Symptomatic human immunodeficiency virus (HIV)–associated neurocognitive disorders that warrant neurological review were accurately predicted using a short, computerized CogState-based screening procedure administered in a HIV community medical clinic. The screening procedure was validated against standard neuropsychological testing.
Keywords: HIV, HAND, neurocognitive screening
Abstract
Background. Human immunodeficiency virus (HIV)–associated neurocognitive disorders (HAND) are not routinely assessed due to the lack of an adequate screening strategy. We aimed to develop a clinically relevant screening procedure for symptomatic HAND, validated against a gold standard neuropsychological (NP) test battery.
Methods. Representative HIV-infected (HIV+) and demographically matched HIV-uninfected (HIV−) participants in an observational study completed a standard evaluation for mood, drug and/or alcohol use, and activities of daily living and a newly designed 20-minute computerized CogState battery that assessed 5 cognitive domains. A subset completed standard NP assessment for 8 cognitive domains. HAND definition on screening and gold standard NP was determined using demographically corrected z scores and the global deficit score (≥ 0.5), applying the Frascati criteria. Participants were blinded to screening results, and the NP examiner was blinded to screening and HIV status.
Results. A total of 254 HIV+ participants were enrolled—mean age, 48.9 ± 10.2 years; median nadir CD4, 270 cells/mL; tertiary educated, 54%; and HIV− controls, 72. HIV+ HAND screening prevalence was 30.7% (HIV-associated dementia, 3.2%; mild neurocognitive disorder, 12.6%; and asymptomatic neurocognitive disorder, 15.0%; HIV− group: 13.9%; P = .004). Of the 75 participants who completed the NP battery, the HAND rate in the HIV+ group was 50.9% vs 43.4% by screening (P > .50). HAND screening vs gold standard NP sensitivity was 76% and specificity was 71%. Clinically relevant HIV-associated dementia and mild neurocognitive disorder sensitivity was 100% and specificity was 98% (positive predictive value 0.92).
Conclusions. Symptomatic HAND warranting neurological review was accurately predicted using a CogState-based screening procedure.
Despite effective combination antiretroviral treatment (cART) with virological suppression, human immunodeficiency virus (HIV)–associated neurocognitive disorders (HAND) remain a major neurological complication associated with HIV infection [1, 2]. Nondemented forms of the disease predominate in the cART era. This clinical profile shift is described by the HAND Frascati diagnostic criteria [3], with the following 3 clinical categories based on disease severity and functional impact: asymptomatic neurocognitive impairment (ANI), symptomatic mild neurocognitive disorder (MND), and HIV-associated dementia (HAD).
Whether nondemented HAND (ANI and MND) represent actual neuropathogenic entities is controversial [4, 5] despite being tested against HIV neuropathological standards with good sensitivity and specificity [6]. Neuropsychological (NP) methods have substantially improved to better identify mild forms of cognitive impairment, and there is strong evidence that even in its mild form, HAND adversely impacts adherence to medication adherence, quality of life, employment, and mortality risk [5]. The potential progression of symptoms over time causes concern in HIV-infected (HIV+) persons [7] and their treating clinicians [8]. There is evidence for progression with past MND/HAD [9, 10], and recent longitudinal studies demonstrate that even the mildest HAND (ANI) risks progression within several years [11, 12].
In some challenges to the statistical grounding of ANI [13] and MND [4], fundamental concepts in quantitative neuropsychology were not optimally rendered [5]. Yet the question of the clinical relevance of ANI remains because this level of neurocognitive impairment is defined by no self-reported functional impact. Although mild HAND may indicate ongoing HIV brain injury, the lack of clear therapeutic guidelines (eg, effectiveness of central nervous system [CNS]-penetrating cART remains controversial) puzzles HIV physicians and may create anxiety in some patients [14]. Moreover, usual HIV biomarkers are often dissociated from these mild forms of HAND (eg, plasma and/or CNS HIV viremia do not necessarily correlate with clinical presentation) [14].
Advocates for HAND screening, while recognizing these controversies, also emphasize that, first and foremost, not all HIV+ persons develop HAND [15]. Second, while comprehensive NP assessment is the gold standard for accurate HAND detection [3], it remains costly and not widely available, even in high-income countries. Third, a screening procedure that streamlines only those needing comprehensive NP assessment is economically and strategically efficient and aligns with best-practice care for HIV+ individuals [16]. Fourth, HAND has been associated with impaired adherence to cART [17], which can lead to poorer virological outcomes. Fifth, longitudinal studies have recently shown that ANI may be an indicator of future deterioration [11, 12]. Sixth, with detection of HAND, practical coping strategies can be instituted (eg, seeking social support and solution-focused coping) [18, 19].
