Abstract
Purpose: Malignant pleural effusion (MPE) is common in patients with advanced cancer. Chemical pleurodesis can be considered for MPE that do not respond to chemotherapy, radiotherapy, or therapeutic thoracentesis. However, it is not yet clear which agent is more effective and safer in chemical pleurodesis.
Methods: This study was designed as a single arm, multicenter, and open-label phase III clinical trial to evaluate efficacy and safety of chemical pleurodesis using mistletoe extraction (ABNOVAviscum® Injection). References of other agents in chemical pleurodesis were investigated to compare efficacy and safety. Efficacy was evaluated by followed up chest X-ray and changes of clinical symptoms and Karnofsky performance scale. Safety was evaluated by serious adverse event (SAE) and changes of laboratory findings. A follow-up period was 4 weeks after last pleurodesis.
Results: Of 62 patients, 49 (79.0%) had complete response, 11 (17.7%) had partial response, and two had no response. Mean response rate was significantly different in this study comparing with reference response rate which was 64% (p <0.0001). There were two SAEs, but all were recovered without sequelas.
Conclusion: The results of this study suggest that mistletoe extraction (ABNOVAviscum® Injection) could be an effective and safe agent of chemical pleurodesis in patients with MPE.
Keywords: malignant pleural effusion, chemical pleurodesis, mistletoe extraction
Introduction
Malignant pleural effusion (MPE) is common in patients with advanced cancer and primary tumors are mainly lung cancer, breast cancer, lymphoma, ovarian cancer and gastric cancer. These malignancies account for 80% of all MPE1–5) and unknown origin MPE is about 10%.6,7) MPE are usually related to disseminate disease and median survival is from 3 to 12 months depending on cell type.5) Management for MPE is primarily focused on the relief of symptoms and the prevention of recurrent pleural effusion. Of treatment modalities for MPE, chemical pleurodesis can be considered for MPE that do not respond to chemotherapy, radiotherapy, or therapeutic thoracentesis. But because the effect of chemical pleurodesis is different according to used agent, it is important to choose a more effective and safer agent in chemical pleurodesis. Although there are many reports about effective and safe chemical agent, it is not yet clear which is better. Of various agents, talc is known as a very effective agent however, small-particle talc could rarely develop acute respiratory distress syndrome. Unfortunately, large-particle talc known as safe agent for acute respiratory distress syndrome is not available in some countries including Korea. Bleomycin is less cost effective and tetracycline commonly used in the past is not available nowadays. Mistletoe extraction which is used in this study has been known as agent having anti-tumoral effect and immune modulation, but efficacy and safety of this agent in chemical pleurodesis for MPE need to be investigated.
Materials and Methods
This study was designed as a single arm, multicenter, and open-label phase III clinical trial to evaluate efficacy and safety of chemical pleurodesis using ABNOVAviscum® Injection (Abnoba GmbH. Germany), and was conducted in single-arm as the setting of the control group was not possible due to the lack of standard remedy for pleural effusions. Therefore, references of other agents in chemical pleurodesis were investigated to compare efficacy and safety and statistical sample size of study group was decided.
This study was carried out ethically and scientifically in accordance with Korea Good Clinical Practice (KGCP) and the Declaration of Helsinki. It was also conducted according to the approved protocol of Institutional Review Board (IRB) of Ministry of Food and Drug Safety (MFDS) and all institutions. After a full explanation, all patients participated in the study have signed the written informed consent form and all process and data of the study were audited by the monitor.
This study was sponsored by ABNOVA Korea and ABNOVA GmbH and there was no other funding agency’s role.
Selection of study population
Inclusion criteria
Male and female aged 20 to 80 years
-
Necessity of chemical pleurodesis among patients with MPE
When repeated thoracostomy for MPE is needed
Even though there is a persistent drainage of MPE during chest tube placement (>300 cc/day)
Full lung expansion must be achieved within 12 to 24 h after pleural drainage8,9)
Expected survival of at least 2 months
Patients who score 50 or more on the Karnofsky performance scale (KPS)10)
Exclusion criteria
Previous attempts at pleurodesis with sclerosing agents
Adverse drug reactions to mistletoe agents
Patients having participated in another clinical study other than the present study
Patients taking immune-suppressive agents
Medical and psychiatric contraindications for the study drug
Not allowed to participate in the study by legal requirements
Not allowed to participate in the study by the Investigator’s judgment
Treatments administered
When pleural effusion was drained and the lung was confirmed of full expansion by a chest X-ray, treatment was performed. Treatment method is as below.
