Abstract
Purpose
Children’s Oncology Group study AHOD03P1 was designed to determine whether excellent outcomes can be maintained for patients with low-risk, pediatric lymphocyte-predominant Hodgkin lymphoma (LPHL) with a strategy of resection alone or minimal chemotherapy.
Patients and Methods
Patients with stage IA LPHL in a single node that was completely resected were observed without further therapy; recurrences were treated with three cycles of doxorubicin/vincristine/prednisone/cyclophosphamide (AV-PC). Patients with unresected stage IA or stage IIA LPHL were treated with three cycles of AV-PC. Patients with less than a complete response (CR) to AV-PC received 21-Gy involved-field radiation therapy (IFRT).
Results
A total of 183 eligible patients were enrolled; 178 were evaluable. Of these, 52 patients underwent complete resection of a single node. There were 13 relapses at a median of 11.5 months; 5-year event-free survival (EFS) was 77% (range, 62% to 87%). A total of 135 patients received AV-PC; 126 were treated at diagnosis and nine at relapse after surgery alone. Eleven patients receiving AV-PC had less than CR and received IFRT. Fourteen first events occurred among 135 patients (12 relapses and two second malignancies). Two relapses occurred in patients who had received IFRT. Five-year EFS was 88.8% (95% CI, 81.8% to 93.2%). Five-year EFS for the entire cohort was 85.5% (95% CI, 79.2% to 90.1%); overall survival was 100%.
Conclusion
Some 75% of highly selected pediatric patients with LPHL may be spared chemotherapy after surgical resection alone. Pediatric LPHL has excellent EFS with chemotherapy that is less intensive than standard regimens; > 90% of patients can avoid radiation therapy. The salvage rate for the few relapses is high, with 100% survival overall.
INTRODUCTION
Lymphocyte-predominant Hodgkin lymphoma (LPHL) is a rare subtype of Hodgkin lymphoma (HL); less than 10% of cases are classified as LPHL in adult and pediatric series.1 Most cases of LPHL demonstrate a nodular pattern. The neoplastic cells of nodular LPHL are lymphocyte-predominant (LP) cells, which are considered to represent a clonal expansion of B cells.2-4 In contrast to Reed-Sternberg cells of classic HL that express CD15 and CD30, LP cells of nodular LPHL express B cell-associated markers such as CD20, CD79a, J-chain, and immunoglobulin transcripts, and typically lack CD15 and CD30.5
The presentation of LPHL is clinically distinct from classic HL.6-8 Patients typically are male (approximately 70%) and pediatric LPHL often occurs at a younger age than classic HL.9 Most patients with LPHL are asymptomatic and have nonbulky, localized disease involving peripheral lymph nodes. Mediastinal involvement is rare.
A standard of care for treatment of low-stage LPHL has yet to be established. There are reports of patients who have been cured with surgery alone, but this approach has never been studied prospectively.10-15 Low-stage LPHL in adults is often treated with radiation therapy (RT) alone.16-21 Pediatric patients have an excellent response to regimens used for low-stage classic HL that use chemotherapy and low-dose radiation.1,22-24 However, classic HL treatment carries the risk for late effects due to exposure to anthracyclines, alkylating agents, and radiation. Second malignant neoplasms (SMNs), including transformation to non-Hodgkin lymphoma (NHL), have been reported.7,10,15,21,22,25-27
Doxorubicin/vincristine/prednisone/cyclophosphamide (AV-PC), commonly known as CHOP, is widely used in NHL treatment. Murphy et al13 described five children with LPHL who received AV-PC without RT following incomplete resection of low-risk LPHL with only one recurrence 6 years posttreatment.
The Children’s Oncology Group (COG) planned the first prospective clinical trial for low-stage pediatric LPHL. A surgery-only/observation strategy was used for patients with resectable disease limited to a single lymph node. AV-PC (three cycles) was the chemotherapy backbone chosen for patients who required chemotherapy at diagnosis or at relapse following observation alone. This regimen was selected based on the data of Murphy et al,13 the similarity of LPHL to NHL, and the potential to limit long-term effects. RT was reserved for patients who had less than a complete response (CR) to chemotherapy. Here we describe the results of this trial, which completed accrual in 2010.
PATIENTS AND METHODS
Patients
This study was conducted at COG institutions from January 2006 through November 2010 (ClinicalTrials.gov identifier, NCT00107198). Written informed consent was obtained according to institutional guidelines and in compliance with Declaration of Helsinki guidelines.
This study was a prospective, nonrandomized trial. Eligible patients were younger than 22 years of age with stage IA/IIA LPHL. Patients with “B” symptoms or bulk disease, defined as a nodal aggregate greater than 6 cm or large mediastinal mass greater than one-third of the thoracic diameter on chest radiograph, were excluded. LPHL histology was confirmed by rapid central pathology review (Revised European American Lymphoma /WHO classification) at study entry.28 Computed tomography (CT) imaging of the neck, chest, abdomen, and pelvis was required for staging and response; positron emission tomography (PET) imaging with 18F-labeled fluorodeoxyglucose was required to confirm complete response.
Treatment Protocol
Chemotherapy.
Patients with stage I LPHL and more than one lymph node or stage II LPHL were treated with three cycles of AV-PC chemotherapy, given every 21 days, as follows: doxorubicin, 50 mg/m2 day 1; vincristine, 1.4 mg/m2 (maximum, 2.8 mg) days 1 and 8; prednisone, 40 mg/m2 days 1-7; cyclophosphamide, 800 mg/m2 day 1. Growth-factor support was not included. Response was assessed after three cycles of AV-PC with CT scan of the primary site and PET scan. Imaging results were confirmed with rapid central review. Response assessment criteria, based primarily on CT scan with PET confirmation of CR, are found in the Appendix (online only).