The lack of an adequate screening strategy has been a major limitation to routine screening. Recommendations from a recent review on HAND screening [20] suggest that an effective screening procedure must possess at least adequate criterion validity across the entire HAND spectrum (ie, ANI, MND, and HAD). However, most currently available screening tools fail to do this [20, 21]. Furthermore, it is important that screening tools be applicable for nonspecialists often lack the time needed to address the demands of an increasingly complex, chronic disease [16, 21]. The interpretation of results for many HAND screening tools is impractical for the nonneuropsychologist [21]. A relevant HAND screening tool must also be relatively simple, with rapid administration while adhering to standard cognitive constructs and reflecting the HAND Frascati criteria [3]. Computerized assessment, administration, and scoring combined with sound NP interpretation of results make such a screening procedure feasible.
Based on these considerations, we designed a study to validate a new screening procedure for the detection of the HAND spectrum; determine the overall screening prevalence of HAND and ANI, MND, and HAD in a representative community-based HIV+ cohort; and determine to what extent HIV biomarkers, history of HIV brain involvement, mild levels of non-HIV neurological confounders, psychiatric confounders, and depressive symptoms moderate HAND prevalence.
METHODS
Study Design
We present baseline data of a prospective observational study for screening cognitive function in a community cohort. All participants provided consent prior to study enrollment. The Bellberry (HREC2011-04-228) ethics committee approved the protocol.
Site Characteristics and Study Population
HIV+ and HIV-uninfected (HIV−) participants attending a community practice with a high HIV caseload in Sydney, Australia (Holdsworth House Medical Practice), completed baseline assessments from October 2011 to October 2012. HIV+ participants had documented infection, medical history, examination, and laboratory results; were fluent in English; and were not intoxicated at the time of their examination visit. HIV− participants had documented HIV-negative testing in the previous 12 months; they were excluded with a history of clinically significant neurological and psychiatric confounders to develop a local NP normative reference. HIV+ participants with a history of HIV brain involvement, mild head injury, nonmajor stroke, hepatitis C virus (HCV) infection, current alcohol use disorder (AUD) or substance use disorder (SUD), or current major depressive disorder were not excluded in order to yield a representative community group.
Subset Who Completed the Gold Standard NP Battery
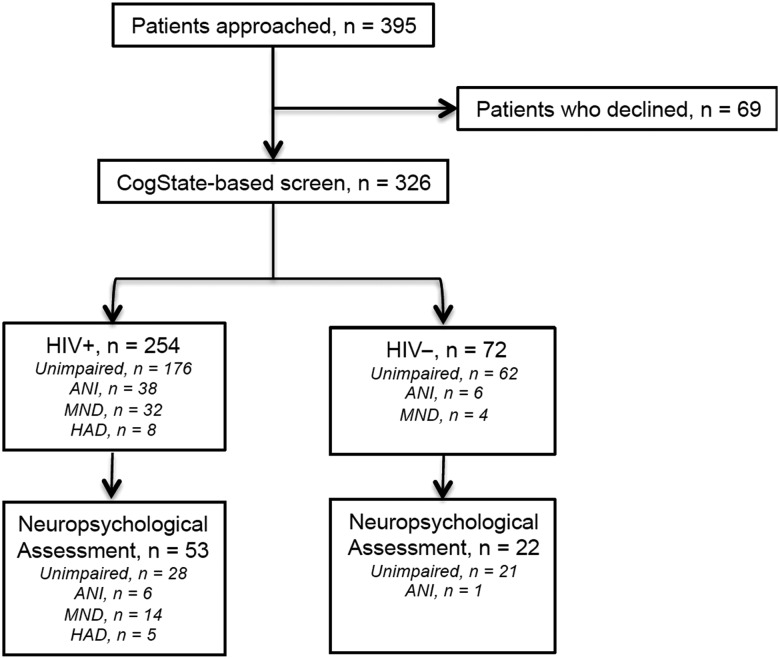
At study entry, all participants completed a brief CogState-based screen. One quarter (N = 75) received the NP assessment: this included those with HIV+ and having symptomatic (MND or HAD) cognitive impairment (defined in Supplementary Table 1) on screen; as well as a random sample of those HIV+ screening asymptomatically impaired or normal, and those who were HIV− (independent of screen status) (Figure 1).
Figure 1.
CONSORT flow diagram of study participation. Abbreviations: ANI, asymptomatic neurocognitive impairment; HAD, HIV-associated dementia; HIV, human immunodeficiency virus; MND, mild neurocognitive disorder.
Study Procedures
Medical Information and Psychological Measures
The practice study coordinator or nurse collected demographic information and medical history. Participants also completed standard self-report questionnaires regarding current mood (a 21-item depression, anxiety, stress scale (DASS-21) [22] and a subsection of the mini international neuropsychiatric interview [23] to determine current (12 months) AUD and SUD. Functional status was evaluated with a standard self-report scale to assess independence in activities of daily living (IADL), which is widely used and validated in the HIV+ population [1, 24]. This scale documented current (within past month) and best ever performance in 17 everyday activities including work efficiency and cognitive and/or physical impairment changes. Decline in at least 2 activities determined a clinically significant decline in ADL independence [24].
