Abstract
Objectives. To review the contributions of the Nurses’ Health Study (NHS) I and NHS II to understanding the role of dietary factors, beverages, body size, and urinary factors in the development of kidney stones.
Methods. We conducted a review of kidney stone–related publications of NHS I and NHS II between 1976 and 2016.
Results. Studies using NHS I and NHS II data have demonstrated the importance of many factors in kidney stone formation and were the first to report that higher dietary calcium was associated with a lower risk of incident kidney stones in women. Data from these cohorts were instrumental in emphasizing that nephrolithiasis is a systemic disease and suggesting that a kidney stone or shared risk factors may lead to hypertension, diabetes, and cardiovascular disease.
Conclusions. Findings from the NHSs have changed the scientific understanding and the clinical practice of stone prevention and have been incorporated into widely consulted textbooks and the American Urological Association Medical Management of Kidney Stones guidelines.
Nephrolithiasis, or kidney stone disease, is common and is a major cause of morbidity. Up to 19% of men and 10% of women in the United States will form a kidney stone at some time,1 a nearly 3-fold increase over the past 4 decades. Stone disease causes tremendous pain and suffering and costs the United States more than $5 billion annually. The incidence rates of stone disease continue to rise in the United States and other countries.1 Recurrence rates are as high as 20%–50% after 5 years.2,3 Because of the high incidence and recurrence rates, identifying modifiable risk factors may reduce the burden of this disease.
In 1997, the first large prospective study of risk factors for incident nephrolithiasis in women was published using data from the Nurses’ Health Study (NHS) I.4 In that study and the many subsequent studies over the past 20 years, findings from NHSs I and II have demonstrated the importance of dietary factors,4–15 beverages,16–18 and urinary factors19,20 in the development of kidney stones (see the box on the next page). Data from NHSs I and II were instrumental in demonstrating that nephrolithiasis is a systemic disorder and that factors such as body size are strongly associated with the risk of stone formation.21,22 In addition, evidence from NHS I and II study cohorts as well as others suggests that kidney stones or shared risk factors may lead to hypertension,23,24 diabetes,24,25 cardiovascular disease,26,27 and chronic kidney disease.28,29 These findings have changed the scientific understanding and the clinical practice of stone prevention and have been incorporated into widely consulted textbooks and the American Urological Association Medical Management of Kidney Stones guidelines.30
Risk Factors for Kidney Stones: Nurses’ Health Studies I and II, United States, 1976–2016
| Factors Associated With Lower Risk | Factors Associated With Higher Risk |
| Dietary calcium (dairy and nondairy) | Supplemental calcium |
| Dietary potassium | Dietary oxalate |
| Caffeine | Sucrose |
| DASH diet | Fructose |
| Total fluid intake | Sugar-sweetened soda |
| Coffee (caffeinated and decaffeinated) | Punch |
| Tea | Body mass index |
| Beer | Diabetes mellitus (type II) |
| Wine | Cholelithiasis |
| Orange juice | Urinary calcium excretion |
| Total urine volume | Urinary oxalate excretion |
| Urinary citrate excretion |
Note. DASH = Dietary Approaches to Stop Hypertension.
A description of the processes of NHSs I and II and the characteristics of their cohorts is provided in the companion article in this issue (Bao et al., p1573).
SELF-REPORTED KIDNEY STONES IN THE NURSES’ HEALTH STUDIES
We initially identified new cases of kidney stones in NHSs I and II by self-report. We next mailed a supplementary questionnaire to women who reported a stone for the first time to obtain additional information about the event, including symptoms and medical evaluation. To demonstrate the reliability of confirming self-reported stones in NHSs I and II, after obtaining permission to collect medical records related to the stone event, we performed a validation study.
