Abstract
Objectives. To review the contribution of the Nurses’ Heath Study (NHS) and the NHS II in identifying risk and protective factors for breast cancer incidence and survival.
Methods. We conducted a narrative review of NHS and NHS II articles on breast cancer incidence and survival published from 1976 to 2016, with a focus on exogenous and endogenous hormones; lifestyle factors, including diet, physical activity, and aspirin use; intermediate markers of risk; and genetic factors.
Results. With the investigation of individual risk factors, as well as their incorporation into risk prediction models, the NHS has contributed to the identification of ways in which women may reduce breast cancer risk, including limiting alcohol consumption, reducing the duration of postmenopausal estrogen-plus-progestin use, avoiding weight gain, and increasing vegetable consumption. In addition, the NHS has helped elucidate the roles of several biomarkers and contributed to the identification of risk alleles.
Conclusions. The NHS has contributed to our understanding of lifestyle, hormonal, and genetic risk factors for breast cancer, highlighting the importance of exposures across the life course, and has helped identify lifestyle changes that may reduce risk and improve survival after a diagnosis of breast cancer.
The Nurses’ Health Study (NHS) originated with the goal of examining use of oral contraceptives and other potential risk factors for breast cancer. Beginning with our first breast cancer publication on use of permanent hair dyes in 1979, we have investigated a wide range of lifestyle factors, medications, and biomarkers, as well as intermediate endpoints and tumor characteristics. Highlights of our findings related to breast cancer, their contributions to public health, and future research directions are described in this article.
EXOGENOUS HORMONES
NHS and NHS II researchers examined the relationships between exogenous hormones and breast cancer risk. We highlight findings related to oral contraceptives and postmenopausal hormone use.
Oral Contraceptives
The NHS began in 1976 with the primary goal of evaluating the long-term consequences of oral contraceptive (OC) use, particularly its potential association with breast cancer risk. In 1986, we published the first NHS investigation on this topic, the largest prospective study at the time, in which we did not observe an overall association between OC use and breast cancer risk, consistent with most previous studies.1 However, we observed that current OC users had an elevated risk, and a subsequent analysis with additional follow-up had similar findings. Our early observations were confirmed in a collaboration of 54 studies, including the NHS, with over 50 000 cases of breast cancer.2 In this collaborative analysis, no overall relationship was observed between duration of use and breast cancer risk, but there was a modest elevated risk among current and recent users of OCs, suggesting a late-stage promotional effect. The establishment of the later NHS II, in 1989, allowed for a more detailed examination of newer, specific OC formulations. Current OC use was again associated with an elevated breast cancer risk (for current vs never use, relative risk [RR] = 1.33; 95% confidence interval [CI] = 1.03, 1.73), with triphasic preparations that include levonorgestrel as the progestin most strongly associated with risk.3 However, given the low absolute risk of breast cancer among younger women, the estimated population attributable risk of current OC use was 1.8%, indicating that OC use is not a major cause of breast cancer. On the basis of these and other studies, the International Agency for Research on Cancer (IARC) concluded that estrogen–progestogen OCs were carcinogenic to humans (group 1). However, the working group also stressed that OC use is protective against other types of cancer, as reported in the NHS, including endometrial and ovarian cancer, and advised women to discuss the risks and benefits of OCs with their clinician.