Cognitive Screen Procedure
A newly designed version of CogState that targets the HAND spectrum was developed by L. A. C. in consultation with P. M., based on findings from the initial Australian CogState HIV study [25, 26]. This CogState version was used to assess 5 cognitive domains across 6 individual tasks: sustained attention, information processing speed, attention, working memory, verbal learning, and verbal memory. Outcome measures were speed (reaction time) and accuracy (see Supplementary Table 2). CogState screen administrators were study staff trained over a half-day by neuropsychologists prior to study commencement. Standardized administration, including verbatim task instructions and preemptive resolution of factors that could bias performance (eg, wearing glasses, documentation of physical incapacities that interfere with tasks, avoiding testing in the overtly unwell), were emphasized.
Gold Standard NP Assessment
The standardized NP battery reflected the range of cognitive functions typically assessed in Australian clinical practice [5], with particular emphasis on HAND classification recommendations for cognitive assessment [3]. NP testing was conducted and scored by J. K., who was blind to HIV and CogState-based screen impairment status.
Impairment Definition
Standard NP raw test scores for each NP measure were transformed into a standard z score using formulas that were developed in the HIV-negative and demographically comparable group for each NP measure (see details about norming procedure in the Supplementary Materials). Next, the z scores were transformed into deficit scores: 0, no impairment (z score ≥ −1.0); 1, mild impairment (z score <−1.0 to −1.5); 2, mild to moderate impairment (z score < −1.5 to −2.0); 3, moderate impairment (z score < −2.0 to −2.5); 4, moderate to severe impairment (z score < −2.5 to −3.0); and 5, severe impairment (z score < −3.0). Individual deficit scores were averaged as a summary score, that is, the global deficit score (GDS, continuous score), and the HAND classification (ANI, MND, or HAD) was derived via the procedure outlined by Blackstone et al [27]. Specifically, GDS ≥ 0.5 and no IADL decline = ANI; GDS ≥ 0.5 and mild to moderate IADL decline = MND or GDS ≥ 0.5–1.5 and any IADL decline = MND; GDS ≥ 1.5 and severe IADL decline = HAD. To differentiate among ANI, MND, and HAD, we used IADL as well as any clinical evidence of IADL decline (eg, medical record, nurse information). Note that a GDS > 0.5 (impairment cutoff) for the screen and a GDS ≥ 0.5 for the NP yielded the best sensitivity and specificity (Supplementary Material; Supplementary Figure 1).
Records of HIV Brain Involvement and Non-HIV–Related Confounds
HIV brain involvement and non-HIV related confounds were self-reported and confirmed on medical records and classified as follows: a history of HIV brain involvement included cases with past HAND and/or CNS opportunistic infections (N = 32, 7 with CNS opportunistic infection that had been treated prior to study entry in the sense that those were not currently active). A history of non-HIV neurological or psychiatric condition included (N = 39): 4 cases with mild stroke, 1 case with mild stroke and SUD, 26 cases with mild to moderate traumatic brain injury, 5 cases with mild to moderate traumatic brain injury and SUD, 1 case with mild traumatic brain injury and AUD, and 2 cases with mild to moderate traumatic brain injury and AUD as well as SUD. In terms of HCV status, we found that 12 HIV+ and no HIV− participants had detectable HCV RNA. Because this small number did not allow for robust statistical analyses, it was not further considered. Mood was investigated separately and in relation to confounders since impact on neurocognitive functions may be systematic or part of HAND clinical presentation [28]. The DASS total score and a clinically relevant cutoff (ie, raw score >13) were used to determine depressed vs nondepressed status.
Statistical Analyses
The study was powered to enroll 250 HIV+ and 70 HIV− participants based on HAND prevalence, approximately 35% in HIV+ and 10% in the HIV− individuals. This rate combines HAND prevalence in nonadvanced HIV infection (20%–30%; [29]), and the inclusion of history of brain HIV involvement and non-HIV confounders to produce a representative cohort—confounders that typically increase cognitive impairment rate [2, 9]. The rate in HIV− participants was a conservative estimate of HAND Frascati criteria applied to a screen for 5 cognitive domains [30], translating into a medium effect size (Cohen d = 0.5) on the CogState GDS (96% power to detect a 0.5 difference between the HIV+ and HIV− sample).
The validity for the screen was based on the capacity of the selected gold standard NP test battery to accurately detect HAND [5].
Aim 1: Screen Validation
We aimed to establish criterion validities between the NP gold standard and CogState-based screen, DAGStat [31] compute overall impairment rate, and HAND severity via sensitivity, specificity, PPP (the probability an individual has HAND given their positive screen result), NPP (the probability an individual does not have HAND given their negative screen result), and overall correct classification rate (CCR).
Aim 2: HAND Screen Prevalence
χ2 C tests were used to compare rates of impairment defined by the GDS between the HIV− and HIV+ groups.
