Abstract
Purpose
The objective of this study was to determine whether the 21-gene Recurrence Score (RS) provides clinically meaningful information in patients with de novo stage IV breast cancer enrolled in the Translational Breast Cancer Research Consortium (TBCRC) 013.
Patients and Methods
TBCRC 013 was a multicenter prospective registry that evaluated the role of surgery of the primary tumor in patients with de novo stage IV breast cancer. From July 2009 to April 2012, 127 patients from 14 sites were enrolled; 109 (86%) patients had pretreatment primary tumor samples suitable for 21-gene RS analysis. Clinical variables, time to first progression (TTP), and 2-year overall survival (OS) were correlated with the 21-gene RS by using log-rank, Kaplan-Meier, and Cox regression.
Results
Median patient age was 52 years (21 to 79 years); the majority had hormone receptor–positive/human epidermal growth factor receptor 2 (HER2)–negative (72 [66%]) or hormone receptor–positive/HER2-positive (20 [18%]) breast cancer. At a median follow-up of 29 months, median TTP was 20 months (95% CI, 16 to 26 months), and median survival was 49 months (95% CI, 40 months to not reached). An RS was generated for 101 (93%) primary tumor samples: 22 (23%) low risk (< 18), 29 (28%) intermediate risk (18 to 30); and 50 (49%) high risk (≥ 31). For all patients, RS was associated with TTP (P = .01) and 2-year OS (P = .04). In multivariable Cox regression models among 69 patients with estrogen receptor (ER)–positive/HER2-negative cancer, RS was independently prognostic for TTP (hazard ratio, 1.40; 95% CI, 1.05 to 1.86; P = .02) and 2-year OS (hazard ratio, 1.83; 95% CI, 1.14 to 2.95; P = .013).
Conclusion
The 21-gene RS is independently prognostic for both TTP and 2-year OS in ER–positive/HER2-negative de novo stage IV breast cancer. Prospective validation is needed to determine the potential role for this assay in the clinical management of this patient subset.
INTRODUCTION
The 21-gene Recurrence Score (RS) is a useful clinical tool for assessing risk of distant recurrence and magnitude of chemotherapy benefit in patients with early-stage estrogen receptor (ER)–positive breast cancer treated with tamoxifen.1-3 The application of the 21-gene RS to clinical practice in patients with ER-positive/node-negative disease has been demonstrated to change treatment recommendations, and the RS has been incorporated into both ASCO and National Comprehensive Cancer Network treatment guidelines for early-stage ER-positive breast cancer.4,5
In metastatic breast cancer, limited level 1 evidence guides clinical decision making; as such, treatment recommendations are largely based on traditional factors, such as ER, progesterone receptor, human epidermal growth factor receptor 2 (HER2), and number and sites of metastases. International consensus guidelines for the treatment of advanced breast cancer have been developed,6,7 yet durability of response to first-line therapy varies, and there are no validated clinical tools for assessing risk of progression of disease or likelihood of achieving a durable response once therapy is initiated. In addition, although survival among patients with metastatic breast cancer has improved, largely due to advances in targeted therapy, there continues to be a wide range in reported outcomes8-12 and there are many unanswered questions related to management strategies, optimal drug sequencing, and the potential for individualized treatment on the basis of predictive markers.
Translational Breast Cancer Research Consortium (TBCRC) 013 was a multicenter prospective registry study with the primary goal of evaluating the role of surgery of the primary tumor in patients with stage IV breast cancer. Patients also provided primary tumor tissue for embedded correlative science aims. The objective of the current analysis was to determine whether the 21-gene RS performed on the primary tumor provides clinically meaningful information in patients with de novo stage IV breast cancer enrolled in TBCRC 013. Further analysis of the role of surgery in this trial is ongoing.
PATIENTS AND METHODS
TBCRC 013 was a multicenter prospective registry study that evaluated the role of surgery of the primary tumor in patients with de novo stage IV breast cancer. Eligibility criteria included de novo stage IV breast cancer with an intact primary tumor (cohort A) or metastatic disease within 3 months of primary breast surgery (cohort B). All patients provided consent for access to formalin-fixed paraffin-embedded tissue from the primary tumor and a metastatic lesion for correlative studies. We aimed to enroll 100 patients with intact tumors and adequate primary tumor tissue for the RS analysis.
From July 2009 to April 2012, 127 eligible patients from 14 institutions were enrolled in the two cohorts (cohort A, n = 112; cohort B, n = 15). Of these, 109 (86%) patients had pretreatment primary tumor diagnostic biopsy samples suitable for 21-gene RS analysis and comprised the RS analysis cohort reported here.
Because this was a registry study, patients were treated according to institutional practice patterns without study-specific intervention. Presenting clinical and pathologic features were determined at the institutional level, which included tumor grade and ER, progesterone receptor, and HER2 status. Treatment regimens and outcomes were reported.
Baseline characteristics were compared by using Fisher's exact test for categorical factors and the Wilcoxon test for continuous factors. Clinical variables, time to first progression (TTP), and 2-year overall survival (OS) were correlated with the 21-gene RS by using log-rank tests, Kaplan-Meier estimates, and Cox regression with medians and 95% CIs. Analyses included all patients (any ER or HER2 status) as well as ER-positive (immunohistochemistry [IHC]) and ER-positive and HER2-negative subsets (IHC, fluorescence in situ hybridization). Exploratory analyses were performed among patients with ER-positive/HER2-negative breast cancer stratified by choice of first-line treatment (endocrine therapy v chemotherapy).
