Abstract
Aim:
The aim of this study was to compare constituents of glass powder, fluoride release, and antimicrobial properties of new atraumatic restorative treatment material with zirconia fillers and conventional glass ionomer cement (GIC) type IX.
Materials and Methods:
Thisin vitro study comparing Zirconomer and Fuji IX was executed in three parts: (1) energy dispersive X-ray microanalysis of glass powders (2) analysis of fluoride release at 1st, 3rd, 7th, 15th, and 30th day, and (3) antimicrobial activity against Streptococcus mutans, Lactobacillus casei, and Candida albicans at 48 hours. Data was analyzed using unpaired t-test and two way analysis of variance followed by least significant difference post hoc test. A P value of < 0.05 was considered statistically significant.
Results:
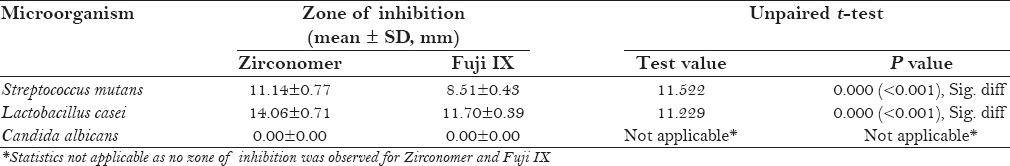
Energy dispersive X-ray microanalysis revealed that, in both Zirconomer and Fuji IX glass powders, mean atomic percentage of oxygen was more than 50%. According to the weight percentage, zirconium in Zirconomer and silica in Fuji IX were the second main elements. Calcium, zinc, and zirconium were observed only in Zirconomer. At all the time intervals, statistically significant higher amount of fluoride release was observed with Zirconomer than Fuji IX. At 48 hours, mean ± standard deviation (SD) of zone of inhibition against Streptococcus mutans was 11.14 ± 0.77 mm and 8.51 ± 0.43 mm for Zirconomer and Fuji IX, respectively. Against Lactobacillus casei, it was 14.06 ± 0.71 mm for Zirconomer and 11.70 ± 0.39 mm for Fuji IX. No antifungal activity was observed against Candida albicans by Zirconomer and Fuji IX.
Conclusion:
Zirconomer had higher antibacterial activity against Streptococcus mutans and Lactobacillus casei, which may be attributed to its composition and higher fluoride release. However, it failed to show antifungal effect againstCandida albicans.
Key words: Dental atraumatic restorative treatment, glass ionomer cements, energy dispersive X-ray analysis, fluoride
INTRODUCTION
Glass ionomer cements (GICs) were introduced by Wilson and Kent in 1970s.[1] Conventional GICs are composed of fluoroaluminosilicate glass powder usually a strontium and calcium salt and polyalkenoic acid liquid, such as polyacrylic, maleic, itaconic, and tricarbalyllic acids.[2,3] GIC is acknowledged to be a biomimetic material because it has mechanical properties similar to dentin.[2] Biocompatibility, fluoride release, and chemical bonding to hard tissues of the tooth render it an ideal material in many restorative situations.[2,4] Its ability to prevent and arrest carious lesions has been supported by numerous systematic reviews and meta-analyses.[5,6] GICs are widely used as the base, liner, luting, and restorative material.[4] Despite many desirable properties, low mechanical strength of conventional GICs make them unsuitable for load bearing areas such as Class I and Class II restorations.[2,3]
To conquer the disadvantage of low mechanical strength, GICs with higher powder liquid ratio such as Fuji IX (GC Corporation, Tokyo, Japan) were developed. These highly viscous GICs with improved mechanical properties are indicated for atraumatic restorative treatment (ART).[7,8]
GIC displays worthy properties for restorative and preventive dentistry, making this a material of choice for ART.[7] The World Health Organization (WHO) recommended ART as a part of Basic package of Oral Care (BPOC) along with oral urgent treatment and affordability of fluoride toothpaste.[8] It consists of excavating infected dentin with exclusive use of hand instruments, followed by sealing the cavities and adjacent pits and fissures with GIC.[7,9,10] Because of hand excavation, cavities treated by ART may have residual infected dentin which may lead to restoration failure.[9] Therefore, success of ART depends upon the performance of GIC.[9,10]
