Abstract
Background
A proper identification of malaria vectors is essential for any attempt to control this disease. Between 40 and 47 Anopheles species have been recorded in Colombia, and eight species complexes have been identified in the last decade. An update of Anopheles species distribution and its relationship with malaria is required, particularly for newly identified members of species complexes.
Methods
A cross-sectional entomological study was conducted at 70 localities in the highest malaria transmission areas in Colombia. In each locality, immature and adult mosquitoes were collected. All specimens were determined using morphological characters and confirmed used restriction profiles of Internal Transcribed Spacer 2 (PCR–RFLP-ITS2), and Cytochrome c Oxidase I (COI) sequence gene. To detect natural Plasmodium infections, enzyme-linked immunosorbent assay and nested PCR analysis were used. Distribution of Anopheles species was spatially associated with malaria incidence.
Results
A total of 1736 larvae and 12,052 adult mosquitoes were determined in the 70 localities. Thirteen Anopheles species were identified. COI sequence analysis suggested 4 new lineages for Colombia: for Anopheles albimanus (An. albimanus B), Anopheles pseudopunctipennis s.l., Anopheles neivai (An. neivai nr. neivai 4), and Anopheles apicimacula. Two members of species complexes were identified, as: Anopheles nuneztovari C, and Anopheles albitarsis I. Another seven species were confirmed. Four mosquitoes were infected with Plasmodium species, An. albimanus B and An. nuneztovari C. In Northwest of Colombia, An. nuneztovari C, An. albimanus, and Anopheles darlingi were present in the municipalities with highest annual parasitic index (API) (>35 cases/1000 inhabitants). In the north of South Pacific coast, with a similar API, An. nuneztovari C were widely distributed inland, and the main species in coastal regions were An. albimanus B and An. neivai s.l. In the South Pacific coast bordering with Ecuador, 3 Anopheles species were found in municipalities with high API (15–88 cases/1000 inhabitants): An. albimanus B, Anopheles calderoni and An. neivai s.l.
Conclusions
In the highest malaria areas of Colombia, 13 Anopheles species and four new lineages were found, which highlights the need for updating the species distribution. A DNA barcode analysis allowed the taxonomic identification to be refined, particularly for species complexes, and to improve the further understanding of their relation with malaria transmission.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-016-1421-4) contains supplementary material, which is available to authorized users.
Keywords: Anopheles, Cytochrome c oxidase I, Colombia, Malaria
Background
According to the 2015 World Malaria Report [1], 17 % of malaria cases reported in the Americas region were from Colombia, where malaria transmission exhibits an endemic/epidemic pattern that maintains unstable endemic transmission levels throughout the country [2]. A decreasing trend in clinical malaria cases has been reported over the past 14 years in Colombia, falling from 144,432 in 2000 [2] to 40,768 in 2014, with a more than 75 % decrease in the incidence of microscopically confirmed malaria during this period of time [1]. Despite this, malaria remains one of the foremost public health concerns in the western region of Colombia where more than 85 % of malaria cases were reported from 2010 to 2015 [3].
The last review of the geographical distribution of Anopheles species in Colombia was published over 4 years ago and mentioned the presence of 40–47 Anopheles species including those belonging to species complexes [4]. Many of the records included in this review took into account data collected more than 50 years ago by the Malaria Eradication Service (Servicio de Erradicacion de la Malaria, SEM).
Environmental, social, economic, and demographic changes have occurred since that time which may have substantially influenced the observed distribution of Anopheles species in Colombia. In addition, it is necessary to differentiate the members of the Anopheles species complexes, which can now be achieved using several molecular tools [5]. A clear example is the recent analysis, based on the sequencing of the mitochondrial DNA marker, Cytochrome c Oxidase I (COI); which showed the presence of two species of the Anopheles (Nyssorhynchus) albitarsis complex in Colombia: An. (Nys.) albitarsis F, in the departments of Meta, Norte de Santander and Vichada; and An. (Nys.) albitarsis I, in departments of Antioquia and Norte de Santander [6]. Based on sequences of molecular markers, COI, and internal transcribed spacer 2 (ITS 2), analysis showed that there are three species of the complex oswaldoi-konderi in Colombia: Anopheles (Nys.) oswaldoi A in department of Amazonas; An. (Nys.) oswaldoi B in departments of Antioquia, Caqueta, Meta, Norte de Santander and Putumayo; and Anopheles sp. nr. konderi in department of Caqueta [7]. However, there are still gaps in knowledge about the distribution of Anopheles mosquitoes in Colombia, as well as the presence of Anopheles species of other species complexes [8].
Of Anopheles species reported in Colombia, 12 have been implicated in malaria transmission. The main malaria vectors are Anopheles (Nys.) darlingi, Anopheles (Nys.) nuneztovari s.l., and Anopheles (Nys.) albimanus [9]. Other species are referred to as vectors of local importance in some areas, or are suspected of being associated with malaria transmission, such as: Anopheles (An.) pseudopunctipennis s.l.; Anopheles (An.) punctimacula s.l.; Anopheles (An.) calderoni; Anopheles (An.) neomaculipalpus; Anopheles (Kerteszia) pholidotus; Anopheles (Ker.) neivai s.l.; Anopheles (Nys.) rangeli; Anopheles (Nys.) benarrochi B; and An. (Nys.) oswaldoi s.l. [9–14].
Studies of malaria vector species in Colombia have usually been performed in just a few localities and usually with the aim to clarify taxonomic identifications or to understand the biology, ecology, and the role in malaria transmission of the species involved. This is the first study to associate spatial vector distributions with intensity of malaria transmission and parasite prevalence. The aim of this entomological study is to describe the relationship between Anopheles species distribution patterns and malaria incidence in the highest malaria transmission region of Colombia.
Methods
Study area
In order to identify and update the Anopheles species present in the highest malaria endemic areas of Colombia, as well to describe spatial distribution patterns and their relationship with the intensity of malaria transmission; a cross-sectional study was conducted in 70 localities, distributed in the Northwest (department of Cordoba) and South Pacific coast regions (department of Valle del Cauca and Nariño) of Colombia. These regions are two of the most important malaria endemic areas in the country. Study localities were distributed as follows: 27 in the Northwest and 43 in the South Pacific coast; names and coordinates of each locality are included in the Additional file 1a. These sites were selected based on high malaria incidence, the ease of access by land or river, and safety. A detailed description of the study areas has been previously published [15].
Adult sampling and identification
Anopheline collections were performed at each locality for a week between May 2011 and November 2012. Human-landing catches were conducted indoors and outdoors in eight households at each locality. Households were selected according to convenience, taking into account: (i) presence of malaria cases, (ii) construction type, and (iii) proximity to wooded areas. Two technicians sampled each household during one night in a period of four consecutive nights, with two households sampled per night. At each household, two collectors were placed: one outdoors and another one indoors. Collections were conducted simultaneously from 18:00 to 24:00 h with collectors’ rotated hourly between indoors and outdoors settings. Samples were made during the first 50 min of every hour. All specimens collected were kept dry over silica gel, separated by site (indoors and outdoors), and date, until the mosquitoes were processed. Mosquitoes were determined using the most recent taxonomic key for Anopheles of Colombia [16].
Larval sampling and identification
At each study site, all larval habitats present in a radius of 1000 m around the households selected for adult sampling were sampled for larvae, using the standard dipping method with a 400 ml ladle according to WHO procedures [17], with a sampling effort of 10 samples-dips per square metre of larval habitat. All third and fourth instars of Anopheline larvae were preserved in 70 % alcohol. In the laboratory, each larva was individually identified to species by morphological characters using the key for Anopheles of Colombia [16].
PCR–RFLP assay
To differentiate An. nuneztovari s.l., An. rangeli, and An. oswaldoi s.l., restriction profiles of internal transcribed spacer 2 (PCR–RFLP-ITS2) were used. Profiles were obtained from the amplification of this gene with the primers as proposed by Collins and Paskewitz [18]: 5,8SD 5′-TGAACTGCAGGACACATGAA-3 and 28SR 3′-TGCTTAAATTTAGGGGGTAGTC-5′. Posterior digestion was performed with the enzyme TaaI (HpyCH4III, Fermentas ®). The products were visualized using 2.5 % agarose gels. Positive controls were: DNA of An. nuneztovari C from department of Cordoba, confirmed by COI sequence for this study (KU925590), and DNA of An. oswaldoi s.l. from department of San Jose del Guaviare and An. rangeli from department of Meta, which had previously been confirmed by ITS2 sequence (KU945816, KU945817). The expected bands for An. rangeli were at 229, 104, 98 and 76 bp, for An. oswaldoi s.l. at 281, 233 and 18 bp, and for An. nuneztovari s.l. at 320 and 220 bp.
