Abstract
Objectives. To review the contribution of the Nurses’ Health Studies (NHS and NHS II) in addressing hypotheses regarding risk factors for and consequences of obesity.
Methods. Narrative review of the publications of the NHS and NHS II between 1976 and 2016.
Results. Long-term NHS research has shown that weight gain and being overweight or obese are important risk factors for type 2 diabetes, cardiovascular diseases, certain types of cancers, and premature death. The cohorts have elucidated the role of dietary and lifestyle factors in obesity, especially sugar-sweetened beverages, poor diet quality, physical inactivity, prolonged screen time, short sleep duration or shift work, and built environment characteristics. Genome-wide association and gene–lifestyle interaction studies have shown that genetic factors predispose individuals to obesity but that such susceptibility can be attenuated by healthy lifestyle choices. This research has contributed to evolving clinical and public health guidelines on the importance of limiting weight gain through healthy dietary and lifestyle behaviors.
Conclusions. The NHS cohorts have contributed to our understanding of the risk factors for and consequences of obesity and made a lasting impact on clinical and public health guidelines on obesity prevention.
Over the past 40 years, few health topics have engendered as much concern, controversy, or debate as obesity. Once a rarity, obesity is now epidemic, and major health organizations consider it a disease. Obesity rates have climbed across the decades despite increasing knowledge about obesity’s health risks and strategies for prevention. When the Nurses’ Health Study (NHS) began in 1976, the national prevalence of overweight (body mass index [BMI; defined as weight in kilograms divided by the square of height in meters] of ≥ 25 to < 30) in women was 24.8%, and obesity (BMI ≥ 30) was 16.8%.1 When NHS II launched in 1989, overweight prevalence in US women still hovered around 25%, but obesity prevalence had climbed to nearly match it.1 Today, approximately two thirds of US women are overweight or obese.
The findings from NHS cohorts have greatly contributed to our understanding of the etiology of obesity, as well as its consequences. Among key findings are the effects of excess weight, even in normal BMI ranges, on the risk of chronic disease morbidity and mortality, the importance of limiting weight gain, and dietary, lifestyle, and genetic determinants of obesity, as well as gene–environment interactions. We have reviewed these and related findings and how they have contributed to obesity knowledge and public health approaches regarding obesity prevention. (For a more inclusive list of NHS and NHS II publications, see the Appendix, available as a supplement to the online version of this article at http://www.ajph.org, and the 2008 textbook Obesity Epidemiology.2)
ASSESSING ANTHROPOMETRICS IN THE NURSES
In brief, in 1976, 121 701 female nurses in the US aged 30 to 55 years enrolled in the NHS. The NHS II was established in 1989, enrolling 116 671 younger female registered nurses. At enrollment and biannually thereafter, questionnaires have been administered in both cohorts to collect and update self-reported medical and lifestyle information; dietary data have been collected every 4 years via a food frequency questionnaire (beginning in 1980 in NHS). Follow-up rates have exceeded 90% in each 2-year cycle. (For additional information on the inception and methods of the NHS, please see Bao et al. [p1573] in this issue.)
With more than 200 000 participants followed up to 40 years, NHS investigators now have millions of self-reported anthropometric measures. Weight is the most commonly assessed measure, appearing as the first or second question on each biennial questionnaire in both cohorts (except for 1984, where it appeared as question 12). Other measures—waist, hip, and upper arm circumferences, childhood and adolescent somatotypes, and recalled weight at age 18 years—have also been assessed.
The first validation study3 in NHS compared self-reported versus technician-based anthropometric measures in a Boston-based subsample of 140 participants in 1986–1987. Technician-measured weight was highly correlated with self-reported weight (r = 0.97), although participants tended to underreport their weight by 1.5 kilograms. Technician-measured and self-reported waist circumferences were nearly identical; hip circumferences tended to be underreported by about 0.5 inch compared with technician measures. A later validation study compared recalled weight at age 18 years with college or other school records in 118 participants of NHS II. Correlations were high between recalled and measured past weight (r = 0.87), height (r = 0.94), and BMI at age 18 years (r = 0.84), although BMI at age 18 years on the basis of self-report was an average 0.5 lower than was that from records.4
OBESITY RISK FACTORS
NHS participants have tended to gain weight through midlife,5 roughly 0.4 kilograms per year.6 Although this pattern of gain is often viewed as normative, it is not inevitable. As NHS research has shown, maintaining a healthy weight is in large part a function of lifestyle choices that can curb the risk of obesity.
Dietary Factors
Nutrients, foods, and beverages.