Postmenopausal Hormone Use
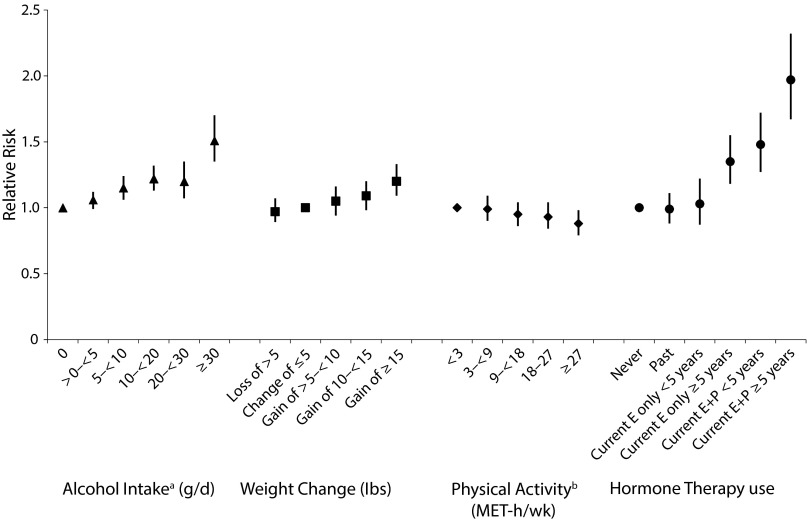
Whereas exogenous estrogens were suspected of increasing breast cancer risk, early studies of postmenopausal hormone therapy (HT) generally did not observe an association; however, these early studies focused on ever versus never use of HT. In 1990, we published the first NHS analysis of postmenopausal HT use and breast cancer risk.4 Although there was no association with past HT use, current users had a 36% higher risk of breast cancer than never users (95% CI = 1.11, 1.67). In our 1995 analysis of type of HT use, both current-estrogen-only and estrogen-plus-progestin HT use were positively associated with risk compared with never use. In a recent NHS–NHS II analysis, the association with estrogen-plus-progestin HT use was stronger among those with longer duration of use (for current use < 5 years, hazard ratio [HR] = 1.48; 95% CI = 1.27, 1.72, and for current use ≥ 5 years, HR = 1.97; 95% CI = 1.67, 2.32; Figure 1).5 In addition, the positive association with current-estrogen-only HT use was apparent only among women who had used it for longer durations (for current use < 5 years, HR = 1.03; 95% CI = 0.87, 1.22, and for current use ≥ 5 years, HR = 1.35; 95% CI = 1.18, 1.55). These observations are supported by results from the Women’s Health Initiative trial of estrogen-plus-progestin use among women aged 50 to 79 years, which was stopped early, largely because of the elevated risk of breast cancer in the estrogen-plus-progestin HT arm. Although the estrogen-alone versus placebo Women’s Health Initiative trial did not observe an association with breast cancer risk, median follow-up was only 6.8 years. Additional information on exogenous hormone use and breast cancer risk may be found in the article by Bhupathiraju et al. in this issue (p1631).
FIGURE 1—
Relative Risks for the Associations Between Selected Risk Factors and Risk of Breast Cancer: The Nurses’ Health Study, United States
Note. E = estrogen; E+P = estrogen plus progestin; MET = metabolic equivalent. Whiskers indicate 95% confidence intervals. The relative risks presented are from studies with different time frames. For exact dates, see source articles.
Source. Sisti et al.,5 Rosner et al.,6 Eliassen et al.,7 and Chen et al.8
a12 grams of alcohol per day is equivalent to approximately 1 drink per day.
b9 MET-hours per week is equivalent to 3 hours of walking at an average pace per week.
ENDOGENOUS HORMONES
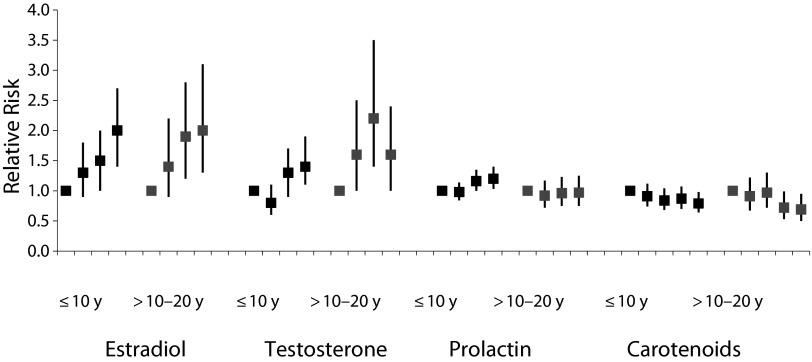
The NHS and NHS II biospecimen collections at multiple time points throughout follow-up have enabled extensive research on the associations between prediagnostic plasma and urinary biomarkers and risk of breast cancer, including analyses by the timing of prediagnostic measurements. The NHS was among the first studies to prospectively demonstrate that circulating sex hormones were associated with postmenopausal breast cancer,9 and recent analyses with additional follow-up have demonstrated positive associations for both estrogens and androgens (Figure 2) among samples collected within 10 years of diagnosis, as well as among those collected 10 to 20 years prior.10 In addition, our unique collection of NHS II premenopausal samples timed in the menstrual cycle has allowed us to explore associations with sex hormones by menstrual phase. In the first large prospective study to examine associations by menstrual phase, estradiol was positively associated with breast cancer risk for follicular levels, whereas androgens were positively associated with risk for both luteal and follicular levels.13 Although these associations were attenuated with further follow-up, in a collaborative reanalysis of 7 prospective studies, including ours, circulating levels of estrogens and androgens were significantly positively associated with risk of breast cancer before age 50 years.
