.847–.896) | 0.906 (.900–.912) | |||
| All ages | 614 | 0.920 (.903–.937) | 0.949 (.938–.960) | 0.867 (.841–.893) | 0.921 (.913–.929) | |||
Serotype diversity is expressed as Simpson's D, which measures the probability that 2 pneumococcal isolates selected at random, with replacement, from a given time period will be different serotypes. The value of this index ranges between 0 and 1, with larger values representing greater diversity.
Abbreviations: CI, confidence interval; IPD, invasive pneumococcal disease; NR, not reported; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
a PCV7 was introduced in September 2006 in the United Kingdom, which was followed by the introduction of PCV13 in April 2010. Moore et al reported their serotype data for the 2006–2010 post-PCV7 period in aggregate, and therefore it was not possible to calculate Simpson's D for the early vs late PCV7 periods.
DISCUSSION
Our data demonstrate rapid changes in pneumococcal serotype diversity following the introduction of PCV7 and PCV13 and provide insight into the dynamics of pneumococcal evolution and ecology. We recognized that selective pressures caused by the widespread use of PCV would decrease the prevalence of vaccine serotypes through type-specific host immunity. We hypothesized that this in turn would open ecologic niches that could be occupied by nonvaccine serotypes with invasive potential that were present at low levels within the population prior to vaccine introduction [25]. The dynamics of serotype distribution and diversity were similar following the introduction of both vaccines, suggesting a strong selective pressure from PCV7 and PCV13. Furthermore, the dynamics were most evident in the youngest children, the group targeted for PCV immunization. In Utah, replacement serotypes that emerged were present, though rarely, in children with IPD before PCV introduction [25]. These serotypes may have been able to outcompete other serotypes due to relative differences in their evolutionary fitness. Thus, our clinical and epidemiological data provide evidence supporting the Red Queen hypothesis, in which there was a reciprocal coevolution of pneumococcal populations in response to changes in human immunity [4]. Moreover, this does not appear to have been a geographically isolated phenomenon, as this finding was supported by multiple studies from the United States, the United Kingdom, Norway, and Spain.
The primary ecological niche of the pneumococci is the nasopharynx of children [26]. Colonization of the nasopharynx is a strong driver of pneumococcal diversity and PCVs are known to alter the serotypes colonizing immunized children and their close contacts [27]. In simulations, immunization results in rapid replacement of vaccine serotypes, which in many of the modeled scenarios was associated with a brief increase in serotype diversity [7]. As demonstrated in the Cobey and Lipsitch model, a short-term increase in serotype diversity was predicted to occur following PCV7 introduction, which would then be followed by a sustained decrease in diversity [7, 28]. Our clinical observations from children with IPD support their mathematical model. Here we showed that following the introduction of both PCV7 and PCV13, a rapid and short-term increase in pneumococcal serotype diversity occurred among children, followed by a decrease in diversity over approximately 3–5 years as new dominant nonvaccine serotypes emerged.
As a consequence of their prioritization for PCV immunization, we hypothesized that young children would be most likely to experience the effects of vaccine-induced selective pressure. In this study, children aged <2 years had the greatest relative increase in serotype diversity following the introduction of both PCV7 and PCV13. This age group also had the greatest relative decline in serotype diversity in the late PCV7 period. Streptococcus pneumoniae colonizes the nasopharynx of healthy individuals, especially young children, who may have colonization rates as high as 75% [29]. Our findings suggest that intense competition emerges among pneumococcal serotypes, particularly in young children, following the introduction of serotype-specific pneumococcal vaccines.
Competition among pneumococcal serotypes is incompletely understood; however, factors that define fitness are likely to include the ability to colonize the nasopharynx, induce inflammation, invade the host, and escape innate and adaptive immune responses [30]. The emergence of serotypes 3, 19A, 7F, and 22F in the early and late PCV7 periods may reflect a combination of selective fitness advantages at the phenotypic and genotypic level. Other serotypes that were infrequently identified in the pre-PCV7 period (eg, serogroup 15) appeared to be unsuccessful in establishing dominance in the early PCV7 era. However, serogroup 15 emerged as the most common cause of IPD following PCV13 introduction, when serotypes 1 and 7F were declining.