That dietary fat was a main cause of obesity was once a widespread belief. However, in an 8-year follow-up of NHS, total fat intake was only weakly related to weight gain.7 Increasing intakes of energy from monounsaturated or polyunsaturated fats were not associated with weight gain, whereas increasing energy intakes from animal, saturated, and, notably, trans fats were positively associated with weight gain, particularly in overweight women.7
As women decreased energy intake from fat through the 1990s,7 total and types of carbohydrates took on new importance. In a 12-year follow-up study in NHS, women who had higher whole grain intake gained less weight than did women with lower intake, whereas those with higher refined grain intake consistently gained more.8 In addition, women who increased their intake of whole grains or total dietary fiber over follow-up had 19% and 34% lower risk, respectively, of becoming obese than did those with the smallest increases in intake. Refined grain intake had opposite effects.8
Other food groups (e.g., fruits, vegetables,9 and nuts10) have also been foci, often owing to controversy about a particular food over time. For example, in the NHS, higher nut consumption was not associated with greater 16-year weight change than was low or no nut consumption.10 In a later NHS II study, women eating nuts at least twice per week gained slightly less weight and had a slightly lower risk of becoming obese than did women who rarely or never ate nuts.
Beverages have also been evaluated in relation to body weight. Alcohol intake in NHS II had a nonlinear relationship with the risk of gaining 5 or more kilograms. Women who were light to moderate drinkers (up to 30 g/day) were less likely to gain weight than were nondrinkers or heavy drinkers.11 By contrast, increasing sugar-sweetened beverage (SSB) or fruit juice intake was associated with greater weight gain, and decreasing consumption with less weight gain.12 In another study, women who increased their daily intake of water, coffee (without added sugar), or a diet beverage by 1 serving lost weight in a given 4-year period, whereas those who increased SSB or fruit juice intake gained weight. Substituting water or coffee for SSBs was associated with an average 0.5-kilogram smaller weight gain.13
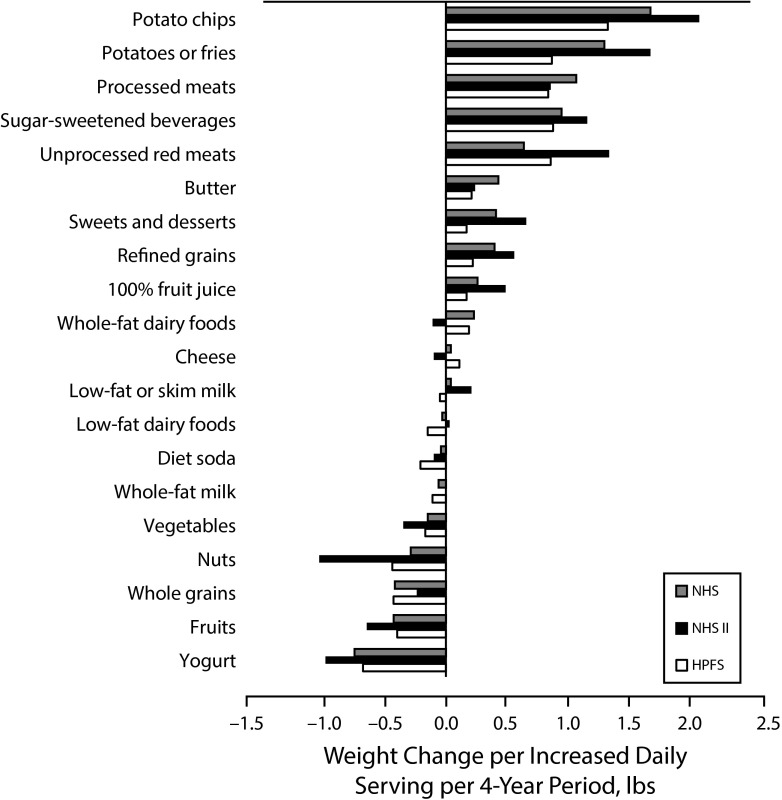
A comprehensive analysis of intake of multiple foods and beverages and weight gain was reported in a 2012 NHS article, which also included the Health Professionals Follow-up Study (Figure 1). Women who increased intakes of potato chips and potatoes, refined grains, sweets or desserts, SSBs, processed and unprocessed red meats, or fried foods experienced greater weight gain, on average, over a 4-year period.6 Conversely, women who increased intakes of vegetables, fruits, whole grains, nuts, or yogurt in the same period experienced less weight gain.
FIGURE 1—
Relationships Between Changes in Food and Beverage Consumption and Weight Change Every 4 Years in the Nurses’ Health Study (NHS; 1986–2006), the NHS II (1991–2003), and the Health Professionals Follow-up Study (HPFS; 1986–2006): United States
Note. Weight changes are shown per increase in daily serving of the food or beverage. All weight changes were adjusted simultaneously for age, baseline body mass index, sleep duration, smoking status, physical activity, television watching, alcohol use, and all the dietary factors shown.
Source. Adapted from Mozaffarian et al.6
Dietary patterns and diet quality.