Cytochrome c oxidase I (COI) sequencing
The DNA Barcoding region of the mitochondrial COI gene was sequenced in the sub-sample of Anopheles species identified by morphology. In each municipality approximately 3 % specimens of the most abundant species, and 100 % specimens of the less abundant species or species included in a complexes were selected for sequencing. The COI gene sequences were generated following the standardized methodology used for the Mosquito Barcoding Initiative of the Walter Reed Biosystematics Unit and the Natural History Museum in London to determine the degree of genetic variability and to seek evidence of species complex. DNA was extracted from the specimens following the tissue DNA extraction protocol provided by the QIAgen DNeasy blood and tissue kit (QIAGEN, Crawley, UK). All buffers were supplied in the kit. The mitochondrial gene cytochrome c oxidase subunit I (COI) was amplified using the primers LCO1490 (GGTCAACAAATCATAAAGATATTGG) and HCO2198 (TAAACTTCAGGGTGACCAAAAAATCA) [19] following the methodology given by Ruiz et al. [20]. PCR products were electrophoresed in 1 % Tris–acetate-EDTA buffer (TAE) and agarose gels, which were stained with ethidium bromide and cleaned using a QIAgen PCR purification kit prior to direct sequencing. PCR products were sequenced with both forward and reverse primers using an ABI-BigDye Terminator (or the Sanger dideoxy method) by the Sequencing and Analysis Service from the Molecular Genetics Institute—SSiGMol of the Universidad Nacional de Colombia in Bogota. The forward and reverse chromatograms were manually corrected using the electropherogram viewer Chromas Lite© (version 2.1.1, Technelysium Pty Ltd., Brisbane, Australia). The sequences were trimmed to 610 bp and multiple alignment was performed using Clustal W in the molecular evolutionary genetics analysis (MEGA) software version 6.0 with default parameters (open gap penalty = 15, extend gap penalty = 6.66). The resulting matrix was manually corrected.
Natural infectivity with Plasmodium species
To detect natural infections with Plasmodium species, the head and thorax of each adult mosquito collected were used, as proposed by Foley et al. [21]. The samples were then processed following the standard protocol using the Enzyme-Linked Immunosorbent Assay (ELISA) kit distributed by the Centers for Disease Control and Prevention (CDC; Atlanta, United States), which detects the circumsporozoite protein (CS) of Plasmodium falciparum, Plasmodium vivax VK210 and P. vivax VK247. The cut-off used was two times the average of the negative control [22]. Positive individuals were re-tested using ELISA for accuracy. To confirm the presence of Plasmodium species, nested PCR analysis was performed following the methodology proposed by Snounou et al. [23]. Because the thoracic material had been used for ELISA and was no longer available, DNA was extracted from abdomens of individual mosquitoes. Briefly, two PCR reactions were performed. In the first, a region that is common to the genus Plasmodium was amplified with the primers 5’rPLU6 5′-TTA AAA TTG TTG CAG TTA AAA CG-3′ and rPLU5 5′-CCT GTT GTT GCC TTA AAC TTC-3′. From the amplified sample, the second PCR was performed with primers specific for P. falciparum species with the primers rFAL1 5′-TTA AAC TGG TTT GGG AAA ACC AAA TAT ATT-3′/rFAL 2 5-′ACA CAA TGA ACT CAA TCA TGA CTA CCC GTC-3′, and/or P. vivax with primers rVIV1 5′-CGC TTC TAG CTT AAT CCA CAT AAC TGA TAC-3′/rVIV2. 5′-ACT TCC AAG CCG AAG CAA AGA AAG TCC TTA-3′.
Spatial distribution of Anopheles species
To describe the spatial distribution patterns, precise geographical coordinates of each immature and adult capture site were recorded using a global positioning system (Garmin GPSMAP®60CSx) and the distribution and proportion of Anopheles species collected in each locality were visualized using the software ArcGis 9.0. Later, spatial distributions of Anopheles species were compared with the number of malaria cases of P. falciparum and P. vivax and the annual parasite index (API) registered during 2011 and 2012 in the National System for Public Health Surveillance of Colombia- SIVIGILA [3] for the municipalities included in this study. In the South Pacific coast, the municipality of Buenaventura was divided in two areas: (1) Cali-Buenaventura road that included localities located over this road, and (2) Pacific plain localities.
Data analysis
Natural infection rate with Plasmodium parasites was calculated as the percentage of mosquitoes infected. To establish the identity percent of the COI sequences obtained in this study, these were compared with available sequences in GenBank [24] and Boldsystems databases [25].
Sequencing analysis
The COI sequences generated for specimens collected in the study sites and identified by morphological characters were aligned with the COI sequences of Anopheles species previously downloaded from GenBank database. All available sequences sharing the same region were included (Additional file 2); however, for the phylogram construction, some sequences were omitted. Chagasia nr. fajardi (GenBank: KF671013) was used as the outgroup in these analyses.
The neighbour joining (NJ) and maximum likelihood (ML) algorithms were implemented in MEGA version 6.0 program. The Tamura 3-parameter plus gamma distribution plus invariable site (T92+G+I) [26] with invariable sites (I = 0.54) and Gamma distribution shape parameter (G = 0.53) was selected as the best evolutionary distance model by MEGA 6 in model selection option [26]. Bootstrap support values (BSV) were generated by 1000 replicates. Bayesian inference (BI) was performed using MrBayes version 3.1.2. [27, 28] using default priors. Two independent analyses were run simultaneously, each with four chains and a temperature parameter value of 0.2 (the default in MrBayes). Parameters and topologies were sampled every 1000 generations. Runs were allowed to continue to 10,000,000 generations until the variance was stabilized below 0.01. Burn-in consisted of the first 25 % of generations (25,000 trees). A consensus phylogram was condensed to eliminate branch points with less than 50 % of probability.
Results
Anopheles species composition
In total, 1736 larvae and 12,052 adult mosquitoes were identified in the 70 localities studied. From this entomological material, 13 species were identified using morphological characteristics and 17 mitochondrial lineages were identified using the sequence COI analysis (Tables 1, 2; Fig. 1). The DNA Barcoding region of the mitochondrial COI gene was obtained for 173 specimens: 90 from the Northwest and, 83 from the South Pacific coast region. The data of specimens used to generate the COI sequence are presented in the Additional file 1b. All sequences were deposited in the GenBank database under the following accession numbers: KU892018-KU892060, KU900755-KU900848, KU925580-KU925615).
Table 1.
Anopheles species collected by human landing catches in the 70 localities of the cross-sectional study in Northwest and South Pacific regions of Colombia
| Species (lineages) | Northwest region | South Pacific coast region | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cordoba | Nariño | Valle | ||||||||||||
| Montelibano | Puerto Libertador | Tierralta | Valencia | Barbacoas | El Charco | Magui Payan | Mosquera | Olaya Herrera | Roberto Payan | Salahonda | Tumaco | Buenaventura | ||
| An. albimanus | 8 | 34 | 221 | 116 | – | – | – | – | – | – | – | – | – | 379 |
| An. albimanus Ba | – | – | – | – | 3 | 175 | – | 3799 | 22 | 2 | 76 | 452 | 330 | 4859 |
| An. albitarsis la | – | – | 4 | 1 | – | – | – | – | – | – | – | – | – | 5 |
| An. apicimacula (NW-SPC, SPC) | – | – | 1 | 1 | – | 2 | – | – | – | – | – | 9 | 2 | 15 |
| An. argyritarsis | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 |
| An. calderoni | – | – | – | – | 4 | 13 | – | 337 | 58 | 1 | 46 | – | 459 | |
| An. darlingi | 6 | 20 | 189 | 7 | – | – | – | – | – | – | – | – | – | 222 |
| An. neivai s.l. | – | – | – | – | – | 1 | – | 1 | – | – | – | 18 | 1.233 | 1253 |
| An. neivai nr. An. neivai 4a | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 |
| An. neomaculipalpus | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 |
| An. nuneztovari Ca | 1026 | 789 | 1647 | 744 | – | – | – | – | – | – | – | – | 501 | 4707 |
| An. pseudopunctipennis s.l. (NW, SPC) | – | – | 12 | 7 | – | – | – | – | – | – | – | – | 22 | 41 |
| An. punctimacula s.l. | 6 | – | 7 | 2 | – | – | – | – | – | – | – | – | – | 15 |
| An. squamifemur | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 |
| An. triannulatus s.l. | 21 | 29 | 24 | 17 | – | – | – | – | – | – | – | – | – | 91 |
| Total | 1067 | 873 | 2105 | 896 | 7 | 191 | 0 | 3800 | 359 | 60 | 77 | 525 | 2092 | 12,052 |
aLineages supported in Culicidae database of Boldsystems (http://www.boldsystems.org) and GenBank databases
NW Northwest of Colombia, SPC South Pacific coast region of Colombia
Table 2.