Aim 3: HIV Biomarkers, Confounds, and Depression Status Analyses
Correlation analysis was used to determine the extent to which confounders were independent from one another. Linear regression analysis was used to assessed the effect of nadir and current CD4, plasma HIV RNA detection, US Centers for Disease Control and Prevention status (C vs other), cART status (on/off), and cART penetration effectiveness score (CPE; high ≥ 7) on the CogState continuous GDS. Confound proportions were compared between the HIV+ and HIV− groups using t test or χ2 test appropriate. In the HIV+ group, point biserial correlations determined the extent of confound independence (yes/no variables were coded into dummy variables as 1/0, respectively). Linear regression analysis was used to assess confounders that had a primary impact on the CogState continuous GDS. Statistical significance was set as P < .05 when interpreting size of effect given the sample size. These analyses were conducted in JMP V.12 (SAS).
RESULTS
Over 12 months, 326 of 395 participants were enrolled (83% recruitment). Of those who did not enroll, demographic and clinical characteristics were similar between the HIV+ and HIV− groups (data not shown). The demographics and clinical characteristics of the 254 HIV+ participants and 72 HIV− who completed the study are outlined in Table 1.
Table 1.
Demographic and Clinical Characteristics in the Study Population Stratified by Human Immunodeficiency Virus Status
| Characteristic | HIV+ n = 254 | HIV− n = 72 | P Value |
|---|---|---|---|
| Age (median y) | 48.5 (15.1) | 48.7 (12.0) | 0.9 |
| Gender (% male) | 99.6 | 97.2 | 0.06 |
| Ethnicity | |||
| White (%) | 86.6 | 90.3 | 0.42 |
| Asian (%) | 5.1 | 8.3 | 0.32 |
| Education | |||
| Primary (%) | 1.1 | 0.0 | 1.0 |
| Secondary (%) | 29.5 | 23.6 | 0.38 |
| Trade school (%) | 17.3 | 13.9 | 0.45 |
| College (%) | 52.0 | 62.5 | 0.12 |
| History of HIV-associated brain involvement (%) | 12.6 | … | … |
| Non-HIV central nervous system condition (%) | 15.3 | … | … |
| Depressive symptoms (depression anxiety stress scale > 13; %) | 26.0 | 19.5 | 0.25 |
| Alcohol use disorder (current 12 mo; %) | 9.2 | 5.6 | 0.33 |
| Substance use disorder (current 12 mo; %) | 19.9 | 2.8 | 0.0005 |
| Hepatitis C virus RNA positive (%)a | 4.1 | 0.0 | 0.04 |
| HIV men-who-have-sex-with-men transmission (%) | 92.9 | ||
| Duration of HIV (mean y) | 14.1 ± 8.6 | … | … |
| Centre for Disease Control category C (%) | 15.4 | … | … |
| Plasma HIV RNA <200 copies/mL (%) | 83.4 | … | … |
| Plasma HIV RNA <50 copies/mL (%) | 78.7 | … | … |
| CD4+ T lymphocyte count (median cells/µL) | 592 (355) | … | … |
| Currently taking cART (%) | 91.7 | … | … |
| High central nervous system penetrating-effectiveness cART regimen (≥7; %)b | 87.0 | … | … |
Data presented as %, median (interquartile range), or mean ± standard deviation.
χ2 analyses were used to assess difference between groups.
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus.
a Prevalence included those with a documented hepatitis C virus test.
b [38].
Aim 1: Screen Validation
A total of 53 HIV+ and 22 HIV− controls completed the brief CogState-based screen and NP assessment. Figure 2 presents the criterion validity indexes of the CogState-based screen compared with the NP gold standard. When considering the whole sample (HIV+ and HIV−), sensitivity was 73% (95% confidence interval [CI], 52%–88%] and specificity was 82% (95% CI, 68%–91%). The CCR was 79% (95% CI, 68–87). The HIV+ group sensitivity was 76% (95% CI, 55%–91%) and specificity was 71% (95% CI, 51%–87%). CCR was 74% (95% CI, 60%–85%). When considering MND/HAD, CogState yielded almost perfect criterion validity (sensitivity = 100%, specificity = 98%; 95% CI, 87%–100%); PPP = 92% (95% CI, 90%–100%), negative predictive value = 100%, and CCR = 98% (95% CI, 90%–100%). The single misclassified case (impaired on the screen and not on the gold standard) had a history of toxoplasmosis, was otherwise high functioning, and had mild to moderate impairment in CogState-screen battery reaction time and 1 of the working memory tasks. On the gold standard, he performed well on all tests except for 1 measure of working memory. We note that another article focuses on this part of the study, with comprehensive details about the neuropsychology procedure. The primary information has been summarized in the supplementary material (Supplementary Table 2).
Figure 2.