RESULTS
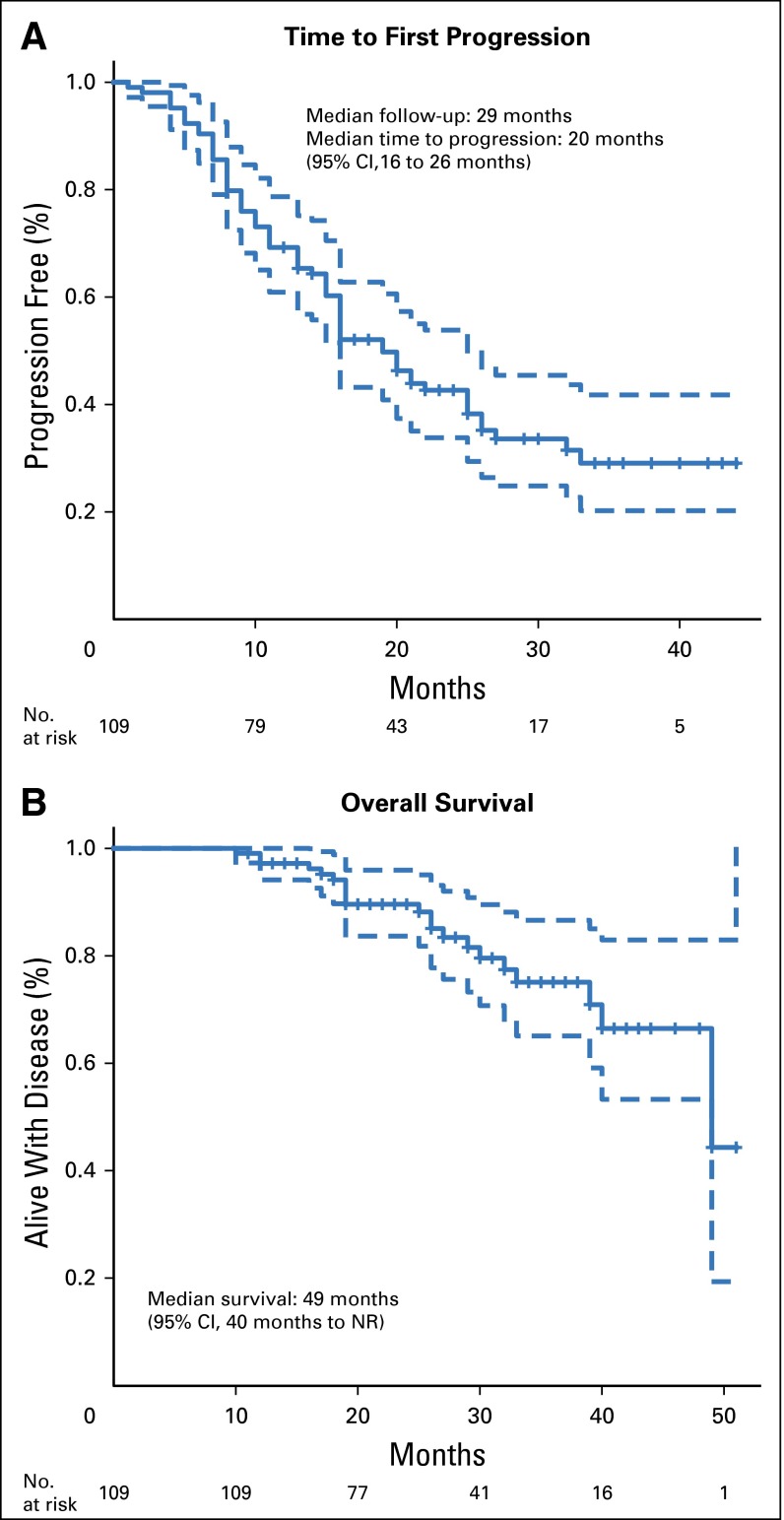
Among the 109 patients in the 21-gene RS analysis cohort, the median patient age was 52 years (range, 21 to 79 years), and the median primary tumor size was 3.1 cm (range, 0.7 to 15.0 cm). The study cohort comprised patients with predominantly ER-positive (84%), HER2-negative (72%), and invasive ductal (86%) cancer, and 50 (46%) patients presented with bone-only metastases (Table 1). The only significant difference between patients enrolled in cohort A (n = 94) and cohort B (n = 15) was the higher frequency of clinical N1 disease in patients in cohort A (85% v 26%; P = .001). There were no significant differences between the 21-gene RS population (n = 109) and the overall TBCRC 013 registry population (n = 127), and no differences in outcome associated with elective surgery at the time of this analysis (data not shown). At a median follow-up of 29 months, median TTP was 20 months (95% CI, 16 to 26 months), and median survival was 49 months (95% CI, 40 months to not reached; Fig 1).
Table 1.
Clinical Characteristics of the 21-Gene RS Population (n = 109)
| No. (%) | |
|---|---|
| Median age at diagnosis, years (range) | 52 (21-79) |
| Median primary tumor size, cm (range) | 3.1 (0.7-15.0) |
| Clinical node status | |
| N1/2 | 77 (71) |
| N0 | 18 (16) |
| Unknown | 14 (13) |
| ECOG status | |
| 0 | 58 (53) |
| 1 | 46 (42) |
| > 1 | 5 (5) |
| Tumor subtype | |
| HR positive/HER2 negative | 72 (66) |
| HR positive/HER2 positive | 20 (18) |
| HR negative/HER2 positive | 10 (9) |
| Triple negative | 7 (6) |
| Site of metastasis at first diagnosis | |
| Bone only | 50 (46) |
| Visceral only | 26 (24) |
| Both (bone and visceral) | 25 (23) |
| Other* | 8 (7) |
| No. of metastatic sites at first diagnosis | |
| Single organ | 65 (60) |
| > 1 organ | 44 (40) |
| First systemic treatment | |
| Chemotherapy | 26 (24) |
| Endocrine therapy | 52 (48) |
| Chemotherapy and endocrine therapy | 3 (3) |
| Chemotherapy plus trastuzumab | 20 (18) |
| Endocrine therapy plus trastuzumab | 6 (6) |
| RS distribution | |
| Low (< 18) | 22 (20) |
| Intermediate (18-30) | 29 (27) |
| High (≥ 31) | 50 (46) |
| Not available | 8 (7) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; RS, Recurrence Score.