Anopheles species collected in the inspected breeding sites of the 70 localities in the cross-sectional study in Northwest and South Pacific regions of Colombia
| Species (lineages) | Northwest region | South Pacific coast region | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cordoba | Nariño | Valle | ||||||||||||
| Montelibano | Puerto Libertador | Tierralta | Valencia | Barbacoas | El Charco | Magui Payan | Mosquera | Olaya Herrera | Roberto Payan | Salahonda | Tumaco | Buenaventura | ||
| An. albimanus | – | – | 30 | 22 | – | – | – | – | – | – | – | – | – | 52 |
| An. albimanus Ba | – | – | – | – | – | 4 | – | – | 4 | – | 8 | 268 | 1 | 285 |
| An. nuneztovari Ca | 22 | 102 | 55 | 35 | – | – | – | – | – | – | – | – | 820 | 1034 |
| An. darlingi | – | – | 6 | 1 | – | – | – | – | – | – | – | – | 7 | |
| An. triannulatus s.l. | 7 | 109 | 51 | 43 | – | – | – | – | – | – | – | – | 210 | |
| An. pseudopunctipennis s.l. (NW, SPC) | – | – | 2 | – | – | – | – | – | – | – | – | – | 50 | 52 |
| An. calderoni | – | – | – | – | – | 2 | – | – | 6 | – | – | 40 | 48 | |
| An. neomaculipalpus | – | 2 | – | 5 | – | – | – | – | – | 1 | – | – | 40 | 48 |
| Total | 29 | 213 | 144 | 106 | 0 | 6 | 0 | 0 | 10 | 1 | 8 | 308 | 911 | 1736 |
aLineages supported in Culicidae database of Boldsystems (http://www.boldsystems.org) and GenBank databases
NW Northwest of Colombia, SPC South Pacific coast region of Colombia
Fig. 1.
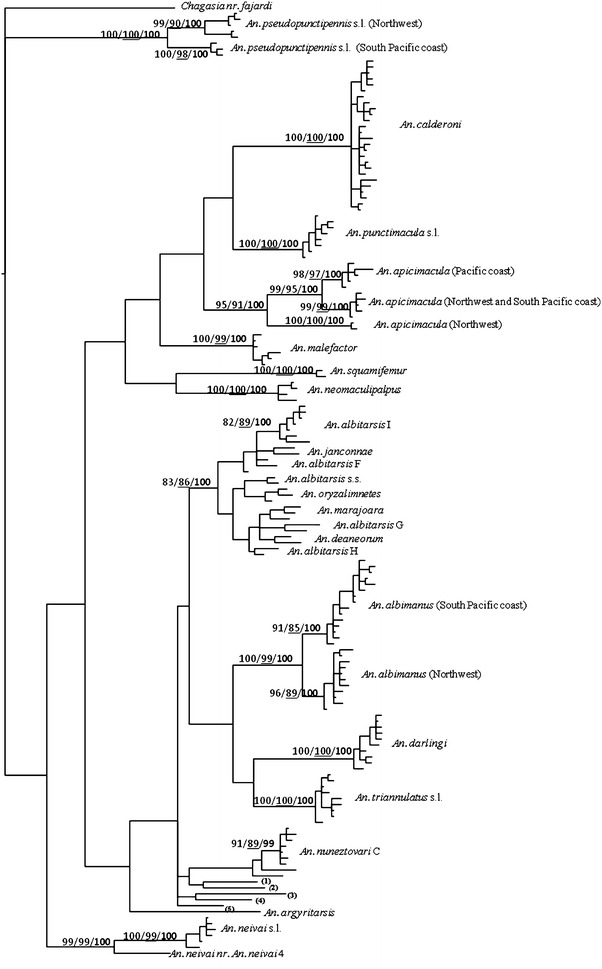
BI topology based on barcode COI sequences of Anopheles species identified in Northwest and South Pacific coast regions of Colombia (First number in each node indicates NJ bootstrap values (in percentages). Second underline number indicates ML bootstrap values. Third number in bold indicates Bayesian posterior probability. Outgroup taxa includes Chagasia nr. fajardi (GenBank KF671013). (1) Anopheles nr. konderi, (2) An. oswaldoi A, (3) An. rangeli, (4) An. oswaldoi B, (5) An. rangeli)
The identity percent with sequences deposited in GenBank and Boldsystems databases was used as an initial approach for Anopheles species confirmation. The results are shown in the Additional file 1b. Interestingly, these results indicate that the sequences of species identified using morphological characters as An. albimanus from the Northwest showed a high percentage of identity (99 %) with sequences of An. albimanus from the Northern region of Colombia (department of Guajira); in contrast, the sequences from the South Pacific coast presented a lower identity percentage (96–97 %) with the same sequences deposited in GenBank. When the comparison in Boldsystems was conducted, all sequences of An. albimanus from the Northwest showed the highest percentage of identity (99.1–99.7 %) with An. albimanus while all sequences from the South Pacific coast showed the highest percentages of identity (99.7–100 %) with An. albimanus B in Boldsystems database. However, information regarding the origin of the COI sequences of An. albimanus B are not presented in the Boldsystem database which prevent any further analysis and comparison with the sequences from this study.
The specimens determined as An. albitarsis s.l. using morphological characters matched with sequences identified as “near janconnae” from the Northwest of Colombia by GenBank (98–99 % of identity) and An. albitarsis I from the same regions included in Boldsystems database (99.5–100 % of identity), a species also confirmed for Colombia.
With respect to An. neivai s.l., the COI sequences are not reported in GenBank database. The sequences from South Pacific coast (14/16) showed the highest percentage of identity with An. neivai in the Boldsystem records (99.7–100 %); nevertheless, in two sequences of specimens collected in the Cali-Buenaventura road, the highest percentage of identity was 94.9 % with An. neivai 4. It is possible that these sequences indicate the presence of another species of subgenus Kerteszia in this region. However, the sequences of An. neivai and An. neivai 4 are not associated with any paper to clarify the taxonomic status of these species.
Anopheles nuneztovari s.l. was initially confirmed using PCR–RFLP-ITS2. Of 380 specimens were included in this assay, 34 of these had been misidentified by morphological characters as An. oswaldoi s.l. and five as An. rangeli; the remaining were identified as An. nuneztovari s.l. The COI sequences generated for An. nuneztovari s.l. allowed confirmation that all specimens collected in the Northwest and the South Pacific coast regions matched with An. nuneztovari C (99.7–100 % of identity) registered previously by Ruiz et al. [20] in the Northeastern Colombia.
In the case of Anopheles apicimacula and Anopheles squamifemur, it was not possible to confirm their morphological determination using COI sequences. The COI sequence of An. squamifemur is present in neither GenBank nor Boldsystems databases, when “Species Level Barcode Records” option was used. However, for An. apicimacula and An. squamifemur in Boldsystems database, using “All barcode records on BOLD” option, 95.8–100 and 92.2–92.5 % identity percent were found respectively, but without further details of sequences origin. Anopheles argyritarsis, An. calderoni, An. darlingi, An. neomaculipalpus, An. pseudopunctipennis s.l., and An. triannulatus s.l. matched already published COI sequences in GenBank and Boldsystems databases.
The data set to sequencing analysis included 1211 COI sequences, of which 436 were used for this analysis because they shared one 610 bp barcode region fragment of which none were identical between them (see Additional file 2). Finally, 160 sequences were used to perform the COI sequences analysis presented in this paper. All sequences were checked for insertions or deletions, finding none. The sequences were translated into amino acids to identify the proper reading frame and to ensure that there were no stop codons, which would indicate the mitochondrial origin of the DNA. The final alignment of the COI gene (partial sequence) had a length of 610 bp corresponding to positions 1463 through 2071 of the An. albitarsis mitochondrion complete genome (GenBank: NC020662).
The ML, NJ, and BI analysis shows very similar topologies (Fig. 1), and indicates the presence of two distinctive lineages at the intraspecific level for An. albimanus, An. pseudopunctipennis s.l., An. neivai s.l., and An. apicimacula. The two lineages of An. albimanus have different geographical distribution. One is found in the Northwest region, and the second is registered in the South Pacific coast, it is called An. albimanus B in Boldsystems database. In all phylograms, these lineages were highly supported (BVS >99). Similarly, for An. pseudopunctipennis s.l., the first lineage included mosquitoes collected in a Northwest region, and the second linage included COI sequences of mosquitoes collected in the South Pacific coast. These two lineages had a BVS = 100 in all analysis. The analysis of COI sequences generated for An. neivai s.l., collected in the South Pacific coast, shows two lineages. The first lineage, included sequences of specimens from the Cali-Buenaventura road (San Cipriano and Kilometro 24–27 localities) in the department of Valle del Cauca, matched with An. neivai 4 in the Boldsystems databases. The second lineage, included the remaining sequences from the South Pacific coast, identified as An. neivai. These lineages were supported with BVS >99 in all analysis. In the case of An. apicimacula, a first lineage included species collected exclusively in the South Pacific coast and the second lineage included specimens from Northwest and South Pacific coast regions; the BVS was >95 in all analysis (Fig. 1).
