Abstract
Objective: Natural orifice transluminal endoscopic surgery (NOTES) has recently become a hot spot in the field of minimally invasive surgery. But, most of the procedures are still in the early stages of development and limited to animal experiments. Transareolar endoscopic surgery could work as a viable intermediate step before thoracic NOTES. Under intravenous anesthesia without endotracheal intubation, transareolar endoscopic thoracic sympathectomy (ETS) with a flexible endoscope has rarely been attempted. The objective of this study is to evaluate the feasibility and safety of this novel minimally invasive technique in managing primary palmar hyperhidrosis (PPH).
Methods: From June 2012 to July 2014, a total of 58 male patients with severe PPH underwent transareolar ETS by use of a flexible endoscope. Under intravenous anesthesia without endotracheal intubation, a flexible endoscope was introduced through the incision on the edge of the areola into the thoracic cavity. The thoracic sympathetic chain was ablated at the level of the fourth rib.
Results: All procedures were successfully performed with a mean operating time of 33.6 ± 8.3 min. All patients regained consciousness rapidly and none of them complained about sore throat after surgery. There were no operative mortality and conversion to open procedure. The symptoms of all patients disappeared as soon as the sympathetic chain was cut off. Fifty six patients (96.6%) were discharged from the hospital on the first postoperative day. The postoperative complications were minor, and no patients developed Horner’s syndrome. At 3 months postoperatively, there was no obvious surgical scar on the chest wall, and none of the patients complained about postoperative pain. Compensatory hyperhidrosis (CH) appeared in 19 patients. No recurrent symptoms were observed in our study. One year follow-up revealed an excellent cosmetic result and degree of satisfaction.
Conclusion: Nonintubated transareolar ETS with a flexible endoscope is a safe, effective and minimally invasive therapeutic procedure, which has the possible advantages of thoracic NOTES and can be performed in routine clinical practice for male PPH patients.
Keywords: primary palmar hyperhidrosis, endoscopic thoracic sympathectomy, areolar approach, intravenous anesthesia
Introduction
Primary palmar hyperhidrosis (PPH) is a disorder characterized by excessive perspiration beyond physiological need, leading to severe psychological, social, and occupational dysfunction.1) The excessive sweating is primarily of the palms, but may involve sole and axilla as well.2) The degree of sweating is variable, ranging in severity from moderate moisture to severe dripping.3) The prevalence of PPH may vary in different population, such as 2.8% in the USA,4) 2.08% in China5) and even higher 12.76% in Japan.6) The cause of PPH still remains unclear. Many evidences imply that PPH could be a genetic disorder with an autosomal dominant mode of transmission.7,8) We have recently identified a novel locus on chromosome 2q31.1, and have provided direct evidence that PPH is a clinically and genetically heterogenous group of disorders.9)
Endoscopic thoracic sympathectomy (ETS) is currently the only effective and sustainable surgical treatment for disabling palmar hyperhidrosis.10) Under general anesthesia with endotracheal intubation, traditional ETS with 2–3 incisions offers a high level of safety and has reached a high therapeutic standard. But, this conventional procedure still leaves visible scars on the chest wall as well as pain on the trocar sites, and sometimes even causes complications associated with endotracheal intubation. Natural orifice transluminal endoscopic surgery (NOTES) has recently attracted great attention for its potential to avoid visible incisions, reduce postoperative pain and improve cosmetic results. However, concerns about problems of closure, postoperative puncture-site fistula, and high risk of infection, impede the clinical application of thoracic NOTES. Therefore, most of the procedures are still limited to the stage of animal experiments.
In recent years, with the continued development of endoscopic surgery, flexible endoscope has been used to diagnose and treat thoracic disease.11) Flexible endoscope will take more and more importance in our daily use in thoracic surgery. Instrument flexibility allows its use through minimally invasive approaches and offers an interesting intra-thoracic navigation. By hiding chest wall incision in the areola, transareolar ETS with a flexible endoscope can achieve the same objectives as thoracic NOTES. By avoiding problems of closure, postoperative fistula, and infection, transareolar ETS is safer than thoracic NOTES and offers better cosmetic results than does traditional ETS. Therefore, transareolar ETS could work as viable intermediate step before thoracic NOTES. Current development in anesthesia using thoracoscopic surgery without endotracheal intubation are safe and feasible for the diagnosis and treatment of pleural diseases.12–14) Such procedures can last up to half an hour with adequate patient tolerance and decreases complications associated with endotracheal intubation. This led us to question whether such anesthetic technique could be applied to the simple thoracic surgery of ETS.