Includes skin, pleura, contralateral axillary lymph nodes, mediastinal lymph nodes, paratracheal lymph nodes, endobronchial lymph nodes, hilar lymph nodes, and prepectoral lymph nodes.
Fig 1.
Time to (A) first progression and (B) overall survival for entire 21-gene Recurrence Score cohort (n = 109). NR, not reached.
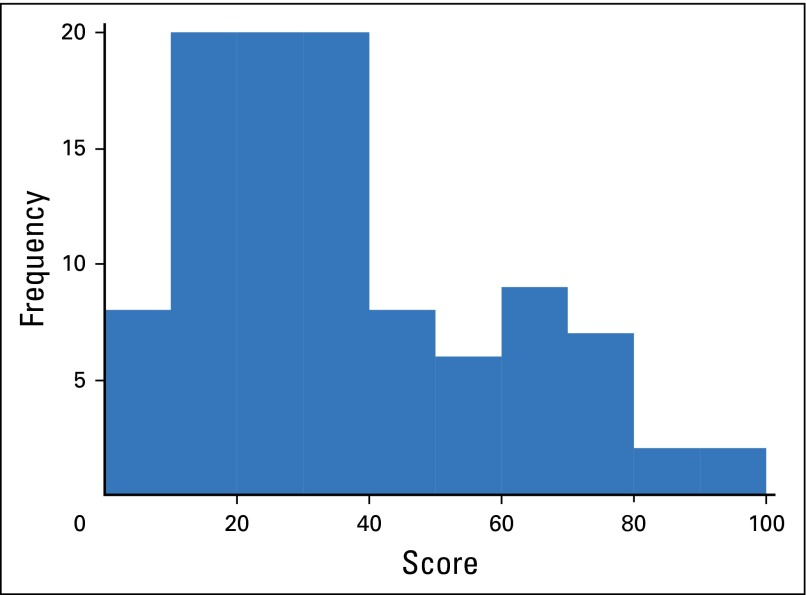
RS results were successfully generated from pretreatment diagnostic biopsy samples of the primary tumor for 101 (93%) patients. The median and mean RS for the population were 30.7 (range, 0 to 100) and 36, respectively; the interquartile range was 19.5 to 49.5. The histogram of RS values is depicted in Appendix Figure A1 (online only), and characteristics of the patients whose samples failed to generate an RS are presented in Appendix Table A1. Risk-group distribution was defined as low (RS < 18), intermediate (RS 18 to 30), and high (RS ≥ 31). Twenty-two (20%) patients had a low-risk RS, all of whom had ER-positive/HER2-negative disease by IHC (Table 1). Among the 29 patients with intermediate-risk RS, 26 had ER-positive/HER2-negative disease and three had ER-positive/HER2-positive disease (IHC/fluorescence in situ hybridization). The remaining 50 (46%) patients had high-risk RS. The high-risk group included 21 patients with ER-positive/HER2-negative tumors, 13 with ER-positive/HER2-positive tumors, 10 with ER-negative/HER2-positive tumors, and six with triple-negative disease (Appendix Fig A2). The only clinical variable found to be correlated with risk group was locally reported tumor grade (Table 2).
Table 2.
Clinical Characteristics by RS
| RS Risk Group, No. (%) | ||||
|---|---|---|---|---|
| Low Risk (RS < 18) | Intermediate Risk (RS 18-30) | High Risk (RS ≥ 31) | P | |
| No. of patients | 22 | 29 | 50 | |
| Median age, years (range) | 58 (38-73) | 52 (29-79) | 50 (21-77) | .16 |
| Median tumor size, cm (range) | 2.6 (0.8-9.0) | 3.0 (0.7-15.0) | 3.5 (1.0-15.0) | .17 |
| Tumor grade* | ||||
| I | 5 (23) | 1 (4) | 1 (2) | < .001 |
| II | 15 (68) | 12 (52) | 8 (18) | |
| III | 2 (9) | 10 (44) | 37 (80) | |
| ECOG status | .33 | |||
| 0 | 21 (96) | 28 (97) | 47 (94) | |
| > 0 | 1 (4) | 1 (3) | 3 (6) | |
| Cohort | .27 | |||
| A | 20 (91) | 22 (76) | 44 (88) | |
| B | 2 (9) | 7 (24) | 6 (12) | |
| Site of first metastasis | .15 | |||
| Bone | 14 (64) | 16 (55) | 18 (36) | |
| Visceral | 2 (9) | 4 (14) | 18 (36) | |
| Both | 1 (5) | 2 (7) | 3 (6) | |
| Other† | 5 (23) | 7 (24) | 11 (22) | |
| No. of metastases | .35 | |||
| 1 | 6 (27) | 11(38) | 23 (46) | |
| > 1 | 16 (73) | 18 (62) | 27 (54) | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; RS, Recurrence Score.
Tumor grade reported locally; missing data for six patients in the intermediate-risk group and four patients in the high-risk group.
Includes mediastinal lymph nodes, paratracheal lymph nodes, endobronchial lymph nodes, hilar lymph nodes, prepectoral lymph nodes, skin, and pleura.