We reviewed medical records from 194 women in NHS I and 858 women in NHS II, and the self-report was confirmed for 96% and 98% of the women, respectively.31 In addition, stone composition reports were available for 78 women in NHS I and 243 women in NHS II; these showed that 77% of NHS I and 79% of NHS II participants had a stone containing at least 50% calcium oxalate.31
TWENTY-FOUR-HOUR URINE COLLECTIONS
We collected 24-hour urine samples from participants with a history of kidney stones, as confirmed by our review of the supplementary questionnaire, and from randomly selected controls in 2 cycles (1994–1999 and 2003–2005). In the first cycle, we asked stone formers and randomly selected controls to perform one 24-hour urine collection. In the second cycle, we asked additional stone formers and controls to perform 2 collections. Those with a history of stones performed the collections after they had been diagnosed with nephrolithiasis. We sent a kit containing all the necessary supplies to individuals agreeing to participate, and participants returned the samples to the laboratory by FedEx. We had previously sent blinded split samples to assess reproducibility; the coefficients of variation for all factors analyzed were less than 10%.
Overall, 1301 NHS I participants and 999 NHS II participants provided at least one 24-hour urine collection. When we examined characteristics for those who did and did not return a sample by case status, we saw only minor differences in the female cohorts for body mass index (BMI; defined as weight in kilograms divided by the square of height in meters) and no important differences in participants’ intake of dietary calcium, sodium, and animal protein.19,20
INCIDENCE RATES
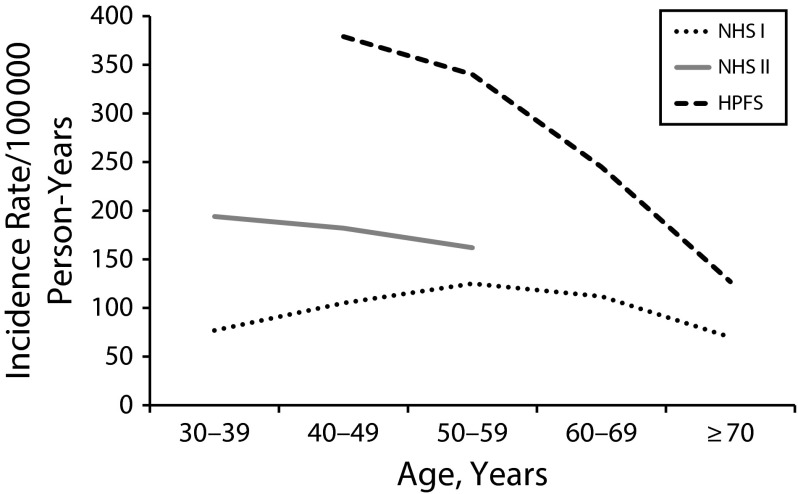
The incidence of nephrolithiasis, defined as the first stone event, varies by age and gender. In women in NHS II, the incidence was higher when women were in their 30s, at approximately 200/105 person-years, and then decreased to approximately 150/105 person-years by the time the women reached age 50 years.
The age-specific incidence was lower in NHS I than in NHS II (likely a result of a cohort effect because NHS I is older than is NHS II), being approximately 80/105 person-years for women in their 30s, increasing to approximately 125/105 person-years by aged 50 years, and then falling to approximately 70/105 person-years by aged 70 years (Figure 1). The incidence rates for the male Health Professionals Follow-Up Study cohort are shown in Figure 1 for comparison. Although changes in calcium balance are well described for perimenopausal women, it is notable that the incidence of nephrolithiasis does not change substantially in that age range.
FIGURE 1—
Age-Specific Incidence Rates for Kidney Stones: Nurses’ Health Studies I and II, United States, 1976–2016
Note. HPFS = Health Professionals Follow-Up Study; NHS = Nurses’ Health Study.
DIETARY RISK FACTORS FOR CALCIUM STONES
Approximately 80% of kidney stones contain calcium, the majority of which are primarily calcium oxalate. For incident kidney stones, it may be that more than 90% of stones contain calcium. Thus, the risk factors for incident stones seen in most large epidemiological studies (including NHSs I and II) likely relate to the development of calcium oxalate stones. Many dietary factors may influence calcium oxalate stone formation, including the consumption of calcium, oxalate, potassium, and other nutrients. Because many dietary factors may be correlated, multivariate adjustment is essential to identify independent associations; therefore, we have multivariate adjusted all results.