FIGURE 2—
Relative Risks for Circulating Biomarkers and Risk of Breast Cancer, by Years Between Blood Collection and Diagnosis: The Nurses’ Health Study (NHS) and NHS II, United States
Note. Estradiol categories are ≤ 4, > 4 to 6, > 6 to 9, and > 9 pg/mL; testosterone categories are ≤ 14, > 14 to 19, > 19 to 26.9, and > 26.9 ng/dL; prolactin categories are ≤ 8.1, > 8.1 to 11.0, > 11.0 to 15.7, and > 15.7 ng/ml; total carotenoids categories are < 729, 729 to < 917, 917 to < 1124, 1124 to < 1379, and ≥ 1379 μg/dL. The relative risks presented are from studies with different time frames. For exact dates, see source articles.
Source. Zhang et al.,10 Tworoger et al.,11 and Eliassen et al.12
We have investigated breast cancer risk with other circulating hormones, including insulin-like growth factor 1 (IGF-1), growth hormone, insulin, C-peptide, leptin, and C-reactive protein, among others. Prolactin, an endogenous hormone involved in breast development, also has been extensively investigated. Although more modest than associations observed for estradiol, prolactin levels measured within 10 years of diagnosis in the NHS and NHS II were positively associated with risk (Figure 2); this association was stronger among postmenopausal women and for estrogen receptor (ER)-positive tumors.11 The inclusion of endogenous hormones (estrone sulfate, testosterone, and prolactin) to both the Gail score and our original Rosner–Colditz model significantly improved risk prediction for breast cancer among postmenopausal women in the NHS, suggesting that inclusion of endogenous hormones in risk models could help better identify women for increased screening or prevention efforts.
WEIGHT AND WEIGHT CHANGE
The association between weight and breast cancer risk is complex, with the relationship varying across the life course. In one of the first detailed studies of childhood and adolescent body size and risk, we observed an inverse association with both pre- and postmenopausal breast cancer,14 which persisted with additional follow-up.15,16 As this strong inverse association has been observed in several other cohorts and across different populations, we explored potential pathways by which early-life body size may affect risk. For example, body fatness in childhood and body mass index (BMI) at age 18 years were inversely associated with adult plasma IGF-1 levels, suggesting that altered IGF-1 levels may be a potential mechanism. Furthermore, early-life body size was inversely associated with percentage of mammographic density (a strong risk factor for breast cancer, discussed in the section “Intermediate Markers of Breast Cancer Risk”) independent of current BMI.
In the first prospective analysis of adult body size and premenopausal breast cancer, higher BMI was inversely associated with risk, consistent with previous case–control studies.17 This early observation was confirmed in a later analysis within the NHS18 and in many other studies. Although higher BMI at age 18 years was inversely associated with both pre- and postmenopausal risk, weight gain after age 18 years was positively associated with risk after menopause, though limited to those who never used HT. In a subsequent analysis with 26 years of follow-up, we observed that among women who never used HT, those who had lost more than 10 kilograms since menopause and maintained their weight loss had a lower risk of breast cancer than women with stable weight since menopause.19 This was the first study to show that women may be able to reduce their risk with lifestyle changes later in life. In 2015, we assessed the association of short-term (4-year) weight change with pre- and postmenopausal breast cancer. Short-term gain was associated with increased breast cancer risk that was stronger for premenopausal women, suggesting that the protective association with current BMI before menopause may be driven by body size earlier in life (Figure 1).6
LIFESTYLE FACTORS
NHS and NHS II researchers examined the relationships between lifestyle factors and breast cancer risk. We highlight findings related to physical activity, diet, aspirin use, and shift work.