The heterogeneous serotype dynamics reported here in response to PCV introduction may be explained, in part, by variations in the relative ability of serotypes to colonize the nasopharynx. The “invasive capacity”—the ratio of the incidence of IPD to the frequency of nasopharyngeal carriage—has been shown to vary up to 50-fold among different serotypes [31]. Serotype 1 has a high invasive capacity and is rarely identified in asymptomatic carriage [32]. Interestingly, identification of serotype 1, a common cause of IPD in Utah in the pre-PCV7 period, did not change substantially following the introduction of PCV7, perhaps because it does not compete for nasopharyngeal carriage and was relatively immune to changing selective pressures in this environment. Conversely, serotype 3 and serogroup 15 have been reported to feature low invasive capacity [32]. It has been proposed that the least invasive serotypes are those that persist in the nasopharynx for prolonged periods [33]. These data suggest that the emergence of serotype 3 and serogroup 15 may reflect, in part, a competitive advantage due to their ability to colonize the nasopharynx for an extended duration. However, further investigation is needed to determine if colonization prevalence may be useful in predicting the emergence of replacement serotypes.
Increasing serotype diversity has been reported following PCV7 introduction in other geographic regions, including Massachusetts, Finland, and the United Kingdom [11, 20]. Hanage et al observed an initial increase in pneumococcal serotype diversity among children colonized with pneumococcus in Massachusetts following introduction of PCV7 [11]. By 2007, the level of diversity had returned to prevaccine levels, leading them to conclude that pneumococcal serotype replacement was complete. Our data suggest that the evolutionary equilibrium that developed after several years of widespread PCV7 use was again disturbed following the introduction of PCV13. The combination of these findings among children colonized with pneumococcus and those with IPD lends credence to the powerful influence that vaccine-induced selective pressure exerts upon pneumococcal evolutionary dynamics.
The increase in IPD attributable to nonvaccine serotypes has not reversed the overall reduction in morbidity and mortality from IPD associated with the widespread use of PCV7 in the United States [34]. The approval of PCV13 has further decreased the burden of IPD and saved additional lives as immunization rates increased [12]. However, the evolutionary niche occupied by PCV13 serotypes has become a site of intense competition between previously rare non-PCV13 serotypes. We speculate that after several years newly dominant serotypes will emerge and may lead to IPD in susceptible populations, especially those with underlying medical conditions [12, 35]. Further surveillance will be necessary to confirm this hypothesis.
The competitive Red Queen dynamics described in this study reveal a limitation of serotype-specific vaccines. For pathogens that rapidly adapt to changes in human immunity, the benefit of reducing disease attributable to vaccine serotypes must be tempered by the potential for selecting for evolutionarily fit nonvaccine serotypes. If elimination of IPD is the goal, vaccines “must contain antigens against specific serotypes that are occurring or can be expected to occur” [36]. In the case of pneumococcus, mathematical modeling informed by biology and clinical observation may be useful in predicting which serotypes will emerge under vaccine selective pressure to become important causes of IPD. In the face of evolutionary selective pressure, alternative approaches to serotype-specific vaccines need to be considered. Possibilities include the development of vaccines that aim to confer universal protection against all pneumococcal serotypes [37], or the regular rotation of different serotype-specific vaccines in response to data derived from active surveillance programs, as is currently done for influenza vaccines [38].
This study has several limitations. First, we cannot causally link the introduction of PCV7 or PCV13 to the changes observed in pneumococcal serotypes. Shifts in the distribution of pneumococcal serotypes are known to occur over time; these changes have not been well explained but could represent loss of virulence in certain serotypes, development of type-specific immunity in populations, or other unmeasured ecological factors [36, 39]. Changes in diversity could also be due to factors other than serotype replacement caused by vaccine-induced selective pressure (eg, unmasking of minority serotypes) [40]. However, the major changes reported in this study began rapidly after introduction of both vaccines, and these findings were substantiated in multiple studies from the United States and Europe [12–24]. Second, we do not have data on the changes in serotypes colonizing the nasopharynxes of children during this period, which would provide additional insight. However, an analysis of data from Norway following the introduction of PCV7 revealed a significant increase in diversity among pneumococcal carriage isolates obtained from children aged <5 years [19].
