Abstract
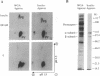
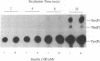
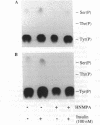
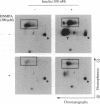
The protein kinase activity of human insulin receptors purified from Sf9 insect cells after infection with a recombinant baculovirus was evaluated. The following experimental observations led to the unexpected conclusion that this receptor protein catalyzes both serine and tyrosine autophosphorylation at significant stoichiometries. (i) Phosphorylation of lectin-purified insulin receptors with [gamma-32P]ATP resulted in rapid receptor tyrosine phosphorylation (7 mol of P per high-affinity binding site) and the delayed onset of insulin-stimulated receptor serine phosphorylation (about 7% of total phosphorylation). The tyrosine kinase inhibitor (hydroxy-2-naphthalenylmethyl)phosphonic acid (HNMPA), which has no effect on protein kinase C or cyclic AMP-dependent protein kinase activities, inhibited both the receptor serine and tyrosine phosphorylation. (ii) Phosphorylation of a synthetic peptide substrate composed of insulin receptor residues 1290-1319 on serines-1305/1306 by partially purified insulin receptors was also inhibited by HNMPA. (iii) Insulin receptors sequentially affinity-purified on immobilized wheat germ agglutinin and immobilized insulin showed no apparent contaminant proteins on silver-stained SDS/polyacrylamide gels yet catalyzed autophosphorylation on receptor serine and tyrosine residues when incubated with [gamma-32P]ATP. These results suggest that the catalytic site of the insulin receptor tyrosine kinase also recognizes receptor serine residues as substrates for the phosphotransfer reaction. Furthermore, insulin-stimulated receptor serine phosphorylation in intact cells may occur in part by an autophosphorylation mechanism subsequent to tyrosine phosphorylation of the insulin receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Ballotti R., Kowalski A., Le Marchand-Brustel Y., Van Obberghen E. Presence of an insulin-stimulated serine kinase in cell extracts from IM-9 cells. Biochem Biophys Res Commun. 1986 Aug 29;139(1):179–185. doi: 10.1016/s0006-291x(86)80096-1. [DOI] [PubMed] [Google Scholar]
- Biener Y., Zick Y. Basic polycations activate the insulin receptor kinase and a tightly associated serine kinase. Eur J Biochem. 1990 Nov 26;194(1):243–250. doi: 10.1111/j.1432-1033.1990.tb19449.x. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Crews C. M., Alessandrini A. A., Erikson R. L. Mouse Erk-1 gene product is a serine/threonine protein kinase that has the potential to phosphorylate tyrosine. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8845–8849. doi: 10.1073/pnas.88.19.8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Fung M. C., Chiu K. Y., Weber T., Chang T. W., Chang N. T. Detection and purification of a recombinant human B lymphotropic virus (HHV-6) in the baculovirus expression system by limiting dilution and DNA dot-blot hybridization. J Virol Methods. 1988 Jan;19(1):33–42. doi: 10.1016/0166-0934(88)90005-5. [DOI] [PubMed] [Google Scholar]
- Gazit A., Yaish P., Gilon C., Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- Geissler J. F., Traxler P., Regenass U., Murray B. J., Roesel J. L., Meyer T., McGlynn E., Storni A., Lydon N. B. Thiazolidine-diones. Biochemical and biological activity of a novel class of tyrosine protein kinase inhibitors. J Biol Chem. 1990 Dec 25;265(36):22255–22261. [PubMed] [Google Scholar]
- Harrison L. C., Itin A. Purification of the insulin receptor from human placenta by chromatography on immobilized wheat germ lectin and receptor antibody. J Biol Chem. 1980 Dec 25;255(24):12066–12072. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis R. E., Cao L., Perregaux D., Czech M. P. Threonine 1336 of the human insulin receptor is a major target for phosphorylation by protein kinase C. Biochemistry. 1990 Feb 20;29(7):1807–1813. doi: 10.1021/bi00459a020. [DOI] [PubMed] [Google Scholar]
- Lewis R. E., Wu G. P., MacDonald R. G., Czech M. P. Insulin-sensitive phosphorylation of serine 1293/1294 on the human insulin receptor by a tightly associated serine kinase. J Biol Chem. 1990 Jan 15;265(2):947–954. [PubMed] [Google Scholar]
- Lindberg R. A., Quinn A. M., Hunter T. Dual-specificity protein kinases: will any hydroxyl do? Trends Biochem Sci. 1992 Mar;17(3):114–119. doi: 10.1016/0968-0004(92)90248-8. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Dunau M. L., Goldman D. A rapid sensitive silver stain for polypeptides in polyacrylamide gels. Anal Biochem. 1981 Jan 1;110(1):201–207. doi: 10.1016/0003-2697(81)90136-6. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Pang D. T., Sharma B. R., Shafer J. A., White M. F., Kahn C. R. Predominance of tyrosine phosphorylation of insulin receptors during the initial response of intact cells to insulin. J Biol Chem. 1985 Jun 10;260(11):7131–7136. [PubMed] [Google Scholar]