Physical Activity
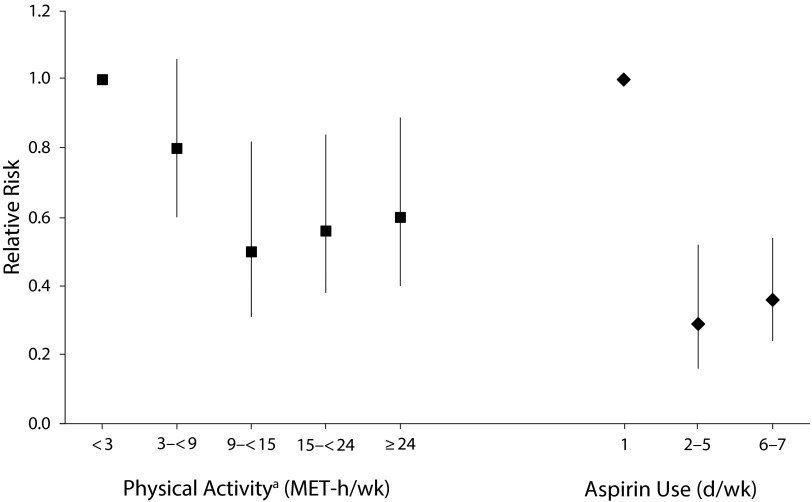
Physical activity has been hypothesized to decrease breast cancer risk by lowering ovarian hormone levels. In an early NHS analysis with over 16 years of follow-up, using repeated measures of physical activity, women who reported participating in at least 7 hours of moderate or vigorous physical activity per week had an 18% lower risk of breast cancer (95% CI = 0.70, 0.97).20 In a follow-up analysis restricted to postmenopausal women, both cumulative and recent physical activity were inversely associated with risk (Figure 1).7 Among younger women in the NHS II, lifetime physical activity was inversely associated with risk of premenopausal breast cancer (RR = 0.77; 95% CI = 0.44, 0.93, comparing the most- with the least-active women). The inverse association was strongest for adolescent activity and for premenopausal risk, but not postmenopausal risk. Reviews by the World Health Organization’s IARC and by the American Institute for Cancer Research and the World Cancer Research Fund International, which included the NHS and other studies, concluded that greater physical activity in adulthood probably reduces the risk of postmenopausal breast cancer. In addition to the association with risk, we have observed that physical activity may be important for survival after breast cancer diagnosis. In the first study of postdiagnostic physical activity, we observed that women who engaged in physical activity for 9 or more metabolic equivalent (MET)-hours per week had a lower risk of death from breast cancer as well as death from any cause21 (Figure 3). For breast cancer survival, the greatest benefit was among those whose physical activity was equivalent to walking 3 to 5 hours per week at an average pace.21 These results suggest that women with breast cancer who follow the US recommendations of at least 30 minutes per day of moderate physical activity for at least 5 days per week may improve their survival, independent of activity level before diagnosis.
FIGURE 3—
Relative Risks for the Associations Between Physical Activity and Aspirin Use After Diagnosis and Risk of Breast Cancer Death: The Nurses’ Health Study, United States
Note. MET = metabolic equivalent. The relative risks presented are from studies with different time frames. For exact dates, see source articles.
Source. Holmes et al.21,22
a9 MET-hours per week is equivalent to 3 hours of walking at an average pace per week.
Diet
To date, the most consistent dietary risk factor for breast cancer risk is alcohol. In one of the first prospective analyses of this association, women consuming both moderate (5–14 g/day) and high (≥ 15 g/day) amounts of alcohol had an elevated risk of breast cancer (RR = 1.3; 95% CI = 1.1, 1.7, and RR = 1.6; 95% CI = 1.3, 2.0, respectively).23 An NHS analysis with additional follow-up8 (Figure 1), as well as multiple subsequent prospective studies, confirmed the association with alcohol, including a pooled analysis of 20 studies. Most recently, we observed that alcohol consumption, both in early and later adult life, was independently associated with risk, even at low levels of consumption in the NHS II (e.g., at 10 g/day, RR = 1.11; 95% CI = 1.00, 1.23, for alcohol consumption between menarche and first pregnancy).24 The IARC classifies alcohol as a group 1 carcinogen for breast and other cancers; we estimated that the population attributable risk of breast cancer because of alcohol intake was 10%.