Remove the maximum amount of pleural fluid.
Mix five amples of ABNOVAviscum® F20mg Injection with 0.9% normal saline to inject the resulting mixture into the pleural space.
Determine a dosing interval (3 to 7 days) according to amounts of newly-generated pleural effusion and carry out the treatment until newly-generated pleural effusion has been resolved (<4 mm/kg/day).
Measures of efficacy and safety data
Efficacy variables
1. Primary efficacy
The response rate (complete response (CR) and partial response (PR)) in pleural effusion were assessed as the primary efficacy variables by a simple X-ray examination at the close-out visit (4 weeks after the last pleurodesis).
‹Classification Criteria for Response Rate (WHO criteria)›13,14)
-
Complete Response:
Response with no replenishment of pleural effusion within 4 weeks after the last pleurodesis
-
Partial Response:
Response that does not need additional drainage since, in chest X-ray findings, there is the replenishment of pleural effusion under 50% of pleural effusion at the pre-treatment, but there are no symptoms
-
No Response
Response that needs additional treatment due to the recurrence of pleural effusion within 4 weeks after the last pleurodesis
Not Evaluable
2. Secondary efficacy
Changes in clinical symptoms and KPS at the pre-treatment and close-out visit were assessed as the secondary efficacy variables.
Safety variables
The safety variables were adverse events newly occurred after injecting the study drug and the results of vital signs, physical examinations and clinical laboratory tests performed at each visit.
Eligible patients: All patients who have had more than one infusion of the investigational agent and of whom the safety related data were verified by the investigator after the infusion.
1. Adverse Events
Adverse Event (AE) means an undesirable and unintended sign or symptom or disease which is occurred in a patient during the study. An AE is not required to the causal relation with the investigational agent.
-
Serious adverse event (SAE) means one of the following AEs resulting from the investigational agent used for the study:
death during the study period;
life- threatening;
persistent or significant disability or decreased function;
admission to hospital or lengthens the period of existing hospitalization;
congenital deformity or anomaly;
clinically important situation
2. Evaluation for other safety data
Liver function tests and kidney function tests at the pre-treatment and close-out visit were performed to observe changing aspects of liver and kidney functions. And basic physical examinations including patient’s height, weight, blood pressure and pulse were performed to observe significant changes at the close-out visit compared to the screening visit, and whether other special changes occurred at the post-treatment by a questionnaire survey was confirmed.
Statistical analysis methods
Analysis of primary efficacy variables was carried out one sample binomial test for evaluating the response rate (CR plus PR) in pleural effusion. 64% of hypothetical proportion14,15) was used and a two-sided test was used. For secondary efficacy variables, at less than 5% of significance level, changes in the KPS using a paired t-test were analyzed. SAS 9.3 was used for a statistical analysis program.
Determination of subject size
The study was to evaluate the response rate in pleural effusion by a simple chest X-ray test at close-out visit.
Level of significance, α = 0.05.
Type II error (β) is 0.2 to maintain 80% of power of the test.
Response rate of reference for other agents in chemical pleurodesis as 64%14,15) and AbnobaViscum®’s expected response rate of 81%15,16) were assumed from previous studies.
The sample size was calculated by using the study size 2.0 program (Creostat HB 2001–2007).
The calculated sample size is 56, and it was estimated that 68 patients were required considering 20% of dropout rate.
Results
Characteristics of patients
68 patients were enrolled and, of them, 6 were dropped out. The median age was 58 years old [range, 33–80 years] and female was 39 (62.9%). The median duration of tumors was 16 months [range, 1–227 months]. Divided by category, ‘under 1 year’ was the highest with 41.9% (26/62 patients) followed by ‘1 to 2 years’ with 21.0% (13/62 patients), ‘2 to 3 years’ with 11.3% (7/62 patients) and others with 25.8%. In the present metastasis state, the metastasis was observed in 61 patients and most frequent in lung with 37 patients (59.7%). The result of patients’ past medical history showed 55 patients (88.7%) with medical history (total 226 cases). In detail, there were 47 cases (20.8%, 47/226) of ‘previous surgery’, 19 (8.4%) of ‘respiratory, thoracic and mediastinal disorders’, 18 (8.0%) of ‘gastrointestinal disorders’, 17 (7.5%) of ‘infections’, and so on (Table 1).