Recently, zirconia reinforced glass ionomer cement known as Zirconomer or white amalgam (Shofu Inc., Kyoto, Japan) has been introduced in dentistry. The inclusion of zirconia fillers in glass component of Zirconomer provides strength and durability similar to amalgam with protective benefits of glass ionomer.[11,12] The manufacturer also claims that it is suitable for ART.[12]
The glass components of GIC critically determine its physical properties; hence, the relationship between the composition of glass and properties should be scrutinized.[13] Fluoride is a well-known anticariogenic agent. Short and long-term fluoride release from dental restorative materials depends upon several factors including the amount of fluoride incorporated and matrices of materials.[14]
Testing of a newer material is always desirable to determine its effectiveness. Literature search has revealed that there is dearth of research on zirconia reinforced glass ionomer cement. In an investigation Patel et al. has evaluated the microleakage of Zirconomer in comparison with amalgam and composite.[11] However, there is no published research on the composition of glass powder, fluoride release, and antimicrobial properties of glass ionomer cement with zirconia fillers to substantiate it for ART procedure. Hence, the present in vitro study was conducted with an aim to compare the constituents of glass powders, fluoride release, and antimicrobial properties of new ART material with zirconia fillers (Zirconomer) and conventional GIC type IX (Fuji IX). The proposed null hypothesis for the present research was that there is no significant difference between new ART material with zirconia fillers and conventional GIC type IX for fluoride release and antimicrobial properties.
MATERIALS AND METHODS
The present in vitro study was executed in three parts: (1) Energy dispersive X-ray (EDX) microanalysis of glass powders, (2) analysis of fluoride release at different time intervals, and (3) antimicrobial activity against Streptococcus mutans, Lactobacillus casei, and Candida albicans at 48 hours.
Glass ionomer cements tested were:
Zirconia reinforced glass ionomer cement (Zirconomer, Shofu Inc., Kyoto, Japan. Lot number: 0813022)
Glass ionomer cement type IX (Fuji IX, GC corporation, Tokyo, Japan. Lot number: 1412161).
Energy dispersive X-ray microanalysis of glass powders
EDX microanalysis of powders of glass ionomer cements was performed using FEI Quanta 200 Scanning Electron Microscope (FEI Company, Hillsboro, Oregon, United States) at the Indian Institute of Information Technology, Design and Manufacturing (IIITDM) Jabalpur, Madhya Pradesh. On Scanning Electron Microscope (SEM), EDX was performed using TEAM™ EDS Software (EDAX Inc., Mahwah, New Jersey, United States). The samples (glass powders) were sprinkled on a carbon-metallic stub. The EDX spectrums were collected from the powder specimens, and elemental analysis (weight% and atomic%) was performed. For each glass ionomer cement, EDX analysis was performed 15 times.
Evaluation of fluoride release
Fifteen specimens of each cement of 6 mm × 8 mm dimensions were prepared using plastic molds. Materials were hand mixed as per the manufacturer's instructions, packed into the molds, and allowed to set at room temperature for 30 minute.
Artificial saliva was prepared by mixing 0.4 gm sodium chloride (NaCl), 1.21 gm potassium chloride (KCl), 0.78 gm disodium anhydrous phosphate (NaH2 PO4.2H2O), 0.005 gm sodium sulphite (Na2S.9H2O), 1.0 gm urea [CO (NH3)3] and 100 mg albumin bovine fraction V in 1000 ml of deionized water.[15] Digital pH meter (Model 1010, Electronics India, Parwanoo, Himachal Pradesh, India) was used to adjust the pH of artificial saliva at 6.7 (average normal pH of human saliva).[16] Set specimens after being removed from the molds were suspended in 5 ml of artificial saliva in tightly closed plastic vials and stored at 37°C (±1°C). After 24 hours, careful removal of specimens from the solution, drying, and transferring into a new vial containing fresh artificial saliva at 37°C (±1°C) was performed and same procedure was repeated on the 3rd, 7th, and 15th day.
