Abstract
Objectives. To provide summary estimates of gastroenteritis risks and illness burden associated with recreational water exposure and determine whether children have higher risks and burden.
Methods. We combined individual participant data from 13 prospective cohorts at marine and freshwater beaches throughout the United States (n = 84 411). We measured incident outcomes within 10 days of exposure: diarrhea, gastrointestinal illness, missed daily activity (work, school, vacation), and medical visits. We estimated the relationship between outcomes and 2 exposures: body immersion swimming and Enterococcus spp. fecal indicator bacteria levels in the water. We also estimated the population-attributable risk associated with these exposures.
Results. Water exposure accounted for 21% of diarrhea episodes and 9% of missed daily activities but was unassociated with gastroenteritis leading to medical consultation. Children aged 0 to 4 and 5 to 10 years had the most water exposure, exhibited stronger associations between levels of water quality and illness, and accounted for the largest attributable illness burden.
Conclusions. The higher gastroenteritis risk and associated burden in young children presents important new information to inform future recreational water quality guidelines designed to protect public health.
Each year there are an estimated 2.2 billion tourist day visits to freshwater and marine beaches in the United States.1 To minimize illness associated with swimming in contaminated water, the US Environmental Protection Agency (EPA) and the World Health Organization publish beach-monitoring guidelines on the basis of Enterococcus spp. fecal indicator bacteria levels.2,3 Elevated Enterococcus levels in recreational waters can indicate potential contamination with fecal waste from sewage or other sources and can be associated with pathogens such as norovirus, Campylobacter spp., Salmonella spp., Cryptosporidium spp., and Giardia spp. Recent waterbody closures at Brazilian Olympic venues4 and Waikiki beach, Hawaii5 illustrate recreational water contamination’s public health and economic reach.
Several cohort studies and randomized trials have measured swimming-related health risks and risks associated with exposure to waters containing elevated fecal indicator bacteria levels,6–17 but individual studies are usually too small to estimate the public health burden associated with water exposure with respect to missed daily activities (work, school, vacation) or medical visits and hospitalizations that result from gastroenteritis. Moreover, individual studies have not typically enrolled enough swimmers to estimate risks separately for children. Children are hypothesized to have a higher risk of swimming-related gastroenteritis than are adults because they spend more time in the water, are more likely to swallow water (the primary mechanism of pathogen exposure), and have less developed immune systems.8,18–20 The EPA and the World Health Organization acknowledge that children could be at higher risk than is the general population but note that evidence for higher risks among children has been inconclusive when viewed across diverse environmental settings such as marine water and freshwater.2,21
We conducted a pooled analysis of 13 prospective cohorts in the United States (84 411 participants).7–14 We coordinated studies to ensure similar designs and measurement methods. This approach enabled us to calculate summary estimates for gastroenteritis risk and related medical and daily activity impacts associated with recreational water exposure and Enterococcus levels; it also enabled us to report for the first time, to our knowledge, separate estimates for children aged 0 to 4 and 5 to 10 years. We had 3 objectives: (1) estimate the risk of diarrhea among beachgoers associated with recreational water exposure, (2) estimate the risk of diarrhea among swimmers associated with Enterococcus levels measured in the water, and (3) estimate the illness burden attributable to recreational water exposure and exposure above regulatory guidelines for the following outcomes: diarrhea, gastrointestinal illness, missed daily activities (work, school, vacation) owing to gastrointestinal illness, and medical consultations owing to gastrointestinal illness.
METHODS
Individual studies took place between 2003 and 2009 and have been described in detail elsewhere.7–14 The beaches represented a range of recreational water conditions across the United States: 4 freshwater beaches were in the Great Lakes region, 4 marine beaches were in Southern California, and the remaining 5 marine beaches were spread along the Gulf Coast, Eastern Seaboard, and Puerto Rico. Nine of the beaches were located near a known source of treated sewage discharge (referred to as “point source”–affected beaches), and the remaining 4 beaches had more diffuse contamination from urban runoff (details are available in Appendix A, available as a supplement to the online version of this article at http://www.ajph.org).