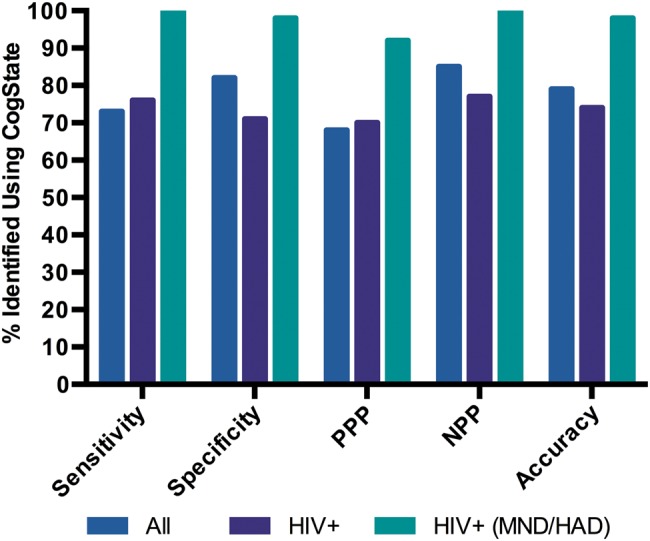
Criterion validity of the CogState-based screen. Sensitivity is the proportion of individuals identified as impaired on both the gold standard and the screen. Specificity is the proportion of individuals identified as unimpaired on both the gold standard and the screen. PPP is the probability an individual has human immunodeficiency virus (HIV)–associated neurocognitive disorders (HAND) given their positive screen result. NPP is the probability an individual does not have HAND given their negative screen result. Abbreviations: HAD, HIV-associated dementia; MND, mild neurocognitive disorder.
Aim 2: Screen HAND Prevalence
Prevalence of HAND in this community sample was 30.7% in HIV+ participants, which was significantly different from the impairment rate in HIV− individuals (13.8%; P = .004; Supplementary Figure 2). The prevalence of the 3 HAND categories in the HIV+ group indicated that ANI (15.0%) and MND (12.6%) were the most common presentations, greater than HAD (3.2%).
Aim 3: HIV Biomarkers, Confound, and Depression Effects on Neurocognitive Performance
Based on univariate analyses results (presented in Supplementary Materials), the multivariate regression analyses included depression levels, HIV brain involvement history, and non-HIV–associated confounders. The overall R2 model was significant (R2 = 0.09; P = .002). HIV brain involvement history remained an independent predictor of neurocognitive performance on the CogState GDS (Std β = 0.25; P < .001), while depression status showed a positive trend (Std β = 0.11; P < .06). For clinical relevance, the depression and HIV brain involvement history data across the HAND categories are presented in Figure 3. Most HAD cases had previous HIV brain involvement, while the proportion in ANI and MND was equivalent. Notably, all cases with HAD reported a clinically significant level of depression.
Figure 3.
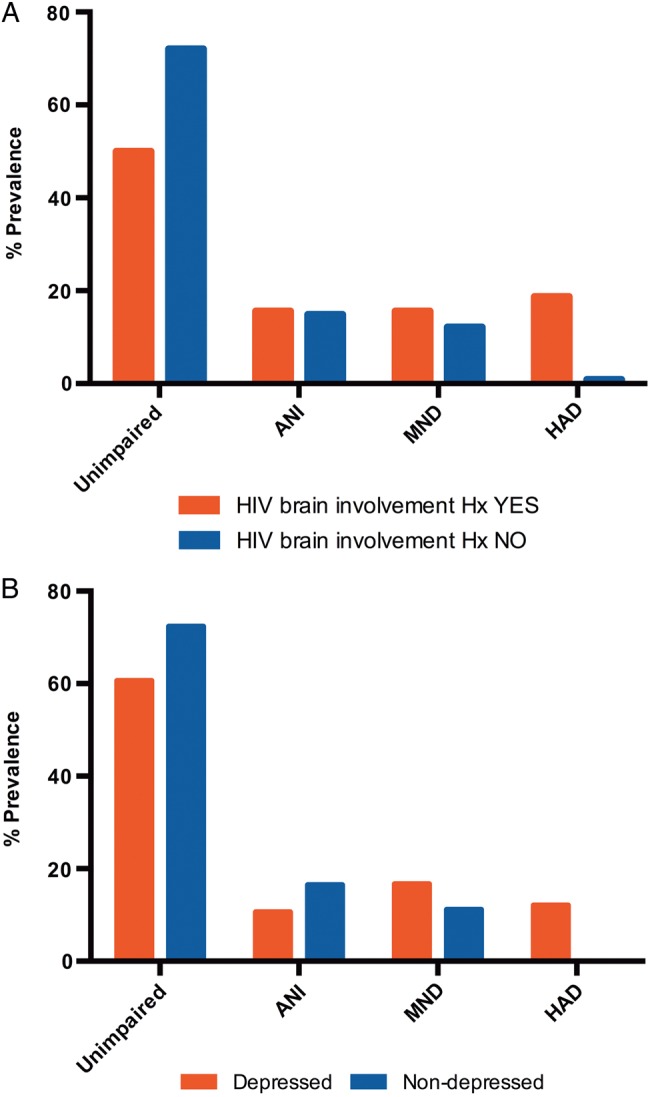
(A) Human immunodeficiency virus (HIV)–associated brain involvement history. (B) Depression categorized by HIV–associated neurocognitive disorders classification. Abbreviations: ANI, asymptomatic neurocognitive impairment; HAD, HIV-associated dementia; HIV, human immunodeficiency virus; Hx, history; MND, mild neurocognitive disorder.