Table 1.
Characteristics of the patients at baseline
| n = 62 | ||
| Age (years old) | Median [range] | 58.00 [33.00~80.00] |
| Age, Category | 30 to 40 years | 3 (4.8%) |
| 40 to 50 years | 9 (14.5%) | |
| 50 to 60 years | 20 (32.3%) | |
| 60 to 70 years | 12 (19.4%) | |
| Over 70 years | 18 (29.0%) | |
| Sex (n) | male:female | 23:39 |
| Primary cancer | Diagnosis | |
| Lung cancer | 28 (45.16%) | |
| Breast cancer | 8 (12.9%) | |
| Colon cancer | 1 (1.61%) | |
| Gastric cancer | 1 (1.61%) | |
| Ovarian cancer | 4 (6.45%) | |
| Pancreatic cancer | 1 (1.61%) | |
| Papillary thyroid cancer | 1 (1.61%) | |
| Plasmacytoma | 1 (1.61%) | |
| Others | 17 (27.41%) | |
| Duration of tumors (month) | Median [range] | 16.00 [1.0~227.0] |
| Duration of tumors, Category | Under 1 | 26 (41.9%) |
| 1 to 2 | 13 (21.0%) | |
| 2 to 3 | 7 (11.3%) | |
| 3 to 5 | 6 (9.6%) | |
| 5 to 10 | 5 (8.1%) | |
| Over 10 | 5 (8.1%) | |
| Present-metastasis state (n) | Existence:None | 61:1 (98.4%:1.6%) |
| Past medical history (n) | Existence:None | 55:7 (88.7%:11.3%) |
n: number of patients
Characteristics of pleural effusion
The average duration of pleural effusion was 2.50 months [range, 1–21 months]. 36 patients (58.1%) had pleural effusion on the right side, 22 (35.5%) on the left side and four (6.4%) on both sides. In the case with both sides, the right side which is the most severe site was selected for treatment. 41 patients (66.1%) had symptoms induced by the pleural effusion: dyspnea in 30 patients (48.4%, 30/62 patients); pleurodynia in 14 (22.6%), cough in seven (11.3%), loss of appetite in two (3.2%) and sleep disturbance in one (1.6%) (Table 2).
Table 2.
Characteristics of pleural effusion
| n = 62 | ||
| Duration of pleural effusions (month) | Mean ± Std [range] | 2.50 ± 4.25 [1.00~21.00] |
| Side of pleural effusions | Right | 36 (58.1%) |
| Left | 22 (35.5%) | |
| Both | 4 (6.4%) | |
| Symptoms related to pleural effusion | Presence:none | 41:21 (66.1%:31.9%) |
| Dyspnea | 30 (48.4%) | |
| Cough | 7 (11.3%) | |
| Pleurodynia | 14 (22.6%) | |
| Sleep disturbance | 1 (1.6%) | |
| Loss of appetite | 2 (3.2%) | |
n: number of patients
Mistletoe-mediated pleurodesis
The dosing interval was determined according to the degree of pleural adhesion and the amount of the newly-generated pleural effusion, and the treatment was conducted until the pleural adhesion occurred. The pleural adhesion occurred mostly after one mistletoe-mediated pleurodesis. Looking at the actual number of treatment times, 50 patients (80.65%) had one pleurodesis, seven (11.29%) had 2, two patients (3.23%) each had 3 and 4, and one patient (1.61%) had 5 times. The average drainage duration and total amount of pleural effusion in the last pleurodesis was 6.27 days [range, 2–15 days] and 103.95 ml [range, 0–820 ml], respectively.
In the last pleurodesis, 33 patients (53.2%, 33/62) had no symptoms, 18 (29.0%) had pleurodynia, 12 (19.3%) had dyspnea, four (6.5%) had cough and two (3.2%, 2/62) had loss of appetite (Table 3).
Table 3.