Studies enrolled beachgoers between May and September, typically on weekends. Interviewers approached people on the beach and enrolled all members of a household who were present and provided consent. Individuals were excluded from participating if they had enrolled in the study in the previous month, if no adult household member was present, or if they did not speak English or Spanish. California cohorts excluded individuals if they were not citizens of the United States, Mexico, or Canada to facilitate follow-up. Participants completed a short survey when leaving the beach to report swim exposure. Interviewers called participants 10 to 14 days after the beach visit to assess outcomes; a knowledgeable member from each household reported the timing and details of illness symptoms for all household participants.
Exposure Definition
We considered 2 types of exposure. First, we determined whether individuals entered the water and, if so, their level of water contact. Consistent with previous studies,7–14 we used graded exposure levels: body immersion swimming (waist depth or greater), head immersion, and swallowed water. The categories were not exclusive; for example, individuals who swallowed water may have been body immersion swimmers. We classified individuals with no reported water contact as nonswimmers.
Second, studies collected water samples from multiple locations at each beach and at multiple times during the day concurrent with participant enrollment. We estimated Enterococcus levels in each sample using a culture-based method, EPA 1600,22 or at 1 beach Enterolert (IDEXX, Westbrook, ME). We also tested water samples for Enterococcus using a quantitative polymerase chain reaction (qPCR) method (EPA 1611).23 The analysis used daily mean log10 Enterococcus per 100 milliliters, averaged over all sampling locations and times (for 3 beaches we used swimmer beach location to assign water quality measurements; Appendix A). We classified Enterococcus exposure in 2 ways: we used quartiles of Enterococcus to assess the relationship between water quality and swimmer diarrhea, and we used a dichotomous measure indicating whether water conditions exceeded EPA regulatory guidelines. For culture methods this was greater than 35 colony-forming units per 100 milliliters, and for qPCR this was greater than 470 calibrator cell equivalents per 100 milliliters2. Our prespecified primary analysis focused on culture method regulatory guidelines, and we included qPCR results in supporting information.
Outcomes
The primary outcome was incident diarrhea, defined as 3 or more loose or watery stools in 24 hours.24 We did not consider individuals who reported an episode of diarrhea or vomiting in the 3 days before enrollment at risk. Secondary outcomes for attributable risk calculations included incident gastrointestinal illness, defined consistently with previous studies10–13 as (1) diarrhea, (2) vomiting, (3) nausea and stomachache, or (4) nausea or stomachache and missed regular activities as a result of illness; days of missed work, school, or vacation owing to any gastrointestinal illness; days of missed paid work owing to gastrointestinal illness; and medical consultations associated with gastrointestinal illness, including telephone consultations, inpatient or outpatient care, and emergency room visits.
For days of missed work, school, or vacation, we included days missed by individuals aged 13 years or older who were caring for other family members even if they were not ill themselves. Participants reported the outcomes during the telephone interview 10 to 14 days following the beach visit. We included incident outcomes in the 10 days following the beach visit.
Statistical Analysis
The study protocol and analysis plan prespecified all choices about exposures, outcomes, effect modifiers, and analysis methods (data are available in the Supplemental Protocol, available as a supplement to the online version of this article at http://www.ajph.org; Open Science Framework registration https://osf.io/i3742). Analyses treated beaches as a fixed effect to derive summary estimates across the 13 cohorts.25 We estimated cumulative incidence ratios (CIRs) using log-linear models with robust SEs clustered at the household level.26 Adjusted models controlled for the following characteristics: age, gender, race, reported chronic gastrointestinal problems at enrollment (irritable bowel syndrome, ulcerative colitis), contact with animals, contact with gastrointestinal illness in the 3 days before enrollment, and consumption of undercooked or raw eggs, meat, or fish in the 3 days before enrollment or at any time during follow-up.
We prespecified the following age categories to examine effect modification: aged 0 to 4, 5 to 10, and older than 10 years. Our rationale was that the intensity of water exposure (time in the water and ingestion of water) was different in these 3 age groups, which would translate into different health risks. We also assessed effect modification by water type (fresh vs marine) and pollution type (nearby source of treated human sewage “point source” vs nonpoint source). We have reported stratified results if there was effect modification on additive or multiplicative scales (P < .2).27 We did not analyze individuals enrolled at beaches that were not measured at follow-up (assumed missing completely at random). Earlier analyses of the cohorts demonstrated that participants lost to follow-up were similar to those who completed follow-up.7–14
Diarrhea associated with water exposure and Enterococcus levels.