For An. nuneztovari s.l., all COI sequences generated in this study are grouped in PM, NJ, and BI analysis, with the sequences denominated An. nuneztovari C and reported in Northeastern of Colombia by Ruiz et al. [20]. Concerning the specimens identified by morphological characteristics as An. albitarsis s.l., the ML, NJ, and BI analysis, which included COI sequences of these mosquitoes and sequences for nine species reported in the Albitarsis group [6], showed that all sequences generated in the Northwest region were confirmed as An. albitarsis I (Fig. 1).
Natural infection of Anopheles with Plasmodium spp. species
Four of 12,027 mosquitoes (0.03 %) were infected with Plasmodium spp. (Table 3). All mosquitoes found positive for CS protein were COI sequenced to confirm the Anopheles species. The Anopheles species infected with Plasmodium species were: An. albimanus B (n = 1) from the South Pacific coast infected with P. falciparum, and An. nuneztovari C (n = 3) with two specimens from the Northwest region infected with P. falciparum and one specimen from the South Pacific coast infected with P. vivax VK247. Every specimen positive by ELISA was also positive by PCR (Table 3).
Table 3.
Number of mosquitoes processed to identify natural infection with Plasmodium spp. using ELISA and infection rate, obtained in 70 localities of the cross-sectional study in Northwest and South Pacific regions of Colombia
| Species (lineages) | Northwest region | South Pacific coast region | # Tested | Positives ELISA | Plasmodium species confirmed by PCR and infection rate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cordoba | Nariño | Valle | ||||||||||||||
| Montelibano | Puerto libertador | Tierralta | Valencia | Barbacoas | El Charco | Magui Payan | Mosquera | Olaya Herrera | Roberto Payan | Salahonda | Tumaco | Buenaventura | ||||
| An. albimanus | 8 | 34 | 221 | 116 | – | – | – | – | – | – | – | – | – | 379 | – | |
| An. albimanus Ba | – | – | – | – | 3 | 174 | 3797 | 22 | 2 | 76 | 451 | 329 | 4854 | 1 | P. falciparum (a) | |
| An. albitarsis la | – | – | 4 | 1 | – | – | – | – | – | – | – | – | – | 5 | – | |
| An. apicimacula (NW-SPC, SPC) | – | – | – | – | – | 2 | – | – | – | – | – | 8 | 1 | 11 | – | |
| An. argyritarsis | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | – | |
| An. calderoni | – | – | – | – | 4 | 13 | – | – | 337 | 58 | 1 | 46 | – | 459 | – | |
| An. darlingi | 6 | 20 | 189 | 7 | – | – | – | – | – | – | – | – | – | 222 | – | |
| An. neivai s.l. | – | – | – | – | – | 1 | – | – | – | – | – | 18 | 1232 | 1251 | – | |
| An. neivai nr. An. neivai 4a | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 | – | |
| An. nuneztovari Ca | 1025 | 786 | 1646 | 742 | – | – | – | – | – | – | – | – | 500 | 4699 | 3 | 2 P. falciparum (b); 1 P.vivax VK247 (c) |
| An. pseudopunctipennis s.l. (NW, SPC) | – | – | 12 | 7 | – | – | – | – | – | – | – | – | 22 | 41 | – | |
| An. punctimacula s.l. | 4 | 7 | 2 | – | – | – | – | – | – | – | – | – | 13 | – | ||
| An. triannulatus s.l. | 21 | 28 | 24 | 17 | – | – | – | – | – | – | – | – | – | 90 | – | |
| Total | 1064 | 869 | 2103 | 892 | 7 | 190 | 0 | 3797 | 359 | 60 | 77 | 523 | 2086 | 12,027 | 4 | 0.03 |
aLineages supported in Culicidae database of Boldsystems (http://www.boldsystems.org) and GenBank databases
NW Northwest of Colombia, SPC South Pacific coast region of Colombia. (a) El Charco, Nariño, (b) Tierralta, Cordoba, (c) Buenaventura, Valle del Cauca
Spatial distribution of Anopheles species in the states of study and its relation to malaria incidence
Regarding the number of malaria cases reported and malaria incidence in the year of sampling, in the Northwest region, particularly in the department of Cordoba (with four municipalities included in this study), the municipality of Valencia reported the lowest number of cases (126), with an annual parasite incidence (API) of 5 cases/1000 inhabitants; while the municipalities of Tierralta, Montelibano, and Puerto Libertador reported a similar number of cases each: 1826 (API: 35 cases/1000 inhabitants), 1419 (API: 86 cases/1000 inhabitants) and 1, 413 (API: 55 cases/1000 inhabitants), respectively. The proportion between P. vivax and P. falciparum infections was similar in the municipalities of study except in Valencia where all infections were only by P. vivax (Fig. 2). In the Northwest region, the most widely distributed species for both adults and larvae was An. nuneztovari C. Other malaria vectors found were An. albimanus and An. darlingi (Fig. 2).
Fig. 2.
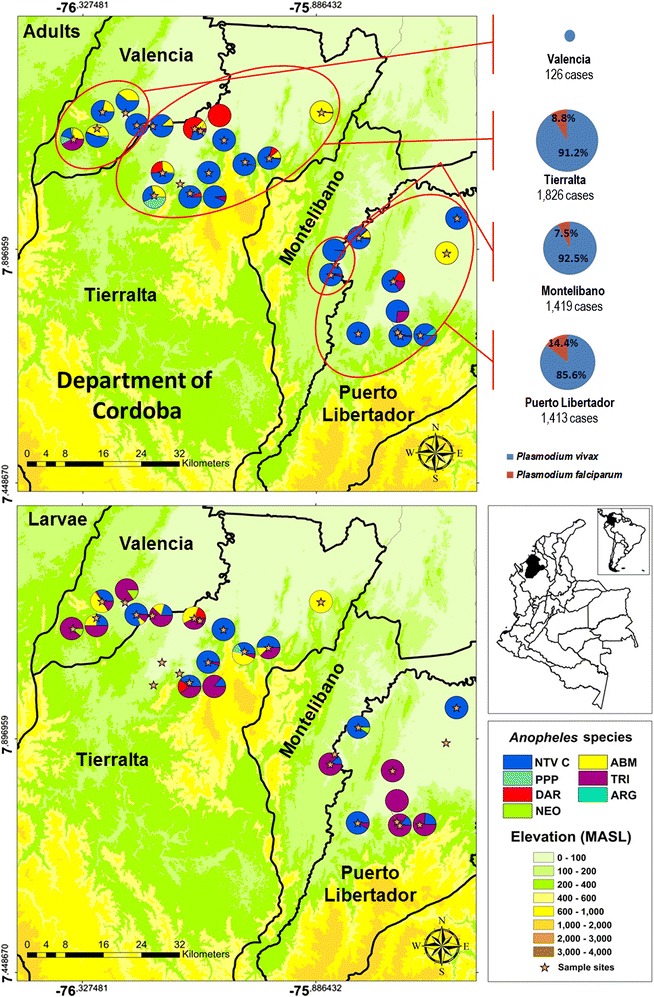
Distribution of Anopheles species found in the Northwest region of Colombia and malaria cases reported. (NTV An. nuneztovari C; ABM An. albimanus; DAR An. darlingi; PPP An. pseudopunctipennis s.l.; TRI An. triannulatus s.l.; ARG An. argyritarsis)
The South Pacific coast is comprised of two areas: (1) the inland area along the Cali-Buenaventura road that includes the foothills of The Andes mountains and, (2) the Pacific plain area that involve the southern portion of Buenaventura and ten municipalities of the department of Nariño. In the first, P. vivax was more prevalent and in the second, P. falciparum was the more prevalent species. In the municipality of Buenaventura, located in the north of this region, an API of 30 cases/1000 inhabitants was registered in the years of mosquito collection. In this area, An. nuneztovari C was the Anopheles species most widely distributed in adult stage, and An. pseudopunctipennis s.l. (Pacific coast lineage) was found in the villages located at the foothills (Fig. 3). In the coastal study sites of Pacific plain area, the predominant species were An. albimanus B (Pacific coast lineage) and An. neivai (An. neivai lineage). In inland in the department of Nariño, An. calderoni was the most important species. A different incidence was observed in the area where An. albimanus B predominates, in which 3220 cases and an API of 27 cases/1000 inhabitants were reported, as compared with the area where An. calderoni predominates, in which 2932 cases and an API of 73 cases/1000 inhabitants were reported (Fig. 3).