Radiation therapy.
Patients with a CR to chemotherapy did not receive RT. Patients with less than CR received 21-Gy involved-field radiation therapy (IFRT); biopsy of residual adenopathy was not required.
Surgery only for totally resected stage I, single-node disease.
Patients with stage I disease and total resection (TR) of a single involved lymph node (confirmed by CT and PET scans) proceeded to observation. If postoperative imaging was not definitive (i.e., findings consistent with either residual lymphoma or postoperative change), CT and PET scans repeated 6-7 weeks postoperatively were used to evaluate TR. Patients with TR after reimaging proceeded to observation. Central review was required to be assigned to observation. Patients with a single node that was partially resected were deemed subtotal resection. Patients who did not have TR received chemotherapy with AV-PC with or without IFRT (AV-PC±IFRT).
Follow-up
Subjects were followed for clinical signs of recurrence and by surveillance imaging. Details of follow-up plans are in the Appendix.
Relapse
All patients had confirmation by biopsy specimen at first relapse before salvage therapy. Patients who relapsed after AV-PC with or without RT were treated per institutional choice. Patients who underwent TR and who had low-risk relapse (stage IA or IIA LPHL) following observation remained in the study and were given AV-PC±IFRT as salvage therapy. The study was amended in March 2013 to close the salvage chemotherapy arm for patients who underwent TR; relapses after this point were treated per institutional choice.
Statistical Methods
Event-free-survival (EFS) was defined as the minimum time from study enrollment to disease relapse/progression, SMN, or death. For EFS among patients receiving AV-PC chemotherapy, the analysis included patients who received AV-PC as upfront therapy as well as patients (with low-risk relapse after observation only) who received AV-PC as salvage therapy. For patients who received AV-PC as upfront therapy, EFS was computed from study enrollment; for patients who received AV-PC after low-risk relapse, EFS was computed from the date of relapse. Overall survival (OS) was defined as the minimum time from study enrollment to death resulting from any cause. Patients without EFS or OS events were censored at date of last contact. EFS and OS estimates were based on the Kaplan-Meier product limit method with CI based on the “exponential” Greenwood equation.29 Patient follow-up data were collected through June 2015.
RESULTS
A total of 188 patients with LPHL were enrolled in the AHOD03P1. Of these, 183 were eligible per protocol criteria; five patients were deemed ineligible because of the presence of bulk disease (n = 2), failure to ensure adequate cardiac function before initiation of chemotherapy (n = 1), and absence of rapid central pathology review (n = 2). Of the 183 eligible patients, five were excluded from treatment outcome analysis because they did not receive protocol-directed treatment.
Clinical characteristics of the 183 eligible patients are summarized in Table 1. The mean age was 12.3 years (range, 4.2 to 20.7 years), the median age was 13.0 years, and 84% of the patients were male. The most common sites of involvement were lymph node groups in the head and neck, axilla, or groin. Mediastinal involvement was rare (n = 7; 3.8%). LPHL was confirmed by central pathology review for all patients.
Table 1.
Clinical Characteristics of Eligible Patients (N = 183)
| Characteristic | No. (%) |
|---|---|
| All patients | 183 (100) |
| Age, years | |
| < 10 | 56 (31) |
| 10-15 | 85 (46) |
| 16-21 | 42 (23) |
| Sex | |
| Male | 153 (84) |
| Female | 30 (16) |
| Stage/assessment at study entry (N = 183), No. | |
| IA, single-node, TR at initial review | 47 |
| IA, single node, equivocal or unknown initially; TR on reimaging at 6-7 weeks | 5 |
| IA, single node, STR or equivocal on reimaging at 6-7 weeks | 3 |
| IA, single node, STR at study entry | 1 |
| IA, more than a single node | 46 |
| IIA | 81 |
| Mediastinal involvement, No. (%) | 7 (3.8) |
| Location of disease | |
| Above the diaphragm | 154 (84) |
| Below the diaphragm | 29 (16) |
Abbreviations: STR, subtotal resection; TR, total resection.
Of the 183 eligible patients, 46 had stage I LPHL, more than one node; 81 had stage II disease, and 56 were categorized by the institution as having stage I LPHL, single node. Fifty-two of 56 patients were confirmed to have TR by central radiology review (47 at enrollment and five on reimaging at week 6). Four of 56 were determined by central review to have less than TR and assigned to receive chemoradiotherapy. Thus, after central review, 52 patients had confirmed TR and were assigned to observation and 131 patients were assigned to receive chemoradiotherapy: 50 had stage I disease and 81 had stage II disease. However, five patients were considered unevaluable: Four did not receive protocol chemotherapy and one did not receive radiotherapy. In summary, 178 evaluable patients received protocol-directed treatment (observation, n = 52; chemoradiotherapy, n = 126).
EFS/OS: Entire Cohort
Of 178 eligible/evaluable patients, 26 have experienced first events (relapse after surgery, relapse after upfront chemoradiotherapy, or SMN). The 5-year EFS estimate for these 178 patients was 85.5% (95% CI, 79.2% to 90.1%). The median follow-up time for the remaining 152 patients was 61.6 months (range, 3.4 to 107.4 months). No deaths have been reported; OS as of this writing was 100%.