In the HIV+ group, depression and confounders were related (Table 2). Correlations were of small size but significant because of the sample size (n = 254). They indicated an association between a history of HIV brain involvement and the presence of depression. Moreover, the presence of depression was associated with current AUD and/or SUD.
Table 2.
Relationships Between Depression Status, Human Immunodeficiency Virus–Related Brain Involvement History, and Other Confounds
| Confounds | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Depressed vs nondepressed (1) | 0.21*** | −0.03 | 0.17** | 0.17** | |
| HIV-associated brain involvement history vs none (2) | 0.21*** | −0.16** | −0.01 | 0.02 | |
| Non-HIV–associated neurological/psychiatric history vs none (3) | −0.03 | −0.16** | −0.03 | 0.05 | |
| Alcohol use disorder within the last 12 months vs none (4) | 0.17** | −0.01 | −0.03 | 0.16** | |
| Substance use disorder within the last 12 months vs none (5) | 0.17** | 0.02 | 0.05 | 0.16** |
Point biserial correlations significance: *P < .05; **P < .01 ***P < .001. All variables were coded as dummy 1/0, with 1 representing an event occurrence. A history of HIV brain involvement included cases with past HIV–associated neurocognitive disorders and/or central nervous system (CNS) opportunistic infections (N = 32, 7 with CNS opportunistic infection that had been treated prior to study entry in the sense that those were not currently active). A history of non-HIV neurological or psychiatric condition included (N = 39): 4 cases with mild stroke, 1 case with mild stroke and Substance use disorder within the last 12 months (SUD), 26 cases with mild to moderate traumatic brain injury, 5 cases with mild to moderate traumatic brain injury and SUD, 1 cases with mild traumatic brain injury and alcohol use disorder within the last 12 months (AUD), and 2 cases with mild to moderate traumatic brain injury and AUD as well as SUD.
Abbreviation: HIV, human immunodeficiency virus.
DISCUSSION
We are the first to develop a screening procedure that yields HAND classifications according to the Frascati criteria, concomitantly documenting cognitive and ADL functioning [3], in a fairly large representative community cohort. Our study demonstrated the feasibility of HAND screening in HIV clinics by nonspecialists who are trained by neuropsychologists. This study does not oppose standard NP testing but rather streamlines testing for patients who need it the most, creating sustainable and targeted care pathways [32].
The screening uptake rate for this type of cohort (83%) suggests that there is a demand in the majority of HIV+ patients for HAND screening and that there is a need to know if these patients are still functioning well cognitively [7]; a normal screen provides a reassuring result. Our study also suggests that psychiatric conditions are complexly linked with the presence of HAND rather than simply additive or superseding. This would suggest a combined care approach for patients with premorbid anxiety-related depression.
With this new screening procedure, we were able to classify HAND to a level similar to that of the gold standard NP assessment. Sensitivity and specificity for MND and HAD (the most clinically relevant level in frontline HIV care) was excellent, exceeding the approximately 85% sensitivity and specificity found in other studies. In our study the only misclassified case had low performance on reaction time; this could be improved in future studies by including reaction time in the NP standard. The screening procedure enabled us to readily target the more advanced patients who most required neurological follow-up and could be of great practical utility in the clinic setting. Future work may assist in delineating the role of this screening procedure in long-term monitoring.
When the entire HAND spectrum was considered, the sensitivity and specificity of the current screening procedure was adequate and in line with the best available screens [20, 21] but less than ideal for HIV frontline care (misclassification rate of 30%). A potential solution for this problem may be to develop new ways of conceptualizing impairment, as recently proposed [33]; however, further validation would be required. An alternative would be to monitor patients with ANI by longitudinally rescreening and managing them in the wider context of their disease (eg, risks for cardiovascular disease, depression, cART adherence, and retention in care). Importantly, in this study, patients with comorbidities were identified but not excluded in order to develop screening tools that are appropriate and implementable in the general HIV clinic setting and applicable to a wide range of patients, including complex cases.
Prevalence of HAND (30.7%) in a relatively healthy, well-educated (even by Australian general population standards) HIV+ community cohort was approaching the hypothesized rate of 35%. It was significantly higher than for the HIV− controls (patients attending the same practice), bringing further confidence in our methods. This result is in contrast to results from a European study [34] that did not show statistical significance between HIV+ and HIV− participants when another version of CogState and different definitions of impairment were used. When we focused only on MND and HAD (15.8%), our rate of impairment was also higher than in a similar community cohort (7%) [35], suggesting that our screening method is powerful, with near perfect criterion validity. Our results question the need to revise the HAND criteria [4, 13, 34]. Rather, our method can be used by carefully implementing relevant criteria by not mixing concepts of tests and individual measures and by empirically grounding the statistical validity of the impairment definition (in screens and test battery) chosen, while using appropriate control groups.