Calcium
Higher urinary calcium increases the risk of calcium stone formation, and higher calcium intake can raise urinary calcium. Thus, for decades, patients with kidney stones were routinely advised to follow a low-calcium diet, but there were no prospective data on the relation between dietary calcium intake and the risk of stone formation. However, in 1997, a study using NHS I data found that higher dietary calcium was associated with a lower risk of incident kidney stones.4 This confirmed a previous study in the all-male Health Professionals Follow-Up Study cohort,6 and similar findings were subsequently published in NHS II.5 NHS participants in the highest quintile of dietary calcium intake had a 30% lower risk of kidney stone formation than did participants in the lowest quintile.4,5 Of note, this relation did not vary by the source of dietary calcium; dairy and nondairy sources of dietary calcium were independently associated with lower risk.14 By contrast, supplemental calcium intake was associated with approximately 20% higher risk of kidney stones in older women4 but was not significantly associated with risk in younger women.5
Although it may seem counterintuitive that higher dietary calcium intake is associated with lower risk, a plausible mechanism is that dietary calcium binds to dietary oxalate in the gut, thereby reducing oxalate absorption. The discrepancy in risk between dietary and supplemental calcium intake may be related to the timing of calcium ingestion, as calcium supplements are commonly taken between meals.
These data from NHSs I and II contributed to a paradigm shift in the understanding of the role of calcium intake in the management of kidney stones. Calcium-restricted diets not only expose patients to a higher risk of kidney stones but may increase the risk of bone loss and osteoporosis in women with higher urine calcium excretion as a result of negative calcium balance. A subsequent randomized control trial in Italy confirmed that dietary calcium restriction was not beneficial for stone prevention; in fact, those in the low-calcium diet group (400 mg/day) had a higher risk of stone recurrence than did those in the “normal” calcium (1200 mg/day), restricted sodium (1150 mg/day), and animal protein (52 g/day) diet group.32 It is now widely accepted that patients with kidney stones should not be instructed to follow a low-calcium diet.
Other Individual Dietary Factors
Calcium intake has received the most attention in relation to incident kidney stones, but many other dietary factors have also been examined.
Although urine oxalate is a well-established and important risk factor for calcium oxalate stone formation, the role of dietary oxalate in the pathogenesis of calcium oxalate nephrolithiasis remains unclear. The major dietary sources for oxalate include spinach, potatoes, and nuts.
The magnitude of association for dietary oxalate, even when comparing substantial differences in intake, was modestly associated with higher risk in NHS I (hazard ratio [HR] = 1.21 for highest compared with lowest quintile) but was not associated with stone formation in NHS II.9 The magnitude of this association is substantially smaller than initially expected but now is less surprising because of the expanded understanding of the complexity of oxalate with regard to bioavailability and absorption of dietary oxalate as well as endogenous production.