EFS/OS: TR Patients
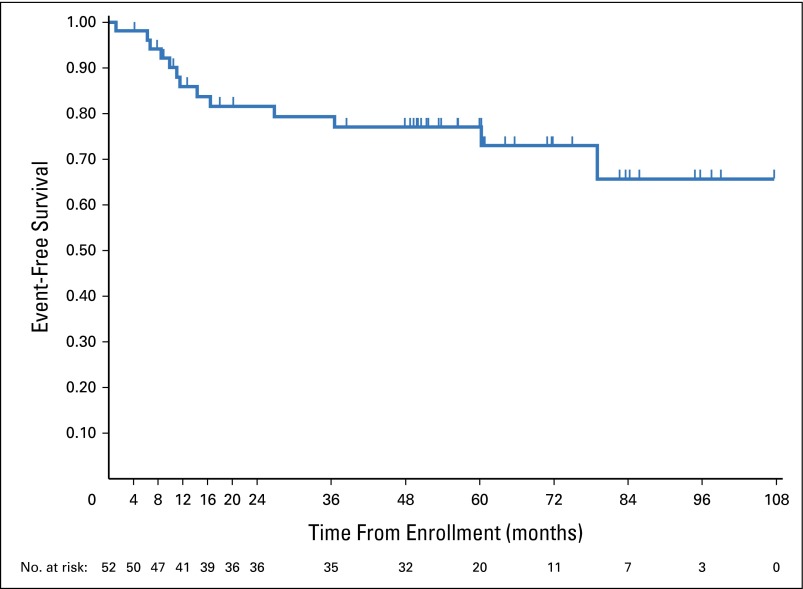
A total of 52 patients were confirmed as having undergone single-node TR and observed without upfront chemotherapy. Thirteen patients have relapsed. The 5-year EFS was 77.1% (95% CI, 62.4% to 86.6%) (Fig 1). Relapses occurred at a median of 11.5 months after study enrollment (range, 1.1 to 79.1 months). The median follow-up among the 39 remaining patients has been 56.3 months (range, 3.9 to 107.4 months).
Fig 1.
Event-free-survival, after observation, in patients who underwent total resection.
Twelve of the relapses were stage IA, occurring at the site or in adjacent nodes or nodal regions; one relapse was stage IIA. Nine of the patients with relapsed disease were treated with AV-PC as salvage chemotherapy per protocol specifications; all were in a CR after chemotherapy and did not receive IFRT. These nine patients are included in the analysis of response to AV-PC. One of the nine patients experienced a second relapse 6 months after treatment with protocol AV-PC. Four patients relapsed after the salvage chemotherapy arm was closed and received chemotherapy per institutional choice. Details of the 13 patients who underwent TR and who relapsed are in Table 2.
Table 2.
Patients Who Underwent Total Resection and Subsequent Disease Relapse
| Time to Relapse (months) | Original Site | Stage at Relapse | Site of Relapse | Method of Relapse Detection |
|---|---|---|---|---|
| 14.3 | Inguinal (right) | IA | Iliac (right) | Imaging (PET/CT imaging and MRI) |
| 11.0 | Submandibular (left) | IA | Cervical (left) | Clinical signs |
| 11.5 | Cervical (right) | IA | Maxilla and/or mandible (right) | Clinical signs and abnormal CT scan at scheduled follow-up |
| 6.2 | Maxilla and/or mandible (right) | IA | Submandibular (right) | Clinical signs |
| 9.9 | Cervical (left) | IA | Cervical and supraclavicular (left) | Abnormal CT scan at scheduled follow-up |
| 1.1 | Cervical (right) | IA | Cervical (right) | Clinical signs |
| 16.5 | Preauricular (right) | IA | Cervical (right) | Clinical signs and abnormal CT scan at scheduled follow-up |
| 8.4 | Cervical (left) | IA | Cervical (left) | Clinical signs and abnormal CT scan at scheduled follow-up |
| 6.7 | Maxilla and/or mandible (left) | IA | Cervical and supraclavicular (left) | Abnormal CT scan at scheduled follow-up |
| 26.8 | Cervical (right) | IIA | Cervical (right and left) | Clinical signs and abnormal CT scan at scheduled follow-up |
| 60.3 | Cervical (right) | IA | Cervical, maxilla/mandible (right) | Clinical signs |
| 79.1 | Cervical (left) | IA | Supraclavicular (left) | Clinical signs |
| 36.6 | Inguinal (left) | IA | Inguinal (left) | Abnormal CT scan at scheduled follow-up |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Response to AV-PC±RT
A total of 126 of the eligible, evaluable patients received AV-PC per protocol at study enrollment and nine patients who underwent TR received AV-PC on protocol (as described in the previous paragraph) at relapse following observation. Thus, a total of 135 patients received AV-PC±RT per protocol; 124 (92%) were in CR confirmed by central review after chemotherapy and did not receive RT. Eleven patients (8%) had less than a CR on central review and were assigned to receive IFRT (Table 3).
Table 3.
Patients Treated With Doxorubicin/Vincristine/Prednisone/Cyclophosphamide Who Had Less Than a Complete Response After Chemotherapy
| Stage | Initial Sites | CT Scan After Chemotherapy | PET Scan After Chemotherapy | Response After Chemotherapy | Relapse?* |
|---|---|---|---|---|---|
| IIA | Parapsoas, iliac, inguinal (left) | Positive | Negative | VGPR | |
| IIA | Femoral, iliac, inguinal (right) | Negative | Positive | VGPR | |
| IIA | Cervical (bilateral), mediastinum, axillary (bilateral), epitrochlear (left) | Positive | Negative | VGPR | |
| IIA | Cervical, supraclavicular (left) | Positive | Positive | VGPR | |
| IA | Preauricular nodes (right) | Positive | Positive | VGPR | |
| IIA | Cervical, supraclavicular (left) | Positive | Negative | VGPR | |
| IIA | Cervical (bilateral) | Positive | Positive | VGPR | |
| IIA | Cervical (bilateral), maxilla/mandible (left), hilar (left), paratracheal (right) | Negative | Positive | VGPR | Yes (see Table 4) |
| IIA | Cervical (right), submandibular | Positive | Negative | VGPR | |
| IIA | Cervical (bilateral) | Negative | Positive | VGPR | |
| IIA | Waldeyer ring, retropharyngeal (bilateral), spinal accessory (right), mediastinum | Negative | Positive | VGPR | Yes (see Table 4) |
Abbreviations: CT, computed tomography; PET, positron emission tomography VGPR, very good partial response.