We estimated the CIR associated with water exposure by comparing diarrhea incidence among swimmers with different levels of water exposure (body immersion, head immersion, swallowed water) with incidence among nonswimmers. We estimated the CIR associated with Enterococcus levels in the water by comparing diarrhea incidence among body immersion swimmers exposed to Enterococcus quartiles 2 to 4 with that in quartile 1. We estimated the CIR associated with swimming exposure above regulatory guidelines by comparing diarrhea incidence among swimmers who were exposed above the guideline (> 35 colony-forming units per 100 ml) with that of nonswimmers and with that of swimmers exposed below the guideline.
Population-attributable risk and attributable fraction.
The population-attributable risk (PAR) represents cases that would be prevented if an exposure were removed. In this study it measures the attributable illness burden in the beachgoer population.28 We estimated the PAR for any water exposure to measure the overall burden of recreational swimming. We estimated a second PAR, assuming no beachgoer swam in water that exceeded the EPA guideline (Enterococcus > 35 colony-forming units per 100 ml), to measure the burden associated with swim exposure above EPA guidelines. We estimated adjusted PARs using predicted outcome probabilities from log-linear models under the observed exposure and covariate distribution and under the counterfactual scenarios, where PAR = Pr(disease) – Pr(disease | no exposure).28 We calculated the population-attributable fraction by dividing the PAR by the baseline risk, which represents the percentage of cases that would be prevented if the exposure were removed. We calculated percentile-based 95% confidence intervals (CIs) for PARs and population-attributable fractions using a nonparametric, clustered bootstrap (1000 iterations) that resampled households with replacement, stratified by beach.29
RESULTS
The 13 cohorts comprised 102 903 beachgoers, of whom 84 411 (82%) completed a follow-up survey and were analyzed. The analysis population included 6580 children aged 0 to 4 years, 10 822 children aged 5 to 10 years, and 65 854 people older than 10 years (n = 1155; 1.4% missing age). More children aged 5 to 10 years immersed their body in water (81%) than did those aged 0 to 4 years (59%) and those older than 10 years (52%), and they spent more time in the water (median = 2 hours compared with 1 hour in other age groups). Swallowing water was more common among children aged 0 to 4 (25%) and 5 to 10 (28%) years than among older beachgoers (9%). A minority of body immersion swimmers at risk for gastroenteritis were exposed to water that exceeded EPA regulatory guidelines for Enterococcus (13% [5870/46 069] using culture methods; 3% [1355/44 857] using qPCR methods). Among 82 463 individuals at risk for gastrointestinal illness, there were 3409 incident diarrhea cases during the 10-day follow-up period (detailed population characteristics and exposure summaries are available in Appendix A).
Incident Diarrhea Associated With Water Exposure and Enterococcus
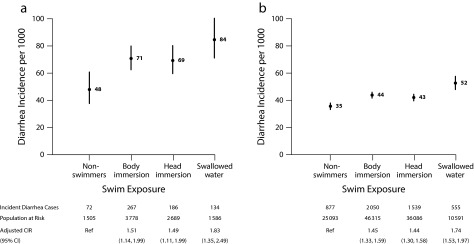
Compared with nonswimmers, diarrhea incidence increased for individuals with body or head immersion and further increased among individuals who swallowed water; children aged 0 to 4 years had the highest incidence of any age group (Figure 1; an extended version of Figure 1 is available in Appendix C, as a supplement to the online version of this article at http://www.ajph.org as). There was low to moderate heterogeneity across beaches (Appendix B, available as a supplement to the online version of this article at http://www.ajph.org). Body immersion swimming was more strongly associated with diarrhea at freshwater beaches (adjusted CIR = 1.70; 95% CI = 1.45, 1.99) than at marine beaches (adjusted CIR = 1.35; 95% CI = 1.21, 1.51; effect modification P = .004 [additive]; P = .05 [multiplicative]; Appendix B). Enterococcus levels were positively associated with diarrhea risk within age strata, with the largest absolute increases in risk among children aged 0 to 4 years (effect modification P = .02 [additive]; P = .08 [multiplicative]; Figure 2; an extended version of Figure 2 is available in Appendix C).
FIGURE 1—
Incident Diarrhea Associated With Water Exposure for (a) Children Aged 0–4 Years and (b) All Ages: Estimates From a Pooled Analysis of 13 US Prospective Cohorts, 2003–2009
Note. CI = confidence interval; CIR = cumulative incidence ratios. We estimated adjusted CIRs with nonswimmers as the reference group.