Higher animal protein intake may increase urinary calcium and decrease urinary citrate, an inhibitor of calcium crystallization, but when studied prospectively, animal protein was not independently associated with a higher risk of incident stone formation.4,5,12 A higher intake of sucrose increases urinary calcium excretion independent of calcium intake. In prospective studies, sucrose and fructose were associated with higher risk in women.4,5,10
Dietary factors that were purported to be inversely associated with the risk of kidney stones include consuming potassium, magnesium, phytate, and fatty acids. Foods rich in potassium commonly contain alkali, which can lead to an increase in urine citrate. Higher potassium intake was associated with lower risk in NHS I.4 Higher magnesium intake may reduce dietary oxalate absorption. In prospective observational studies, higher dietary magnesium was not associated with a lower risk of stone formation in women.4,5 Phytate, commonly found in cold cereals, dark bread, and beans, binds to calcium in the gastrointestinal tract, reducing calcium absorption, and in the urine phytate may act as an inhibitor of calcium crystal formation.5 NHS II participants in the highest quintile of phytate intake had a 36% lower risk of stone formation.5
Data from NHSs I and II have also been vital in elucidating the relation between caffeine intake and kidney stones. Caffeine may increase urinary calcium excretion; yet, in both NHSs I and II, those in the highest quintile of caffeine intake had approximately 30% lower risk of kidney stone formation.15 Additional understanding of this relation came from an analysis of 24-hour urine data from NHS participants. Although caffeine intake was indeed associated with slightly higher urine calcium, the urine volume was higher and the urine oxalate was lower, so the supersaturation (i.e., the likelihood of crystal formation) of calcium oxalate and uric acid was overall reduced.15 The mechanism for higher urinary volume induced by caffeine is not completely understood but may, in part, be related to natriuresis being mediated by caffeine’s inhibition of adenosine receptors.33
Fatty acid intake has been inconsistently associated with kidney stones. For example, higher levels of arachidonic and linoleic acid intake were not associated with the risk of incident kidney stones in women of all ages.13 Greater intake of n-3 fatty acids was not associated with risk in NHS II but was associated with moderately higher risk in NHS I.13 Total energy intake was not found to be significantly associated with risk in younger or older women.34
Vitamin C can be metabolized to oxalate, which could increase the risk of calcium oxalate stones. However, in the late 1990s, data from the NHS I cohort showed that there was no significant association between vitamin C intake and the risk of kidney stone formation for women.8 More recently, additional data demonstrated that total vitamin C and supplemental vitamin C intake were not significantly associated with the risk of kidney stones in NHSs I or II, although there was a higher risk among men.35
Dietary Pattern
Although the examination of individual nutrients provides valuable insights, it may be easier for individuals trying to prevent recurrent stones to follow certain dietary patterns than to modify individual nutrients. The Dietary Approaches to Stop Hypertension diet, which is high in fruits and vegetables, moderate in low-fat dairy products, and low in red and processed meats, is a novel potential means of kidney stone prevention.
In a large prospective observational study of NHSs I and II, a higher Dietary Approaches to Stop Hypertension score (meaning a diet more closely resembling the Dietary Approaches to Stop Hypertension diet) was associated with a 40% to 45% lower risk of incident kidney stone formation.11 The strong, inverse relation in NHSs I and II is notable, considering the differences in some individual dietary factors that have been observed between older and younger women.
FLUID INTAKE AND BEVERAGES
Nephrolithiasis is a disease driven by the urinary concentration of lithogenic factors. Thus, fluid intake, the main determinant of urine volume, plays a critical role in kidney stone formation. Total fluid intake has been consistently found to be one of the other strongest protective factors against kidney stone formation.36 In NHS II, participants who had at least 2.7 liters of daily fluid intake had a 32% lower risk of stone formation compared with those with less than 1.5 liters of daily fluid intake.5 This protection was even greater in NHS I, with a nearly 40% lower risk in the highest fluid intake group.4,16,17
Although higher intake of any beverage will increase urine volume, the type of beverage consumed may influence risk beyond just the volume of the beverage consumed. Sugar-sweetened soda and punch consumption is associated with a higher risk of stone formation. By contrast, consumption of coffee (caffeinated and decaffeinated), tea, beer, wine, and orange juice is associated with lower risk.16–18 These findings from NHSs I and II have refuted previous lore about several beverages and have provided guidance for clinical recommendations.
NONDIETARY FACTORS
There is substantial evidence that nephrolithiasis is a systemic disorder. In addition to the well-known conditions that lead to calcium kidney stone formation such as primary hyperparathyroidism and Crohn’s disease, studies examining NHS I and II data have identified other conditions associated with risk, including obesity, type 2 diabetes mellitus, and cholelithiasis.
Obesity and weight gain are both independently associated with a higher risk of kidney stone formation, and the magnitude of association is much larger in women than in men.21,22 Compared with women who weighed less than 150 pounds, the risk for those who weighed more than 220 pounds was 89% higher in older women and 92% higher in younger women even after adjusting for potential confounders, including dietary factors.22 Furthermore, weight gain of 35 pounds since early adulthood (age 18 years) and higher BMI were both associated with substantially higher kidney stone risk. Participants who gained 35 pounds since age 18 years were at 70% and 82% higher risk for kidney stones in NHS I and NHS II, respectively. Compared with women with a BMI of 21 to 23, the multivariate relative risk was more than 2-fold higher for women with a BMI of 35 or greater.22 This risk was not explained by dietary factors or history of diabetes.