In this article, we describe here the first use in China of a flexible endoscope through areolar incision to perform ETS under intravenous anesthesia without endotracheal intubation.
Patients and Methods
Patient selection
Between June 2012 and July 2014, 58 male patients with severe PPH (mean age of 24.3 years; range 17~48) underwent nonintubated transareolar ETS with flexible endoscope devices exclusively at our institution. Among them, 15 cases had positive family history, and 31 cases (53.4%) received non-surgical treatments before operation. The Distribution of hyperhidrosis was: 17 cases (29.3%) had palmar hyperhidrosis only; seven cases (12.1%) had palmar hyperhidrosis and axillary hyperhidrosis; 19 cases (32.8%) had palmar hyperhidrosis and plantar hyperhidrosis; 15 cases (25.9%) had palmar hyperhidrosis, axillary hyperhidrosis and plantar hyperhidrosis. This study was approved by the institutional review board and ethics committee of the First Affiliated Hospital of Fujian Medical University (No. 2012-17). All the patients received a preoperative routine blood examination, cardiological consulting and chest computed tomography scan to exclude lung, pleural and heart diseases. A detailed medical history, the degree of symptoms and distribution of excessive sweating were also documented. Informed consent was signed by all patients at least 1 day before operation after careful explanation of the procedure and goals of the study. Criteria for inclusion in this study were: age ≥16 years and ≤50 years; male patients with severe palmar hyperhidrosis; absence of thoracic surgery history and severe chest wall deformity. Criteria for exclusion were: plantar, axillary hyperhidrosis without palmar hyperhidrosis; lung, pleural and heart diseases that could increase surgical risk; secondary hyperhidrosis including hyperthyroidism, acute and chronic infections, malignancy, and immunologic disorders.
Surgical procedure
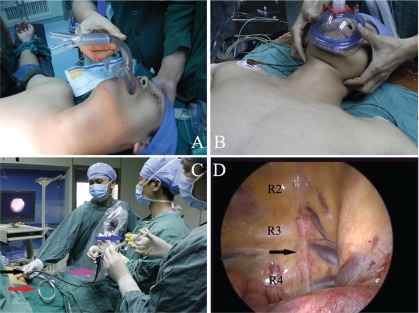
Surgery was performed under intravenous anesthesia with laryngeal mask or face mask (Figs. 1A and 1B), and endotracheal intubation was not needed. All patients were given propofol (1.5 mg/kg, i.v. injection) and fentanyl (50 µg, i.v. injection). Oxygen was delivered via laryngeal mask or face mask during the operation. The patient was placed on the operating table in a semi-sitting position thus exposing areola to allow a sequential bilateral procedure without the need for turning. A palmar temperature probe (Infrared thermometer GM700, Shenzhen, China) was placed on the thenar eminence and taped in place. Before operation, palmar temperature was kept less than 30°C by immersing the hand in water at 4°C. Before skin incision, baseline palmar temperature was recorded. The transareolar incision was characterized by a 5 mm incision in the fourth intercostals space on the edge of the areola. Then, a long trocar (20 cm length, 4.6 mm internal diameter, 0.4 mm wall thickness) was inserted into the thoracic cavity as a guide for a flexible endoscope (BF-MP60: 4.4 mm outer diameter, 2.0 mm inner diameter of instrumental channel, Olympus Medical Systems, Tokyo, Japan). The trocar was also used to establish pneumothorax, which was achieved by insufflating the thoracic cavity with carbon dioxide at 8 mmHg (Fig. 1C).
Fig. 1.
(A) and (B) show intravenous anesthesia with laryngeal mask and face mask, and endotracheal intubation was not required. (C) A flexible endoscope was introduced into the thoracic cavity through a long trocar. The coloured arrows indicate the following: black, 20-cm long trocar; red, pipe of carbon dioxide; pink, flexible endoscope; blue, hot biopsy forceps. (D) An endoscopic view of right thoracic cavity. The black arrow shows the sympathetic chain.