Dietary patterns and dietary quality also emerged as themes of obesity research in NHS cohorts. Women who increased their adherence to a Western pattern (high intakes of red and processed meats, refined grains, sweets or desserts, SSBs, and potatoes) gained the most weight across 8 years of follow-up. In parallel, women who increased their adherence to a prudent pattern (high intakes of fruits, vegetables, whole grains, fish, poultry, and salad dressing) gained the least weight.14
In a recent study of dietary quality characterized by established healthy diet indices (i.e., a Mediterranean-style diet, the Alternate Healthy Eating Index, and the Dietary Approaches to Stop Hypertension diet), higher or increasing adherence to any of these indices was associated with less weight gain in a given 4-year interval through midlife, with greater benefits observed in overweight women.15 (For additional information on dietary assessments in the NHS, please see Hu et al. in this issue [p1567].)
Physical Activity, Sedentariness, and Sleep
Higher levels of physical activity were associated with both prevention of weight gain16 and long-term weight maintenance after intentional weight loss. Among the major findings in NHS II are that women with low physical activity levels (e.g., < 30 min/day) who increased to high levels (≥ 30 min/day) had significantly less weight gain. However, if physical activity remained low, or fell from high to low, women had an elevated risk of gaining weight.16 In NHS II, jogging or running appeared best for limiting weight gain, although brisk walking and bicycle riding were also inversely related to weight gain.
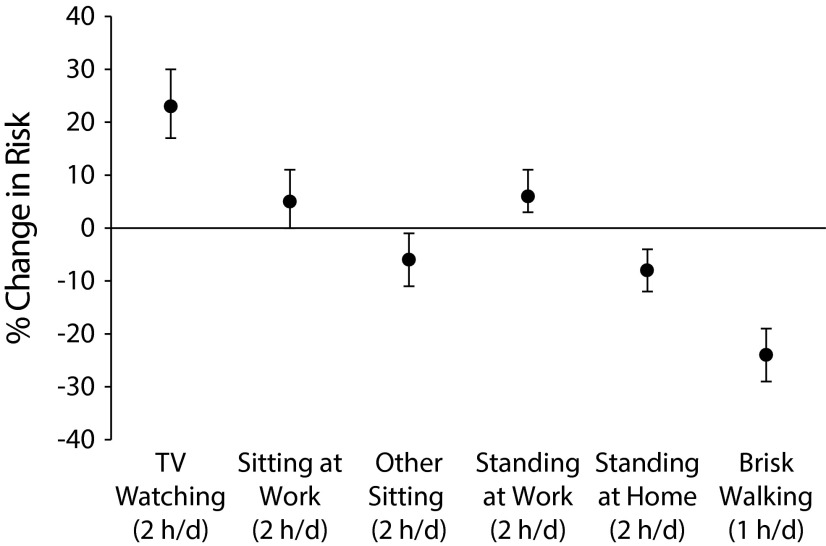
In addition, sedentariness plays an important role in obesity: television watching and other sedentary activities at home or work increased the risk of becoming obese in NHS.17 Conversely, each 2-hour daily increment spent standing or walking was associated with 9% lower risk, whereas an hour per day of brisk walking was associated with 24% lower risk (Figure 2).17
FIGURE 2—
Percentage Changes in the Risk of Developing Obesity Associated With Television Watching, Other Sedentary Behaviors, and Walking Among Nonobese Women in the Nurses’ Health Study: United States, 1992–1998
Note. Analyses were adjusted for age, smoking, alcohol consumption, and dietary covariates. All sedentary behavior variables were included simultaneously in the model. Other sitting included reading, mealtime, and at a desk. Vertical bars indicate 95% confidence intervals.
Source. Adapted from Hu et al.17
Habitual sleep duration was first assessed in NHS in 1986. Across 16 years of follow-up, women who slept 5 or fewer hours per night were 32% more likely, and women who slept 6 hours were 12% more likely to gain 15 or more kilograms than were those sleeping 7 to 8 hours.18 In addition, in NHS II, more years of rotating night shift work (i.e., less nighttime rest) were associated with an increased risk of weight gain and obesity over 18 years of follow-up.19
Genetics and Gene–Lifestyle Interactions
As one of the founding members of the Genetic Investigation of Anthropometric Traits Consortium (https://www.broadinstitute.org/collaboration/giant/index.php/Main_Page), NHS cohorts have substantially contributed to understanding the genetic determinants of obesity (see Appendix). Through genome-wide association studies, Genetic Investigation of Anthropometric Traits investigators have identified hundreds of novel common variants associated with weight and BMI, waist circumference, waist to hip circumference ratio, height, and macronutrient intake.