Diabetes is also an independent risk factor for incident kidney stones. Among those with type 2 diabetes, the risk was 29% higher in NHS I and 60% higher in NHS II.25 The findings were independent of BMI, dietary factors, age, and thiazide medication use. The mechanism may be related to changes in urine composition stemming from the effect of insulin resistance on ammoniagensis. Furthermore, an additional analysis in the same study showed that a history of kidney stones is also a risk factor for developing diabetes.25
Gallstones and kidney stones, although composed of unrelated substances, are independently bidirectionally associated.37 There is a higher risk of kidney stones in women who have previously had gallstones, and women who have had kidney stones are at higher risk for developing gallstones. There have been multiple proposed mechanisms to account for these findings, but more studies are needed to identify shared mechanisms underlying both conditions.
Menopause may be associated with a higher urinary calcium excretion. However, data from the NHSs suggest that after accounting for age and other factors there is no significant change in risk with natural menopause. This is not the case for surgical menopause, which is associated with a 39% higher risk. The difference in risk between surgical and natural menopause may be related to changes in bone turnover. After surgical menopause, there is a higher urinary excretion of bone resorption markers, which represents a higher rate of bone loss. Although no published studies have examined urinary calcium excretion in this setting, there is likely higher urinary calcium excretion as well. Additionally, there was no association found between past or current postmenopausal hormone use or the duration of use with kidney stones.38
Although a study from the Women’s Health Initiative suggested that higher physical activity may reduce the risk of self-reported kidney stones, a carefully controlled analysis that included confirmed cases in NHSs I and II found no association.34
URINE FACTORS
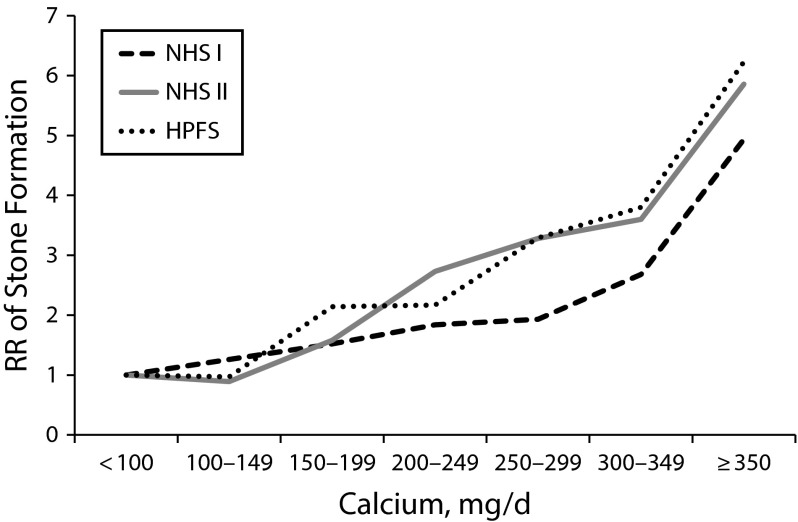
The 24-hour urine composition plays an integral part in the management of patients with kidney stones. Data from NHSs I and II have contributed substantially to our knowledge of risk even within the reference range values by challenging commonly held beliefs of what constitutes normal. Before work in NHSs I and II, there were very few adequately sized studies of the 24-hour urine components of individuals with and without kidney stones. Data from the 2300 NHS I and II participants who performed 24-hour urine collections underscored that the urinary factors are continuously distributed variables, and the risk for stone formation begins to rise within the traditional normal range, so setting specific dichotomous thresholds for normal is not appropriate. For example, kidney stone risk begins to increase and may become 2- to 3-fold higher even in ranges of calcium excretion considered normal (Figure 2).19,20
FIGURE 2—
Twenty-Four–Hour Urine Calcium Excretion and Multivariate-Adjusted Relative Risks for History of Kidney Stones: Nurses’ Health Studies I and II, United States, 1976–2016
Note. HPFS = Health Professionals Follow-Up Study; NHS = Nurses’ Health Study; RR = relative risk. The sample size was n = 2237 cases and n = 1113 controls.