Empty cells indicate no relapse to date.
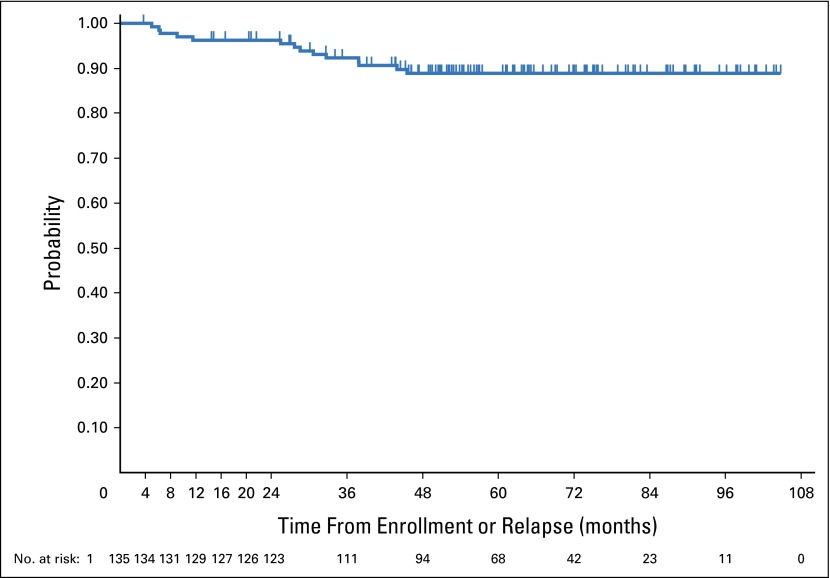
There had been 14 events among 135 patients as of this writing; 12 had relapses and two developed NHL as a first event. Median time to an event (from study entry for those receiving AV-PC upfront or from first relapse for those receiving AV-PC as salvage therapy) was 28.1 months (range, 5.1 to 45.5 months). The median follow-up among the 121 remaining patients was 62.2 months (range, 3.5 to 104.5 months). The 5-year EFS estimate is 88.8% (95% CI, 81.8% to 93.2%) (Fig 2).
Fig 2.
Event-free-survival in patients treated with doxorubicin/vincristine/prednisone/cyclophosphamide with or without radiation therapy.
Details of relapses after chemotherapy are given in Table 4. Seven patients relapsed at low-stage (I or II) disease; four patients relapsed at stage III disease and one at stage IV disease. No patient had B symptoms at relapse. Two patients had received IFRT as part of initial therapy for stage II disease. One patient, who had undergone TR, relapsed 6 months after receiving AV-PC as salvage treatment.
Table 4.
Patients Who Relapsed After Doxorubicin/Vincristine/Prednisone/Cyclophosphamide With or Without RT
| Original Stage | Original Site | Upfront RT? | Time to Relapse (months) | Stage at Relapse | Sites of Relapse | Method of Relapse Detection |
|---|---|---|---|---|---|---|
| IIA | Cervical, parotid (right) | No | 28 | IIA | Maxilla/mandible/supraclavicular (right) | Clinical signs |
| IIA | Maxilla, submental (left); axillary (bilateral) | No | 5 | IA | Submandibular (left) | Clinical signs |
| IA | Cervical (left) | No | 38 | IIIA | Cervical/maxilla/submandibular (left), supraclavicular, axilla (right), anterior mediastinum | Clinical signs and abnormal CT scan at scheduled follow-up |
| IA | Cervical (right) | No | 26 | IIIA | Cervical (right), Waldeyer ring, peripancreatic, spleen | Clinical signs and abnormal CT scan at scheduled follow-up |
| IIA | Cervical (left), paratracheal (right) | No | 29 | IIA | Cervical (bilateral) | Clinical signs |
| IA | Cervical (right) | No | 44 | IIIA | Cervical (left), axillary (bilateral); external lilac/pelvic sidewall (right) | Clinical signs and abnormal CT scan at scheduled follow-up |
| I A | Multiple nodes above diaphragm (7 groups) | No | 38 | IIIA | Cervical (bilateral), supraclavicular (bilateral), mediastinum, mesentery | Abnormal CT scan at scheduled follow-up |
| IIA | Cervical (bilateral); maxilla, hilar (left); paratracheal (right) | Yes | 33 | IIA | Infraclavicular, axillary (right) | Clinical signs |
| IA* | Cervical (left) | No | 6 | IA | Cervical (left) | Clinical signs and abnormal CT scan at scheduled follow-up |
| IA | Cervical, supraclavicular (left) | No | 9 | IVA | Cervical (left), bone (right acetabulum) | Clinical signs |
| IIA | Waldeyer ring, retropharyngeal (bilateral), spinal accessory (right) | Yes | 30 | IIA | Cervical (bilateral) | Abnormal CT scan at scheduled follow-up |
| IIA | Axillary, epitrochlear, subpectoral (right) | No | 6 | IIA | Axillary, epitrochlear (right) | Clinical signs |
Abbreviations: CT, computed tomography; RT, radiation therapy.
A patient who underwent TR and who had relapse of disease 8.4 months after observation.