Dietary fat intake was long hypothesized to account for higher rates of breast cancer in affluent countries, largely on the basis of the strong international correlations with breast cancer incidence and animal studies in which dietary fat promoted mammary tumors. Although positive associations between dietary fat and breast cancer risk were seen in some case–control studies, we did not observe an association between dietary fat intake and risk in the NHS in the first decade of follow-up.25,26 This null association is consistent with a 2001 pooled analysis of 8 prospective cohort studies as well as with the Women’s Health Initiative, as neither observed significant associations between fat intake and breast cancer risk.
A prudent dietary pattern (characterized by higher intakes of fruits, vegetables, whole grains, low-fat dairy, fish, and poultry) was inversely associated with ER-negative breast tumors in the NHS, largely driven by an inverse association with fruit and vegetable intake.27 In a later NHS analysis with additional follow-up, vegetable intake was more modestly associated with ER-negative tumors, whereas fruit intake was not associated. In a subsequent pooled analysis of 20 cohort studies, including the NHS and NHS II, fruit intake was not significantly associated with risk; however, vegetable intake was inversely associated with ER-negative tumors, consistent with our previous observations. Carotenoids are hypothesized to be some of the components of vegetables that influence breast cancer risk because of their potential inhibition of tumor progression and proliferation. In the NHS nested case–control study, we observed that higher levels of plasma carotenoids, including α-carotene, β-carotene, and lycopene, were inversely associated with breast cancer risk (Figure 2).12,28 In a subsequent pooled analysis of 8 prospective cohort studies, including the NHS, inverse associations between circulating carotenoids were stronger for ER-negative than for ER-positive tumors, providing further evidence for a role of carotenoids in the prevention of ER-negative breast cancer.29
The NHS has contributed to the recognition that early-life factors are important in the investigation of breast cancer risk. For example, using a 131-item high school food frequency questionnaire in the NHS II, we pioneered research into adolescent diet and breast cancer.30 We observed higher risks of premenopausal breast cancer among women with greater consumption of red meat in high school (RR comparing extreme quintiles = 1.43; 95% CI = 1.05, 1.94; P trend < .01), and lower risks among women with higher intakes of fiber (RR comparing extreme quintiles = 0.84; 95% CI = 0.70, 1.01; P trend = .04) and fruit (RR comparing extreme quintiles = 0.76; 95% CI = 0.62, 0.93) during adolescence.
Aspirin
In analyses in NHS and NHS II, we did not observe an association between use of aspirin (or other nonsteroidal anti-inflammatory drugs) and risk of breast cancer, including among regular aspirin users and users of long duration (> 20 years).31 Although aspirin use was not associated with incidence, recent data from the NHS suggest that aspirin use after breast cancer diagnosis may improve survival. In an analysis of over 4000 women who had been diagnosed with breast cancer, aspirin use was associated with a lower risk of distant recurrence and breast cancer death.22 For example, compared with nonusers, women who used aspirin 7 days per week had a 64% lower risk of breast cancer death (95% CI = 0.24, 0.54; Figure 3). This was the first analysis to report better survival for those women with breast cancer who take aspirin. We explored the biology behind this association, finding that the breast cancer survival–aspirin association is not mediated by COX-2, as it is in colon cancer. Our study and others provided the impetus for the current Aspirin for Breast Cancer randomized trial to investigate whether aspirin use in women with breast cancer will reduce rates of recurrence and improve survival.
Shift Work
Occupational exposure to rotating night shifts may influence subsequent breast cancer risk through suppression of melatonin by direct exposure to light at night. In the first prospective study of shift work and breast cancer risk, we observed a positive association between number of years working on rotating night shifts and risk of breast cancer in the NHS; the relative risk among women who worked 30 or more years on night shifts was 1.36 (95% CI = 1.04, 1.78).32 In the NHS II, the associations between long-term shift work and risk were similar or suggestively stronger.33 In 2007, the IARC concluded that night shift work was a probable human carcinogen.34 The NHS and NHS II studies provided the only prospective data on this potential association. However, additional research is needed to understand how the timing, duration, and type of shift work affects breast cancer risk over a woman’s lifespan.
INTERMEDIATE MARKERS OF BREAST CANCER RISK
NHS and NHS II researchers examined intermediate markers of breast cancer risk, including predictors of these markers as well as the relationship with breast cancer. We highlight findings related to benign breast disease and mammographic density.