The 24-hour urine results from NHSs I and II further demonstrated the difference between stone-forming and non–stone-forming individuals. For example, higher urinary calcium and oxalate levels were associated with higher risk of kidney stones, whereas citrate and total volume were inversely associated with risk.19,20 Although studies from the 1970s suggested that higher urine uric acid was a risk factor for calcium oxalate stones, more data were needed. The results from the large 24-hour urine collection in NHSs I and II showed that there was no higher risk of kidney stones with increasing urine uric acid excretion. In fact, in NHS II it was found that there was a strong inverse association (HR = 0.16) when comparing the highest uric acid excretion category (≥ 800 mg/day) with the lowest (< 400 mg/day).20
There are known racial differences in stone risk, with Black women having a substantially lower incidence of kidney stones than do White women, but few studies had characterized the differences in 24-hour urine composition of Black and White women. NHSs I and II found that the adjusted mean 24-hour urinary calcium excretion was 35% (65 mg) less in Black women. This large difference in urinary calcium likely accounts for a substantial proportion of the difference in incidence rates.39
KIDNEY STONES AND RISK OF IMPORTANT CONDITIONS
A history of kidney stones is also relevant for its association with multiple important conditions, including hypertension, coronary heart disease, and wrist fracture.
There is a positive association between a history of kidney stones and incident hypertension. Women with a history of kidney stones had a 24% higher risk of developing hypertension than did women who had no history of kidney stones independent of common risk factors for both conditions. However, participants who had hypertension were not at increased risk for subsequently developing kidney stones.23
Women and men with a history of kidney stones are at higher risk for coronary heart disease, defined as fatal or nonfatal myocardial infarction or coronary revascularization; the risk is higher in women than in men. In the pooled analysis of NHSs I and II, the HR for coronary heart disease among women with a history of kidney stones was 1.30. This positive association persisted after adjusting for a large number of potential confounders, including diabetes, hypertension, hyperlipidemia, and BMI.26 These results are consistent with those found in other cohorts. For example, kidney stones were also associated with a higher risk of cardiovascular events, specifically acute myocardial infarction, coronary interventions, and stroke, in a cohort of men and women from Canada.27 The observed association may in part be explained by impaired calcification regulation.
Women who form calcium kidney stones commonly have higher urinary calcium, and this may be associated with lower bone mineral density. A study of bone mineral density scores showed an inverse relation to urinary calcium excretion in individuals who form kidney stones but not in those without kidney stones.40 In an analysis of NHS postmenopausal women without baseline osteoporosis, a history of kidney stones was associated with an 18% higher risk of wrist fracture, but there was no association with hip fracture.41 These findings were consistent with those from the Health Improvement Network cohort of men and women, which found a higher incident fracture risk in women aged 30 to 79 years who formed kidney stones.42
LIMITATIONS
Studies using data from NHSs I and II have limitations. The design of the cohorts is observational, and these studies cannot rule out confounding by unknown or unmeasured factors. Much of the data collected from participants were self-reported; however, in the analyses of kidney stones, we only considered reports of kidney stones as cases when they were confirmed by at least a supplementary questionnaire (the validation study using medical records demonstrated that > 90% of self-reported events were confirmed by medical record review).
Potential misclassification, such as discrepancies between food frequency questionnaire measurements and actual nutrient intake, can be expected to be random with respect to a subsequent risk of incident kidney stones. There are also some differences in findings between NHSs I and II that are not readily explained. These types of studies can only suggest potential mechanisms. Finally, the generalizability of results of these studies may be limited because cohort participants are all women and are predominantly White.