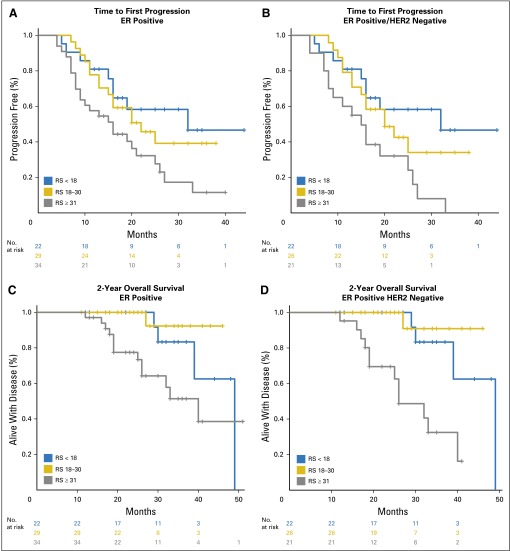
When stratified by RS, patients with low- and intermediate-risk scores had improved TTP and 2-year OS compared with patients with high-risk scores. This was true when all patients were included in the analysis, yet the difference was most pronounced among the ER-positive/HER2-negative subset where median TTP was not reached among those with low-risk scores and 2-year OS was 100% for both the low- and the intermediate-risk groups (Fig 2; Table 3). In univariable analysis, tumor grade was not significantly associated with OS (P = .22) or TTP (P = .05). In multivariable Cox regression models that included age and RS as continuous variables and adjusted for tumor size and site of first metastatic disease (bone only v other), the 21-gene RS was independently prognostic for TTP and 2-year OS in patients with ER-positive/HER2-negative stage IV disease (Table 4).
Fig 2.
Median time to (A and B) first progression and (C and D) 2-year overall survival by risk group among patients with ER-positive (n = 85) and ER-positive/HER2-negative (n = 69) de novo stage IV breast cancer. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; RS, Recurrence Score.
Table 3.
Median TTP and 2-Year OS by Risk Group Among Patients With De Novo Stage IV Breast Cancer
| RS Risk Group | ||||
|---|---|---|---|---|
| Low Risk (RS < 18) | Intermediate Risk (RS 18-30) | High Risk (RS ≥ 31) | Log-Rank P | |
| TTP, months, median (range) | ||||
| All patients (n = 101) | NR (16-NR) | 22 (16-NR) | 16 (9-25) | .010 |
| ER positive (n = 85) | 32 (16-NR) | 22 (16-NR) | 15 (9-25) | .007 |
| ER positive/HER2 negative (n = 69) | NR (16-NR) | 20 (16-NR) | 15 (8-27) | .003 |
| 2-Year OS, % | ||||
| All patients (n = 101) | 100 (78-100) | 100 (78-100) | 80 (69-93) | .035 |
| ER positive (n = 85) | 100 (78-100) | 100 (78-100) | 77 (64-94) | .008 |
| ER positive/HER2 negative (n = 69) | 100 (78-100) | 100 (75-100) | 69 (51-93) | < .001 |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; NR, not reached; OS, overall survival; RS, Recurrence Score; TTP, time to first progression.
Table 4.
Multivariable Cox Regression Models for TTP and 2-Year OS Among Patients With ER-Positive/HER2-Negative De Novo Stage IV Disease
| TTP | 2-Year OS | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| RS, 50 points | 5.36 (1.28 to 22.51) | .022 | 20.58 (1.89 to 224.2) | .013 |
| RS, 10 points | 1.40 (1.05 to 1.86) | .022 | 1.83 (1.14 to 2.95) | .013 |
| Age at diagnosis, years | 0.99 (0.96 to 1.02) | .660 | 1.01 (0.97 to 1.06) | .655 |
| Tumor size, cm | 1.07 (0.94 to 1.22) | .311 | 1.00 (0.79 to 1.25) | .972 |
| Site first metastases | 0.57 (0.28 to 1.16) | .123 | 0.83 (0.28 to 2.48) | .737 |
NOTE. Adjusted Cox regression models with Recurrence Score and age as continuous variables. Site of first metastases: bone-only v other.
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival; RS, Recurrence Score; TTP, time to first progression.
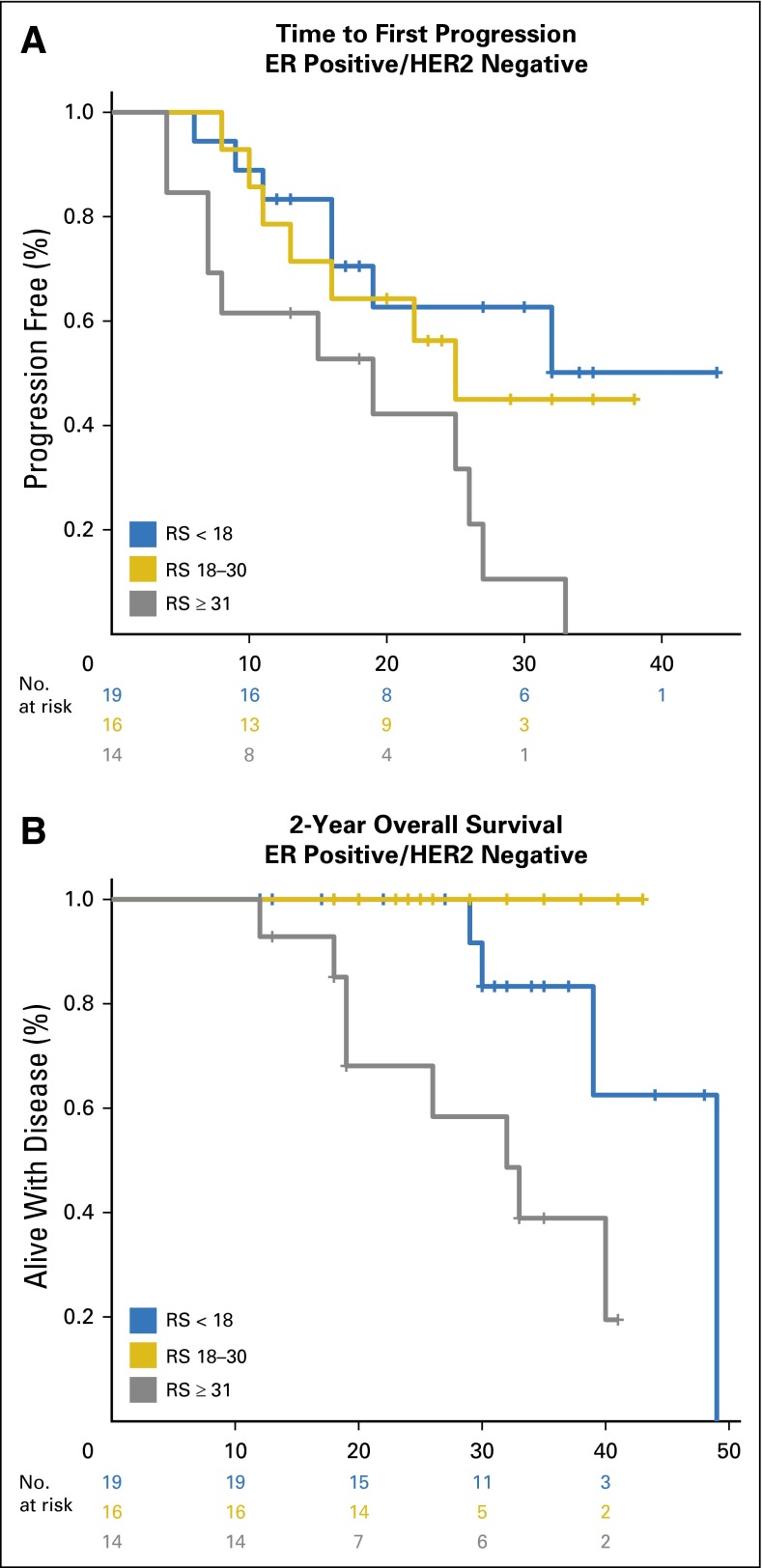
In an exploratory analysis to determine whether the 21-gene RS may be useful in predicting response to therapy in this cohort, we examined the 69 patients in the ER-positive/HER2-negative group by first-line treatment received (Appendix Table A2). Because this was a registry study, patients were selected for treatment at the discretion of their treating physician. Forty-nine (71%) patients received first-line endocrine therapy, and 20 (29%) received first-line chemotherapy. Despite the correlation between tumor grade and risk group, there was no association between tumor grade and the decision to proceed with first-line chemotherapy (Appendix Table A2). Patients who received first-line chemotherapy were younger (median