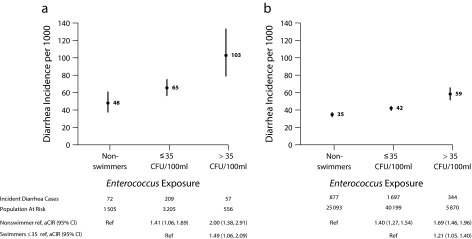
FIGURE 2—
Incident Diarrhea Among Beachgoers Associated with Enterococcus Levels Above and Below Regulatory Guidelines for (a) Children Aged 0–4 Years and (b) All Ages: Estimates From a Pooled Analysis of 13 US Prospective Cohorts, 2003–2009
Note. aCIR = adjusted cumulative incidence ratios; CFU = colony-forming units; CI = confidence interval. We estimated aCIRs using 2 reference groups: nonswimmers and swimmers exposed below Environmental Protection Agency regulatory guidelines for Enterococcus: > 35 CFU/100 ml.
Swimmers exposed to Enterococcus levels above EPA guidelines had higher diarrhea incidence than did nonswimmers and swimmers exposed below the guideline in all age groups. Children aged 0 to 4 years exposed to water above regulatory guidelines experienced 103 episodes per 1000 compared with 48 episodes per 1000 among nonswimmers (adjusted CIR = 2.00; 95% CI = 1.38, 2.91; Figure 2). Swim exposure above the regulatory guideline increased diarrhea incidence only at beaches with a known point source of human fecal pollution (effect modification P = .09 [additive]; P = .16 [multiplicative]; Appendix C). The relationships between culture- and qPCR-based measures of Enterococcus and incident diarrhea episodes were similar and showed increased diarrhea risk at higher quartiles of Enterococcus levels (i.e., no clear risk threshold; Appendix C).
The exposure-response relationship was more consistently monotonic for Enterococcus measured using qPCR methods versus culture methods across different analyses, even when restricted to beaches without a known point source of pollution (Appendix C). The relationships between swim exposure, Enterococcus exposure, and more broadly defined gastrointestinal illness were very similar to diarrhea (Appendix D, available as a supplement to the online version of this article at http://www.ajph.org). Negative control analyses30,31 showed no association between Enterococcus levels and diarrhea incidence among nonswimmers, and sensitivity analyses showed that the results were robust to different definitions of exposure, modeling approach, and lengths of follow-up (Appendix E, available as a supplement to the online version of this article at http://www.ajph.org).
Estimates of Attributable Illness Burden
Among beachgoers, 8.6 diarrhea episodes per 1000 (21% of all episodes) and 4.9 days of missed activities (work, school, or vacation) per 1000 (9% of all days missed) were attributable to swimming (Table 1). Removing swim exposure would not reduce cases of gastroenteritis that result in missed paid work or medical consultations. The diarrhea illness burden attributable to swimming among children aged 5 to 10 years was more than double that of those older than 10 years, and children aged 0 to 4 years had higher attributable risks than did those older than 10 years for diarrhea, gastrointestinal illness, and days of missed work, school, or vacation. For example, the attributable risk for gastrointestinal illness among children aged 0 to 4 years was 22.4 cases per 1000 compared with 7.8 cases per 1000 among individuals older than 10 years (Table 1).