IMPACT ON GUIDELINES AND LITERATURE
The vast and informative findings on the basis of data from the NHS I and II cohorts related to nephrolithiasis has led to substantial and important changes in the care of patients with kidney stones. The compelling findings for dietary, nondietary, and urinary risk factors have contributed to our knowledge of kidney stone formation.
These data have influenced clinical practice and past and ongoing scientific studies. For example, findings from NHSs I and II were cited many times in the clinical practice guidelines on the medical management of kidney stones from the American Urological Association.30 These findings also serve as the basis for commonly used clinical resources, such as UpToDate, and major medical texts, such as Harrison’s Principles of Internal Medicine.43
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (grants T32 DK007527 to M. L. P., R01DK094910 to E. N. T., K24DK091417 to G. C. C., and CA186107, CA176726, and CA167552).
HUMAN PARTICIPANT PROTECTION
No protocol approval was necessary for this review, because no human participants were involved.
REFERENCES
- 1.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coe FL, Keck J, Norton ER. The natural history of calcium urolithiasis. JAMA. 1977;238(14):1519–1523. [PubMed] [Google Scholar]
- 3.Rule AD, Lieske JC, Li X, Melton LJ, 3rd, Krambeck AE, Bergstralh EJ. The ROKS nomogram for predicting a second symptomatic stone episode. J Am Soc Nephrol. 2014;25(12):2878–2886. doi: 10.1681/ASN.2013091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curhan GC, Willett W, Speizer F, Spiegelman D, Stampfer M. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126(7):497–504. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women (Nurses’ Health Study II) Arch Intern Med. 2004;164(8):885–891. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 6.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328(12):833–838. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- 7.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J Urol. 1996;155(6):1847–1851. [PubMed] [Google Scholar]
- 8.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol. 1999;10(4):840–845. doi: 10.1681/ASN.V104840. [DOI] [PubMed] [Google Scholar]
- 9.Taylor EN, Curhan GC. Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol. 2007;18(7):2198–2204. doi: 10.1681/ASN.2007020219. [DOI] [PubMed] [Google Scholar]
- 10.Taylor EN, Curhan GC. Fructose consumption and the risk of kidney stones. Kidney Int. 2008;73(2):207–212. doi: 10.1038/sj.ki.5002588. [DOI] [PubMed] [Google Scholar]
- 11.Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol. 2009;20(10):2253–2259. doi: 10.1681/ASN.2009030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15(12):3225–3232. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 13.Taylor EN, Stampfer MJ, Curhan GC. Fatty acid intake and incident nephrolithiasis. Am J Kidney Dis. 2005;45(2):267–274. doi: 10.1053/j.ajkd.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Taylor EN, Curhan GC. Dietary calcium from dairy and nondairy sources, and risk of symptomatic kidney stones. J Urol. 2013;190(4):1255–1259. doi: 10.1016/j.juro.2013.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Caffeine intake and the risk of kidney stones. Am J Clin Nutr. 2014;100(6):1596–1603. doi: 10.3945/ajcn.114.089987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Stampfer MJ. Prospective study of beverage use and the risk of kidney stones. Am J Epidemiol. 1996;143(3):240–247. doi: 10.1093/oxfordjournals.aje.a008734. [DOI] [PubMed] [Google Scholar]
- 17.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med. 1998;128(7):534–540. doi: 10.7326/0003-4819-128-7-199804010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol. 2013;8(8):1389–1395. doi: 10.2215/CJN.11661112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59(6):2290–2298. doi: 10.1046/j.1523-1755.2001.00746.x. [DOI] [PubMed] [Google Scholar]
- 20.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 2008;73(4):489–496. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 21.Curhan GC, Willett WC, Rimm EB, Speizer FE, Stampfer MJ. Body size and risk of kidney stones. J Am Soc Nephrol. 1998;9(9):1645–1652. doi: 10.1681/ASN.V991645. [DOI] [PubMed] [Google Scholar]