Change of symptoms related to pleural effusion in the last pleurodesis
| (n = 62) | Pre-treatment | Post-treatment |
|---|---|---|
| No symptom | 21 (31.9%) | 33 (53.2%) |
| Dyspnea | 30 (48.4%) | 12 (19.3%) |
| Cough | 7 (11.3%) | 4 (6.5%) |
| Pleurodynia | 14 (22.6%) | 18 (29.0%) |
| Sleep disturbance | 1 (1.6%) | 0 |
| Loss of appetite | 2 (3.2%) | 2 (3.2%) |
n: number of patients
Efficacy evaluation
-
Primary efficacy variables
There were 49 CRs (79.03%), 11 PRs (17.74%), and 2 NRs (3.23%) out of the total 62 patients, resulting in overall response rate of 96.77% (60/62 patients). A binominal test for CR and PR was performed with a reference response rate of 64.0%. There was significant difference between the study and reference response rate (p <0.0001).14,15)
-
Secondary efficacy variables
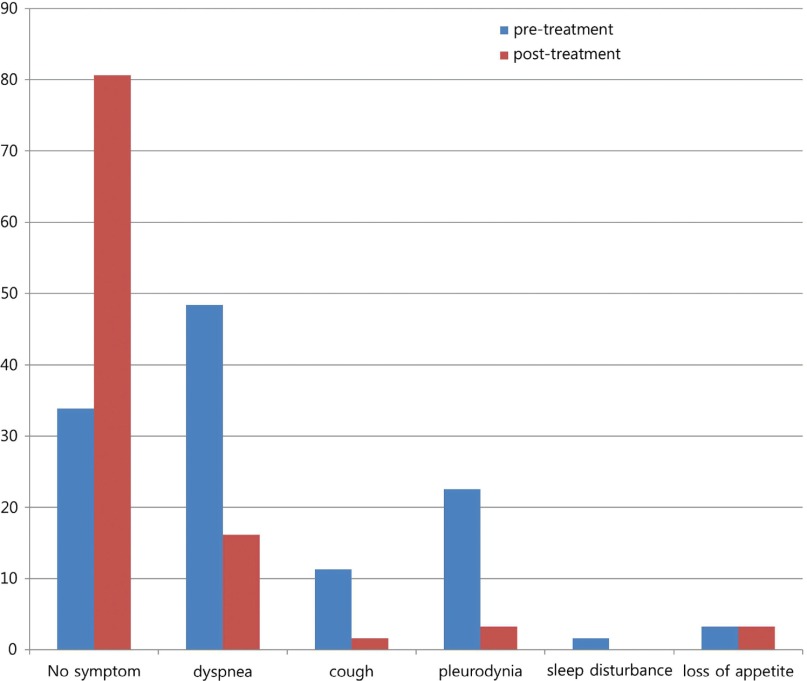
In the evaluation for change of clinical symptoms conducted at the close-out visit, 50 patients (80.7%, 50/62 patients) had no symptoms, 10 (16.1%) had dyspnea, two (3.2%) had pleurodynia, two (3.2%) had loss of appetite and one (1.6%) had cough (Fig. 1). And average changes in the KPS at the close-out visit compared to the screening visit were not statistically significant (1.48 ± 13.14%, p = 0.38).
Fig. 1.
Change of symptoms related to pleural effusion at the close-out visit. In the evaluation for change of clinical symptoms conducted at the close-out visit, the proportion of patients without symptom was increased from 31.9% to 80.7%, and the proportion of dyspnea, cough, and pleurodynia was decreased from 48.4% to 16.1%, from 11.3% to 1.6%, and from 22.6% to 3.2%, respectively.
Safety evaluation
AE means an undesirable and unintended sign or symptom or disease which is occurred in a patient during the study and an AE is not required to the causal relation with the investigational agent. During the conduct of the study, 309 cases AEs occurred in 61 out of the total 68 patients including six patients dropped out, resulting in 89.71% of incidence rate (IR). The most frequent AE was gastrointestinal disorders (25.89%, 80 cases). In detail, constipation (22 cases) was the most frequent AE, followed by nausea (17), dyspepsia (9), vomiting (9), and diarrhea (8). The second most frequent AE was general symptoms and localized symptoms around administration site (72 cases, 23.30%). In detail, pyrexia (39 cases) was the second most frequent AE, followed by pain (13) and chills (7). Of 309 cases, there were 42 cases AEs that cannot exclude casual relationship with the study drug in 27 patients (IR, 39.71%). Of these 42 cases of AEs, the most frequent AE was general symptoms and localized symptoms around administration site (32 cases, 76.19%). In detail, the most frequent AE was pyrexia (27), followed by chills (3), fatigue (1), and pain (1). These AEs were fully recovered. And two SAE that cannot exclude casual relationship with the study drug occurred in one patient (IR, 1.47%). A serious pleuritis and pain were included in SAE and recovered without sequela.