Not all participants underwent NP testing, and that is a limitation of our study. While testing all participants would have been optimal, it was not possible due to limited resources. However, we devised a strategy to ensure that normal performance as well as the entire HAND spectrum was adequately represented (ANI, MND, and HAD). This meant that the specificity and sensitivity of the screening compared with the comprehensive NP testing was based on optimal power and had strong validity and adequate generalizability to the rest of the sample. The cross-sectional assessment has yet to be validated longitudinally, especially for those with ANI. The population in the community clinic was predominantly white, men who have sex with men, virologically well controlled, and well educated. Although similar to other Australian settings, our results may not directly translate to other HIV clinical settings. Nevertheless, the CogState screening battery was chosen for its ease of use in a community clinic and because it is readily adaptable for other language and cultural settings.
Finally, past HIV-associated head injury but no common HIV biomarkers were associated with current HAND, indicating a need for new markers that could be identified in HIV HAND management. The analyses of the CPE score should be viewed cautiously as the majority of patients had high CPE scores and channelling bias is also possible, which was the case for another very large retrospective study [36]. The effect of cART cannot be reliably established in nonrandomized prospective studies [37].
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the treating physicians at Holdsworth House Medical Practice who enrolled patients in this study: Andrew Gowers, Jane Hunt, Kate Bessey, David Austin, and Ercel Ozser; the study coordinators and nursing staff at Holdsworth House Medical Practice: Shikha Agrawal, Kate Beileiter, Shakira Watts, Rosie Warren, and Dr Danielle Horley; the participating patients at Holdsworth House Medical Practice who so generously donated their time for the study; ViiV Healthcare for the unrestricted study grant that enabled us to undertake the study; and CogState Sciences for providing the modified CogState screening battery and online scoring system.
Financial support. An unrestricted study grant was provided by ViiV Healthcare. CogState Science provided the modified CogState battery and online scoring system gratis as per other investigator-led Australian studies.
Potential conflicts of interest. M. B. has received research funding from ViiV Healthcare, Gilead Sciences, Bristol-Myers Squibb, Abbvie, Janssen, Amgen, and Merck Sharp & Dohme; travel sponsorship from Abbvie, Gilead Sciences, ViiV Healthcare, Bristol-Myers Squibb, and Merck Sharp & Dohme; and has served on advisory boards for Abbvie, Merck Sharp & Dohme, Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare, and Eli Lilly. A. J. has received travel sponsorship from Gilead Sciences. D. Q. has received travel sponsorship from Gilead Sciences, ViiV Healthcare, Bristol-Myers Squibb, and Merck Sharp & Dohme and has served on advisory boards for MSD, Bristol-Myers Squibb, Gilead Sciences, and ViiV Healthcare. P. M. (an author of this study based on his intellectual contributions) is head of CogState Science and facilitated delivery of the CogState battery and online scoring system. B. B. has received grant support from Biogen Idec, ViiV, Merck Sharpe & Dohme, National Health and Medical Research Council (NHMRC), and National Institutes of Health; speaker fees from ViiV Healthcare, Novartis, Biogen Idec, and Boehringer Ingelheim; and royalties from Oxford University Press and Cambridge University Press. L. A. C. is funded by a NHMRC of Australia Career Development fellowship (APP1045400); has received honoraria from Abbvie Ltd, CogState Ltd, and ViiV Healthcare; and received partial salary support in 2012 from Merck Sharp Dome and CogState Ltd. (unrelated to the current study); and has received research support from Abbvie Ltd, ViiV Healthcare, the Australian National Association of People Living with Human Immunodeficiency Virus/AIDS, and Gilead Sciences. CogState did not pay her for the current study or any other commercial entity. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cysique LA, Heaton RK, Kamminga J et al. HIV-associated neurocognitive disorder in Australia: a case of a high-functioning and optimally treated cohort and implications for international neuroHIV research. J Neurovirol 2014; 20:258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Clifford DB, Franklin DR Jr et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antinori A, Arendt G, Becker JT et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gisslén M, Price RW, Nilsson S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis 2011; 28:356–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cysique LA, Bain MP, Lane TA, Brew BJ. Management issues in HIV-associated neurocognitive disorders. Neurobehav HIV Med 2012; 4:63–73. [Google Scholar]
- 6.Cherner M, Cysique L, Heaton RK et al. Neuropathologic confirmation of definitional criteria for human immunodeficiency virus-associated neurocognitive disorders. J Neurovirol 2007; 13:23–8. [DOI] [PubMed] [Google Scholar]
- 7.Hopcroft L, Bester L, Clement D et al. “My body's a 50 year-old but my brain is definitely an 85 year-old”: exploring the experiences of men ageing with HIV-associated neurocognitive challenges. J Int AIDS Soc 2013; 16:18506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright EJ. The Four Seasons approach to the management of modern HIV medicine in high-income countries. Sex Health 2012; 9:491–2. [DOI] [PubMed] [Google Scholar]