Toxicity
The AV-PC chemotherapy regimen was well tolerated. Seven patients (5.1%) experienced grade 3 or 4 fever and neutropenia. No unexpected toxicities were reported.
Second Malignant Neoplasms
To date, three SMNs have been reported. Two patients developed NHL (diffuse large B-cell lymphoma [DLBCL]) as a first event following chemotherapy with AV-PC without IFRT. Both had stage II LPHL in the head and neck at presentation. One developed DLBCL at 12 months from initial treatment, the other at 45 months from initial treatment.
One patient developed a chest-wall Ewing sarcoma (ES) as a second event, 18 months after enrollment in the study. Initially, the patient had stage II LPHL of maxillary, submental, and axillary nodes, and received AV-PC chemotherapy without IFRT. He had biopsy specimen-proven LPHL recurrence in a submandibular node 3 months after completing chemotherapy and was treated with chemotherapy and IFRT. Thirteen months after the LPHL relapse, he presented with a chest-wall, localized ES. He was given standard treatment of ES including chemotherapy, complete resection of the chest wall tumor, and RT. As of this writing, he remains in remission, more than 6 years after his LPHL relapse and more than 5 years after his ES diagnosis.
DISCUSSION
In the past, patients with low-risk LPHL have had excellent outcomes when using treatment regimens designed for classic HL. However, this approach has exposed children and young adults to long-term risks associated with chemotherapy and RT.30,31 Overtreating LPHL is a concern because patients may have a higher risk for morbidity and mortality from treatment-related toxicity than from recurrent disease.6,7,17-19,21,32,33 RT doses > 25 Gy can cause impaired bone growth and soft-tissue deficits among younger children,34 cardiopulmonary toxicity due to mediastinal radiation,35-39 and an increased risk for second malignancies.40-48 Although pediatric RT protocols currently use low-dose radiation, the risk of developing SMN persists.49,50 Alkylating agents and anthracycline chemotherapy can also cause infertility51-53 and cardiac failure.54 Therefore, it is imperative to consider late toxicities when planning treatment of pediatric LPHL.
Many adult patients with low-stage LPHL receive local RT alone, typically to a dose of 30 Gy.16-21 Pediatric trials have shown that a chemotherapy-only approach is efficacious.13,24,55 We previously demonstrated that patients with LPHL treated with cyclophosphamide, vincristine sulfate, prednisone, procarbazine hydrochloride, doxorubicin hydrochloride, bleomycin, and vinblastine sulfate had a 5-year EFS of 96%; however, this chemotherapy regimen would be considered fairly intensive for low-risk patients by today’s standards.1 A retrospective British and French analysis reported a relapse-free survival rate of 74% and OS of 100% in 51 children with stage I/II LPHL treated with three cycles of cyclophosphamide/vinblastine/prednisone without RT55; this regimen is currently under investigation in a European pediatric trial. AV-PC, as used in our study, resulted in a 5-year EFS of 88.8% and OS of 100%, the best results, to our knowledge, reported for patients with LPHL treated with chemotherapy, reserving RT for a minority. For the first time, to our knowledge, our study shows prospectively that excellent EFS can be obtained while avoiding RT for a large majority of patients with low-stage LPHL who are given an effective chemotherapy regimen, using a carefully applied response evaluation comprising both anatomic and functional criteria.
Some patients with LPHL who have limited disease may be cured with surgery alone.12-14 Retrospective series included patients with a variety of clinical stages of disease; in addition, the extent of surgical resection was not uniform. In contrast, AHOD03P1 used a resection-alone strategy for a clearly defined group: patients with stage IA LPHL in a single node that was completely resected at diagnosis. Our results demonstrate that 75% of these patients can be spared chemotherapy or radiation. Surgery-only can be a successful strategy for a clearly defined population of pediatric patients with LPHL with resectable disease; relapses have been localized and treatable with limited chemotherapy.
It is noteworthy that recurrences in the TR group were at the site, or in adjacent nodes or nodal regions to the initially resected node. The implication of this observation is that even with efforts to limit the surgery-only strategy to those with a single node and TR, occult (microscopic) disease may be present in adjacent nodes or in an adjacent regional chain; such disease is usually eradicated with either chemotherapy or IFRT. This may impact outcomes in studies that either extend the surgical approach to those with more than one identified node at diagnosis or that use involved site radiation therapy,56 which should be designed to include nodes in adjacent regions.
The surgery-only strategy was limited to patients with involvement of a single node because of concern that if a second node was clinically involved, the likelihood was high that additional nodes may have subclinical involvement. TR status of our patients was confirmed by rapid central radiology review; this is only feasible in the context of a clinical trial. Therefore, our results may not be entirely reproducible when TR status is determined on an institutional basis or if clinicians use surgery-only for patients with more than one involved node. If clinicians extend the surgery-only approach beyond single-node LPHL, there may be a significantly higher risk for local recurrence. It is also necessary to consider the potential morbidity of more extensive surgical procedures.
Our results are excellent to date, but we recognize that it is critical to continue monitoring these patients. Obtaining biopsy specimens of suspicious adenopathy during follow-up is necessary to differentiate between recurrent LPHL and NHL. The development of NHL following a diagnosis of LPHL may represent a second malignancy or the transformation of LPHL to DLBCL over time.25-27 Chemotherapy may either prevent subsequent NHL or play a role in its emergence. With only three SMNs, it is not possible to give attribution nor to be confident that others will not emerge over time. Similarly, late relapses of LPHL can occur; multiple relapses have also been reported. Longer follow-up is needed, particularly for the patients treated with upfront chemotherapy, to determine if the EFS curves remain stable.