TABLE 1—
Population-Attributable Risk Among Beachgoers Owing to Body Immersion Swimming: Estimates From a Pooled Analysis of 13 US Prospective Cohorts, 2003–2009
| Predicted Incidence,a per 1000 |
||||||
| Outcome | No. Events | No. at Risk | Observed Exposure | No Swim Exposure | Population-Attributable Riskb (95% CI) | Population-Attributable Fraction,c % (95% CI) |
| Diarrhea, episodes | ||||||
| All ages | 3409 | 82 463 | 41 | 33 | 8.6 (6.6, 10.6) | 21 (16, 25) |
| Age stratified, y | ||||||
| 0–4 | 398 | 6394 | 62 | 48 | 14.7 (3.8, 25.3) | 23 (6, 40) |
| 5–10 | 393 | 10 633 | 37 | 20 | 16.9 (7.3, 26.1) | 46 (20, 69) |
| > 10 | 2585 | 64 295 | 40 | 33 | 6.9 (4.8, 8.9) | 17 (12, 22) |
| Gastrointestinal illness,d episodes | ||||||
| All ages | 5024 | 82 463 | 61 | 52 | 9.2 (6.6, 11.8) | 15 (11, 19) |
| Age stratified, y | ||||||
| 0–4 | 562 | 6394 | 88 | 66 | 22.4 (9.9, 34.8) | 25 (12, 40) |
| 5–10 | 697 | 10 633 | 66 | 54 | 11.8 (–2.6, 24.7) | 18 (–4, 37) |
| > 10 | 3716 | 64 295 | 58 | 50 | 7.8 (5.4, 10.1) | 14 (9, 17) |
| Missed daily activities,e days | ||||||
| All ages | 4551 | 82 463 | 55 | 50 | 4.9 (0.4, 9.2) | 9 (1, 17) |
| Age stratified, y | ||||||
| 0–4 | 445 | 6394 | 70 | 43 | 27.1 (7.7, 44.5) | 39 (12, 61) |
| 5–10 | 691 | 10 633 | 65 | 58 | 7.2 (–17.0, 29.6) | 11 (–26, 45) |
| > 10 | 3377 | 64 295 | 53 | 48 | 4.3 (0.1, 8.9) | 8 (0, 17) |
| Missed paid work,f days | ||||||
| All agesg,h | 1051 | 82 463 | 13 | 13 | −0.6 (–2.8, 1.3) | NA |
| Medical visits, events | ||||||
| All agesg,h | 915 | 82 463 | 11 | 11 | 0.4 (–1.3, 2.1) | 4 (–12, 18) |
Note. CI = confidence interval; NA = not applicable (owing to negative attributable risk estimate).
Predicted incidence per 1000 among all beachgoers under the empirical distribution of swim exposure (observed) and under a counterfactual scenario in which nobody entered the water. Estimates are from a multivariable regression model adjusted for a range of potential confounders and beach-level fixed effects.
Population-attributable risk is the number of events per 1000 beachgoers that would be prevented if swimming exposure were removed from the population. The proportion exposed to body immersion swimming was all ages (56%), 0–4 years (59%), 5–10 years (81%), > 10 years (52%).
Population-attributable fraction is the percentage of events among beachgoers attributable to body immersion swimming.
Gastrointestinal illness was defined as (1) diarrhea, (2) vomiting, (3) stomach cramps and missed daily activities, or (4) nausea and missed daily activities.
Includes days of school, work, or vacation missed because of gastrointestinal illness.
Includes work days missed because of gastrointestinal illness.
Includes telephone consultations, outpatient visits, and emergency room visits owing to gastrointestinal illness.
Outcome incidence was too rare to calculate age-stratified estimates.
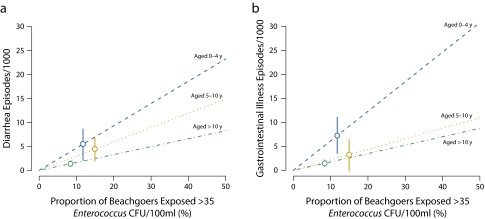
The risk attributable to swimming in water that exceeded EPA Enterococcus guidelines was modified by age, with the highest levels of exposure and preventable illness burden among children aged 0 to 4 and 5 to 10 years (Figure 3). The attributable risk for this higher level of exposure was small and not distinguishable from zero for missed days of paid work and medical consultations (Appendix F, available as a supplement to the online version of this article at http://www.ajph.org). The illness burden attributable to swimming in water that exceeded EPA guidelines was low because a minority of beachgoers (10%) were exposed above the guideline (Figure 3) despite a large increase in risk associated with exposure (aged 0–4 years: risk difference = 55 episodes per 1000; aged 5–10 years: 34 per 1000; older than 10 years: 20 per 1000—comparing swimmers above the guideline vs nonswimmers in Figure 2).
FIGURE 3—
Age-Stratified Attributable Risk Among Beachgoers From Swimming in Water With Enterococcus Levels That Exceed Environmental Protection Agency Regulatory Guidelines of Enterococcus > 35 CFU/100 ml for (a) Diarrhea and (b) Gastrointestinal Illness: Estimates From a Pooled Analysis of 13 US Prospective Cohorts, 2003–2009
Note. CFU = colony-forming units. Circles mark the observed levels of exposure and corresponding population-attributable risk estimates with 95% confidence intervals. Dashed lines show adjusted model predictions over a range of exposure levels assuming the same risk relationship within age strata for different proportions exposed.