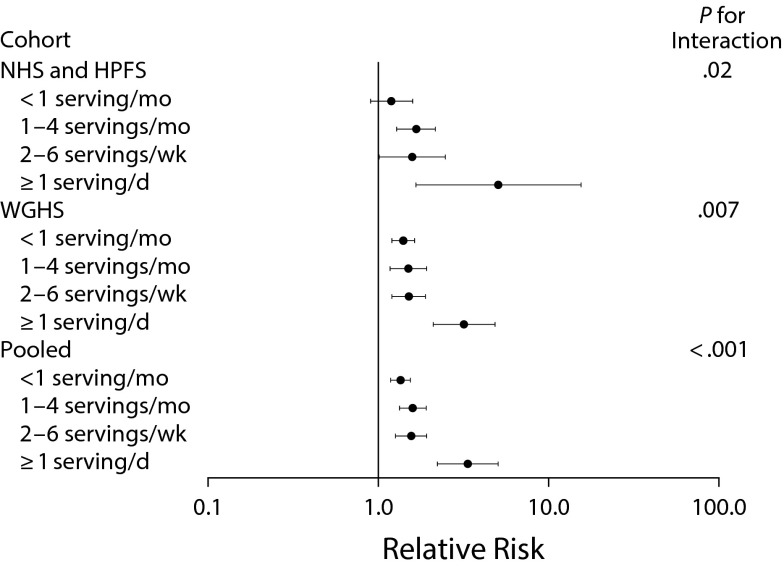
Studies on interactions between lifestyle factors and genetic predisposition to obesity have also been a research focus. In a study that included the Health Professionals Follow-up Study and NHS as discovery cohorts and the Women’s Genome Health Study as the replication cohort, SSB intake strongly interacted with the genetic risk of obesity. Increasing genetic risk at 32 BMI-related loci, coupled with higher SSB consumption, resulted in an exponentially higher risk of obesity (Figure 3).20 In a similar analysis, a genetic risk score interacted with fried food consumption, showing that frequent consumption of fried food magnified genetic risk.21 In addition, in NHS and the Health Professionals Follow-up Study, genetic risk interacted with television viewing,22 physical activity,22 and, most recently, vitamins B intake23 on obesity risk.
FIGURE 3—
Relative Risk of Developing Obesity Among Nonobese Men and Women, per Increment of 10 Obesity Risk Alleles, According to Intake of Sugar-Sweetened Beverages in the Nurses’ Health Study (1980–1998), Health Professionals Follow-up Study (1986–1998), and Women’s Genome Health Study (1992–1998): United States
Note. Shown are the relative risks of incident obesity, adjusted for age, source of genotyping data, physical activity, time spent watching television, smoking, alcohol intake, and other dietary covariates. Horizontal bars indicate 95% confidence intervals.
Source. Reprinted with permission from Qi et al. 20
NHS investigators have also led and contributed to consortia-based analyses on gene–lifestyle interactions, notably in the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (http://www.chargeconsortium.com; see Appendix). Studies have included, for example, genetic predisposition to higher BMI or obesity interacting with dietary patterns, macronutrients, and physical activity. In each case, results indicate that poor lifestyle choices exacerbate genetic risk (or, conversely, healthy lifestyle choices mitigate genetic risk).
Environmental Exposures and Built Environment
Several NHS substudies have examined so-called obesogens and obesity risk, including blood and urine biomarkers of these exposures. Among these, for example, was a study of urinary concentrations of metabolites of bisphenol A and phthalates—byproducts of plastics and other consumer goods. Higher levels of several of these metabolites were associated with greater weight gain in both NHS cohorts, implicating these endocrine disruptors in the obesity epidemic.24 These intriguing findings are currently being expanded in new areas of research (e.g., metabolomics).
The built environment (urban sprawl, walkability, etc.) has also been implicated in obesity risk. NHS participants living in higher-density counties (i.e., lower sprawl) had lower BMI and higher physical activity, including more hours per week spent walking, bicycling, and jogging or running, than did participants living in lower-density counties.25 In a subset of older NHS participants, increasing density (whether defined by population, intersections, or facilities density [e.g., post offices, restaurants]) was associated with higher odds of meeting physical activity recommendations by walking; in addition, a higher density of physical activity facilities was associated with a 31% lower odds of overweight or obesity.26
EXCESS WEIGHT AND WEIGHT GAIN, AND MORBIDITY AND MORTALITY
Most NHS research has focused on weight and weight gain in relation to major chronic conditions: type 2 diabetes, cardiovascular diseases, cancers, and mortality. However, weight and weight gain, as well as circulating concentrations of related markers (e.g., estrogens, adiponectin, C-reactive protein, insulin-like growth factor-I), have also been linked to a host of other disease outcomes in the NHS, including gallstones, infertility, asthma, cataract, psoriasis, and others not discussed here (see Appendix).