- Paul J. I., Tavaré J., Denton R. M., Steiner D. F. Baculovirus-directed expression of the human insulin receptor and an insulin-binding ectodomain. J Biol Chem. 1990 Aug 5;265(22):13074–13083. [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- Pillay T. S., Siddle K. Insulin-stimulated serine/threonine phosphorylation of the insulin receptor: paucity of threonine 1348 phosphorylation in vitro indicates the involvement of more than one serine/threonine kinase in vivo. Biochem Biophys Res Commun. 1991 Sep 16;179(2):962–971. doi: 10.1016/0006-291x(91)91912-v. [DOI] [PubMed] [Google Scholar]
- Prigent S. A., Stanley K. K., Siddle K. Identification of epitopes on the human insulin receptor reacting with rabbit polyclonal antisera and mouse monoclonal antibodies. J Biol Chem. 1990 Jun 15;265(17):9970–9977. [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saperstein R., Vicario P. P., Strout H. V., Brady E., Slater E. E., Greenlee W. J., Ondeyka D. L., Patchett A. A., Hangauer D. G. Design of a selective insulin receptor tyrosine kinase inhibitor and its effect on glucose uptake and metabolism in intact cells. Biochemistry. 1989 Jun 27;28(13):5694–5701. doi: 10.1021/bi00439a053. [DOI] [PubMed] [Google Scholar]
- Seger R., Ahn N. G., Boulton T. G., Yancopoulos G. D., Panayotatos N., Radziejewska E., Ericsson L., Bratlien R. L., Cobb M. H., Krebs E. G. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6142–6146. doi: 10.1073/pnas.88.14.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson S. E., White M. F., Kahn C. R. Nonphosphorylatable substrate analogs selectively block autophosphorylation and activation of the insulin receptor, epidermal growth factor receptor, and pp60v-src kinases. J Biol Chem. 1989 May 15;264(14):7831–7836. [PubMed] [Google Scholar]
- Smith D. M., King M. J., Sale G. J. Two systems in vitro that show insulin-stimulated serine kinase activity towards the insulin receptor. Biochem J. 1988 Mar 1;250(2):509–519. doi: 10.1042/bj2500509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Sale G. J. Evidence that a novel serine kinase catalyses phosphorylation of the insulin receptor in an insulin-dependent and tyrosine kinase-dependent manner. Biochem J. 1988 Dec 15;256(3):903–909. doi: 10.1042/bj2560903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S., White M. F., Kahn C. R. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988 Mar 5;263(7):3440–3447. [PubMed] [Google Scholar]
- Tavaré J. M., Clack B., Ellis L. Two-dimensional phosphopeptide analysis of the autophosphorylation cascade of a soluble insulin receptor tyrosine kinase. The tyrosines phosphorylated are typical of those observed following phosphorylation of the heterotetrameric insulin receptor in intact cells. J Biol Chem. 1991 Jan 25;266(3):1390–1395. [PubMed] [Google Scholar]
- Tavaré J. M., Dickens M. Changes in insulin-receptor tyrosine, serine and threonine phosphorylation as a result of substitution of tyrosine-1162 with phenylalanine. Biochem J. 1991 Feb 15;274(Pt 1):173–179. doi: 10.1042/bj2740173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Kuppuswamy D., Visvanathan A., Pike L. J. Substrate specificity and kinetic mechanism of human placental insulin receptor/kinase. Biochemistry. 1987 Mar 10;26(5):1428–1433. doi: 10.1021/bi00379a033. [DOI] [PubMed] [Google Scholar]
- White M. F., Takayama S., Kahn C. R. Differences in the sites of phosphorylation of the insulin receptor in vivo and in vitro. J Biol Chem. 1985 Aug 5;260(16):9470–9478. [PubMed] [Google Scholar]
- Whittaker J., Okamoto A. K., Thys R., Bell G. I., Steiner D. F., Hofmann C. A. High-level expression of human insulin receptor cDNA in mouse NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5237–5241. doi: 10.1073/pnas.84.15.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasa Y., Seino S., Whittaker J., Kakehi T., Kosaki A., Kuzuya H., Imura H., Bell G. I., Steiner D. F. Insulin-resistant diabetes due to a point mutation that prevents insulin proreceptor processing. Science. 1988 May 6;240(4853):784–787. doi: 10.1126/science.3283938. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]
- Zick Y., Grunberger G., Podskalny J. M., Moncada V., Taylor S. I., Gorden P., Roth J. Insulin stimulates phosphorylation of serine residues in soluble insulin receptors. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1129–1135. doi: 10.1016/s0006-291x(83)80260-5. [DOI] [PubMed] [Google Scholar]