Fig. 3.
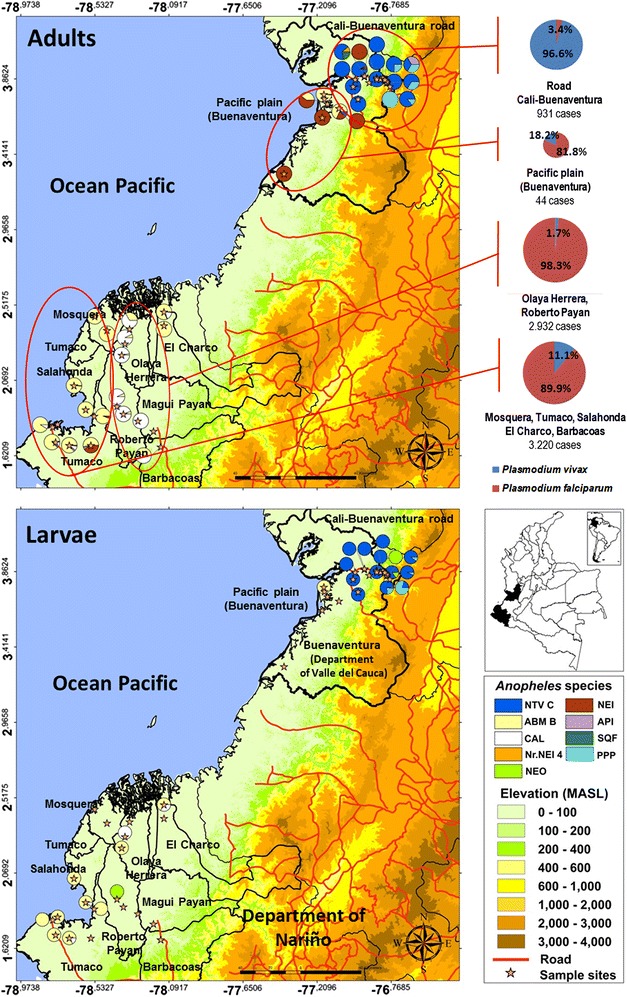
Distribution of Anopheles species found in the South Pacific coast region of Colombia and malaria cases reported (NTV An. nuneztovari C; NEI An. neivai s.l.; Nr. NEI 4: An. neivai nr. An. neivai 4; ABM B An. albimanus B; API An. apicimacula; NEO An. neomaculipalpus; PPP An. pseudopunctipennis s.l.; SQF An. squamifemur)
Discussion
During this study, using PCR–RFLP-ITS 2 and morphological characters, 13 formally named Anopheles species were confirmed in the endemic malaria region of Colombia, however, the NJ, ML, and BI analysis using COI barcode sequences genes revealed the presence of four new mitochondrial lineages. One new lineage of An. albimanus, called An. albimanus B in the Boldsystem databases, was found in the South Pacific coast. One new lineage of An. pseudopunctipennis s.l., was found in the Northwest and the South Pacific coast. One new lineage for An. neivai (An. neivainr. An. neivai 4) was found in South Pacific coast and one new lineage for An. apicimacula that include sequences from Northwest and South Pacific coast regions.
Regarding the association of Anopheles species with malaria distribution, the department of Cordoba, located in the Northwest region of Colombia, contributes with 18 % of malaria cases reported annually in the country [2]. This region was the study area with the highest number of Anopheles species, ten out of the 13 species identified. An important aspect of the current study is that in this area three primary malaria vectors were registered: An. nuneztovari s.l. (=An. nuneztovari C), An. darlingi and An. albimanus (northwest lineage). This may partly explain the local high malaria burden. Both An. nuneztovari s.l. and An. darlingi are anthropophilic species with an important biting activity in evening early hours [29–38]. During this period, people are still active and unprotected by LLINs, the main measure used for the control of malaria vectors in this region. Interestingly, the results show significant differences in relation to the predominant species in adult stage and immature stage. Predominant species in adults was An. nuneztovari C (85 % of collections), whereas An. nuneztovari C and An. triannulatus s.l. were collected in similar proportions in immature stages. Differences in Anopheles species composition in adult and immature stages may be explained by the presence of cows that provides blood sources for the zoophilic species like An. triannulatus s.l. [39–41].
With respect to another Anopheles species registered in the Northwest region, in this study, the presence of An. albitaris I (n = 5) was confirmed. This species had been reported in the same region by Gutierrez et al. [42] as An. albitarsis near janconae; however, afterwards it was confirmed by Ruiz et al. [6] as An. albitaris I. According to the results of this study, the distribution of An. albitaris I was extended to others localities in the Northwest region. The malaria vector status of An. albitaris I is unknown in South America [6]. Additionally, morphological characters and COI sequence analysis confirmed the presence of other Anopheles species in low densities: An. pseudopunctipennis s.l. (northwest lineage) (n = 19), An. punctimacula s.l. (n = 16), An. apicimacula (northwest lineage) (n = 1), An. argyritarsis (n = 1), and, An. neomaculipalpus (n = 1).
In relation to the role that Anopheles species play in malaria transmission in the Northwest region, three primary vectors were registered: An. nuneztovari C, An. darlingi and An. albimanus. In this study, two specimens of An. nuneztovari C were found infected with P. falciparum; however, previous studies conducted in the same region reported An. nuneztovari s.l. as infected with P. vivax VK247. The taxonomic determination of species within this species complex was not clarified in these studies [34, 43]. It is likely that the species corresponded to An. nuneztovari C from the results found in the present study. Other species reported as positive for Plasmodium infection were An.darlingi infected with P. vivax VK247 [34, 43, 44], and An. triannulatus s.l. infected with P. vivax VK247 [43]; however, the role of the latter species in the Northwest region is not yet clear. Other Anopheles species registered in Northwest region, An. pseudopunctipennis s.l., An. punctimacula s.l., and, An. neomaculipalpus are considered malaria vector in America [45–47], however, in this region their importance in the sustaining of malaria transmission is unknown.
During the past ten years, in Buenaventura (department of Valle del Cauca), API fluctuated between nine and 167 cases/1000 inhabitants and in ten municipalities of department of Nariño, with the highest malaria transmission, this index ranged between 22 and 100 cases/1000 inhabitants, with a prevalence of P. vivax inland, along the Cali-Buenaventura road, and P. falciparum in the Pacific plain in the South Pacific region. In this region, seven Anopheles species were identified. The most abundant Anopheles species in the area along the Cali-Buenaventura road were An. nuneztovari C and An. pseudopunctipennis s.l. (Pacific coast lineage). Anopheles neivai s.l. was identified with evidence of two lineages (An. neivai and An. neivainr. An. neivai 4). In the Pacific plain area, An. albimanus B was the most important species along the coast; while inland An. calderoni, An. neivai, and An. apicimacula (Northwest-Pacific coast and only Pacific coast lineages) were confirmed. Different composition in adults and larval stages of Anopheles species were observed in the South Pacific coast. As evidenced in the maps (Fig. 3), in coastal localities An. albimanus B and An. neivai were registered in adult state, whereas inland An. nuneztovari C, An. pseudopunctipennis and An. calderoni were registered. Anopheles neivai were collected in adult but not in immature states in the coastal towns. This can be explained by the difficulty of sampling in the water that is accumulated in the axils of bromeliads leaves, An. neivai s.l. larval habitat [48–51]. Sampling of bromeliads could only be carried out in three localities where the species was present in the adult stage. In general, these Colombian Pacific coastal localities are surrounded by mangroves, which host several species of bromeliads.
In the study sites of the South Pacific coast, two specimens were found infected with Plasmodium species: An. albimanus B from the Pacific plain region was infected with P. falciparum; and An. nuneztovari C from the Cali-Buenaventura road was infected with P. vivax VK247. In various studies carried out in recent years in the same region, An. albimanus was reported infected with P. falciparum [52] and with P. vivax VK210 [52, 53]. Additionally, in this region another two Anopheles species have been reported infected with Plasmodium:Anopheles neivai infected with P. vivax VK210, P. vivax VK 247 and P. falciparum [52, 53], and An. calderoni infected with P. vivax VK210 and P. falciparum [14, 44]. The previous results provides evidence to confirm the status as malaria vectors of: An. nuneztovari C, An. albimanus B, An. neivai, and An. calderoni in the Southwest of Colombia, due to their presence and wide distribution in a region with active malaria transmission, anthropophilic behaviour, and detection of natural infection with Plasmodium species [14, 44, 53–55].