Type 2 Diabetes
Across the initial 8 years of NHS, incident diabetes risk in women with high normal BMI (23–23.9) was 3.6 or more times the risk of those with BMI less than 22.27 Weight gain after age 18 years was also a strong risk factor: compared with those who maintained a stable weight through 1984 the relative risk (RR) of diabetes exceeded 17 for those who gained 35 or more kilograms.27 In extended follow-up, women with an attained BMI of 35 or more versus less than 22 had an age-adjusted RR of 93.2. In addition, whereas adult weight gain continued to be predictive of diabetes, weight loss of 5 or more kilograms since age 18 years was associated with nearly 50% lower risk.28
Body fat distribution—assessed by waist circumference or waist to hip ratio—was found to be an independent predictor of diabetes risk: a waist to hip ratio of 0.88 or more versus less than 0.72 was associated with greater than 3 times the risk of diabetes; a waist circumference of 38 or more versus less than 28 inches was linked to greater than 6 times the risk of diabetes.29 These observations support the inclusion of anthropometric indices of central obesity in clinical “action levels” for weight management.29
Hypertension and Cardiovascular Disease
By 1992, nearly 20% of NHS participants had developed hypertension.30 Both BMI at age 18 years and midlife BMI were significantly associated with incident hypertension. In addition, weight loss over the long term (i.e., from age 18 years) as well as medium term (i.e., from 1976) were associated with a lower, and weight gain with a higher, risk of hypertension. Results from NHS II were similar: 11% higher odds of hypertension per kilogram per meter squared and 20% higher odds per 4.5-kilogram gain.31
The first article to examine obesity and the risk of coronary heart disease in NHS appeared in 1990.32 Across 8 years of follow-up, compared with those with a BMI less than 21, each 2-BMI increment increased heart disease risk: at 29 or more, women had more than 3 times the risk of heart disease after adjusting for age and smoking, and nearly twice the risk even after additionally adjusting for mediators such as hypertension and diabetes.32 In addition, gaining 10 or more kilograms from age 18 years versus maintaining weight within 3 kilograms, conferred a more than 60% higher risk of heart disease.
In a follow-up article published in 1995 investigating this association across a longer, 14-year follow-up, women who were able to remain within 5 kilograms of their age 18 years weight through adulthood had a significantly lower risk of heart disease than did those who gained 5 or more kilograms. These weight gain–heart disease relationships held even among women within the “normal” BMI range, reinforcing the idea that weight gain through middle adulthood of more than a few kilograms significantly raises heart disease risk, even if those gains are not enough to explicitly classify an individual as overweight or obese. These findings suggested that the then-current US weight guidelines were likely “falsely reassuring” to the large proportion of women who were within the normal BMI range.33
Subsequently, higher waist to hip ratios and larger waist circumferences were shown to be associated with a significantly higher risk of heart disease, independent of BMI.34 Again, risk increases were similar even in normal weight women, suggesting an independent role of central adiposity in heart disease.34
Beyond heart disease, BMI and weight change have also been implicated in stroke risk.35 Women with BMI of 27 or more versus less than 21 had approximately 42% higher risk. Similar to heart disease, weight gain since age 18 years, compared with weight stability, was related to an increased risk of ischemic stroke. Although the leanest women appeared to have a higher risk of hemorrhagic stroke than did the heaviest women, the inverse relationship was driven by smoking status: lean smokers, but not lean nonsmokers, had a higher risk of hemorrhagic stroke than did their heavier counterparts.35
Cancers