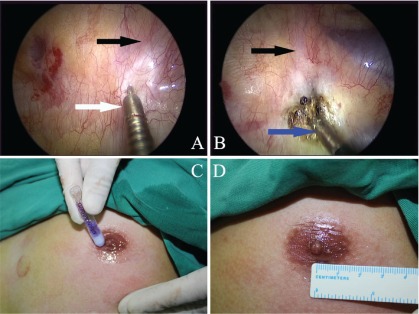
Usually, the first rib can not be seen in the thoracic cavity, so the uppermost rib that could be visualized is the second rib, followed by the third and fourth ribs (Fig. 1D). Before cutting the sympathetic chain, 2% lidocaine was injected into both sides of the sympathetic chain through the endoscopic syringe (Alton Medical Equipment C., Ltd., Shanghai, China) (Fig. 2A). Once the sympathetic chain was identified, a hot biopsy forceps (Alton Medical Equipment C., Ltd., Shanghai, China) was used to grasp and ablate the sympathetic chain crossing the fourth rib (R4) (Fig. 2B). The tissue adjacent to R4 was also interrupted laterally for approximately 2 cm to include any accessory nerve fibers (the nerve of Kuntz). The depth of the ablation would be to the periosteum of the rib. A palmar temperature increase of 1.5°C confirmed adequate sympathectomy. These measures would make sure that the sympathetic chain was ablated completely. After the sympathectomy was completed, the endoscope devices were then removed. A mild vacuum suction applied to re-expand the lung. At the same time, the anesthesiologist ventilated the patient manually, exerting continuous positive pressure for a few seconds, to prevent pneumothorax. At the end of the procedure, the incision was tightly pressed for a few seconds to make sure there was no active bleeding. The 5-cm incision was closed with Dermabond Skin Adhesive (Ethicon LLC, USA) (Figs. 2C and 2D). No sutures or dressing was needed. The entire procedure was then repeated on the opposite side without changing the position of the patient or the operation setting.
Fig. 2.
(A) The lidocaine was injected to the pleura adjacent to the nerve via endoscopic syringe before ablating the sympathetic chain. The black arrow shows the sympathetic chain. The white arrow shows the endoscopic syringe. (B) The R4 sympathetic chain was ablated by the hot biopsy forceps. The blue arrow shows the hot biopsy forceps. (C) Transareolar incision was closing with Dermabond Skin Adhesive. (D) A 5 mm incision was hidden in the areola after closing.
Follow-up and data collection
The operating time, resuscitation time, sore throat, palmar temperature rise, resolution of palmar hyperhidrosis, hospital stay, and complications were collected after surgery. The operating time was calculated from the time of skin incision to the application of the dressing over the incision. This excluded anesthesia induction and reversal time.
The patients were followed up for between 12 and 22 months. The mean duration was 14.2 ± 3.1 months. Patients were required to complete a detailed questionnaire (Table 1). Resolution of symptoms, cosmetic results, postoperative pain, compensatory hyperhidrosis (CH), and satisfaction scores were evaluated by hospital visits, telephone or e-mail.
Table 1.
Follow-up questionnaire
| Question response |
| 1. Results after operation Hands: worse/unchanged/become warm and dry Axillae: worse/unchanged/become warm and dry Feet: worse/unchanged/become warm and dry |
| 2. Postoperative pain Visual analogue scale: from 0 ‘no pain’ to 10 ‘worst pain imaginable’; mild (1–4)/moderate (5–7)/severe (8–10) |
| 3. Cosmetic results Verbal response scale: 1 dissatisfied/2 accepted/3 satisfied/4 perfect |
| 4. Compensatory hyperhidrosis No/yes Level: none/mild/moderate/severea Location: back, abdomen, lower extremities, thorax, other part of body |
| 5. Satisfaction Analogue visual scale: very satisfied (9–10)/satisfied (6–8)/dissatisfied (3–5)/very dissatisfied (0–2) |
aThe degree of postoperative compensatory hyperhidrosis was classified as mild, moderate, or severe, according to Cerfolio.10)
Results
All procedures were successfully performed. The mean operating time (mean ± SD) was 33.6 ± 8.3 (range, 26~68) min. During the operation, the vital signs of all patients were stable, and none of them needed conversion to endotracheal intubation. All patients regained consciousness rapidly. The mean resuscitation time (mean ± SD) of laryngeal mask anesthesia and face mask anesthesia was 5.1 ± 0.8 min and 4.6 ± 0.9 min, respectively. No patients complained about sore throat after surgery. There were no operative mortality and conversion to open procedure. The palms of all patients became dry and warm after surgery. The average rise in probe temperature after nerve ablation was 2.2°C. It usually occurred 1 to 5 min after the ablation. Hospital stay was very short, with 56 patients (96.6%) discharged on the first postoperative day, and the rest discharged on postoperative day 2. Postoperative pneumothorax was found on the chest X-ray in three patients, one of them resolved with pleural drainage and others did not need further intervention. No patients had intraoperative bleeding. Horner’s syndrome was not observed in the study. None of the patients had an infection of his wound.