A fluoride ion selective electrode (Orion Star A214, Thermo Fisher Scientific Inc., Waltham, Massachusetts, United States) was used for measuring the fluoride release at Madhya Pradesh Public Health Engineering Department (PHED), Dindori, Madhya Pradesh. Calibration of the electrode was performed immediately before as well as during the experiment to ensure precise readings. Standard solutions of 0.50, 1.00, 10.00, 50.00 and 100 ppm fluoride concentrations were used to calibrate the instrument as per the manufacturer's instruction. The fluoride concentration in each sample was recorded in ppm.
Assessment of antimicrobial properties
Fifteen samples of each cement were tested for antimicrobial activity against Streptococcus mutans (ATCC 25175), Lactobacillus casei (ATCC 393), and Candida albicans (ATCC 90028). For the evaluation of the antimicrobial effect, agar diffusion test was performed at Excellent Bio Research Solutions Private Limited, Jabalpur, Madhya Pradesh. For Streptococcus mutans and Lactobacillus casei, Mueller-Hinton agar (Himedia Laboratory Pvt. Ltd, Mumbai, India) and for Candida albicans, Sabouraud Dextrose Agar (Himedia Laboratory Pvt. Ltd, Mumbai, India) was used. On the designated agar plate, 4 wells measuring 6 mm were made using the blunt end of a micropipette tip. Specimens of 6 mm × 5 mm dimensions were prepared as per the method discussed earlier. The wells were completely filled by set specimens. The culture plates were placed in the incubator at 37°C (±1°C). After 48 hours, diameters of zone of inhibition were recorded by a digital Vernier caliper in millimetres around the specimens.
Statistical analysis
Mean and standard deviation of weight and atomic percentages of main constituents (elements) in glass powder, fluoride release, and zones of inhibition were calculated. Data was further analyzed using unpaired t-test and two-way analysis of variance (ANOVA) followed by least significant difference (LSD) posthoc test. The P value <0.05 was considered statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 21 (IBM Corporation, Armonk, New York, USA).
RESULTS
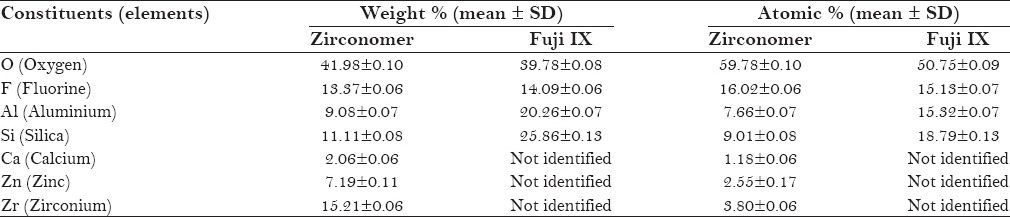
Figures 1–4 show SEM images and EDX spectrums of Zirconomer and Fuji IX, respectively. In both Zirconomer and Fuji IX, weight and atomic percentages of element oxygen were higher than that of other elements (fluorine, aluminium, and silica). Mean atomic percentage of oxygen was more than 50 in both the groups. Mean ± SD atomic percentage of fluorine was 16.02 ± 0.06 and 15.13 ± 0.07 in Zirconomer and Fuji IX, respectively. According to the weight percentage, zirconium in Zirconomer and silica in Fuji IX were the second main elements. Calcium, zinc, and zirconium were observed only in Zirconomer [Table 1].
Figure 1.
Scanning electron microscope image of Zirconomer glass powder
Figure 4.
Energy dispersive X-ray spectrum of Fuji IX glass powder
Table 1.
Weight and atomic percentages of main constituents (elements) of glass powder of Zirconomer and Fuji IX identified with energy dispersive X-ray microanalysis
Two-way ANOVA (interaction effect) showed significant difference in fluoride release between Zirconomer and Fuji IX (F = 5034.697, P < 0.001). When LSD post-hoc test was applied, it showed that for all the time intervals statistically significant maximum fluoride release was observed in Zirconomer than Fuji IX. In addition, in Zirconomer statistically significant maximum fluoride release was observed at 7th day followed by the 3rd day, 1st day, 15th day, and 30th day. Similar pattern was observed in Fuji IX [Figure 5].
Figure 5.