As predicted by the Red Queen hypothesis, we observed clinical evidence suggestive of vaccine-induced selective pressure that resulted in serotype replacement in children following the introduction of both PCV7 and PCV13 within the US childhood immunization schedule. These evolutionary dynamics were characterized by an increase in serotype diversity after introduction of the vaccine and were followed by the establishment of an apparent new equilibrium and the emergence of a limited number of dominant nonvaccine serotypes that were present at low levels before vaccine introduction. The effect was greatest in young children, who were also the population targeted for immunization. Serotype-specific immunization of children appears to be a powerful driver of serotype replacement within the pneumococcal population, leading to changes in the dominant types responsible for IPD both in immunized children and in nonimmunized populations. The evolutionary dynamics underlying the observed changes in serotype diversity and replacement have not been fully elucidated and deserve further investigation. Ultimately, alternative vaccine strategies, perhaps informed by evolutionary dynamics, may be needed to disrupt the antagonistic coevolution of pathogens and humans observed following the introduction of serotype-specific vaccines.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Dr Nels C. Elde for his scientific guidance and thorough review of the manuscript.
Author contributions. C. S., K. A., A. T. P., A. J. B., L. J. F., and C. L. B. developed the study design. K. A. and C. L. B. coordinated the data collection. E. O. M. performed the pneumococcal serotyping. C. S. conducted the Simpson's D analysis. C. S., K. A., A. T. P., A. J. B., L. J. F., and C. L. B interpreted the data. C. S., K. A., A. T. P., A. J. B., and C. L. B. wrote the first draft of the manuscript. All authors contributed substantively to the review and revision of the final version.
Financial support. This work was supported by grants from the National Institute of Allergy and Infectious Diseases (grant number U01A1082482 to K. A. and C. L. B.; grant number U01 AI074419-01 to C. L. B.); the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant number UL1RR025764 to C. L. B.); the National Center for Research Resources (grant number P20 RR016448 to L. J. F.); and the Centers for Disease Control and Prevention (U18-IP000303 to C. S., K. A., A. T. P., A. J. B., C. L. B.). This project was further supported by the University of Utah, Department of Pediatrics through the Children's Health Research Center and the Pediatric Clinical and Translational Research Scholars Program, the H. A. and Edna Benning Presidential Endowment, and the Primary Children's Hospital Foundation.
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Whitney CG, Farley MM, Hadler J et al. . Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348:1737–46. [DOI] [PubMed] [Google Scholar]
- 2.Singleton RJ, Hennessy TW, Bulkow LR et al. . Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska Native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 2007; 297:1784–92. [DOI] [PubMed] [Google Scholar]
- 3.Byington CL, Samore MH, Stoddard GJ et al. . Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups. Clin Infect Dis 2005; 41:21–9. [DOI] [PubMed] [Google Scholar]
- 4.Jefferies JM, Clarke SC, Webb JS, Kraaijeveld AR. Risk of Red Queen dynamics in pneumococcal vaccine strategy. Trends Microbiol 2011; 19:377–81. [DOI] [PubMed] [Google Scholar]
- 5.Neu HC. The crisis in antibiotic resistance. Science 1992; 257:1064–73. [DOI] [PubMed] [Google Scholar]
- 6.Van Valen L. Molecular evolution as predicted by natural selection. J Mol Evol 1974; 3:89–101. [DOI] [PubMed] [Google Scholar]
- 7.Cobey S, Lipsitch M. Niche and neutral effects of acquired immunity permit coexistence of pneumococcal serotypes. Science 2012; 335:1376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson EH. Measurement of diversity. Nature 1949; 163:688. [Google Scholar]
- 9.Ampofo K, Pavia AT, Stockmann CR et al. . Evolution of the epidemiology of pneumococcal disease among Utah children through the vaccine era. Pediatr Infect Dis J 2011; 30:1100–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Zhou J. Re-parameterization of multinomial distributions and diversity indices. J Stat Plan Inference 2010; 140:1731–8. [Google Scholar]
- 11.Hanage WP, Finkelstein JA, Huang SS et al. . Evidence that pneumococcal serotype replacement in Massachusetts following conjugate vaccination is now complete. Epidemics 2010; 2:80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore MR, Link-Gelles R, Schaffner W et al. . Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black S, France EK, Isaacman D et al. . Surveillance for invasive pneumococcal disease during 2000–2005 in a population of children who received 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2007; 26:771–7. [DOI] [PubMed] [Google Scholar]
- 14.Lacapa R, Bliss SJ, Larzelere-Hinton F et al. . Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin Infect Dis 2008; 47:476–84. [DOI] [PubMed] [Google Scholar]