This study did not specify a salvage regimen for patients who relapsed after chemotherapy with or without RT. Although relapses are uncommon, it would be helpful to have data regarding the efficacy of salvage regimens for LPHL that do not include stem cell transplantation. Recent data have demonstrated the prognostic importance of variation in histology for adult and pediatric patients with LPHL.57,58 It will be valuable to examine the biopsy specimens of the patients in our study to see if initial histologic findings can predict risk for relapse or transformation to NHL.
Given the universal positivity for CD20, there is significant interest in incorporating rituximab, a chimeric anti-CD20 monoclonal antibody, into LPHL regimens. It has been used in adults for newly diagnosed and relapsed disease.59,60 Most patients respond to single-agent rituximab, but the majority relapse. Advani et al61 reported the long-term results of single-agent rituximab in 39 adult patients. All patients had either a CR or PR after four weekly doses; the most durable responses were seen in patients treated with maintenance rituximab every 6 months for 2 years. Twenty-three of 39 patients experienced a relapse; however, nine of 23 had evidence of transformation to aggressive B-cell NHL. There are no published pediatric trials that include rituximab. In addition, data regarding long-term effects of rituximab on immune function are lacking.
In conclusion, this is the first prospective study for pediatric patients with low-risk LPHL that minimized exposure to chemotherapy and RT. Many patients with stage IA, single-node disease were curable with surgical resection alone; relapses were localized and responsive to chemotherapy. In addition, 92% of patients who were treated with three cycles of AV-PC chemotherapy avoided upfront RT. Overall, these results confirm that children and young adults with low-risk LPHL can be successfully treated with a regimen designed to minimize their risk for late effects; OS remains 100% to date. We hope that future clinical trials will extend the approach of treating LPHL across all age groups with regimens to maintain excellent cure rates while minimizing exposure to both chemotherapy and RT.
Acknowledgment
We thank Sharon B. Murphy, MD, for encouraging us in the development of this protocol. Dr. Murphy recognized several decades ago that lymphocyte-predominant Hodgkin lymphoma is a clinically distinct entity from classical Hodgkin lymphoma and that selected patients may be cured by surgery alone.
Appendix
Response Definitions
Complete response (CR): resolution of pathologic palpable adenopathy, computed tomography (CT) scan showing at least 80% reduction in the product of the perpendicular diameters (PPD) of each nodal mass, negative positron emission tomography scan
Very good partial response (VGPR): at least 60% reduction in the product of the perpendicular diameter (PPD) of each of the areas of measurable disease on CT scan
Partial response (PR): at least 50% reduction in the PPD of each of the areas of measurable disease
Stable disease (SD): less than a partial response but not progressive disease
-
Progressive disease (PD): including any of the following:
At least 50% increase in the PPD of any of the involved nodes or nodal masses
At least 50% increase in the in the PPD of any of the focal organ lesions
New lesion(s)
Follow-Up Observations
Observation-only (total resection) patients:
Patients were followed with physical examinations every 3 months for 24 months, then every 6 months for an additional 3 years (to month 60 after enrollment). After month 60, patients were seen annually.
Computed tomography (CT) scans of involved sites were required every 6 months for the first 2 years after enrollment.
Patients who received chemotherapy with or without radiation:
Patients were followed with physical examinations every 3 months for 24 months, then every 6 months for an additional 3 years (to month 60 after enrollment). After month 60, patients were seen annually.
The study originally required CT scans of involved sites every 6 months for the first 2 years after completion of therapy, then yearly until month 60 (5 years after completion of therapy). However, the protocol was amended in March 2012 to eliminate surveillance CT scans after the month-18 CT scan, based on data from an earlier clinical trial of pediatric Hodgkin lymphoma.
Chest radiographs were required between scheduled CT scans.
Footnotes
Supported by Grant No. U10CA098543 and U10CA098413 from the US National Institutes of Health to the Children’s Oncology Group.
Presented in part at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012; the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013; and the Childhood, Adolescent and Young Adult Hodgkin Lymphoma 2nd International Symposium, Berlin, Germany, May 7-10, 2014.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Burton E. Appel, David C. Hodgson, Peter F. Ehrlich, Louis S. Constine, Cindy L. Schwartz
Provision of study materials or patients: Cindy L. Schwartz
Collection and assembly of data: Burton E. Appel, Allen B. Buxton, Robert E. Hutchison, Cindy L. Schwartz
Data analysis and interpretation: Burton E. Appel, Lu Chen, Allen B. Buxton, David C. Hodgson, Louis S. Constine, Cindy L. Schwartz
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Minimal Treatment of Low-Risk, Pediatric Lymphocyte-Predominant Hodgkin Lymphoma: A Report From the Children's Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Burton E. Appel
Consulting or Advisory Role: United Therapeutics
Lu Chen
No relationship to disclose
Allen B. Buxton
No relationship to disclose