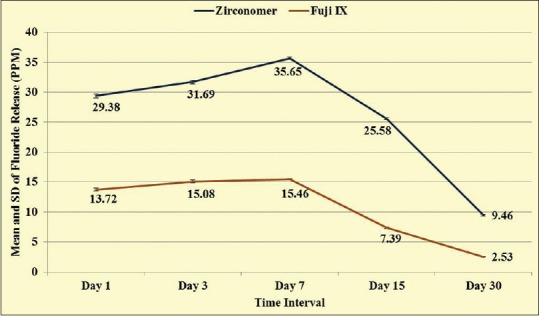
Mean and standard deviation of fluoride release from Zirconomer and Fuji IX at different time intervals
Figure 2.

Scanning electron microscope image of Fuji IX glass powder
Figure 3.
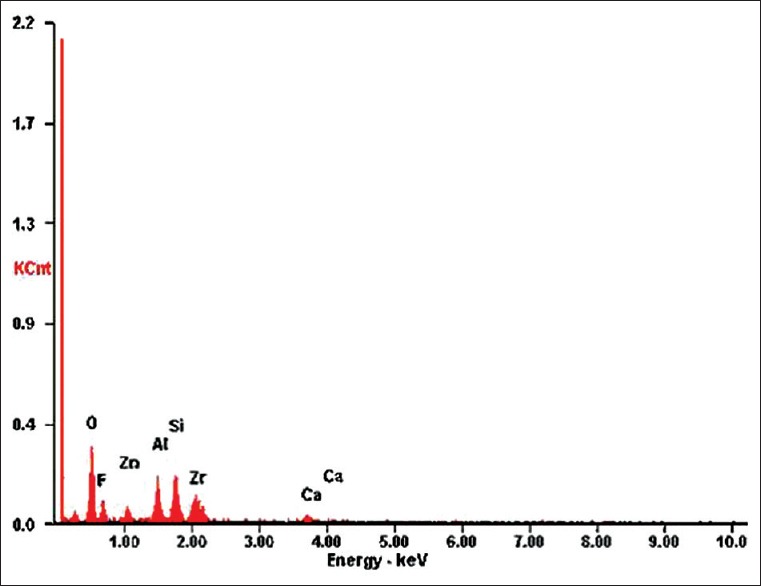
Energy dispersive X-ray spectrum of Zirconomer glass powder
At 48 hours, mean ± SD of zone of inhibition against Streptococcus mutans was 11.14 ± 0.77 mm and 8.51 ± 0.43 mm for Zirconomer and Fuji IX, respectively. Against Lactobacillus casei, it was 14.06 ± 0.71 mm for Zirconomer and 11.70 ± 0.39 mm for Fuji IX. Zirconomer has shown statistically significant larger zone of inhibition than Fuji IX against Streptococcus mutans and Lactobacillus casei. No antifungal activity was observed against Candida albicans by Zirconomer and Fuji IX [Table 2].
Table 2.
Comparison of zone of inhibition against various microorganisms between Zirconomer and Fuji IX
DISCUSSION
The crystalline dioxide of zirconium is known as zirconia. In 1969, zirconium oxide was first used for medical purposes in orthopedics as a new material for hip head replacement. In contemporary dentistry, it has become a popular substitute to alumina as a biomaterial and is used for fabricating aesthetic orthodontic brackets, endodontic posts, crown and bridge restorations, and implant abutments.[17]
The present research was conducted on EDX microanalysis, fluoride release, and antimicrobial properties of new ART material with zirconia fillers (Zirconomer) in comparison with established ART material Fuji IX. Fuji IX is the most frequently reported material in several in vivo and in vitro studies.[7,8,18] A literature search revealed that there is no published research on the abovementioned aim, hence our research is considered to be the pioneer on these aspects.