BMI and other measures of adiposity have been studied in relation to breast, colorectal, endometrial, ovarian, kidney, pancreatic, and other cancers.36,37 Overall, higher BMI and increases in BMI over the life course are associated with a higher risk of cancer.36 With respect to postmenopausal breast cancer, weight and weight gain elevated risk, but the magnitude of risk was modified by the use of menopausal hormone therapy. In a 16-year follow-up of NHS, obese women who had never used hormone therapy had a 59% higher risk of postmenopausal breast cancer compared with their lean counterparts. Among never users of hormone therapy, weight gain since age 18 years was associated with incident and fatal breast cancer after menopause. This relationship was subsequently supported across 26 years of follow-up.37 Furthermore, weight loss since age 18 years was associated with a lower risk of breast cancer in all postmenopausal women. Weight change since menopause showed a similar pattern: gaining or losing 10 or more kilograms was associated with higher or lower risk, respectively, of postmenopausal breast cancer, with more pronounced associations among never users of hormone therapy.37
With respect to other cancers, the risk of large, but not small, adenomas in the distal colon more than doubled in women with BMI of 29 or higher versus less than 21 across 6 years of follow-up. A significant association between larger body shape at age 5 years and a higher risk of distal adenoma was also subsequently observed, as was the association of weight gain over the life course with an increased risk of colorectal cancers overall.36 In NHS cohorts, weight change since age 18 years and higher adult BMI were significantly associated with elevated endometrial cancer risk, and in postmenopausal women the association was stronger among nonusers of hormone therapy. In a recent NHS study, women who gained weight over the life course, whether initially lean or heavy, also had an elevated endometrial cancer risk.36 In long-term follow-up, the risk of cancers of the kidney and pancreas were also significantly higher with higher BMI and weight gain.36
Quality of Life
NHS5,38 and NHS II5 studies have examined weight and weight change in relation to quality of life, as captured by the Short-Form 36 Health Survey, which assesses 8 domains of physical and emotional health in relation to activities of daily living or usual roles. In a cross-sectional study of BMI and Short-Form 36 physical quality of life dimensions from 1992, obese women had lower levels of physical functioning and vitality than did women with BMI of 21 to 23. In a study using a second Short-Form 36 assessment from 1996, women who gained 2.25 kilograms or more were more likely to experience decreased levels of physical functioning and vitality and increased bodily pain, irrespective of baseline weight.38 Conversely, losing 2.25 or more kilograms in overweight women was associated with improved physical functioning, vitality, and pain.38 In another study including NHS II with follow-up through 2000–2001, gaining 6.75 or more kilograms in a given 4-year interval was associated with lower physical quality of life.5 In addition to physical functioning, bidirectional relationships between obesity and depression have also been observed in NHS.39
NHS investigators have extended this research into successful aging, defined as being free of major chronic conditions and having no substantial cognitive, physical, or mental limitations at age 70 years. Consistent with the findings for quality of life, excess body weight, as well as weight gain since age 18 years, significantly predicted diminished chances of successful aging.40
Mortality
Some researchers have suggested that excess weight is protective against mortality, but this “obesity paradox” is likely observed because of confounding by smoking and existing or preclinical conditions that lead to weight loss preceding death (i.e., reverse causation).41 When these methodological issues are correctly accounted for, as in NHS analyses, BMI in the overweight or obese range is associated with a higher risk of premature death among generally healthy individuals at baseline42,43 and in those with type 2 diabetes.44
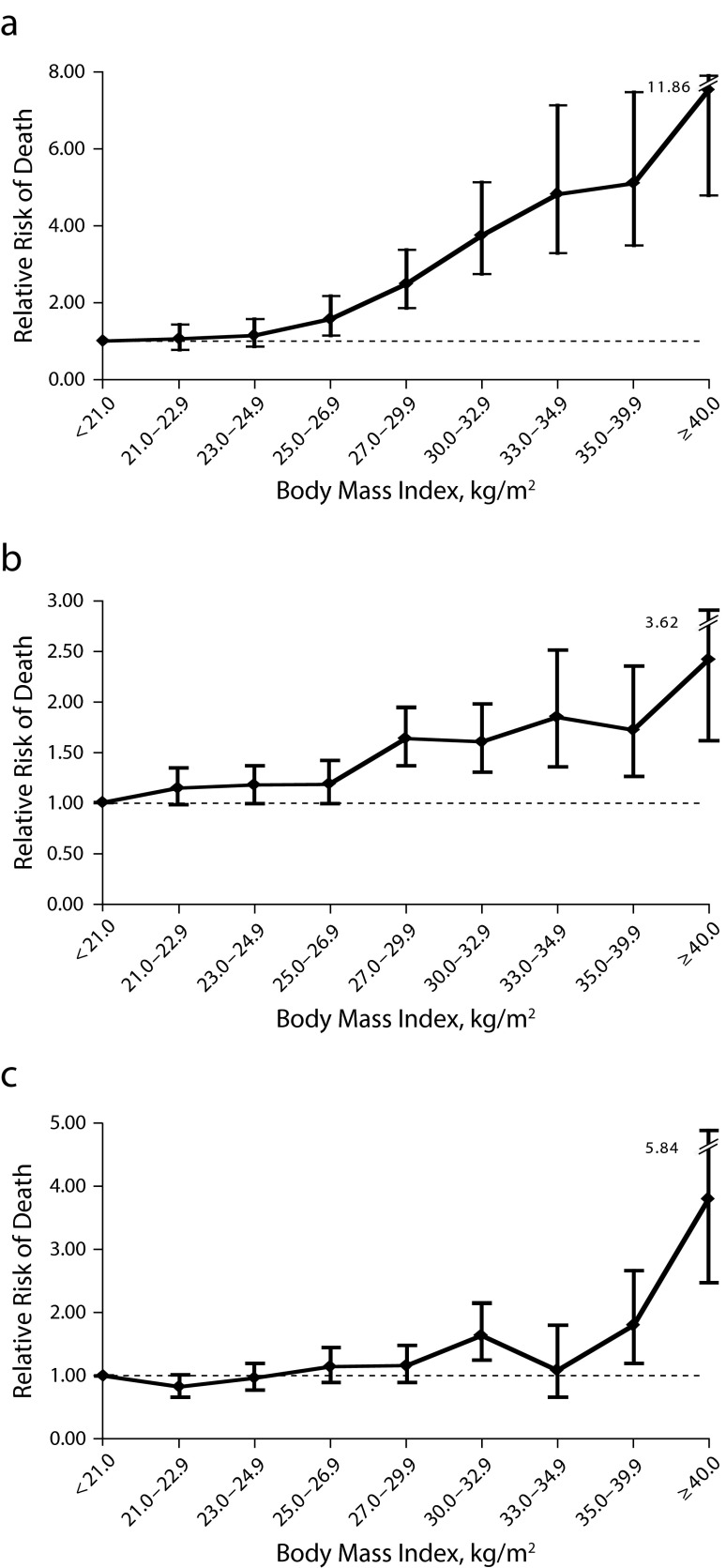
Among never smokers, there are monotonic increases in the risk of all-cause, cardiovascular, and cancer mortality from excess body weight (Figure 4), weight gain, or central obesity. In ever smokers, a J-shaped curve appears more typical, in which both low normal BMI (< 19–22) and obesity are associated with higher mortality risk. Overweight or obesity is consistently among the strongest risk factors for premature death in the NHS, accounting for some 22% (in nonsmokers; 14% overall) of the population-attributable risk of all-cause mortality.45
FIGURE 4—
Relative Risk of Death From (a) Cardiovascular Disease, (b) Cancer, and (c) Other Causes According to Body Mass Index Among Women in the Nurses’ Health Study Who Had Never Smoked: United States, 1976–2000
Note. Analyses were adjusted for age, parental history of coronary heart disease, menopausal status, hormone use, physical activity, and alcohol intake. Vertical bars indicate 95% confidence intervals.