age, 50 v 54 years), had larger primary tumors, and had more visceral and multiorgan disease, yet these differences were not statistically significant. Eighty-five percent of the patients who received first-line chemotherapy had intermediate-risk (n = 10) or high-risk (n = 7) RS values, which suggests that physicians are appropriately selecting many patients for more-aggressive treatment; however, 61% of the patients who received first-line endocrine therapy also had intermediate- or high-risk RS values, which highlights the opportunity for clinical decision-making tools to affect treatment decisions in this setting (Appendix Table A2).
In this exploratory analysis, both TTP and 2-year OS were shorter among patients with ER-positive/HER2-negative breast cancer and high-risk RS values who received first-line endocrine therapy, whereas there was no difference by RS in TTP or 2-year OS among those with ER-positive/HER2-negative disease and high-risk RS values who received first-line chemotherapy (Appendix Figs A3 and A4; Appendix Table A3). Although exploratory, these findings suggest that a high-risk RS may be a surrogate for relative endocrine resistance in de novo stage IV disease, which leads to the hypothesis that RS may be a tool to select patients with ER-positive/HER2-negative de novo stage IV breast cancer who may benefit from first-line chemotherapy. In this cohort, use of RS ≥ 31 to select first-line chemotherapy or first-line endocrine therapy would have resulted in a treatment change for 17 (25%) patients. However, these findings require prospective validation before being incorporated into clinical practice.
DISCUSSION
In metastatic breast cancer, the goals of care are to optimize both length and quality of life. Several advances have been made, particularly for HER2-positive and luminal-like subtypes, and survival has improved; however, median OS is still reported as 2 to 3 years.8-12 The use of treatment guidelines, primarily in early-stage breast cancer, has been associated with significant improvements in survival,13 yet for metastatic breast cancer, limited level 1 evidence exists, and only recently have international consensus guidelines been developed.6,7 In ER-positive/HER2-negative breast cancer, endocrine therapy is the preferred option, even in the presence of visceral disease, unless there is concern or proof of endocrine resistance or of disease needing a fast response.7 We demonstrate in the current study that the 21-gene RS, when performed on the primary tumor in patients with ER-positive/HER2-negative breast cancer, is independently prognostic for both TTP and 2-year OS in de novo stage IV disease, which leads to the hypothesis that this molecular profile may be useful in the clinical management of this patient subset.
We also demonstrate that the natural history of de novo ER-positive stage IV breast cancer differs from metastatic disease that recurs after adjuvant therapy. At a median follow-up of 29 months, the TTP for the whole cohort was 20 months (95% CI, 16 to 26 months), and median survival was 49 months (95% CI, 40 months to not reached). Among the 85 patients with ER-positive disease, the median TTP ranged from 32 months for those with a low-risk score to 15 months for those with a high-risk score. This difference was even more pronounced in the ER-positive/HER2-negative cohort where the median TTP for patients with a low-risk score had not been reached at a median follow-up of 29 months. This information could potentially be used in discussing treatment options and expectations in this patient cohort, specifically, with respect to the expected duration of response to first-line therapy and subsequent need for treatment modifications.