DISCUSSION
Our results provide, to our knowledge, the largest summary of the gastroenteritis risk and related public health burden associated with recreational water exposure in the United States. Increased risk at the highest Enterococcus levels supports their use as a monitoring indicator for diarrhea risk among beachgoers, although the association between Enterococcus and illness was weaker at beaches without an identified source of human fecal pollution. The higher diarrhea risk among younger children associated with water exposure and larger illness burden attributable to swimming exposure were consistent with longer water contact times and a greater propensity to swallow water. An earlier analysis of this study’s freshwater cohorts reported higher swimming-associated gastrointestinal illness risk among children younger than 10 years compared with the overall population,8 but our findings provide evidence for heightened sensitivity among children across a broader set of beach conditions, including marine water and diffuse, “nonpoint” sources of pollution from urban runoff. Our analysis also highlights for the first time, to our knowledge, the largest attributable burden among the youngest children, those aged 0 to 4 years.
The illness burden attributable to swimming was substantial (21% of diarrhea episodes; Table 1). Yet, the burden attributable to swimming in water that exceeded EPA guidelines was smaller (5% of diarrhea episodes; Appendix F) because just 10% of beachgoers were exposed above the guideline. This result illustrates that attaining EPA water quality guidelines is protective of public health. On the basis of the relationships we estimated, the illness burden would be considerably higher if water quality were worse and a higher percentage of beachgoers were exposed above the guideline (Figure 3). Nevertheless, swimmers exposed to low levels of Enterococcus had higher diarrhea rates than did nonswimmers (Figure 2), which could result from noninfectious causes of diarrhea associated with swimming (e.g., swallowing excess salt water), outcome reporting bias, or pathogens present in recreational waters that do not covary with Enterococcus.32 Our results suggest that recreational water exposure generally leads to less severe gastroenteritis because episodes leading to a medical consultation were not associated with water exposure (Table 1).
Our study had some limitations. First, we did not randomize beachgoers to swimming exposure, and if healthier individuals were more likely to swim then we could have underestimated the risk associated with swimming. The magnitude of association between swim exposure and diarrhea we estimated was similar to those reported in trials that randomized participants to enter the ocean,6,15 so we expect this was not a significant source of bias. Second, we matched daily averages of Enterococcus levels to swimmers, which could lead to random error in their exposure and bias estimates toward the null.33 Third, we measured outcomes using participant report, which could be subject to recall errors or other reporting biases.
The negative control exposure analyses suggested that unmeasured sources of measurement or confounding bias were unlikely to explain the associations between Enterococcus and incident diarrhea (Appendix E). Excess incidence among swimmers occurred mainly within 2 days after exposure (Appendix E), but pathogen-specific outcome measurements would be necessary to make definitive conclusions about etiology. Finally, we limited the analysis to gastroenteritis and related impacts, but extending similar analyses to additional endpoints such as ear infections or eye infections could increase estimates of public health burden.34 These limitations notwithstanding, the large study population, broad range of beach and water conditions, stability of the findings across many sensitivity and negative control analyses, and internal consistency of the results all lend credibility to our findings.
The synthesis of data across 13 cohorts creates many opportunities for research beyond our prespecified objectives. For example, an analysis of the freshwater cohorts included in this study identified beachgoers older than 55 years as a high-risk group for gastroenteritis,8 which suggests there could be additional age categories beyond younger children that are more sensitive to exposure than is the general population. A more detailed characterization of swimmer exposure could help inform quantitative microbial risk assessment models,2 and more detailed comparisons of risk associated with Enterococcus measured by qPCR versus culture methods could inform future regulatory guidelines.
PUBLIC HEALTH IMPLICATIONS
Recreational water exposure was associated with an increased risk of acute gastroenteritis resulting in missed daily activities—but not medical visits—with the highest risk and attributable burden among younger children. Combining information from 13 cohorts enabled us to estimate, for the first time to our knowledge, risk among the youngest children (aged 0–4 and 5–10 years), who had higher levels of exposure (time in the water and swallowed water). We found that 87% of swimmers were exposed to water that met EPA regulatory guidelines on the basis of levels of cultured Enterococcus, but if more swimmers were exposed to poor water quality the attributable risk for incident diarrhea would increase substantially, particularly among children aged 0 to 4 years.