Although this study included data about Anopheles species composition at locality level, the relationship between the presence of Anopheles species and the specific locality burden of malaria was difficult to determine, due mainly to difficulties in the reporting system. Although, in Colombia it is mandatory to report malaria cases by locality of origin, it is common to find the locality origin of the infection mixed up with the locality where the patient lives, or with the locality in which the diagnosis was made. Also, patients could refuse to give information about the locality in which they probably got the infection. These situations make the analysis by localities difficult. For this reason, the relation between Anopheles species composition and malaria prevalence is presented at municipality level.
Anopheles species with two lineages identified in this study were: Anopheles albimanus, An. pseudopunctipennis s.l., An. neivai s.l., and An. apicimacula. Some considerations about taxonomic status are put forward here: Anopheles albimanus shows two lineages: Northwest, An. albimanus and Pacific coast, An. albimanus B, each lineage with different geographical distribution. The differences between An. albimanus populations of the Caribbean regions (North of Colombia) and the Pacific coast had previously been reported by Narang et al. [56] using allozyme variability and chromosomal analysis to characterize An. albimanus populations collected in 11 localities from Colombia (north and Pacific coast), and were confirmed by Gutierrez et al. [57], who defined two distinctive groups corresponding to haplotypes from the Caribbean and Pacific coast regions by analysis of COI and microsatellite. However, the reported data of hybridization and backcrosses that included North and South Pacific coast populations of An. albimanus, showed that hybrid females and males were fertile and had normal ovaries and testes, indicating the absence of cryptic speciation for this species in Colombia [56]. It is necessary to carry out studies in vector competence to identify differences in infection susceptibility by strains of Plasmodium, because both lineages are present in regions with different malaria prevalence.
Similarly, evidence to support two lineages of An. pseudopunctipennis s.l. with different geographical distribution was found: one linage conformed by specimens collected in the Northwest region and the other by specimens collected from the Southwest in the Pacific region. Several studies have attempted to evaluate the taxonomic status of An. pseudopunctipennis s.l. Estrada-Franco et al. [58] used isoenzyme electrophoresis and rDNA restriction fragment length polymorphisms (RFLPs) and Coetzee et al. [59] used cross-mating experiments, suggested that this species is actually a complex of three species: An. pseudopunctipennis A, represented by specimens from central Mexico; An. pseudopunctipennis B, represented by specimens from the Andes of Peru and Bolivia [58]; and An. pseudopunctipennis C, represented by specimens collected on the island of Grenada in the Lesser Antilles [59]. However, based on the evidence of isozyme analyses of 42 populations of this species collected in ten countries in North, Central, and South America, Manguin et al. [60] suggested that An. pseudopunctipennis s.l. is a single species with three geographical populations represented in: (1). North America (United States and Mexico) and Guatemala; (2). Belize and South America (Colombia, Ecuador, Peru, Chile, and Argentina); and (3). Grenada Island. Additionally, Manguin et al. [60] suggested that the first and second geographic population converge in Southern Mexico and Northern Central America on the border between Belize and Guatemala. However, it should be noted that in the study by Manguin et al. [60], the samples included for analysis from Colombia came from the South of the country with no representation of specimens of this species from the North, which would probably be more like those found in Central America: a hypothesis that should be tested.
Anopheles neivai s.l. was another species that showed two lineages; however, in contrast to An. albimanus and An. pseudopunctipennis s.l., these lineages were found in the same region. The limitation of this finding is that all specimens identified by morphological characters as An. neivai s.l., in localities where the second lineage (An. neivai nr. An. neivai 4) is present, were not sequenced and the lineage An. neivai were confirmed in the same area, leaving untested the sympatry of the two lineages in the South Pacific coast region.
Regarding An. apicimacula, the findings partially support the results reported by Gomez et al. [61] who inferred the existence of two lineages, Atlantic coast and Pacific coast. The results of the present study show two lineages with a BVS >95, the first included the COI sequence of specimens collected exclusively in Pacific coast, and the second included the sequence generated for specimens collected in the Northwest and the South Pacific coast. In the same sequence analysis, a third lineage was observed with a BVS = 100, which only included sequences generated by Gomez et al. [61] using specimens collected in the Northwest region, which were grouped in the original study as An. apicimacula Caribbean lineage (Fig. 1). These results suggest that is necessary perform an analysis that includes more COI sequences of Northwest region to clarify because the second lineage include the Northwest and South Pacific coast sequences and confirm the different populations of An. apicimacula present in Colombia.
This study provides evidence about the richness of Anopheles species found in malaria transmission regions in Colombia and establishes that several species incriminated as malaria vectors are sympatric. This condition is an important aspect to consider designing control strategies, given the fact that species can exhibit different biting behaviours that could diminish the effectiveness of traditional control measures when applied to large scale.
Conclusions
In the malaria endemic areas of the Northwest and South Pacific coast regions of Colombia, 13 Anopheles species and four new lineages were confirmed using COI sequences analysis. The DNA barcode analyses refine the taxonomic identification process, particularly for species complexes, which can lead to advancements in the understanding of the relationship between species complexes and malaria transmission. The result provides evidence about Anopheles species richness, and the different composition and relative abundance presented in each study regions. In the Northwest and South Pacific regions two dominant vector species were identified, An. nuneztovari C and An. albimanus B. Both were naturally infected with Plasmodium species. However, other species present in low abundance have also been incriminated as malaria vectors in Colombia (9, 14), such as An. darlingi, An. calderoni and An. neivai s.l., and they might also sustain malaria transmission in these regions. The knowledge of local vector distribution will be useful for planning targeted intervention strategies for malaria control and elimination.
Authors’ contributions
MLQ and MLA participated in study coordination, data analysis and manuscript draft. LO participated in the determination of specimens, data and sequence analysis and drafted the manuscript. PXP participated in the determination of specimens, carried out the ELISAs and data management. MC participated in the determination of specimens and data management. DMC, FGC and JAL contributed to the coordination of the fieldwork and collection of data. SH was involved during the project conception and reviewed the manuscript. MLQ and JCB contributed to the project concept, design of the study and participated in writing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Special thanks are given to Marco F Suarez for facilitating fieldwork, Margarita Peñaloza, Carlos Murcia, Laura Fonseca and Elizabeth Ruiz for facilitating laboratory work and Pilar Perez (Instituto Departamental de Salud de Nariño), and Ruby Hernandez (Secretaria de Desarrollo de la Salud de Cordoba, Cordoba, Colombia), for logistics support to fieldwork. We gratefully acknowledge Dr. Jose Usme-Ciro (Universidad de la Salle, Bogota, Colombia) for his guidance to carry out the sequence analyses included in this study and Amy R Krystosik and Edison Soto for a critical review of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
The datasets supporting the conclusions of this article are included within the article and its additional files.
Ethical approval
This study was approved by the National Institutes of Health (NIH), September 15, 2011, Protocol DMID 11-0046, entomological cross sectional study of malaria transmission dynamics in non-Amazonian regions of Latin America, and by the Ethical Committee of the Facultad de Medicina, Universidad Nacional de Colombia, CE-079, Act No. 1 of June 20, 2011.
Funding
This project was funded by the NIAID ICEMR (research Grant U19AI089802), COLCIENCIAS (research Grant 360-2011) and Instituto Nacional de Salud of Colombia (CTIN28-2010). The funders had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- API
annual parasitic index
- BI
bayesian inference
- BLAST
basic local alignment search tool
- BOLD
barcode of life data system
- bp
base pair
- BVS
bootstrap support value
- CDC
Centers for Disease Control and Prevention
- CLAIM
Latin American Research Center in Malaria
- COI
cytochrome c oxidase subunit I gene
- CS
circumsporozoite protein
- DNA
deoxyribonucleic acid
- ELISA
enzyme-linked immunosorbent assay
- IRS
indoor residual spraying of insecticides
- ITS2
internal transcribed spacer 2
- LLIN
long-lasting insecticidal nest
- MEGA
molecular evolutionary genetics analysis
- ML
maximum likelihood
- NIH
National Institutes of Health
- NJ
neighbour joining
- nr
near
- PCR
polymerase chain reaction
- RFLP
restriction fragment length polymorphisms
- RFLP-ITS2
restriction profiles of the internal transcribed spacer 2
- SEM
Servicio de Erradicación de la Malaria
- SIVIGILA
Sistema Nacional de Vigilancia en salud pública de Colombia
- TAE
tris-acetate-EDTA buffer
- WHO
World Health Organization
Additional files
10.1186/s12936-016-1421-4 a: Localities included in a cross sectional study in Northwest and South Pacific coast regions. b: List of Anopheles specimens using to generate COI sequence and identity percent with COI sequences deposited in GenBank and Boldsystems databases.
10.1186/s12936-016-1421-4 Anopheles species and GenBank accession numbers of COI sequences used to sequence analysis. In bold accession numbers assigned to COI sequences generated in this study by GenBank database.