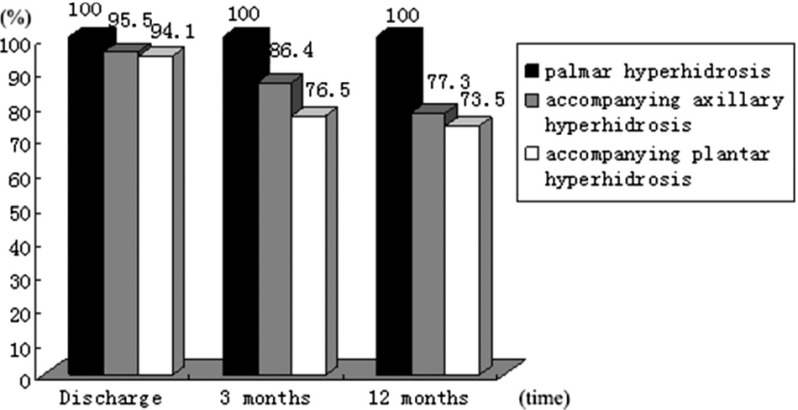
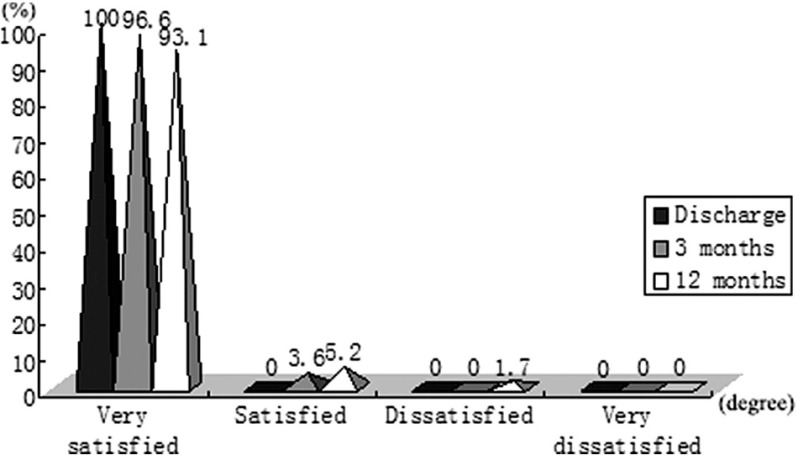
Follow-up was 100% completed. Forty-nine patients (84.5%) returned the questionnaire immediately. Another nine patients returned the questionnaire after a reminder or repeated mailing. The effectiveness at the time of discharge, 3 months and 12 months is presented in Fig. 3. No recurrent symptoms were observed in our study. Postoperative pain at the time of discharge affected 17 patients (29.3%). Among them, 15 cases were considered mild (88.2%) and two cases were considered moderate (11.8%), according to visual analogue scale (VAS). No patient required analgesia. None of the patients complained about postoperative pain 3 months after surgery. In our study, CH occurred in 19 patients (32.8%). Among them, 16 cases were mild and 3 cases were moderate. The most frequent locations were the back (52.6%), thorax (47.4%), abdomen (31.6%) and lower extremities (26.3%). As far as the evolution over time, three patients (15.8%) reported improvement, 15 patients (78.9%) had no change and one patient (5.3%) got worse. Our procedure had an excellent cosmetic result and degree of satisfaction (Fig. 4) during follow-up. The verbal response scale (mean ± SD) at the time of discharge, 3 months and 12 months after surgery was 3.66 ± 0.42, 3.74 ± 0.44 and 3.86 ± 0.35, respectively.