- 15.Guevara M, Barricarte A, Gil-Setas A et al. . Changing epidemiology of invasive pneumococcal disease following increased coverage with the heptavalent conjugate vaccine in Navarre, Spain. Clin Microbiol Infect 2009; 15:1013–9. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Trallero E, Marimon JM, Ercibengoa M, Vicente D, Perez-Yarza EG. Invasive Streptococcus pneumoniae infections in children and older adults in the north of Spain before and after the introduction of the heptavalent pneumococcal conjugate vaccine. Eur J Clin Microbiol Infect Dis 2009; 28:731–8. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan SL, Barson WJ, Lin PL et al. . Serotype 19A Is the most common serotype causing invasive pneumococcal infections in children. Pediatrics 2010; 125:429–36. [DOI] [PubMed] [Google Scholar]
- 18.Wenger JD, Zulz T, Bruden D et al. . Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J 2010; 29:251–6. [DOI] [PubMed] [Google Scholar]
- 19.Vestrheim DF, Hoiby EA, Bergsaker MR, Ronning K, Aaberge IS, Caugant DA. Indirect effect of conjugate pneumococcal vaccination in a 2+1 dose schedule. Vaccine 2010; 28:2214–21. [DOI] [PubMed] [Google Scholar]
- 20.Flasche S, Van Hoek AJ, Sheasby E et al. . Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med 2011; 8:e1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999–2011(1.). Emerg Infect Dis 2013; 19:1074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guevara M, Ezpeleta C, Gil-Setas A et al. . Reduced incidence of invasive pneumococcal disease after introduction of the 13-valent conjugate vaccine in Navarre, Spain, 2001–2013. Vaccine 2014; 32:2553–62. [DOI] [PubMed] [Google Scholar]
- 23.Moore CE, Paul J, Foster D et al. . Reduction of invasive pneumococcal disease 3 years after the introduction of the 13-valent conjugate vaccine in the Oxfordshire region of England. J Infect Dis 2014; 210:1001–11. [DOI] [PubMed] [Google Scholar]
- 24.Richter SS, Diekema DJ, Heilmann KP, Dohrn CL, Riahi F, Doern GV. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent conjugate vaccine in the United States. Antimicrob Agents Chemother 2014; 58:6484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byington CL, Hulten KG, Ampofo K et al. . Molecular epidemiology of pediatric pneumococcal empyema from 2001 to 2007 in Utah. J Clin Microbiol 2010; 48:520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meats E, Brueggemann AB, Enright MC et al. . Stability of serotypes during nasopharyngeal carriage of Streptococcus pneumoniae. J Clin Microbiol 2003; 41:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien KL, Millar EV, Zell ER et al. . Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis 2007; 196:1211–20. [DOI] [PubMed] [Google Scholar]
- 28.Cobey S, Lipsitch M. Pathogen diversity and hidden regimes of apparent competition. Am Nat 2013; 181:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan TQ. Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin Microbiol Rev 2012; 25:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberger DM, Trzcinski K, Lu YJ et al. . Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog 2009; 5:e1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yildirim I, Hanage WP, Lipsitch M et al. . Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine 2010; 29:283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brueggemann AB, Peto TE, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis 2004; 190:1203–11. [DOI] [PubMed] [Google Scholar]
- 33.Crook DW, Brueggemann A, Sleeman K, Peto TEA. Epidemiology of the carrier state. In: Tuomanen E, Mitchell TJ, Morrison D, Spratt B. The pneumococcus. Washington, DC: ASM Press, 2004. [Google Scholar]
- 34.Pulido M, Sorvillo F. Declining invasive pneumococcal disease mortality in the United States, 1990–2005. Vaccine 2010; 28:889–92. [DOI] [PubMed] [Google Scholar]
- 35.Stockmann C, Byington CL. PCV13 in the USA: early successes and potential challenges. Lancet Infect Dis 2016; 15:254–6. [DOI] [PubMed] [Google Scholar]
- 36.Finland M, Barnes MW. Changes in occurrence of capsular serotypes of Streptococcus pneumoniae at Boston City Hospital during selected years between 1935 and 1974. J Clin Microbiol 1977; 5:154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moffitt KL, Malley R. Next generation pneumococcal vaccines. Curr Opin Immunol 2011; 23:407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell CA, Jones TC, Barr IG et al. . Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine 2008; 26(suppl 4):D31–4. [DOI] [PubMed] [Google Scholar]
- 39.Prins-van Ginkel AC, Berbers GA, Grundeken LH et al. . Dynamics and determinants of pneumococcal antibodies specific against 13 vaccine serotypes in the pre-vaccination era. PLoS One 2016; 11:e0147437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipsitch M. Bacterial vaccines and serotype replacement: lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg Infect Dis 1999; 5:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.