Benign Breast Disease
To study the association between benign breast disease (BBD) and risk of breast cancer, we initiated a collection of BBD specimens from participants who had subsequently developed breast cancer as well as those who had not. We demonstrated that specific classes of benign lesions are associated with higher subsequent risk of breast cancer. For example, compared with women with nonproliferative BBD, women with proliferative disease without atypia had a twofold increased risk and women with atypical hyperplasia had a fourfold increased risk of breast cancer.35,36 We observed that the increased risk persists for over 10 years after biopsy and that only approximately 60% of subsequent cancers develop in the ipsilateral breast, suggesting that BBD is a generalized marker of risk rather than a precursor lesion.36 The inclusion of type of BBD to the Rosner–Colditz risk prediction model improved risk classification compared with a model with only a yes–no BBD classification.37 In addition, features such as radial scars were associated with risk independent of these general BBD categories, and the presence of predominant type 1 lobules in BBD (a marker of greater involution) was associated with a decreased risk of breast cancer compared with BBD with no type 1 lobules.
We recently expanded our BBD research to the Growing Up Today Study, a cohort comprising children of NHS II participants, in which we assessed potential predictors of BBD in young women. This study provides a unique opportunity to prospectively examine early-life exposures in relation to a marker of breast cancer risk. We observed that higher consumption of alcoholic beverages during adolescence was positively associated with risk of BBD, whereas higher carotenoid consumption was inversely associated with BBD risk.38,39
Mammographic Density
Percentage of mammographic density (i.e., the proportion of breast tissue on a mammogram that is radiodense and appears light) is one of the strongest risk factors for breast cancer and a potential intermediate marker of risk. The NHS–NHS II collection of mammograms from women in the nested case–control studies of breast cancer has allowed extensive investigations of risk factors for high density, as well as further elucidating the relationship between density and risk. Contrary to the long-standing hypothesis that high density may reflect greater exposure to endogenous estrogen, we observed that circulating estradiol levels in postmenopausal women were not associated with percentage density40; however, women with both high density and hormones levels were at particularly high risk of breast cancer. This suggests that both biomarkers may be useful in identifying and targeting women for chemoprevention and additional screening. We also demonstrated that, although percentage density is the strongest single measure of breast density associated with breast cancer risk, the dense and nondense areas on the mammogram are both strongly and independently associated with risk.41 These novel findings suggest that nondense area (i.e., breast fat) is protective against breast cancer. Together with basic science data, it appears that adipocytes may play a role in breast cancer that was not previously appreciated, opening new avenues of research for prevention. We also observed that percentage density was more strongly associated with ER-negative than with ER-positive breast cancer. Because few risk factors are identified for ER-negative breast cancer, this finding may be especially important to better understand predictors of this more aggressive subtype.
GENETICS
The first National Institutes of Health–funded genome-wide association study (GWAS) of breast cancer was conducted in the NHS and identified a common breast cancer–associated variant in FGFR2.42,43 To overcome the inherent issue of multiple comparisons in GWASs, investigators across the world have formed large international consortia that include genetic data from thousands of women with and without breast cancer. NHS–NHS II investigators contributed to and led many of the consortial efforts, such as the Breast Cancer Association Consortium and the Breast and Prostate Cancer Cohort Consortium. Through these and other GWAS consortia, more than 90 common (minor allele frequency > 0.05) risk loci for breast cancer have been identified; the variants at these loci explain approximately 16% of the familial risk of breast cancer.44
CONCLUSIONS
Over the last 40 years, research in the NHS has contributed greatly to our understanding of risk factors for breast cancer. The Rosner–Colditz breast cancer prediction model, developed on the basis of risk factors identified in the NHS and other studies, was recently validated in the California Teachers’ Cohort, where it outperformed the Gail model.45 As our continuing research in the NHS and NHS II has identified new risk factors for breast cancer, we are currently evaluating whether additional factors, including hormone levels, mammographic density, and genetic polymorphisms, improve our ability to identify women at highest risk for breast cancer. Importantly, research in the NHS has identified ways in which women may reduce their risk of breast cancer, including limiting adult weight gain, reducing the duration of estrogen-plus-progestin HT use, limiting alcohol consumption, and increasing consumption of vegetables.