- 22.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293(4):455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 23.Madore F, Stampfer MJ, Willett WC, Speizer FE, Curhan GC. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis. 1998;32(5):802–807. doi: 10.1016/s0272-6386(98)70136-2. [DOI] [PubMed] [Google Scholar]
- 24.Krambeck AE, Gettman MT, Rohlinger AL, Lohse CM, Patterson DE, Segura JW. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol. 2006;175(5):1742–1747. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 25.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68(3):1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 26.Ferraro PM, Taylor EN, Eisner BH et al. History of kidney stones and the risk of coronary heart disease. JAMA. 2013;310(4):408–415. doi: 10.1001/jama.2013.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander RT, Hemmelgarn BR, Wiebe N et al. Kidney stones and cardiovascular events: a cohort study. Clin J Am Soc Nephrol. 2014;9(3):506–512. doi: 10.2215/CJN.04960513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(4):804–811. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander RT, Hemmelgarn BR, Wiebe N et al. Kidney stones and kidney function loss: a cohort study. BMJ. 2012;345:e5287. doi: 10.1136/bmj.e5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearle MS, Goldfarb DS, Assimos DG et al. Medical management of kidney stones: AUA guideline. J Urol. 2014;192(2):316–324. doi: 10.1016/j.juro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Taylor EN, Stampfer MJ, Mount DB, Curhan GC. DASH-style diet and 24-hour urine composition. Clin J Am Soc Nephrol. 2010;5(12):2315–2322. doi: 10.2215/CJN.04420510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borghi L, Schianchi T, Meschi T et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346(2):77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 33.Ming Z, Lautt WW. Caffeine-induced natriuresis and diuresis via blockade of hepatic adenosine-mediated sensory nerves and a hepatorenal reflex. Can J Physiol Pharmacol. 2010;88(11):1115–1121. doi: 10.1139/y10-090. [DOI] [PubMed] [Google Scholar]
- 34.Ferraro PM, Curhan GC, Sorensen MD, Gambaro G, Taylor EN. Physical activity, energy intake and the risk of incident kidney stones. J Urol. 2015;193(3):864–868. doi: 10.1016/j.juro.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferraro PM, Curhan GC, Gambaro G, Taylor EN. Total, dietary, and supplemental vitamin C intake and risk of incident kidney stones. Am J Kidney Dis. 2016;67(3):400–407. doi: 10.1053/j.ajkd.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol. 1996;155(3):839–843. [PubMed] [Google Scholar]
- 37.Taylor EN, Chan AT, Giovannucci EL, Curhan GC. Cholelithiasis and the risk of nephrolithiasis. J Urol. 2011;186(5):1882–1887. doi: 10.1016/j.juro.2011.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattix Kramer HJ, Grodstein F, Stampfer MJ, Curhan GC. Menopause and postmenopausal hormone use and risk of incident kidney stones. J Am Soc Nephrol. 2003;14(5):1272–1277. doi: 10.1097/01.asn.0000060682.25472.c3. [DOI] [PubMed] [Google Scholar]
- 39.Taylor EN, Curhan GC. Differences in 24-hour urine composition between Black and White women. J Am Soc Nephrol. 2007;18(2):654–659. doi: 10.1681/ASN.2006080854. [DOI] [PubMed] [Google Scholar]
- 40.Asplin JR, Bauer KA, Kinder J et al. Bone mineral density and urine calcium excretion among subjects with and without nephrolithiasis. Kidney Int. 2003;63(2):662–669. doi: 10.1046/j.1523-1755.2003.00763.x. [DOI] [PubMed] [Google Scholar]
- 41.Taylor EN, Feskanich D, Paik JM, Curhan GC. Nephrolithiasis and risk of incident bone fracture. J Urol. 2016;195(5):1482–1486. doi: 10.1016/j.juro.2015.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denburg MR, Leonard MB, Haynes K et al. Risk of fracture in urolithiasis: a population-based cohort study using the health improvement network. Clin J Am Soc Nephrol. 2014;9(12):2133–2140. doi: 10.2215/CJN.04340514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curhan G. Nephrolithiasis. In: Kasper D, editor. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill; 2015. [Google Scholar]