EDX is a reproducible, reliable, and precise technique to identify and quantify major components present in a material. Identification of constituents in materials leads to understanding of its various physical, biological, chemical, and mechanical properties. Still, it has limitations for precise detection of low molecular weight elements such as carbon, hydrogen, and oxygen. The proportion of ionizing episodes that results in emission of X-rays decreases with elements of smaller atomic number.[13] The EDX microanalysis revealed difference in elements of glass powders of Zirconomer and Fuji IX. In addition, at all the time intervals, statistically significant higher amount of fluoride release was observed with Zirconomer than Fuji IX. Systematic reviews and meta-analyses have provided evidence that fluoride release from GICs inhibits caries development, progression, and secondary caries instigation.[5,6] Fluoride release from GICs depends upon various factors including material composition, powder-liquid ratio, pH, and temperature of external environment.[18,19] pH and temperature of external environment (experimental conditions) were similar for both the materials in the present study. As discussed earlier, both the materials differed in their composition. In addition, Zirconomer has higher powder liquid ratio (8:1 P/L)[12] than Fuji IX (3.6:1 P/L).[1,8] These factors may be responsible for observed differences in fluoride release.
In the present study, fluoride release from GICs was first increased from day 1 to day 7 and then decreased on day 15 and 30. Similar results have been reported by Upadhyay et al.[19] and Mousavinasab and Meyers.[20] Higher fluoride release in the first few days is a normal feature of GICs.[19,20,21] This “Burst Effect” reduces the amount of residual bacteria in restored cavities and also supports the remineralization of enamel and dentin.[19,20]
In the present investigation, antimicrobial activity of GICs was assessed against Streptococcus mutans, Lactobacillus casei, and Candida albicans using agar diffusion test. Agar diffusion test is a relatively inexpensive, rapid, easy, and widely accepted screening method to assess antimicrobial properties of restorative materials.[18,22] It is well-established that Streptococcus mutans and Lactobacillus casei play a major role in the etiology of dental caries.[23,24] In the present investigation, Zirconomer had higher antibacterial activity against Streptococcus mutans and Lactobacillus casei than Fuji IX at 48 hours. Antibacterial activity of GICs is directly related with the amount of fluoride release.[1,25,26,27] Hence, in the present research, dissimilarity in fluoride release in both the materials may be a reason for observed antibacterial activity.
In contrast with our research, studies conducted by Mittal et al.[18] and Prabhakar et al.[27] have shown that Fuji IX has no effect against Streptococcus mutans and Lactobacillus casei. However, similar to the present investigation, Shashibhushan et al.,[1] El-Baky and Hussien,[22] and Luczaj-Cepowicz et al.[25] have shown that Fuji IX inhibits the growth of both the bacteria. Differences in methodology to assess antibacterial activity may be a reason for these contrasting observations.
Numerous studies have proposed Candida albicans as a pathogen for dental caries.[23,28,29] The organic acid and enzyme produced by Candida albicans can dissolve the hydroxyapatite of dental hard tissues and degrade the dentinal collagen.[23,29] Investigations by Cassanho et al.[30] and Bhavana et al.[31] have shown that glass ionomer cements did not have any effect on the growth of Candida albicans. In a study by Dastjerdie et al., GICs demonstrated weak antifungal effect against Candida albicans.[32] Nevertheless, Candida albicans was taken as the test microbe in the present research to investigate whether new ART material Zirconomer had any antifungal effect. In the present study, no antifungal effect was generated by Zirconomer and Fuji IX.
Limitations
The present research is in vitro in nature which does not reflect the actual status of fluoride release and antimicrobial properties in oral cavity. Furthermore, it does not differentiate between bacteriostatic and bactericidal effects of Zirconomer and Fuji IX because the agar diffusion test does not provide information on viability of microorganisms within the zones of inhibition.[22] Therefore, further in vivo studies on the abovementioned aspects are recommended.