- 9.Cysique LA, Maruff P, Brew BJ. Variable benefit in neuropsychological function in HIV-infected HAART-treated patients. Neurology 2006; 66:1447–50. [DOI] [PubMed] [Google Scholar]
- 10.Stern Y, McDermott MP, Albert S et al. Factors associated with incident human immunodeficiency virus-dementia. Arch Neurol 2001; 58:473–9. [DOI] [PubMed] [Google Scholar]
- 11.Grant I, Franklin DJ, Deutsch R et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 2014; 10:2055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rourke S, Rachlis A, Gill J et al. Relative risk and factors associated with progression to symptomatic HAND. Seattle, WA: Conference on Retroviruses and Opportunistic Infections, 2015. [Google Scholar]
- 13.Meyer AC, Boscardin WJ, Kwasa JK, Price RW. Is it time to rethink how neuropsychological tests are used to diagnose mild forms of HIV-associated neurocognitive disorders? Impact of false-positive rates on prevalence and power. Neuroepidemiology 2013; 41:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nightingale S, Winston A, Letendre S et al. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol 2014; 13:1139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brew BJ. AIDS dementia complex. Oxford: Oxford University Press, 2001. [Google Scholar]
- 16.Cysique LA, Murray JM, Dunbar M, Jeyakumar V, Brew BJ. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med 2010; 11:642–9. [DOI] [PubMed] [Google Scholar]
- 17.Ettenhofer ML, Foley J, Castellon SA, Hinkin CH. Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology 2010; 74:1217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slater LZ, Moneyham L, Vance DE, Raper JL, Mugavero MJ, Childs G. Support, stigma, health, coping, and quality of life in older gay men with HIV. J Assoc Nurses AIDS Care 2013; 24:38–49. [DOI] [PubMed] [Google Scholar]
- 19.Hansen NB, Harrison B, Fambro S, Bodnar S, Heckman TG, Sikkema KJ. The structure of coping among older adults living with HIV/AIDS and depressive symptoms. J Health Psychol 2013; 18:198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zipursky AR, Gogolishvili D, Rueda S et al. Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS. AIDS 2013; 27:2385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamminga J, Cysique L, Lu G, Batchelor J, Brew BJ. Validity of cognitive screens for HIV-associated neurocognitive disorder: a systematic review and an informed screen selection guide. Curr HIV/AIDS Rep 2013; 10:342–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the depression anxiety stress scales (DASS) with the Beck depression and anxiety inventories. Behav Res Ther 1995; 33:335–43. [DOI] [PubMed] [Google Scholar]
- 23.Sheehan DV, Lecrubier Y, Sheehan KH et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33. [PubMed] [Google Scholar]
- 24.Heaton RK, Marcotte TD, Mindt MR et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 2004; 10:317–31. [DOI] [PubMed] [Google Scholar]
- 25.Cysique LA, Maruff P, Darby D, Brew BJ. The assessment of cognitive function in advanced HIV-1 infection and AIDS dementia complex using a new computerised cognitive test battery. Arch Clin Neuropsychol 2006; 21:185–94. [DOI] [PubMed] [Google Scholar]
- 26.Maruff P, Thomas E, Cysique L et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol 2009; 24:165–78. [DOI] [PubMed] [Google Scholar]
- 27.Blackstone K, Moore DJ, Franklin DR et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol 2012; 26:894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cysique L, Deutsch R, Atkinson J et al. Incident major depression does not affect neuropsychological functioning in HIV-infected men. J Int Neuropsychol Soc 2007; 13:1–11. [DOI] [PubMed] [Google Scholar]
- 29.White DA, Heaton RK, Monsch AU. Neuropsychological studies of asymptomatic human immunodeficiency virus-type-1 infected individuals: The HNRC Group. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc 1995; 1:304–15. [DOI] [PubMed] [Google Scholar]
- 30.Ingraham L, AIken C. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology 1996; 10:120–4. [Google Scholar]
- 31.Mackinnon A. A spreadsheet for the calculation of comprehensive statistics for the assessment of diagnostic tests and inter-rater agreement. Comput Biol Med 2000; 30:127–34. [DOI] [PubMed] [Google Scholar]
- 32.Barnett JH, Lewis L, Blackwell AD, Taylor M. Early intervention in Alzheimer's disease: a health economic study of the effects of diagnostic timing. BMC Neurol 2014;14:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brouillette M-J, Mayo N, Fellow LK et al. A better screening tool for HIV-associated neurocognitive disorders: is it what clinicians need? AIDS 2015; 29:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonnell J, Haddow L, Daskalopoulou M et al. Minimal cognitive impairment in UK HIV-positive men who have sex with men: effect of case definitions and comparison with the general population and HIV-negative men. J Acquir Immune Defic Syndr 2014; 67:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCombe JA, Vivithanaporn P, Gill MJ, Power C. Predictors of symptomatic HIV-associated neurocognitive disorders in universal health care. HIV Med 2013; 14:99–107. [DOI] [PubMed] [Google Scholar]
- 36.Caniglia EC, Cain LE, Justice A et al. Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology 2014; 83:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gates TM, Cysique LA. The Chronicity of HIV Infection Should Drive the Research Strategy of NeuroHIV Treatment Studies: A Critical Review. CNS Drugs 2016; 30:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med 2010; 18:45–55. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.