Guidelines state that treatment choice in metastatic breast cancer should take into account hormone receptor and HER2 status, tumor burden (number and site of metastases), patient age, performance status, comorbidities, menopausal status, and need for rapid disease/symptom control. Because we performed the 21-gene RS on all-comers, the majority of patients, not surprisingly, with HER2-positive tumors, and all patients with triple-negative tumors had high-risk RS results. Of note, we do not advocate for this approach because treatment algorithms in patients with hormone receptor–negative disease and HER2-positive disease differ substantially from those with hormone receptor–positive disease; however, this analysis provides proof of principle that RS results differ substantially by breast cancer subtype. In this data set, the median RS values ranged from 23 (0 to 59) to 62 (33 to 73) for patients with ER–positive/HER2-negative disease and triple-negative disease, respectively (Appendix Fig A2). Of note, the median RS was also correlated with tumor grade, ranging from 12 (7 to 33) among patients with grade 1 tumors to 33 (4 to 50) among those with grade 3 tumors, yet no relationship existed between RS risk group and other clinical factors typically considered when treatment recommendations are made (Table 2).
When we limited the analysis to only patients with ER-positive/HER2-negative disease, the distribution of low-, intermediate-, and high-risk scores was 32%, 38%, and 30%, respectively, similar to the distribution of scores seen in early-stage disease, and again, we see the correlation between tumor grade and risk group (Appendix Table A4). However, on exploration of first-line treatment choices made independently by physicians and patients, no significant association with tumor grade and the decision to proceed with first-line chemotherapy or endocrine therapy was found, which highlights the potential for the 21-gene RS to provide clinically meaningful information for this patient cohort, although we acknowledge that this requires further prospective study and validation.
Patients with ER-positive/HER2-negative disease who received first-line chemotherapy tended to be younger, and were more likely to have larger primary tumors, and visceral disease and/or more than one site of metastatic disease compared with those who received first-line endocrine therapy (Appendix Table A2). Although these comparisons were not statistically significant, they are consistent with the expected biases toward more-aggressive treatment in younger women with greater disease burden. Further exploratory analysis of TTP and survival in this cohort when examined by first-line treatment demonstrated that both outcomes were inferior among ER-positive/HER2-negative patients with high-risk RS results who received first-line endocrine therapy, whereas there was no difference by RS in TTP or 2-year OS among ER-positive/HER2-negative patients who received first-line chemotherapy (Appendix Figs A3 and A4; Appendix Table A3). Although exploratory, these findings suggest that a high-risk RS may be a surrogate for relative endocrine resistance in de novo stage IV disease. When selected by clinical criteria, 15% of patients who received first-line chemotherapy had low-risk RS, which suggests that endocrine therapy may have been more appropriate, and perhaps more importantly, 61% of patients who received first-line endocrine therapy had intermediate- or high-risk scores, which suggests that these patients may have disease that is less responsive to endocrine therapy, a hypothesis that requires testing in a prospective clinical trial.
In summary, the TBCRC 013 registry population provides new insights into the natural history of de novo stage IV breast cancer. The majority of women with de novo stage IV breast cancer have ER-positive/HER2-negative disease and experience durable responses to first-line physician-directed therapy. However, within this population, which represented more than one-third of patients enrolled in PALOMA-3,14 the potential to individualize treatment on the basis of predictive markers remains an unmet clinical need. In the ER-positive/HER2-negative cohort, 30% of patients had a high-risk RS, which is somewhat higher than that seen in the setting of node-negative disease. If a high-risk RS was considered an indication for chemotherapy and a low-risk RS considered a contraindication to chemotherapy, first-line treatment decisions would have differed for 25% of the study population, with the potential to affect both OS and quality of life. Given the growing body of evidence that demonstrates the ability of the 21-gene RS to predict prognosis and benefit from chemotherapy in both early-stage node-positive and node-negative disease,1-3,15,16 these findings suggest that biology is the major determinant of outcome and warrant further prospective investigation.
Appendix
Fig A1.
Histogram of Recurrence Scores among all patients with de novo stage IV breast cancer.
Fig A2.
Correlation between Recurrence Score (RS) and tumor subtype by immunohistochemistry. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
Fig A3.
Exploratory analysis of (A) time to first progression and (B) 2-year overall survival among patients with ER-positive/HER2-negative de novo stage IV breast cancer treated with first-line endocrine therapy (n = 49). ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; RS, Recurrence Score.
Fig A4.
Exploratory analysis of (A) time to first progression and (B) 2-year overall survival among patients with ER-positive/HER2-negative de novo stage IV breast cancer treated with first-line chemotherapy (n = 20). ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; RS, Recurrence Score.
Table A1.
Characteristics of Eight Patients for Whom RS Values Were Not Generated
| No. (%) | |
|---|---|
| Median age at diagnosis, years (range) | 49 (43-60) |
| Median primary tumor size, cm (range) | 3.4 (1.4-7.0) |
| Clinical node status | |
| Clinically suspicious | 8 (100) |
| Nothing suspicious | 0 (0) |
| Unknown | 0 (0) |
| ECOG status | |
| 0 | 5 (62.5) |
| 1 | 3 (37.5) |
| > 1 | 0 (0) |
| Tumor subtype | |
| HR positive/HER2 negative | 3 (37.5) |
| HR positive/HER2 positive | 4 (50) |
| HR negative/HER2 positive | 0 (0) |
| Triple negative | 1 (12.5) |
| Site of metastasis at first diagnosis | |
| Bone only | 2 (25) |
| Visceral only | 3 (37.5) |
| Both (bone and visceral) | 1 (12.5) |
| Other* | 2 (25) |
| No. of metastatic sites at first diagnosis | |
| Single organ | 4 (50) |
| > 1 organ | 4 (50) |
| First systemic treatment | |
| Chemotherapy | 3 (37.5) |
| Endocrine therapy | 2 (25) |
| Chemotherapy and endocrine therapy | 0 (0) |
| Chemotherapy plus trastuzumab | 3 (37.5) |
| Endocrine therapy plus trastuzumab | 0 (0) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; RS, Recurrence Score.