Based on the results, it can be concluded that new ART material Zirconomer had higher antibacterial activity against Streptococcus mutans and Lactobacillus casei, which may be attributed to its composition and higher fluoride release. However, it failed to show antifungal effect against Candida albicans.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Shashibhushan KK, Basappa N, Subba Reddy VV. Comparison of antibacterial activity of three fluorides- and zinc-releasing commercial glass ionomer cements on strains of mutans streptococci: An in vitro study. J Indian Soc Pedod Prev Dent. 2008;26:S56–61. [PubMed] [Google Scholar]
- 2.Tyas MJ. Clinical evaluation of glass-ionomer cement restorations. J Appl oral Sci. 2006;14:10–3. doi: 10.1590/s1678-77572006000700003. [DOI] [PubMed] [Google Scholar]
- 3.Sayyedan FS, Fathi M, Edris H, Doostmohammadi A, Mortazavi V, Shirani F. Fluoride release and bioactivity evaluation of glass ionomer: Forsterite nanocomposite. Dent Res J. 2013;10:452–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, Sakagami H, Morales-Luckie RA, Nakajima H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J Appl oral Sci. 2015;23:321–8. doi: 10.1590/1678-775720140496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raggio DP, Tedesco TK, Calvo AFB, Braga MM. Do glass ionomer cements prevent caries lesions in margins of restorations in primary teeth.: A systematic review and meta-analysis? J Am Dent Assoc. 2016;147:177–85. doi: 10.1016/j.adaj.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Tedesco TK, Bonifacio CC, Calvo AFB, Gimenez T, Braga MM, Raggio DP. Caries lesion prevention and arrestment in approximal surfaces in contact with glass ionomer cement restorations-A systematic review and meta-analysis. Int J Paediatr Dent. 2016;26:161–72. doi: 10.1111/ipd.12174. [DOI] [PubMed] [Google Scholar]
- 7.Hesse D, Bonifacio CC, Guglielmi Cde A, Franca Cd, Mendes FM, Raggio DP. Low-cost glass ionomer cement as ART sealant in permanent molars: A randomized clinical trial. Braz Oral Res. 2015;29:1–9. doi: 10.1590/1807-3107BOR-2015.vol29.0063. [DOI] [PubMed] [Google Scholar]
- 8.Calvo AFB, Kicuti A, Tedesco TK, Braga MM, Raggio DP. Evaluation of the relationship between the cost and properties of glass ionomer cements indicated for atraumatic restorative treatment. Braz Oral Res. 2016;30:e8. doi: 10.1590/1807-3107BOR-2016.vol30.0008. [DOI] [PubMed] [Google Scholar]
- 9.Tuzuner T, Ulusu T. Effect of antibacterial agents on the surface hardness of a conventional glass-ionomer cement. J Appl oral Sci. 2012;20:45–9. doi: 10.1590/S1678-77572012000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konde S, Raj S, Jaiswal D. Clinical evaluation of a new art material: Nanoparticulated resin-modified glass ionomer cement. J Int Soc Prev Community Dent. 2012;2:42–7. doi: 10.4103/2231-0762.109361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel MU, Punia SK, Bhat S, Singh G, Bhargava R, Goyal P, et al. An in vitro evaluation of microleakage of posterior teeth restored with amalgam, composite and zirconomer-A stereomicroscopic study. J Clin Diagn Res. 2015;9:ZC65–7. doi: 10.7860/JCDR/2015/13024.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zirconomer Zirconia Reinforced Restorative [Internet] [Last cited 2016 Mar 11]. Available from: http://www.shofu.com.sg/downloads/pdf/Zirconomer Brochure.pdf .