The changes of weight (p = 0.0001), systolic blood pressure (p = 0.0216), and pulse (p = 0.0008) were statistically significant, but not clinically significant. In a clinical laboratory test at the close-out visit compared to the screening visit, there were no significant changes in total bilirubin (p = 0.055), alanine transaminase (ALT) (p = 0.90), aspartate aminotransferase (AST) (p = 0.53), creatinine (p = 0.19), and blood urea nitrogen (BUN) (p = 0.52).
Discussion
An MPE is common in patients with advanced cancer and occurs as a result of obstruction of the pleural lymphatic drainage which results from the metastases of pleural and mediastinal lymph node in lung cancer, breast cancer, or gastric cancer. Although survival of patients with MPE is relatively poor, systemic chemotherapy, radiation treatment, or therapeutic thoracentesis have been attempted to treat MPE and the chemical pleurodesis can be considered for the patients who do not respond to above modalities. However, the effect of chemical pleurodesis is different according to used agent, so it is important to choose a more effective and safer agent in chemical pleurodesis. In this study, mistletoe extraction (ABNOVAviscum® Injection) was used as chemical agent for chemical pleurodesis in patients with MPE to investigate its therapeutic efficacy and safety, in order to overcome the problems of the existing agents. Mistletoe extraction (ABNOVAviscum® Injection) is known as agent having anti-tumoral effect and immune modulation and has been used in some cancer patients in various ways. And the mechanism of mistletoe-mediated pleurodesis is known as stimulation of antitumor immunity rather than mechanical sclerosis.17)
The primary efficacy suggested in this study was response rate of mistletoe-mediated pleurodesis in MPE by a simple X-ray test at the close-out visit (4 weeks after the last pleurodesis), resulting in overall response rate of 96.77% (60/62 patients). In other word, mistletoe-mediated pleurodesis showed a higher response rate than other chemical pleurodesis in patients with MPE and there was significant difference between this study and a reference response rate (p <0.0001).14,15) The secondary efficacy variables were changes in clinical symptoms (dyspnea, cough, sleep disturbance, pain, etc.) and in the KPS at the pre-treatment and close-out visit. Several studies with other agents reported pleurodesis related clinical symptoms were developed as chest pain (18% to 36%), fever (8% to 41%), nausea and vomiting (5%).18) In the evaluation for change of clinical symptoms conducted at the close-out visit, it was investigated that 50 patients (80.65%, 50/62 patients) had no symptoms. In other words, the proportion of patients without symptom was increased from 31.9% to 80.7%, and the proportion of dyspnea, cough, and pleurodynia was decreased from 48.4% to 16.1%, from 11.3% to 1.6%, and from 22.6% to 3.2%, respectively (Fig. 1). This suggests that clinical symptoms tended to be improved after mistletoe-mediated pleurodesis. There was no significant improvement in KPS at the close-out visit, but this might be interpreted as few damage of mistletoe-mediated pleurodesis itself to patients.
The safety variables assessed in this study were AEs and change in physical measurements and vital signs and laboratory test at the pre-treatment and post-treatment. In this study, of the total 309 cases AEs, there were 42 cases AEs that cannot exclude casual relationship with the study drug and, of 42 cases, the most frequent AE was pyrexia. Comparing the occurred AEs to the existing drug safety profile of AbnobaViscum® Injection, we confirmed that pyrexia of 32.35% out of the total 68 patients was an expectable AE and that pyrexia occurred due to an infusion of AbnobaViscum® Injection does not need to be treated with an antifebrile, because pleural inflammation is a therapeutic response generating in the mechanism of pleural adhesion and is known to cause the adhesion.19,20) Although antibiotics were administrated to the concerned patients to treat the inflammation caused by elevation of inflammatory factors (creative protien (CRP) etc.), the elevation of the inflammatory factors (white blood cell (WBC), erythrocyte sedimentation rate (ESR), CRP etc.) after the pleurodesis is an expected response, hence it is advised not to administer antibiotics.21) The two cases of SAE mentioned in results recovered without sequela. The changes in height, weight, blood pressure and pulse at the close-out visit compared to the screening visit were statistically significant, but these changes were not clinically significant. There were no significant changes in clinical laboratory tests performed at the close-out visit compared to the screening visit.
Conclusion
The results of this study suggest that mistletoe extraction (ABNOVAviscum® Injection) could be an effective and safe agent of chemical pleurodesis in patients with MPE.
Disclosure Statement
This study was granted by Abnoba Korea and Abnoba GmbH and it was presented at the 15th World Conference on Lung cancer in 2013.