Contributor Information
Martha L. Ahumada, Email: mahumada@ins.gov.co
Lorena I. Orjuela, Email: loriza1983@gmail.com
Paula X. Pareja, Email: paxipa82@gmail.com
Marcela Conde, Email: marcelaconde79@gmail.com.
Diana M. Cabarcas, Email: marcelita1102@gmail.com
Eliana F. G. Cubillos, Email: elgalindocu@gmail.com
Jorge A. Lopez, Email: titoandres78@hotmail.com
John C. Beier, Email: jbeier@med.miami.edu
Sócrates Herrera, Email: sherrera@inmuno.org.
Martha L. Quiñones, Email: mlquinonesp@unal.edu.co
References
- 1.WHO. World malaria report. Geneva: World Health Organization; 2015. http://www.who.int/malaria/publications. Accessed 2 Jan 2016.
- 2.Padilla JC, Álvarez G, Montoya R, Chaparro P, Herrera S. Epidemiology and control of malaria in Colombia. Mem Inst Oswaldo Cruz. 2011;106(Suppl 1):114–122. doi: 10.1590/s0074-02762011000900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sistema Nacional de Vigilancia en Salud Pública—SIVIGILA Vigilancia Rutinaria. http://www.ins.gov.co/lineas-de-accion/Subdireccion-Vigilancia/sivigila/Paginas/vigilancia-rutinaria.aspx. Accessed 25 Jan 2016.
- 4.Montoya-Lerma J, Solarte YA, Giraldo-Calderón GI, Quiñones ML, Ruiz-López F, González R. Malaria vector species in Colombia-a review. Mem Inst Oswaldo Cruz. 2011;106(Suppl 1):223–238. doi: 10.1590/S0074-02762011000900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris DE. Genetic markers for study of the anopheline vectors of human malaria. Int J Parasitol. 2002;32:1607–1615. doi: 10.1016/S0020-7519(02)00189-3. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Lopez F, Wilkerson RC, Conn JE, McKeon SN, Levin DM, Quiñones ML, et al. DNA barcoding reveals both known and novel taxa in the Albitarsis Group (Anopheles: Nyssorhynchus) of Neotropical malaria vectors. Parasit Vectors. 2012;5:44. doi: 10.1186/1756-3305-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz-Lopez F, Wilkerson RC, Ponsonby DJ, Herrera M, Sallum MAM, Velez ID, et al. Systematics of the oswaldoi complex (Anopheles, Nyssorhynchus) in South America. Parasit Vectors. 2013;6:324. doi: 10.1186/1756-3305-6-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brochero H, Quiñones ML. Retos de la entomología médica para la vigilancia en salud pública en Colombia: reflexión para el caso de malaria. Biomédica. 2008;28:18–24. doi: 10.7705/biomedica.v28i1.105. [DOI] [PubMed] [Google Scholar]
- 9.Olano VA, Brochero HL, Sáenz R, Quiñones ML, Molina JA. Mapas preliminares de la distribución de especies de Anopheles vectores de malaria en Colombia. Biomédica. 2001;21:402–408. [Google Scholar]
- 10.Quiñones ML, Suarez MF, Rodríguez A, Fleming GA, Galvis LE. Comportamiento de Anopheles (Kerteszia) lepidotus Zavortink, 1973, y su incriminación como posible vector de malaria en el departamento del Tolima, Colombia. Biomédica. 1984;4:5–13. doi: 10.7705/biomedica.v4i1.1876. [DOI] [Google Scholar]
- 11.Quiñones ML, Ruiz F, Calle DA, Harbach RE, Linton YM. Incrimination of Anopheles (Nyssorhynchus) rangeli and An. (Nys.) oswaldoi as natural vectors of Plasmodium vivax in Southern Colombia. Mem Inst Oswaldo Cruz. 2006;101:617–623. doi: 10.1590/S0074-02762006000600007. [DOI] [PubMed] [Google Scholar]
- 12.Orjuela LI, Herrera M, Erazo H, Quiñones ML. Especies de Anopheles presentes en el departamento del Putumayo y su infección natural con Plasmodium. Biomédica. 2013;33:42–52. doi: 10.1590/S0120-41572013000100006. [DOI] [PubMed] [Google Scholar]
- 13.Escovar JE, González R, Quiñones ML, Wilkerson RC, Ruiz F, Harrison BA. Morphology of the larvae, male genitalia and DNA sequences of Anopheles (Kerteszia) pholidotus (Diptera: Culicidae) from Colombia. Mem Inst Oswaldo Cruz. 2014;109:473–479. doi: 10.1590/0074-0276130596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orjuela LI, Ahumada ML, Avila I, Herrera S, Beier JC, Quiñones ML. Human biting activity, spatial-temporal distribution and malaria vector role of Anopheles calderoni in the southwest of Colombia. Malar J. 2015;14:256. doi: 10.1186/s12936-015-0764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conde M, Pareja PX, Orjuela LI, Ahumada ML, Durán S, Jara JA, et al. Larval habitat characteristics of the main malaria vectors in the most endemic regions of Colombia: potential implications for larval control. Malar J. 2015;14:476. doi: 10.1186/s12936-015-1002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González R, Carrejo N. Introducción al estudio taxonómico de Anopheles de Colombia claves y notas de distribución. 2. Cali: Programa Editorial de la Universidad del Valle; 2009. [Google Scholar]
- 17.Service MW. Mosquito ecology: field sampling methods. Chapman and Hall. 2nd ed. Essex: Elsevier Science Publishers; 1993.
- 18.Collins FH, Paskewitz SM. A review of the use of ribosomal DNA (rDNA) to differentiate among cryptic Anopheles species. Insect Mol Biol. 1996;5:1–9. doi: 10.1111/j.1365-2583.1996.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 19.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 20.Ruiz F, Linton YM, Ponsonby DJ, Conn JE, Herrera M, Quiñones ML, et al. Molecular comparison of topotypic specimens confirms Anopheles (Nyssorhynchus) dunhami Causey (Diptera: Culicidae) in the Colombian Amazon. Mem Inst Oswaldo Cruz. 2010;105:899–903. doi: 10.1590/s0074-02762010000700010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foley DH, Harrison G, Murphy JR, Dowler M, Rueda LM, Wilkerson RC. Mosquito bisection as a variable in estimates of PCR-derived malaria sporozoite rates. Malar J. 2012;11:145. doi: 10.1186/1475-2875-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beier JC, Asiago CM, Onyango FK, Koros JK. ELISA absorbance cut-off method affects malaria sporozoite rate determination in wild Afrotropical Anopheles. Med Vet Entomol. 1988;2:259–264. doi: 10.1111/j.1365-2915.1988.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 23.Snounou GS, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-B. [DOI] [PubMed] [Google Scholar]
- 24.GenBank National Center for Biotechnology Information. http://www.blast.ncbi.nlm.nih.gov/Blast.cgi. Accessed Oct 2015.
- 25.Barcode of Life Data System-BOLD. http://www.boldsystems.org/index.php/IDS_OpenldEngine. Accessed Oct 2015.