Exposure to water with Enterococcus levels above EPA regulatory guidelines was associated with increased diarrhea risk in all age groups at beaches with known point sources of human fecal pollution but not at beaches without an identified pollution source. The higher gastroenteritis risk and associated burden in the youngest children and the absence of an association between Enterococcus levels and gastroenteritis risk at beaches with no identified pollution source presents important new information to inform future recreational water quality guidelines designed to protect public health.
ACKNOWLEDGMENTS
This study was funded by the National Institutes of Health (NIH; grant R03-HD076066).
We are grateful to Jenny Liang and Meghana Bhimarao at the University of California, Berkeley for help creating maps of the study beaches and to Sharon Nappier, John Ravenscroft, and Andrey Egorov at the US Environmental Protection Agency (EPA) for helpful comments on a draft of this article.
Note. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the EPA. NIH had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; or decision to submit the article for publication.
HUMAN PARTICIPANT PROTECTION
The 13 study protocols were each reviewed and approved by institutional review boards at the University of California, Berkeley, the University of North Carolina, Chapel Hill, and the Centers for Disease Control and Prevention and received ethical approval from the US Environmental Protection Agency human subjects official. All participants provided written informed consent.
REFERENCES
- 1.Houston JR. The economic value of beaches—a 2013 update. Shore & Beach. 2013;81(1):3–11. [Google Scholar]
- 2.US Environmental Protection Agency. Recreational Water Quality Criteria. Washington, DC: Office of Water; 2012. [Google Scholar]
- 3.Guidelines for Safe Recreational Water Environments. Volume 1: Coastal and Fresh Waters. Geneva, Switzerland: World Health Organization; 2003. [PubMed] [Google Scholar]
- 4.Concerns raised over water quality at the Rio Olympics. Lancet Infect Dis. 2015;15(9):987. doi: 10.1016/S1473-3099(15)00264-9. [DOI] [PubMed] [Google Scholar]
- 5.Roig S. Sewage spill closes Honolulu’s Waikiki beach through Wednesday. 2015. Available at: http://www.reuters.com/article/2015/08/26/us-usa-hawaii-sewage-idUSKCN0QU26L20150826. Accessed September 17, 2015.
- 6.Wade TJ, Pai N, Eisenberg JNS, Colford JM. Do U.S. Environmental Protection Agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environ Health Perspect. 2003;111(8):1102–1109. doi: 10.1289/ehp.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wade TJ, Calderon RL, Sams E et al. Rapidly measured indicators of recreational water quality are predictive of swimming-associated gastrointestinal illness. Environ Health Perspect. 2006;114(1):24–28. doi: 10.1289/ehp.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wade TJ, Calderon RL, Brenner KP et al. High sensitivity of children to swimming-associated gastrointestinal illness: results using a rapid assay of recreational water quality. Epidemiology. 2008;19(3):375–383. doi: 10.1097/EDE.0b013e318169cc87. [DOI] [PubMed] [Google Scholar]
- 9.Colford JM, Wade TJ, Schiff KC et al. Water quality indicators and the risk of illness at beaches with nonpoint sources of fecal contamination. Epidemiology. 2007;18(1):27–35. doi: 10.1097/01.ede.0000249425.32990.b9. [DOI] [PubMed] [Google Scholar]
- 10.Wade TJ, Sams E, Brenner KP et al. Rapidly measured indicators of recreational water quality and swimming-associated illness at marine beaches: a prospective cohort study. Environ Health. 2010;9:66. doi: 10.1186/1476-069X-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colford JM, Schiff KC, Griffith JF et al. Using rapid indicators for Enterococcus to assess the risk of illness after exposure to urban runoff contaminated marine water. Water Res. 2012;46(7):2176–2186. doi: 10.1016/j.watres.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold BF, Schiff KC, Griffith JF et al. Swimmer illness associated with marine water exposure and water quality indicators: impact of widely used assumptions. Epidemiology. 2013;24(6):845–853. doi: 10.1097/01.ede.0000434431.06765.4a. [DOI] [PubMed] [Google Scholar]