Source. Reprinted with permission from Hu et al.43
CONCLUSIONS
Forty years of NHS research have revealed excess adiposity as one of the most important risk factors for chronic disease morbidity and mortality. Most women, the nurses of the NHS among them, gain weight through middle adulthood. Adult weight gain has many adverse health implications: the risk of heart disease, diabetes, and certain cancers is substantially elevated with higher weight, even among those classified at the upper limit of normal weight. Limiting weight gain and obesity—even in the face of genetic predisposition, childhood size, or adolescent weight—is possible through healthy diet, physical activity, and other positive lifestyle choices, which have consistently been shown to be the best preventive measures against most chronic morbidity and mortality. The public health messages from these studies are clear: even small improvements in diet quality, small increases in time spent physically active and decreases in time spent sitting, are significantly inversely associated with weight gain and obesity and the risk of chronic disease and mortality.
Although the NHS is a largely homogenous population (mainly White, higher socioeconomic status, originally all nurses), NHS research has had a widespread impact on clinical and dietary guidelines for obesity prevention, largely because of the studies’ considerable strengths: large sample sizes, now 40 years of follow-up, and detailed and repeated assessments of diet and lifestyle, anthropometrics, and disease incidence. Innovative hypotheses and analyses have allowed investigators to map the effects of weight and changing weight across the life course.
Genome-wide association study data has enabled the discovery of genetic determinants of obesity and gene–lifestyle interactions. Through this research, NHS cohorts have helped define a desirable BMI range for a variety of disease outcomes, contributing to guidelines for weight-related prevention or management of chronic disease. NHS research has identified dietary determinants of obesity (e.g., SSBs and poor diet quality), which have provided important justifications for the inclusion in the current US Dietary Guidelines of a focus on healthy eating patterns and reducing added sugars for the prevention of obesity and obesity-related chronic diseases.
ACKNOWLEDGMENTS
Research on Nurses’ Health Studies (NHSs) cohorts is supported by the National Institutes of Health (grants P01 CA87969, P01 CA055075, P30 DK46200, R01 DK58845, R01 HL034594, R01 HL060712, R01 CA050385, UM1 CA176726, and U54 CA155626).
We thank the participants of the NHSs for their longstanding support as well as our colleagues working in these studies for their considerable contributions.
HUMAN PARTICIPANT PROTECTION
No institutional review board approval was necessary because this was a review and therefore no human participants were involved in this study.
Footnotes
See also Galea and Vaughan, p. 1531.