- 26.Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol Biol Evol. 1992;9:678–687. doi: 10.1093/oxfordjournals.molbev.a040752. [DOI] [PubMed] [Google Scholar]
- 27.Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 28.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 29.Panday RS. Anopheles nuneztovari and malaria transmission in Surinam. Mosq News. 1977;37:728–737. [Google Scholar]
- 30.Brochero H, Rey G, Buitrago LS, Olano VA. Biting activity and breeding sites of Anopheles species in the municipality Villavicencio, Meta, Colombia. J Am Mosq Control. 2005;21:182–186. doi: 10.2987/8756-971X(2005)21[182:BAABSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Brochero H, Pareja PX, Ortiz G, Olano VA. Sitios de cría y actividad de picadura de especies de Anopheles en el municipio de Cimitarra, Santander, Colombia. Biomédica. 2006;26:269–277. doi: 10.7705/biomedica.v26i2.1416. [DOI] [PubMed] [Google Scholar]
- 32.Harris AF, Matias-Arnez A, Hill N. Biting time of Anopheles darlingi in the Bolivian Amazon and implications for control of malaria. Trans R Soc Trop Med Hyg. 2006;100:45–47. doi: 10.1016/j.trstmh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Parra-Henao G, Alarcón EP. Observaciones sobre la bionomía de Anopheles spp. (Diptera: Culicidae) en el municipio Valencia, departamento Córdoba, Colombia. Bol Mal Salud Amb. 2008;48:95–98. [Google Scholar]
- 34.Gutiérrez LA, González JJ, Gómez GF, Castro MI, Rosero DA, Luckhart S, et al. Species composition and natural infectivity of anthropophilic Anopheles (Diptera: Culicidae) in the states of Cordoba and Antioquia, Northwestern Colombia. Mem Inst Oswaldo Cruz. 2009;104:1117–1124. doi: 10.1590/s0074-02762009000800008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmerman RH, Lounibos LP, Nishimura N, Galardo AKR, Galardo CD, Arruda ME. Nightly biting cycles of malaria vectors in a heterogeneous transmission area of eastern Amazonian Brazil. Malar J. 2013;12:262. doi: 10.1186/1475-2875-12-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiemann DJ, Quiñones ML, Hankeln T. Anthropophilic Anopheles species composition and malaria in Tierradentro, Cordoba, Colombia. Mem Inst Oswaldo Cruz. 2014;109:384–387. doi: 10.1590/0074-0276130483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno M, Saavedra MP, Bickersmith SA, Lainhart W, Tong C, Alava F, et al. Implications for changes in Anopheles darlingi biting behaviour in three communities in the peri-Iquitos region of Amazonian Peru. Malar J. 2015;14:290. doi: 10.1186/s12936-015-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahumada ML, Pareja PX, Buitrago LS, Quiñones ML. Comportamiento de picadura de Anopheles darlingi Root, 1926 (Diptera: Culicidae) y su asociación con la transmisión de malaria en Villavicencio (Colombia) Biomédica. 2013;33:241–250. [PubMed] [Google Scholar]
- 39.Rubio-Palis. Anopheles (Nyssorhynchus) de Venezuela. Taxonomía, bionomía, ecología e importancia médica. Esc. Mal. San. Amb.“Dr. Arnoldo Gabaldon”, Maracay; 2000.
- 40.Silva-do-Nascimento TF, Rona LD, Peixoto AA, Lourenço-de-Oliveira R. Molecular divergence in the timeless and cpr genes among three sympatric cryptic species of the Anopheles triannulatus complex. Mem Inst Oswaldo Cruz. 2011;106(Suppl 1):218–222. doi: 10.1590/S0074-02762011000900027. [DOI] [PubMed] [Google Scholar]
- 41.Herrera-Varela M, Orjuela LI, Peñalver C, Conn JE, Quiñones ML. Anopheles species composition explains differences in Plasmodium transmission in La Guajira, northern Colombia. Mem Inst Oswaldo Cruz. 2014;109:955–959. doi: 10.1590/0074-0276140126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutiérrez LA, Orrego LM, Gómez GF, López A, Luckhart S, Conn JE, et al. A new mtDNA COI gene lineage near An. janconnae of the Albitarsis complex from Caribbean Colombia. Mem Inst Oswaldo Cruz. 2010;105:1019–1025. doi: 10.1590/S0074-02762010000800011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naranjo-Díaz N, Rosero DA, Rúa-Uribe G, Luckhart S, Correa MM. Abundance, behavior and entomological inoculation rates of anthropophilic anophelines from a primary Colombian malaria endemic area. Parasit Vectors. 2013;6:61. doi: 10.1186/1756-3305-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naranjo-Díaz N, Altamiranda M, Luckhart S, Conn JE, Correa MM. Malaria vectors in ecologically heterogeneous localities of the Colombian Pacific region. PLoS One. 2014;9:e103769. doi: 10.1371/journal.pone.0103769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.González-Cerón M, Rodríguez MH, Nettel JC, Villarreal C, Kain KC, Hernández JE. Differential susceptibilities of Anopheles albimanus and Anopheles pseudopunctipennis to infections with coindigenous Plasmodium vivax variants VK210 and VK247 in Southern Mexico. Infect Immun. 1999;67:410–412. doi: 10.1128/iai.67.1.410-412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleming G. Biology and ecology of malaria vectors in the Americas. Washington: Pan American Health Organization; 1986. PNSP/86–72.
- 47.Moreno JE, Rubio-Palis Y, Páez E, Pérez A, Sánchez V, Vaccari E. Anopheles (Anopheles) neomaculipalpus: a new malaria vector in the Amazon basin? Med Vet Entomol. 2005;19:329–332. doi: 10.1111/j.1365-2915.2005.00572.x. [DOI] [PubMed] [Google Scholar]
- 48.Astaiza R, Murillo C, Fajardo P. Biología de Anopheles (Kerteszia) neivai H., D. & K., 1913 (Diptera: Culicidae) en la costa Pacífica de Colombia II. Fluctuación de la población adulta. Rev Saúde Pública. 1988;22:101–108. doi: 10.1590/S0034-89101988000200005. [DOI] [PubMed] [Google Scholar]
- 49.Solarte Y, Hurtado C, Gonzalez R, Alexander B. Man-biting activity of Anopheles (Nyssorhynchus) albimanus and An. (Kerteszia) neivai (Diptera: Culicidae) in the Pacific lowlands of Colombia. Mem Inst Oswaldo Cruz. 1996;91:141–146. doi: 10.1590/S0074-02761996000200002. [DOI] [PubMed] [Google Scholar]
- 50.Murillo C, Astaiza R, Fajardo P. Biología de Anopheles (Kerteszia) neivai H., D. & K., 1913 (Diptera: Culicidae) en la Costa Pacífica de Colombia. I. Fluctuación de la población larval y características de sus criaderos. Rev Saúde Pública. 1988;22:94–100. doi: 10.1590/S0034-89101988000200004. [DOI] [PubMed] [Google Scholar]
- 51.Marrelli MT, Malafronte RS, Sallum MAM, Natal D. Kerteszia subgenus of Anopheles associated with the Brazilian Atlantic rainforest: current knowledge and future challenges. Malar J. 2007;6:127. doi: 10.1186/1475-2875-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gutiérrez LA, Naranjo N, Jaramillo LM, Muskus C, Luckhart S, Conn JE, et al. Natural infectivity of Anopheles species from the Pacific and Atlantic regions of Colombia. Acta Trop. 2008;107:99–105. doi: 10.1016/j.actatropica.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 53.Escovar JE, González R, Quiñones ML. Anthropophilic biting behaviour of Anopheles (Kerteszia) neivai Howard, Dyar & Knab associated with Fishermen’s activities in a malaria-endemic area in the Colombian Pacific. Mem Inst Oswaldo Cruz. 2013;108:1057–1064. doi: 10.1590/0074-0276130256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fajardo P, Alzate A. Anopheles nuneztovari como vector de malaria en el Bajo Calima, Buenaventura. Colombia Médica. 1987;18:14–18. [Google Scholar]
- 55.Quiñones ML, Suarez MF, Fleming G. Distribución y bionomía de los anofelinos de la costa pacífica de Colombia. Colombia Médica. 1987;18:19–24. [Google Scholar]
- 56.Narang SK, Seawright JA, Suarez MF. Genetic structure of natural populations of Anopheles albimanus in Colombia. J Am Mosquito Control Assoc. 1991;7:437–445. [PubMed] [Google Scholar]
- 57.Gutiérrez LA, Naranjo NJ, Cienfuegos AV, Muskus CE, Luckhart S, Conn JE, et al. Population structure analyses and demographic history of the malaria vector Anopheles albimanus from the Caribbean and the Pacific regions of Colombia. Malar J. 2009;8:259. doi: 10.1186/1475-2875-8-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Estrada-Franco JG, Lanzaro GC, Ma MC, Walker-Abbey A, Romans P, Galvan-Sanchez C, et al. Characterization of Anopheles pseudopunctipennis sensu lato from three countries of neotropical America from variation in allozymes and ribosomal DNA. Am J Trop Med Hyg. 1993;49:735–745. doi: 10.4269/ajtmh.1993.49.735. [DOI] [PubMed] [Google Scholar]
- 59.Coetzee M, Estrada-Franco JG, Wunderlich CA, Hunt RH. Cytogenetic evidence for a species complex within Anopheles pseudopunctipennis theobald (Diptera: Culicidae) Am J Trop Med Hyg. 1999;60:649–653. doi: 10.4269/ajtmh.1999.60.649. [DOI] [PubMed] [Google Scholar]
- 60.Manguin S, Robert DR, Peyton EL, Fernandez-Salas I, Barreto M, Fernandez R, et al. Biochemical systematics and population genetic structure of Anopheles pseudopunctipennis, vector of malaria in Central and South America. Am J Trop Med Hyg. 1995;53:362–377. doi: 10.4269/ajtmh.1995.53.362. [DOI] [PubMed] [Google Scholar]
- 61.Gomez GF, Bickersmith SA, Gonzalez R, Conn JE, Correa MM. Molecular taxonomy provides new insights into Anopheles species of the neotropical Arribalzagia series. PLoS One. 2015;10:e0119488. doi: 10.1371/journal.pone.0119488. [DOI] [PMC free article] [PubMed] [Google Scholar]