- 13.Guedes OA, Borges AH, Bandeca MC, Nakatani MK, de Araujo Estrela CR, de Alencar AHG, et al. Chemical and structural characterization of glass ionomer cements indicated for atraumatic restorative treatment. J Contemp Dent Pract. 2015;16:61–7. doi: 10.5005/jp-journals-10024-1636. [DOI] [PubMed] [Google Scholar]
- 14.Mungara J, Philip J, Joseph E, Rajendran S, Elangovan A, Selvaraju G. Comparative evaluation of fluoride release and recharge of pre-reacted glass ionomer composite and nano-ionomeric glass ionomer with daily fluoride exposure: An in vitro study. J Indian Soc Pedod Prev Dent. 2013;31:234–9. doi: 10.4103/0970-4388.121820. [DOI] [PubMed] [Google Scholar]
- 15.Gopikrishnan S, Melath A, Ajith VV, Mathews NB. A comparative study of bio degradation of various orthodontic arch wires: An in vitro study. J Int Oral Health. 2015;7:12–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Baliga S, Muglikar S, Kale R. Salivary pH: A diagnostic biomarker. J Indian Soc Periodontol. 2013;17:461–5. doi: 10.4103/0972-124X.118317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madfa AA, Al-Sanabani FA, Al-Qudami NH, Al-Sanabani JS, Amran AG. Use of zirconia in dentistry: An overview. Open Biomater J. 2014;5:1–9. [Google Scholar]
- 18.Mittal S, Soni H, Sharma DK, Mittal K, Pathania V, Sharma S. Comparative evaluation of the antibacterial and physical properties of conventional glass ionomer cement containing chlorhexidine and antibiotics. J Int Soc Prev Community Dent. 2015;5:268–75. doi: 10.4103/2231-0762.161754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upadhyay S, Rao A, Shenoy R. Comparison of the amount of fluoride release from nanofilled resin modified glass ionomer, conventional and resin modified glass ionomer cements. J Dent. 2013;10:134–40. [PMC free article] [PubMed] [Google Scholar]
- 20.Mousavinasab SM, Meyers I. Fluoride release by glass ionomer cements, compomer and giomer. Dent Res J. 2009;6:75–81. [PMC free article] [PubMed] [Google Scholar]
- 21.Rao BS, Moosani GK, Shanmugaraj M, Kannapan B, Shankar BS, Ismail PM. Fluoride release and uptake of five dental restoratives from mouthwashes and dentifrices. J Int Oral Health. 2015;7:1–5. [PMC free article] [PubMed] [Google Scholar]
- 22.El-Baky RMA, Hussien SM. Comparative antimicrobial activity and durability of different glass ionomer restorative materials with and without chlorohexidine. J Adv Biotechnol Bioeng. 2013;1:14–21. [Google Scholar]
- 23.Lai G, Li M. The possible role of Candida albicans in the progression of dental caries. Int Res J Microbiol. 2011;2:504–6. [Google Scholar]
- 24.Karpinski TM, Szkaradkiewicz AK. Microbiology of dental caries. J Biol Earth Sci. 2013;3:M21–4. [Google Scholar]
- 25.Luczaj-Cepowicz E, Marczuk-Kolada G, Zalewska A, Pawinska M, Leszczynska K. Antibacterial activity of selected glass ionomer cements. Postep Hig Med Dosw. 2014;68:23–8. doi: 10.5604/17322693.1086069. [DOI] [PubMed] [Google Scholar]
- 26.Chau NPT, Pandit S, Cai JN, Lee MH, Jeon JG. Relationship between fluoride release rate and anti-cariogenic biofilm activity of glass ionomer cements. Dent Mater. 2015;31:e100–8. doi: 10.1016/j.dental.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Prabhakar AR, Prahlad D, Kumar SR. Antibacterial activity, fluoride release, and physical properties of an antibiotic modified glass ionomer cement. Pediatr Dent. 2013;35:411–5. [PubMed] [Google Scholar]
- 28.Al-hebshi NN, Abdulhaq A, Quadri MFA, Tobaigy FM. Salivary carriage of Candida species in relation to dental caries in a population of Saudi Arabian primary school children. Saudi J Dent Res. 2015;6:54–9. [Google Scholar]
- 29.Metwalli KH, Khan SA, Krom BP, Jabra-Rizk MA. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog. 2013;9:e1003616. doi: 10.1371/journal.ppat.1003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassanho ACA, Fernandes AM, de Oliveira LD, Carvalho CAT, Jorge AOC, Koga-Ito CY. In vitro activity of zinc oxide-eugenol and glass ionomer cements on Candida albicans. Braz Oral Res. 2005;19:134–8. doi: 10.1590/s1806-83242005000200011. [DOI] [PubMed] [Google Scholar]
- 31.Bhavana V, Chaitanya KP, Gandi P, Patil J, Dola B, Reddy RB. Evaluation of antibacterial and antifungal activity of new calcium-based cement (Biodentine) compared to MTA and glass ionomer cement. J Conserv Dent. 2015;18:44–6. doi: 10.4103/0972-0707.148892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dastjerdie EV, Oskoui M, Sayanjali E, Tabatabaei FS. In-vitro comparison of the antimicrobial properties of glass ionomer cements with zinc phosphate cements. Iran J Pharm Res. 2012;11:77–82. [PMC free article] [PubMed] [Google Scholar]