Fig. 3.
Effectiveness of transareolar ETS. ETS: endoscopic thoracic sympathectomy
Fig. 4.
Satisfaction degree of transareolar ETS. ETS: endoscopic thoracic sympathectomy
Discussion
Thoracoscopic sympathetic ganglion resection is an effective treatment for patients suffering from PPH. Its therapeutic mechanism might be to interrupt the transmission of impulses from sympathetic ganglia to eccrine sweat glands.15) Under general anesthesia with endotracheal intubation, conventional ETS is usually used in the treatment of patients with severe PPH. Although widely accepted, the procedure has some shortcomings to be improved. To begin with, endotracheal intubation is easy to cause postoperative sore throat, dysphagia, and even possible cardiopulmonary complications. Next, the cost of anesthesia with endotracheal intubation can run as high as 5000 RMB, which is more than nonintubated one. Finally, traditional ETS via 2–3 incisions still leaves visible scars on the chest wall that results in a permanent cosmetic defect, postoperative wound-related pain, numbness or paraesthesia. It is reported that the frequency of dysesthesias and pain in the thoracic wall is variable (5%–78%) and the main reason for these chronic postoperative problems is intercostal nerve injury when the trocars are introduced into the intercostal space.16) As PPH is prevalent in young people, cosmetic outcome is an important consideration in ETS. Attempts at further improving cosmetic outcome and reducing access trauma have resulted in the development of a series of minimally invasive surgical procedures, including single port ETS.17) As for the ‘single port’ surgery, the name of the procedure may be somewhat misleading. The ‘single port’ is actually larger than the traditional incision and thus allows two surgical instruments to operate in the same port. The procedure may lead to increased postoperative pain and poor cosmetic results.
In order to overcome the shortcomings mentioned above, we performed transareolar ETS with a flexible endoscope. The results are in line with previous reports of ETS.18,19) Drawing on the method of intravenous sedation used for gastroscopy, we used intravenous anesthesia instead of general anesthesia with endotracheal intubation. As we only used fentanyl and propofol, the patients did not experience or remember pain but are capable of breathing on their own during operation. Moreover, 2% lidocaine was injected to the pleura adjacent to the nerve via endoscopic syringe to prevent pleural reaction and arrhythmia while ablating the sympathetic chain. All patients waked up quickly from anesthesia, and none of them complained about sore throat after surgery. The effectiveness of palmar hyperhidrosis was 100% with a rate of total satisfaction (satisfied plus very satisfied) of 98.3% at the time of 12 months postoperatively. Complications were minor, and none of them required open surgery. Pneumothorax was the most common early postoperative complication, and only 1 case required a pleural drainage for one day in our study. However, an exertion of continuous positive pressure for a few seconds in coordination with the anesthesiologist and the application of a mild suction before the closing of the skin incision are essential to prevent pneumothorax. The frequency of Horner’s syndrome is variable (0.5%–17%), and its pathogenesis is direct or indirect lesions of the stellate ganglion.20) In our study, no patients developed Horner’s syndrome, the possible reason was that the sympathetic level of R4 transection is far from the stellate ganglion. Incidence rates of recurrence vary considerably and have been described as ranging 1%–27%.21) The most common causes are incomplete thoracic sympathectomy, anatomic variability of the sympathetic chain, or nerve regeneration. In our series, there was no recurrence observed during the follow-up period. The reason was that the sympathetic ganglion and the nerve of Kuntz were completely ablated. CH is the most undesirable side effect of ETS, which occurs in the literature at a rate of 3% to 98%.10) This wide variability may be attributable to different level of nerve ablation, emotional stress, or, more importantly, to a variety of definitions of CH. In our experience, CH occurred in 32.8% of patients and most of them were mild and moderate. The most affected areas were back, thorax, abdomen and lower extremities. But the symptoms were not severe enough to interfere with lifestyle, and this required no further treatment. The pathogenesis of CH is still unknown. It is postulated that this phenomenon is caused by a temperature-regulating compensatory mechanism in the body and is correlated with the extent of sympathectomy.15)
The newest trend in the field of thoracic surgery, thoracic NOTES are emerging as alternatives to the traditional endoscopic thoracic surgery with the advantages of reducing postoperative pain and avoiding visible scars on the chest wall. Thoracic NOTES includes transtracheal, transesophageal, transoral, or transumbilical endoscopic access to the thoracic cavity.22–25) However, concerns about longer operative time, complicated surgical skills, and serious complications impede the widespread adoption of this new procedure. So far, most of thoracic NOTES procedures are still in the early stages of development and limited to animal experiments. However, our procedure avoids the risk of postoperative puncture-site fistula, mediastinitis, or diaphragmatic hernia—the shortcomings of thoracic NOTES, and could work as a viable intermediate step before thoracic NOTES.