Robert E. Hutchison
No relationship to disclose
David C. Hodgson
No relationship to disclose
Peter F. Ehrlich
No relationship to disclose
Louis S. Constine
Travel, Accommodations, Expenses: IBA
Cindy L. Schwartz
No relationship to disclose
REFERENCES
- 1.Appel BE, Chen L, Buxton A, et al. Impact of low-dose involved-field radiation therapy on pediatric patients with lymphocyte-predominant Hodgkin lymphoma treated with chemotherapy: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012;59:1284–1289. doi: 10.1002/pbc.24258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marafioti T, Hummel M, Anagnostopoulos I, et al. Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal-center B cells. N Engl J Med. 1997;337:453–458. doi: 10.1056/NEJM199708143370703. [DOI] [PubMed] [Google Scholar]
- 3.Ohno T, Stribley JA, Wu G, et al. Clonality in nodular lymphocyte-predominant Hodgkin’s disease. N Engl J Med. 1997;337:459–466. doi: 10.1056/NEJM199708143370704. [DOI] [PubMed] [Google Scholar]
- 4.Braeuninger A, Küppers R, Strickler JG, et al. Hodgkin and Reed-Sternberg cells in lymphocyte predominant Hodgkin disease represent clonal populations of germinal center-derived tumor B cells. Proc Natl Acad Sci USA. 1997;94:9337–9342. doi: 10.1073/pnas.94.17.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris NL. Shades of gray between large B-cell lymphomas and Hodgkin lymphomas: Differential diagnosis and biological implications. Mod Pathol. 2013;26:S57–S70. doi: 10.1038/modpathol.2012.182. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 6.Bodis S, Kraus MD, Pinkus G, et al. Clinical presentation and outcome in lymphocyte-predominant Hodgkin’s disease. J Clin Oncol. 1997;15:3060–3066. doi: 10.1200/JCO.1997.15.9.3060. [DOI] [PubMed] [Google Scholar]
- 7.Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: Report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J Clin Oncol. 1999;17:776–783. doi: 10.1200/JCO.1999.17.3.776. [DOI] [PubMed] [Google Scholar]
- 8.Nogová L, Reineke T, Brillant C, et al. Lymphocyte-predominant and classical Hodgkin’s lymphoma: A comprehensive analysis from the German Hodgkin Study Group. J Clin Oncol. 2008;26:434–439. doi: 10.1200/JCO.2007.11.8869. [DOI] [PubMed] [Google Scholar]
- 9.Shankar A, Daw S. Nodular lymphocyte predominant Hodgkin lymphoma in children and adolescents–a comprehensive review of biology, clinical course and treatment options. Br J Haematol. 2012;159:288–298. doi: 10.1111/bjh.12055. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Franssila KO, Saxén E. Hodgkin’s disease, lymphocytic predominance nodular. Increased risk for subsequent non-Hodgkin’s lymphomas. Cancer. 1983;51:2293–2300. doi: 10.1002/1097-0142(19830615)51:12<2293::aid-cncr2820511221>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Hansmann ML, Zwingers T, Böske A, et al. Clinical features of nodular paragranuloma (Hodgkin’s disease, lymphocyte predominance type, nodular) J Cancer Res Clin Oncol. 1984;108:321–330. doi: 10.1007/BF00390466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellegrino B, Terrier-Lacombe MJ, Oberlin O, et al. Lymphocyte-predominant Hodgkin’s lymphoma in children: Therapeutic abstention after initial lymph node resection--a Study of the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:2948–2952. doi: 10.1200/JCO.2003.01.079. [DOI] [PubMed] [Google Scholar]
- 13.Murphy SB, Morgan ER, Katzenstein HM, et al. Results of little or no treatment for lymphocyte-predominant Hodgkin disease in children and adolescents. J Pediatr Hematol Oncol. 2003;25:684–687. doi: 10.1097/00043426-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Mauz-Körholz C, Gorde-Grosjean S, Hasenclever D, et al. Resection alone in 58 children with limited stage, lymphocyte-predominant Hodgkin lymphoma-experience from the European network group on pediatric Hodgkin lymphoma. Cancer. 2007;110:179–185. doi: 10.1002/cncr.22762. [DOI] [PubMed] [Google Scholar]
- 15.Biasoli I, Stamatoullas A, Meignin V, et al. Nodular, lymphocyte-predominant Hodgkin lymphoma: A long-term study and analysis of transformation to diffuse large B-cell lymphoma in a cohort of 164 patients from the Adult Lymphoma Study Group. Cancer. 2010;116:631–639. doi: 10.1002/cncr.24819. [DOI] [PubMed] [Google Scholar]
- 16.Schlembach PJ, Wilder RB, Jones D, et al. Radiotherapy alone for lymphocyte-predominant Hodgkin’s disease. Cancer J. 2002;8:377–383. doi: 10.1097/00130404-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Wirth A, Yuen K, Barton M, et al. Long-term outcome after radiotherapy alone for lymphocyte-predominant Hodgkin lymphoma: A retrospective multicenter study of the Australasian Radiation Oncology Lymphoma Group. Cancer. 2005;104:1221–1229. doi: 10.1002/cncr.21303. [DOI] [PubMed] [Google Scholar]
- 18.Tsai HK, Mauch PM. Nodular lymphocyte-predominant Hodgkin lymphoma. Semin Radiat Oncol. 2007;17:184–189. doi: 10.1016/j.semradonc.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Chen RC, Chin MS, Ng AK, et al. Early-stage, lymphocyte-predominant Hodgkin’s lymphoma: Patient outcomes from a large, single-institution series with long follow-up. J Clin Oncol. 2010;28:136–141. doi: 10.1200/JCO.2009.24.0945. [DOI] [PubMed] [Google Scholar]
- 20.King MT, Donaldson SS, Link MP, et al. Management of nodular lymphocyte predominant Hodgkin lymphoma in the modern era. Int J Radiat Oncol Biol Phys. 2015;92:67–75. doi: 10.1016/j.ijrobp.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Eichenauer DA, Plütschow A, Fuchs M, et al. Long-term course of patients with stage IA nodular lymphocyte-predominant Hodgkin lymphoma: A report from the German Hodgkin Study Group. J Clin Oncol. 2015;33:2857–2862. doi: 10.1200/JCO.2014.60.4363. [DOI] [PubMed] [Google Scholar]