References
- 1).Kastelik JA. Management of malignant pleural effusion. Lung 2013; 191: 165-75. [DOI] [PubMed] [Google Scholar]
- 2).Gompelmann D, Eberhardt R, Herth FJ. Advanced malignant lung disease: what the specialist can offer. Respiration 2011; 82: 111-23. [DOI] [PubMed] [Google Scholar]
- 3).Sears D, Hajdu SI. The cytologic diagnosis of malignant neoplasms in pleural and peritoneal effusions. Acta Cytol 1987; 31: 85-97. [PubMed] [Google Scholar]
- 4).DiBonito L, Falconieri G, Colautti I, et al. The positive pleural effusion. A retrospective study of cytopathologic diagnoses with autopsy confirmation. Acta Cytol 1992; 36: 329-32. [PubMed] [Google Scholar]
- 5).Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65 Suppl 2: ii32-40. [DOI] [PubMed] [Google Scholar]
- 6).Salyer WR, Eggleston JC, Erozan YS. Efficacy of pleural needle biopsy and pleural fluid cytopathology in the diagnosis of malignant neoplasm involving the pleura. Chest 1975; 67: 536-9. [DOI] [PubMed] [Google Scholar]
- 7).Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 1985; 56: 905-9. [DOI] [PubMed] [Google Scholar]
- 8).Diacon AH, Wyser C, Bolliger CT, et al. Prospective randomized comparison of thoracoscopic talc poudrage under local anesthesia versus bleomycin instillation for pleurodesis in malignant pleural effusions. Am J Respir Crit Care Med 2000; 162: 1445-9. [DOI] [PubMed] [Google Scholar]
- 9).Antunes G, Neville E, Duffy J, et al. BTS guidelines for the management of malignant pleural effusions. Thorax 2003; 58 Suppl 2: ii29-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Burrows CM, Mathews WC, Colt HG. Predicting survival in patients with recurrent symptomatic malignant pleural effusions: an assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest 2000; 117: 73-8. [DOI] [PubMed] [Google Scholar]
- 11).Medford AR, Maskell N. Pleural effusion. Postgrad Med J 2005; 81: 702-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Lee YC, Light RW. Management of malignant pleural effusions. Respirology 2004; 9: 148-56. [DOI] [PubMed] [Google Scholar]
- 13).WHO Handbook for reporting results of cancer treatment. Geneva(Switzerland): World Health Organization Offset Publication No. 4 8; 1979: 23. [Google Scholar]
- 14).Kessinger A, Wigton RS. Intracavitary bleomycin and tetracycline in the management of malignant pleural effusions: a randomized study. J Surg Oncol 1987; 36: 81-3. [DOI] [PubMed] [Google Scholar]
- 15).Walker-Renard PB, Vaughan LM, Sahn SA. Chemical pleurodesis for malignant pleural effusions. Ann Intern Med 1994; 120: 56-64. [DOI] [PubMed] [Google Scholar]
- 16).Kim JJ, Lee SK, Im J, et al. Viscum album therapy in malignant pleural effusion. Korean J Thorac Cardiovasc Surg 2004; 37: 978-82. [Google Scholar]
- 17).Stumpf C, Büssing A. Stimulation of antitumour immunity by intrapleural instillation of a Viscum album L. extract. Anticancer Drugs 1997; 8 Suppl 1: S23-6. [DOI] [PubMed] [Google Scholar]
- 18).Parulekar W, Di Primio G, Matzinger F, et al. Use of small-bore vs large-bore chest tubes for treatment of malignant pleural effusions. Chest 2001; 120: 19-25. [DOI] [PubMed] [Google Scholar]
- 19).Rodriguez-Panadero F, Antony VB. Pleurodesis: state of the art. Eur Respir J 1997; 10: 1648-54. [DOI] [PubMed] [Google Scholar]
- 20).Hurewitz AN, Wu CL, Mancuso P, et al. Tetracycline and doxycycline inhibit pleural fluid metalloproteinases. A possible mechanism for chemical pleurodesis. Chest 1993; 103: 1113-7. [DOI] [PubMed] [Google Scholar]
- 21).Ukale V, Agrenius V, Widström O, et al. Inflammatory parameters after pleurodesis in recurrent malignant pleural effusions and their predictive value. Respir Med 2004; 98: 1166-72. [DOI] [PubMed] [Google Scholar]