As we enter the fifth decade of the Nurses’ Health Studies, we are seeking new ways to further our understanding of breast cancer etiology and prevention. Our recent work has expanded to examine breast cancer risk factor associations by molecular subtype (e.g., luminal A or basal-like); continued tumor tissue collection in both the NHS and NHS II will allow further exploration of risk and survival by subtypes. Lastly, as reproductive and lifestyle exposures evolve over time, it is crucial to include younger generations of women in cohort studies, particularly as early-life exposures may be most relevant for breast cancer risk. The expansion of the cohorts to include the Nurses’ Health Study 3 (nhs3.org) will help further our understanding of breast cancer etiology, prevention, and survival.
ACKNOWLEDGMENTS
The Nurses’ Health Study and Nurses’ Health Study II are supported by research grants from the National Cancer Institute, National Institutes of Health (UM1CA186107, P01CA87969, R01CA49449, UM1CA176726, and R01CA67262).
We thank the participants of the Nurses’ Health Study and Nurses’ Health Study II for their continuing contributions. We thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
HUMAN PARTICIPANT PROTECTION
The Nurses’ Health Study protocols have been approved by the Brigham and Women’s Hospital institutional review board and accepted by the Harvard T. H. Chan School of Public Health.
Footnotes
See also Galea and Vaughan, p. 1531.
REFERENCES
- 1.Lipnick RJ, Buring JE, Hennekens CH et al. Oral contraceptives and breast cancer. A prospective cohort study. JAMA. 1986;255(1):58–61. [PubMed] [Google Scholar]
- 2.Breast cancer and hormonal contraceptives: further results. Collaborative Group on Hormonal Factors in Breast Cancer. Contraception. 1996;54(3 suppl):1S–106S. doi: 10.1016/s0010-7824(15)30002-0. [DOI] [PubMed] [Google Scholar]
- 3.Hunter DJ, Colditz GA, Hankinson SE et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2496–2502. doi: 10.1158/1055-9965.EPI-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colditz GA, Stampfer MJ, Willett WC, Hennekens CH, Rosner B, Speizer FE. Prospective study of estrogen replacement therapy and risk of breast cancer in postmenopausal women. JAMA. 1990;264(20):2648–2653. [PubMed] [Google Scholar]
- 5.Sisti JS, Collins LC, Beck AH, Tamimi RM, Rosner BA, Eliassen AH. Reproductive risk factors in relation to molecular subtypes of breast cancer: results from the Nurses’ Health Studies. Int J Cancer. 2016;138(10):2346–2356. doi: 10.1002/ijc.29968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosner B, Eliassen AH, Toriola AT et al. Short-term weight gain and breast cancer risk by hormone receptor classification among pre- and postmenopausal women. Breast Cancer Res Treat. 2015;150(3):643–653. doi: 10.1007/s10549-015-3344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170(19):1758–1764. doi: 10.1001/archinternmed.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hankinson SE, Willett WC, Manson JE et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90(17):1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Tworoger SS, Eliassen AH, Hankinson SE. Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat. 2013;137(3):883–892. doi: 10.1007/s10549-012-2391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tworoger SS, Eliassen AH, Zhang X et al. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res. 2013;73(15):4810–4819. doi: 10.1158/0008-5472.CAN-13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliassen AH, Liao X, Rosner B, Tamimi RM, Tworoger SS, Hankinson SE. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am J Clin Nutr. 2015;101(6):1197–1205. doi: 10.3945/ajcn.114.105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliassen AH, Missmer SA, Tworoger SS et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98(19):1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 14.Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and breast carcinoma risk. Cancer. 1999;85(11):2400–2409. doi: 10.1002/(sici)1097-0142(19990601)85:11<2400::aid-cncr15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171(11):1183–1194. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baer HJ, Colditz GA, Rosner B et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res. 2005;7(3):R314–R325. doi: 10.1186/bcr998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willett WC, Browne ML, Bain C et al. Relative weight and risk of breast cancer among premenopausal women. Am J Epidemiol. 1985;122(5):731–740. doi: 10.1093/oxfordjournals.aje.a114156. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z, Hankinson SE, Colditz GA et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278(17):1407–1411. [PubMed] [Google Scholar]
- 19.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 20.Rockhill B, Willett WC, Hunter DJ, Manson JE, Hankinson SE, Colditz GA. A prospective study of recreational physical activity and breast cancer risk. Arch Intern Med. 1999;159(19):2290–2296. doi: 10.1001/archinte.159.19.2290. [DOI] [PubMed] [Google Scholar]