REFERENCES
- 1.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272(3):205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 2.Hu FB. Obesity Epidemiology. Oxford, UK: Oxford University Press; 2008. [Google Scholar]
- 3.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19(8):570–572. [PubMed] [Google Scholar]
- 5.Pan A, Kawachi I, Luo N et al. Changes in body weight and health-related quality of life: 2 cohorts of US women. Am J Epidemiol. 2014;180(3):254–262. doi: 10.1093/aje/kwu136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field AE, Willett WC, Lissner L, Colditz GA. Dietary fat and weight gain among women in the Nurses’ Health Study. Obesity (Silver Spring) 2007;15(4):967–976. doi: 10.1038/oby.2007.616. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr. 2003;78(5):920–927. doi: 10.1093/ajcn/78.5.920. [DOI] [PubMed] [Google Scholar]
- 9.Bertoia ML, Mukamal KJ, Cahill LE et al. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three prospective cohort studies. PLoS Med. 2015;12(9):e1001878. doi: 10.1371/journal.pmed.1001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA. 2002;288(20):2554–2560. doi: 10.1001/jama.288.20.2554. [Comment in JAMA. 2003;290(1):38–39; author reply 39–40] [DOI] [PubMed] [Google Scholar]
- 11.Wannamethee SG, Field AE, Colditz GA, Rimm EB. Alcohol intake and 8-year weight gain in women: a prospective study. Obes Res. 2004;12(9):1386–1396. doi: 10.1038/oby.2004.175. [DOI] [PubMed] [Google Scholar]
- 12.Schulze MB, Manson JE, Ludwig DS et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 13.Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes (Lond) 2013;37(10):1378–1385. doi: 10.1038/ijo.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulze MB, Fung TT, Manson JE, Willett WC, Hu FB. Dietary patterns and changes in body weight in women. Obesity (Silver Spring) 2006;14(8):1444–1453. doi: 10.1038/oby.2006.164. [DOI] [PubMed] [Google Scholar]
- 15.Fung TT, Pan A, Hou T et al. Long-term change in diet quality is associated with body weight change in men and women. J Nutr. 2015;145(8):1850–1856. doi: 10.3945/jn.114.208785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mekary RA, Feskanich D, Malspeis S, Hu FB, Willett WC, Field AE. Physical activity patterns and prevention of weight gain in premenopausal women. Int J Obes (Lond) 2009;33(9):1039–1047. doi: 10.1038/ijo.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 18.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16(3):643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8(12):e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi Q, Chu AY, Kang JH et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367(15):1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi Q, Chu AY, Kang JH et al. Fried food consumption, genetic risk, and body mass index: gene–diet interaction analysis in three US cohort studies. BMJ. 2014;348:g1610. doi: 10.1136/bmj.g1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi Q, Li Y, Chomistek AK et al. Television watching, leisure time physical activity, and the genetic predisposition in relation to body mass index in women and men. Circulation. 2012;126(15):1821–1827. doi: 10.1161/CIRCULATIONAHA.112.098061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang T, Zheng Y, Qi Q et al. DNA methylation variants at HIF3A locus, B-vitamin intake, and long-term weight change: gene–diet interactions in two US cohorts. Diabetes. 2015;64(9):3146–3154. doi: 10.2337/db15-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes (Lond) 2014;38(12):1532–1537. doi: 10.1038/ijo.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James P, Troped PJ, Hart JE et al. Urban sprawl, physical activity, and body mass index: Nurses’ Health Study and Nurses’ Health Study II. Am J Public Health. 2013;103(2):369–375. doi: 10.2105/AJPH.2011.300449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troped PJ, Starnes HA, Puett RC et al. Relationships between the built environment and walking and weight status among older women in three U.S. States. J Aging Phys Act. 2014;22(1):114–125. doi: 10.1123/japa.2012-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colditz GA, Willett WC, Stampfer MJ et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 1990;132(3):501–513. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- 28.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122(7):481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Carey VJ, Walters EE, Colditz GA et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol. 1997;145(7):614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z, Willett WC, Manson JE et al. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128(2):81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 31.Field AE, Byers T, Hunter DJ et al. Weight cycling, weight gain, and risk of hypertension in women. Am J Epidemiol. 1999;150(6):573–579. doi: 10.1093/oxfordjournals.aje.a010055. [DOI] [PubMed] [Google Scholar]
- 32.Manson JE, Colditz GA, Stampfer MJ et al. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322(13):882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 33.Willett WC. Weight, weight change, and coronary heart disease in women: risk within the ‘normal’ weight range. JAMA. 1995;273(6):461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 34.Rexrode KM, Carey VJ, Hennekens CH et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280(21):1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 35.Rexrode KM, Hennekens CH, Willett WC et al. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997;277(19):1539–1545. doi: 10.1001/jama.1997.03540430051032. [DOI] [PubMed] [Google Scholar]
- 36.Song M, Willett WC, Hu FB et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383–2395. doi: 10.1002/ijc.29981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 38.Fine JT, Colditz GA, Coakley EH et al. A prospective study of weight change and health-related quality of life in women. JAMA. 1999;282(22):2136–2142. doi: 10.1001/jama.282.22.2136. [DOI] [PubMed] [Google Scholar]
- 39.Pan A, Sun Q, Czernichow S et al. Bidirectional association between depression and obesity in middle-aged and older women. Int J Obes (Lond) 2012;36(4):595–602. doi: 10.1038/ijo.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Q, Townsend MK, Okereke OI, Franco OH, Hu FB, Grodstein F. Adiposity and weight change in mid-life in relation to healthy survival after age 70 in women: prospective cohort study. BMJ. 2009;339:b3796. doi: 10.1136/bmj.b3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willett WC, Hu FB, Thun M. Overweight, obesity, and all-cause mortality. JAMA. 2013;309(16):1681. doi: 10.1001/jama.2013.3075. [DOI] [PubMed] [Google Scholar]
- 42.Manson JE, Willett WC, Stampfer MJ et al. Body weight and mortality among women. N Engl J Med. 1995;333(11):677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 43.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351(26):2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 44.Tobias DK, Pan A, Jackson CL et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370(3):233–244. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]