Includes contralateral axillary lymph nodes.
Table A2.
Clinical Characteristics of the ER-Positive/HER2-Negative Patients in the 21-Gene RS Population Stratified by First-Line Therapy
| Group, No. (%) | ||||
|---|---|---|---|---|
| Characteristic | All ER-Positive/HER2-Negative Patients (n = 69) | Endocrine Therapy (n = 49) | Chemotherapy (n = 20) | P |
| Median age at diagnosis, years (range) | 53 (21-79) | 54 (31-79) | 50 (21-62) | .05 |
| Primary tumor size, cm (median) | 3.0 (0.7-15) | 2.6 (0.7-15) | 3.5 (1.2-9) | .09 |
| ECOG status | .29 | |||
| 0 | 36 (52) | 23 (47) | 13 (65) | |
| 1 | 29 (42) | 22 (45) | 7 (35) | |
| > 1 | 4 (6) | 4 (8) | 0 (0) | |
| Site of metastasis at first diagnosis | .26 | |||
| Bone only | 38 (55) | 29 (59) | 9 (45) | |
| Visceral only | 14 (20) | 9 (18) | 5 (25) | |
| Both (bone and visceral) | 13 (19) | 7 (14) | 6 (30) | |
| Other* | 4 (6) | 4 (8) | 0 (0) | |
| Number of metastatic sites at first diagnosis | .43 | |||
| Single organ | 43 (62) | 32 (65) | 11 (55) | |
| Multiple organs | 26 (38) | 17 (35) | 9 (45) | |
| Recurrence score | .14 | |||
| Low (< 18) | 22 (32) | 19 (39) | 3 (15) | |
| Intermediate (18-30) | 26 (38) | 16 (33) | 10 (50) | |
| High (≥ 31) | 21 (30) | 14 (29) | 7 (35) | |
| Tumor Grade† | .49 | |||
| I | 6 (10) | 5 (11) | 1 (6) | |
| II | 29 (46) | 22 (49) | 7 (39) | |
| III | 28 (44) | 18 (40) | 10 (56) | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; RS, Recurrence Score.
Includes mediastinal lymph nodes, endobronchial lymph nodes, skin, and pleura.
Locally reported tumor grade missing for 6 patients in the ER+/HER2- group.
Table A3.
Exploratory Analysis of Median TTP and 2-Year OS Among Patients With ER-Positive/HER2-Negative De Novo Stage IV Breast Cancer by First-Line Therapy
| RS Risk Group | ||||
|---|---|---|---|---|
| Low Risk (RS < 18) | Intermediate Risk (RS 18-30) | High Risk (RS ≥ 31) | Log-Rank P | |
| TTP, months, median (range) | ||||
| First-line endocrine (n = 49) | NR (19-NR) | 25 (15-NR) | 15 (7-NR) | .007 |
| First-line chemotherapy (n = 20) | 15 (5-NR) | 18 (13-NR) | 13 (9-NR) | .61 |
| 2-Year OS, % | ||||
| First-line endocrine (n = 49) | 100 (75-100) | 100 (66-100) | 68 (47-100) | .002 |
| First-line chemotherapy (n = 20) | 100 (16-100) | 100 (40-100) | 71 (45-100) | .604 |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; NR, not reached; OS, overall survival; RS, Recurrence Score; TTP, time to first progression.
Table A4.
Clinical Characteristics by RS Among Patients With ER-Positive/HER2-Negative De Novo Stage IV Breast Cancer
| RS Risk Group, No. (%) | ||||
|---|---|---|---|---|
| Low Risk (RS < 18) | Intermediate Risk (RS 18-30) | High Risk (RS ≥ 31) | P | |
| No. of patients | 22 | 26 | 21 | |
| Median age, years (range) | 58 (38-73) | 52 (26-79) | 50 (21-77) | .246 |
| Median tumor size, cm (range) | 2.6 (0.8-9) | 3.1 (0.7-15) | 3.5 (1-9) | .352 |
| ECOG status | .651 | |||
| 0 | 9 (41) | 16 (62) | 11 (52) | |
| > 0 | 13 (59) | 10 (38) | 10 (48) | |
| Cohort | .001 | |||
| A | 20 (91) | 19 (73) | 21 (100) | |
| B | 2 (9) | 7 (27) | 0 (0) | |
| Site of first metastasis | .397 | |||
| Bone | 14 (64) | 14 (54) | 10 (48) | |
| Visceral | 5 (23) | 6 (23) | 3 (14) | |
| Both | 2 (9) | 4 (15) | 7 (33) | |
| Other* | 1 (4) | 2 (8) | 1 (5) | |
| No. of metastases | .580 | |||
| 1 | 16 (73) | 16 (62) | 11 (52) | |
| > 1 | 6 (27) | 10 (38) | 10 (48) | |
| Tumor grade† | ||||
| I | 5 (23) | 0 (0) | 1 (5) | < .001 |
| II | 15 (68) | 11 (52) | 3 (15) | |
| III | 2 (9) | 10 (48) | 16 (80) | |
Abbreviations: ER, estrogen receptor; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; RS, Recurrence Score.
Includes mediastinal lymph nodes, endobronchial lymph nodes, skin, and pleura.