Compared with traditional ETS, this newly designed procedure is more minimally invasive and has several potential advantages. Firstly, the endoscope is so flexible that the head part of it can reach every site of the thoracic cavity. Secondly, the endoscopic syringe, hot biopsy forceps and vacuum suction can be used through the instrumental channel without the need for extra ports, which avoids the inconvenience caused by the thoracoscope and other surgical instruments operating together through one single incision in conventional single-port ETS. Thirdly, with endoscopic instruments operated in a single incision through instrumental channel, our procedure greatly reduces postoperative wound-related pain, numbness and paraesthesia resulted from the injury of intercostal nerve around the incision. Lastly, the newly designed procedure achieves a perfect cosmetic result by hiding incisions in the areola. Three months later, there were no obvious surgical scars on the chest wall of the patients.
While an improvement over the traditional method, the described procedure still has some flaws. First of all, as this was the primary evaluation of such a technique, we restricted the criteria for inclusion to male patients. Moreover, the flexible endoscope was too thin and soft, lacking force transmission when it entered the thoracic cavity. So a long trocar was needed to guide its operative direction during operation. Finally, this study had a small number of patients and a short follow-up time. More patients with a longer follow-up are needed to evaluate the long-term outcomes of this procedure.
Conclusion
This study demonstrates that nonintubated transareolar ETS with a flexible endoscope is a safe, effective and minimally invasive therapeutic procedure for PPH, which gives an excellent cosmetic and clinical outcome. This procedure is a promising approach and can be performed in routine clinical practice for male patients.
Disclosure Statement
Jianfeng Chen, Jianbo Lin, Yuanrong Tu, Min Lin, Xu Li, and Fancai Lai have no conflicts of interest or financial ties to disclose.
Acknowledgments
This work was supported by Natural Science Foundation of China [No. 81070906], Natural Science Foundation of Fujian Province [No. 2013J01303] and Key Program of Scientific Research of Fujian Province [No. 2015-ZQN-ZD-22].
References
- 1).Sato K, Kang WH, Saga K, et al. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol 1989; 20: 713-26. [DOI] [PubMed] [Google Scholar]
- 2).Adar R, Kurchin A, Zweig A, et al. Palmar hyperhidrosis and its surgical treatment: a report of 100 cases. Ann Surg 1977; 186: 34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Lai YT, Yang LH, Chio CC, et al. Complications in patients with palmar hyperhidrosis treated with transthoracic endoscopic sympathectomy. Neurosurgery 1997; 41: 110-3; discussion 113-5. [DOI] [PubMed] [Google Scholar]
- 4).Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol 2004; 51: 241-8. [DOI] [PubMed] [Google Scholar]
- 5).Lai FC, Tu YR, Li YP, et al. Nation wide epidemiological survey of primary palmar hyperhidrosis in the People’s Republic of China. Clin Auton Res 2015; 25: 105-8. [DOI] [PubMed] [Google Scholar]
- 6).Fujimoto T, Kawahara K, Yokozeki H. Epidemiological study and considerations of primary focal hyperhidrosis in Japan: from questionnaire analysis. J Dermatol 2013; 40: 886-90. [DOI] [PubMed] [Google Scholar]