- 13.Yau VM, Schiff KC, Arnold BF et al. Effect of submarine groundwater discharge on bacterial indicators and swimmer health at Avalon Beach, CA, USA. Water Res. 2014;59:23–36. doi: 10.1016/j.watres.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 14.Wade TJ, Sams EA, Haugland R . Report on 2009 National Epidemiologic and Environmental Assessment of Recreational Water Epidemiology Studies. Washington, DC: US Environmental Protection Agency; 2010. [Google Scholar]
- 15.Fleisher JM, Fleming LE, Solo-Gabriele HM et al. The BEACHES study: health effects and exposures from non-point source microbial contaminants in subtropical recreational marine waters. Int J Epidemiol. 2010;39(5):1291–1298. doi: 10.1093/ije/dyq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorevitch S, Pratap P, Wroblewski M et al. Health risks of limited-contact water recreation. Environ Health Perspect. 2012;120(2):192–197. doi: 10.1289/ehp.1103934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamparelli CC, Pogreba-Brown K, Verhougstraete M et al. Are fecal indicator bacteria appropriate measures of recreational water risks in the tropics: a cohort study of beach goers in Brazil? Water Res. 2015;87:59–68. doi: 10.1016/j.watres.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Dufour AP, Evans O, Behymer TD, Cantú R. Water ingestion during swimming activities in a pool: a pilot study. J Water Health. 2006;4(4):425–430. [PubMed] [Google Scholar]
- 19.Koehler KM, Lasky T, Fein SB et al. Population-based incidence of infection with selected bacterial enteric pathogens in children younger than five years of age, 1996–1998. Pediatr Infect Dis J. 2006;25(2):129–134. doi: 10.1097/01.inf.0000199289.62733.d5. [DOI] [PubMed] [Google Scholar]
- 20.Nwachuku N, Gerba CP. Health risks of enteric viral infections in children. Rev Environ Contam Toxicol. 2006;186:1–56. doi: 10.1007/0-387-32883-1_1. [DOI] [PubMed] [Google Scholar]
- 21.Addendum to Guidelines for Safe Recreational Water Environments. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 22.Method 1600: Enterococci in Water by Membrane Filtration Using Membrane-Enterococcus Indoxyl-Beta-D-Glucoside Agar (mEI) Washington, DC: US Environmental Protection Agency; 2006. [Google Scholar]
- 23.US Environmental Protection Agency. Method 1611: enterococci in water by TaqMan® quantitative polymerase chain reaction (qPCR) assay. 2012. Available at: https://www.epa.gov/sites/production/files/2015-08/documents/method_1611_2012.pdf. Accessed May 31, 2016.
- 24.Baqui AH, Black RE, Yunus M, Hoque AR, Chowdhury HR, Sack RB. Methodological issues in diarrhoeal diseases epidemiology: definition of diarrhoeal episodes. Int J Epidemiol. 1991;20(4):1057–1063. doi: 10.1093/ije/20.4.1057. [DOI] [PubMed] [Google Scholar]
- 25.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3(4):486. [Google Scholar]
- 26.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 27.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014;3(1):33–72. [Google Scholar]
- 28.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49(3):865–872. [PubMed] [Google Scholar]
- 29.Greenland S. Interval estimation by simulation as an alternative to and extension of confidence intervals. Int J Epidemiol. 2004;33(6):1389–1397. doi: 10.1093/ije/dyh276. [DOI] [PubMed] [Google Scholar]
- 30.Lipsitch M, Tchetgen ET, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383–388. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold BF, Ercumen A, Benjamin-Chung J, Colford JM., Jr Negative controls to detect selection bias and measurement bias in epidemiologic studies. Epidemiology. May 11, 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 32.Wu J, Long SC, Das D, Dorner SM. Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J Water Health. 2011;9(2):265–278. doi: 10.2166/wh.2011.117. [DOI] [PubMed] [Google Scholar]
- 33.Fleisher JM. The effects of measurement error on previously reported mathematical relationships between indicator organism density and swimming-associated illness: a quantitative estimate of the resulting bias. Int J Epidemiol. 1990;19(4):1100–1106. doi: 10.1093/ije/19.4.1100. [DOI] [PubMed] [Google Scholar]
- 34.Fleisher JM, Kay D, Wyer MD, Godfree AF. Estimates of the severity of illnesses associated with bathing in marine recreational waters contaminated with domestic sewage. Int J Epidemiol. 1998;27(4):722–726. doi: 10.1093/ije/27.4.722. [DOI] [PubMed] [Google Scholar]