Locally reported tumor grade missing for five patients in the intermediate-risk group and one patient in the high-risk group.
Footnotes
Written on behalf of the Translational Breast Cancer Research Consortium.
Supported in part by the Translational Breast Cancer Research Consortium and its supporters (Avon Foundation, The Breast Cancer Research Foundation, Susan G. Komen), Genomic Health, and the National Institutes of Health/National Cancer Institute, Cancer Center Support Grant P30 CA008748 (to M.G.).
Presented at the 2013 ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2013.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Tari A. King, Amy Pratt Sing, Larry Norton, Monica Morrow, Clifford A. Hudis
Administrative support: Tari A. King, Jaclyn P. Lyman, Camilla Boafo, Amy Pratt Sing, E. Shelley Hwang, Larry Norton, Monica Morrow, Clifford A. Hudis
Provision of study materials or patients: Tari A. King, Jaclyn P. Lyman, Camilla Boafo, Amy Pratt Sing, E. Shelley Hwang, Michael D. Alvarado, Minetta C. Liu, Judy C. Boughey, Kandace P. McGuire, Catherine H. Van Poznak, Lisa K. Jacobs, Ingrid M. Meszoely, Helen Krontiras, Gildy V. Babiera, Monica Morrow, Clifford A. Hudis
Collection and assembly of data: Tari A. King, Jaclyn P. Lyman, Mithat Gonen, Amy Voci, Marina De Brot, Camilla Boafo, Amy Pratt Sing, E. Shelley Hwang, Michael D. Alvarado, Minetta C. Liu, Judy C. Boughey, Kandace P. McGuire, Catherine H. Van Poznak, Lisa K. Jacobs, Ingrid M. Meszoely, Helen Krontiras, Gildy V. Babiera
Data analysis and interpretation: Tari A. King, Jaclyn P. Lyman, Mithat Gonen, Amy Pratt Sing, Larry Norton, Monica Morrow, Clifford A. Hudis
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prognostic Impact of 21-Gene Recurrence Score in Patients With Stage IV Breast Cancer: TBCRC 013
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Tari A. King
No relationship to disclose
Jaclyn P. Lyman
No relationship to disclose
Mithat Gonen
No relationship to disclose
Amy Voci
No relationship to disclose
Marina De Brot
No relationship to disclose
Camilla Boafo
No relationship to disclose
Amy Pratt Sing
Employment: Genomic Health
Stock or Other Ownership: Genomic Health
E. Shelley Hwang
Consulting or Advisory Role: Genomic Health, Pfizer
Travel, Accommodations, Expenses: Genomic Health
Michael D. Alvarado
Honoraria: Genomic Health
Speakers’ Bureau: Genentech
Research Funding: Carl Zeiss
Minetta C. Liu
Research Funding: Eisai (Inst), Seattle Genetics (Inst), Celgene (Inst), Veridex (Inst), Clearbridge Biomedics (Inst), Novartis (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: Genentech
Judy C. Boughey
Research Funding: Myriad Genetics (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending: Methods and Materials for Assessing Chemotherapy Responsiveness and Treating Cancer (Inst)
Kandace P. McGuire
No relationship to disclose
Catherine H. Van Poznak
Research Funding: Bayer (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Lisa K. Jacobs
No relationship to disclose
Ingrid M. Meszoely
No relationship to disclose
Helen Krontiras
No relationship to disclose
Gildy V. Babiera
No relationship to disclose
Larry Norton
Travel, Accommodations, Expenses: Genentech, Celgene
Monica Morrow
Honoraria: Genomic Health
Clifford A. Hudis
Consulting or Advisory Role: Merck, Genentech, Novartis, Eli Lilly, Pfizer
Other Relationship: The Breast Cancer Research Foundation
REFERENCES
- 1.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 2.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (ed 20) Fort Washington, PA: National Comprehensive Cancer Network; 2014. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1) Breast. 2012;21:242–252. doi: 10.1016/j.breast.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Breast. 2014;23:489–502. doi: 10.1016/j.breast.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Andre F, Slimane K, Bachelot T, et al. Breast cancer with synchronous metastases: Trends in survival during a 14-year period. J Clin Oncol. 2004;22:3302–3308. doi: 10.1200/JCO.2004.08.095. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso F. Metastatic breast cancer patients: The forgotten heroes! Breast. 2009;18:271–272. doi: 10.1016/j.breast.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Foukakis T, Fornander T, Lekberg T, et al. Age-specific trends of survival in metastatic breast cancer: 26 years longitudinal data from a population-based cancer registry in Stockholm, Sweden. Breast Cancer Res Treat. 2011;130:553–560. doi: 10.1007/s10549-011-1594-z. [DOI] [PubMed] [Google Scholar]
- 11.Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19:2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundquist M, Eriksson Z, Tejler G, et al. Trends in survival in metastatic breast cancer. Eur J Cancer. 2010;8:191. [Google Scholar]
- 13.Hébert-Croteau N, Brisson J, Latreille J, et al. Compliance with consensus recommendations for systemic therapy is associated with improved survival of women with node-negative breast cancer. J Clin Oncol. 2004;22:3685–3693. doi: 10.1200/JCO.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Turner CT, Ro J, Andre F, et al. PALOMA3: A double-blind, phase III trial of fulvestrant with or without palbociclib in pre- and post-menopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer that progressed on prior endocrine therapy. J Clin Oncol 33, 2015 (suppl; abstr LBA502)
- 15.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene Recurrence Score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J Clin Oncol. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 16.Tang G, Cuzick J, Costantino JP, et al. Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor-positive breast cancer: Recurrence Score alone and integrated with pathologic and clinical factors. J Clin Oncol. 2011;29:4365–4372. doi: 10.1200/JCO.2011.35.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]