- 7).Ro KM, Cantor RM, Lange KL, et al. Palmar hyperhidrosis: evidence of genetic transmission. J Vasc Surg 2002; 35: 382-6. [DOI] [PubMed] [Google Scholar]
- 8).Kaufmann H, Saadia D, Polin C, et al. Primary hyperhidrosis—evidence for autosomal dominant inheritance. Clin Auton Res 2003; 13: 96-8. [DOI] [PubMed] [Google Scholar]
- 9).Chen J, Lin M, Chen X, et al. A novel locus for primary focal hyperhidrosis mapped on chromosome 2q31. 1. Br J Dermatol 2015; 172: 1150-3. [DOI] [PubMed] [Google Scholar]
- 10).Cerfolio RJ, De Campos JR, Bryant AS, et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg 2011; 91: 1642-8. [DOI] [PubMed] [Google Scholar]
- 11).Assouad J, Fénane H, Masmoudi H, et al. Flexible endoscope in thoracic surgery: CITES or cVATS? Rev Pneumol Clin 2013; 69: 294-7. [DOI] [PubMed] [Google Scholar]
- 12).Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 2012; 93: 1049-54. [DOI] [PubMed] [Google Scholar]
- 13).Mineo TC, Sellitri F, Tacconi F, et al. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17: 761-8. [DOI] [PubMed] [Google Scholar]
- 14).Hung MH, Cheng YJ, Chan KC, et al. Nonintubated uniportal thoracoscopic surgery for peripheral lung nodules. Ann Thorac Surg 2014; 98: 1998-2003. [DOI] [PubMed] [Google Scholar]
- 15).Li X, Tu YR, Lin M, et al. Endoscopic thoracic sympathectomy for palmar hyperhidrosis: a randomized control trial comparing T3 and T2-4 ablation. Ann Thorac Surg 2008; 85: 1747-51. [DOI] [PubMed] [Google Scholar]
- 16).Dumont P, Denoyer A, Robin P. Long-term results of thoracoscopic sympathectomy for hyperhidrosis. Ann Thorac Surg 2004; 78: 1801-7. [DOI] [PubMed] [Google Scholar]
- 17).Lardinois D, Ris HB. Minimally invasive video-endoscopic sympathectomy by use of a transaxillary single port approach. Eur J Cardiothorac Surg 2002; 21: 67-70. [DOI] [PubMed] [Google Scholar]
- 18).Jeganathan R, Jordan S, Jones M, et al. Bilateral thoracoscopic sympathectomy: results and long-term follow-up. Interact Cardiovasc Thorac Surg 2008; 7: 67-70. [DOI] [PubMed] [Google Scholar]
- 19).Liu Y, Yang J, Liu J, et al. Surgical treatment of primary palmar hyperhidrosis: a prospective randomized study comparing T3 and T4 sympathicotomy. Eur J Cardiothorac Surg 2009; 35: 398-402. [DOI] [PubMed] [Google Scholar]
- 20).Singh B, Moodley J, Allopi L, et al. Horner syndrome after sympathectomy in the thoracoscopic era. Surg Laparosc Endosc Percutan Tech 2006; 16: 222-5. [DOI] [PubMed] [Google Scholar]
- 21).Rodríguez PM, Freixinet JL, Hussein M, et al. Side effects, complications and outcome of thoracoscopic sympathectomy for palmar and axillary hyperhidrosis in 406 patients. Eur J Cardiothorac Surg 2008; 34: 514-9. [DOI] [PubMed] [Google Scholar]
- 22).Liu YH, Liu HP, Wu YC, et al. Feasibility of transtracheal thoracoscopy (natural orifice transluminal endoscopic surgery). J Thorac Cardiovasc Surg 2010; 139: 1349-50. [DOI] [PubMed] [Google Scholar]
- 23).Rolanda C, Silva D, Branco C, et al. Peroral esophageal segmentectomy and anastomosis with single transthoracic trocar: a step forward in thoracic NOTES. Endoscopy 2011; 43: 14-20. [DOI] [PubMed] [Google Scholar]
- 24).Ko PJ, Chu Y, Wu YC, et al. Feasibility of endoscopic transoral thoracic surgical lung biopsy and pericardial window creation. J Surg Res 2012; 175: 207-14. [DOI] [PubMed] [Google Scholar]
- 25).Zhu LH, Wang W, Yang S, et al. Transumbilical thoracic sympathectomy with an ultrathin flexible endoscope in a series of 38 patients. Surg Endosc 2013; 27: 2149-55. [DOI] [PubMed] [Google Scholar]