- 21.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 22.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28(9):1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Hennekens CH, Speizer FE. Moderate alcohol consumption and the risk of breast cancer. N Engl J Med. 1987;316(19):1174–1180. doi: 10.1056/NEJM198705073161902. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Colditz GA, Rosner B et al. Alcohol intake between menarche and first pregnancy: a prospective study of breast cancer risk. J Natl Cancer Inst. 2013;105(20):1571–1578. doi: 10.1093/jnci/djt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Hennekens CH, Speizer FE. Dietary fat and the risk of breast cancer. N Engl J Med. 1987;316(1):22–28. doi: 10.1056/NEJM198701013160105. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Hunter DJ, Stampfer MJ et al. Dietary fat and fiber in relation to risk of breast cancer. An 8-year follow-up. JAMA. 1992;268(15):2037–2044. [PubMed] [Google Scholar]
- 27.Fung TT, Hu FB, Holmes MD et al. Dietary patterns and the risk of postmenopausal breast cancer. Int J Cancer. 2005;116(1):116–121. doi: 10.1002/ijc.20999. [DOI] [PubMed] [Google Scholar]
- 28.Tamimi RM, Colditz GA, Hankinson SE. Circulating carotenoids, mammographic density, and subsequent risk of breast cancer. Cancer Res. 2009;69(24):9323–9329. doi: 10.1158/0008-5472.CAN-09-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eliassen AH, Hendrickson SJ, Brinton LA et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst. 2012;104(24):1905–1916. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frazier AL, Ryan CT, Rockett H, Willett WC, Colditz GA. Adolescent diet and risk of breast cancer. Breast Cancer Res. 2003;5(3):R59–R64. doi: 10.1186/bcr583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Smith-Warner SA, Collins LC, Rosner B, Willett WC, Hankinson SE. Use of aspirin, other nonsteroidal anti-inflammatory drugs, and acetaminophen and postmenopausal breast cancer incidence. J Clin Oncol. 2012;30(28):3468–3477. doi: 10.1200/JCO.2012.42.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schernhammer ES, Laden F, Speizer FE et al. Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health Study. J Natl Cancer Inst. 2001;93(20):1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 33.Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006;17(1):108–111. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- 34.Straif K, Baan R, Grosse Y et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 35.London SJ, Connolly JL, Schnitt SJ, Colditz GA. A prospective study of benign breast disease and the risk of breast cancer. JAMA. 1992;267(7):941–944. [PubMed] [Google Scholar]
- 36.Collins LC, Baer HJ, Tamimi RM, Connolly JL, Colditz GA, Schnitt SJ. Magnitude and laterality of breast cancer risk according to histologic type of atypical hyperplasia: results from the Nurses’ Health Study. Cancer. 2007;109(2):180–187. doi: 10.1002/cncr.22408. [DOI] [PubMed] [Google Scholar]
- 37.Tamimi RM, Rosner B, Colditz GA. Evaluation of a breast cancer risk prediction model expanded to include category of prior benign breast disease lesion. Cancer. 2010;116(21):4944–4953. doi: 10.1002/cncr.25386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berkey CS, Willett WC, Frazier AL et al. Prospective study of adolescent alcohol consumption and risk of benign breast disease in young women. Pediatrics. 2010;125(5):e1081–e1087. doi: 10.1542/peds.2009-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boeke CE, Tamimi RM, Berkey CS et al. Adolescent carotenoid intake and benign breast disease. Pediatrics. 2014;133(5):e1292–e1298. doi: 10.1542/peds.2013-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99(15):1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 41.Pettersson A, Hankinson SE, Willett W, Lagiou P, Trichopoulos D, Tamimi RM. Non-dense mammographic area and risk of breast cancer. Breast Cancer Res. 2011;13(5):R100. doi: 10.1186/bcr3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter DJ, Kraft P, Jacobs KB et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39(7):870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas G, Jacobs KB, Yeager M et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40(3):310–315. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]
- 44.Michailidou K, Beesley J, Lindstrom S et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat Genet. 2015;47(4):373–380. doi: 10.1038/ng.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosner BA, Colditz GA, Hankinson SE, Sullivan-Halley J, Lacey JV, Jr, Bernstein L. Validation of Rosner–Colditz breast cancer incidence model using an independent data set, the California Teachers Study. Breast Cancer Res Treat. 2013;142(1):187–202. doi: 10.1007/s10549-013-2719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]