- 22.Karayalcin G, Behm FG, Gieser PW, et al. Lymphocyte predominant Hodgkin disease: Clinico-pathologic features and results of treatment--the Pediatric Oncology Group experience. Med Pediatr Oncol. 1997;29:519–525. doi: 10.1002/(sici)1096-911x(199712)29:6<519::aid-mpo1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson SS, Link MP, Weinstein HJ, et al. Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin’s disease. J Clin Oncol. 2007;25:332–337. doi: 10.1200/JCO.2006.08.4772. [DOI] [PubMed] [Google Scholar]
- 24.Hall GW, Katzilakis N, Pinkerton CR, et al. Outcome of children with nodular lymphocyte predominant Hodgkin lymphoma - a Children’s Cancer and Leukaemia Group report. Br J Haematol. 2007;138:761–768. doi: 10.1111/j.1365-2141.2007.06736.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang JZ, Weisenburger DD, Vose JM, et al. Diffuse large B-cell lymphoma arising in nodular lymphocyte predominant Hodgkin lymphoma: A report of 21 cases from the Nebraska Lymphoma Study Group. Leuk Lymphoma. 2004;45:1551–1557. doi: 10.1080/1042819031000149421. [DOI] [PubMed] [Google Scholar]
- 26.Al-Mansour M, Connors JM, Gascoyne RD, et al. Transformation to aggressive lymphoma in nodular lymphocyte-predominant Hodgkin’s lymphoma. J Clin Oncol. 2010;28:793–799. doi: 10.1200/JCO.2009.24.9516. [DOI] [PubMed] [Google Scholar]
- 27.Eyre TA, Gatter K, Collins GP, et al. Incidence, management, and outcome of high-grade transformation of nodular lymphocyte predominant Hodgkin lymphoma: Long-term outcomes from a 30-year experience. Am J Hematol. 2015;90:E103–E110. doi: 10.1002/ajh.23989. [DOI] [PubMed] [Google Scholar]
- 28.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer, Lyon, France; 2008. [Google Scholar]
- 29.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley & Sons, New York, NY: 1980. [Google Scholar]
- 30.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng AK. Current survivorship recommendations for patients with Hodgkin lymphoma: Focus on late effects. Blood. 2014;124:3373–3379. doi: 10.1182/blood-2014-05-579193. [DOI] [PubMed] [Google Scholar]
- 32.Savage KJ, Skinnider B, Al-Mansour M, et al. Treating limited-stage nodular lymphocyte predominant Hodgkin lymphoma similarly to classical Hodgkin lymphoma with ABVD may improve outcome. Blood. 2011;118:4585–4590. doi: 10.1182/blood-2011-07-365932. [DOI] [PubMed] [Google Scholar]
- 33.Xing KH, Connors JM, Lai A, et al. Advanced-stage nodular lymphocyte predominant Hodgkin lymphoma compared with classical Hodgkin lymphoma: A matched pair outcome analysis. Blood. 2014;123:3567–3573. doi: 10.1182/blood-2013-12-541078. [DOI] [PubMed] [Google Scholar]
- 34.Krasin MJ, Constine LS, Friedman DL, et al. Radiation-related treatment effects across the age spectrum: Differences and similarities or what the old and young can learn from each other. Semin Radiat Oncol. 2010;20:21–29. doi: 10.1016/j.semradonc.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 36.Heidenreich PA, Schnittger I, Strauss HW, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J Clin Oncol. 2007;25:43–49. doi: 10.1200/JCO.2006.07.0805. [DOI] [PubMed] [Google Scholar]
- 37.Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308–1315. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 38.Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117:412–418. doi: 10.1182/blood-2010-06-291328. [DOI] [PubMed] [Google Scholar]
- 39.Ng AK. Review of the cardiac long-term effects of therapy for Hodgkin lymphoma. Br J Haematol. 2011;154:23–31. doi: 10.1111/j.1365-2141.2011.08713.x. [DOI] [PubMed] [Google Scholar]
- 40.Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med. 1996;334:745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 41.Wolden SL, Lamborn KR, Cleary SF, et al. Second cancers following pediatric Hodgkin’s disease. J Clin Oncol. 1998;16:536–544. doi: 10.1200/JCO.1998.16.2.536. [DOI] [PubMed] [Google Scholar]
- 42.Swerdlow AJ, Barber JA, Hudson GV, et al. Risk of second malignancy after Hodgkin’s disease in a collaborative British cohort: The relation to age at treatment. J Clin Oncol. 2000;18:498–509. doi: 10.1200/JCO.2000.18.3.498. [DOI] [PubMed] [Google Scholar]
- 43.Ng AK, Bernardo MVP, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: Long-term risks and risk factors. Blood. 2002;100:1989–1996. doi: 10.1182/blood-2002-02-0634. [DOI] [PubMed] [Google Scholar]
- 44.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 45.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol. 2007;25:1489–1497. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 46.Constine LS, Tarbell N, Hudson MM, et al. Subsequent malignancies in children treated for Hodgkin’s disease: Associations with gender and radiation dose. Int J Radiat Oncol Biol Phys. 2008;72:24–33. doi: 10.1016/j.ijrobp.2008.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turcotte LM, Whitton JA, Friedman DL, et al. Risk of subsequent neoplasms during the fifth and sixth decades of life in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2015;33:3568–3575. doi: 10.1200/JCO.2015.60.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Brien MM, Donaldson SS, Balise RR, et al. Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol. 2010;28:1232–1239. doi: 10.1200/JCO.2009.24.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omer B, Kadan-Lottick NS, Roberts KB, et al. Patterns of subsequent malignancies after Hodgkin lymphoma in children and adults. Br J Haematol. 2012;158:615–625. doi: 10.1111/j.1365-2141.2012.09211.x. [DOI] [PubMed] [Google Scholar]
- 51.Hobbie WL, Ginsberg JP, Ogle SK, et al. Fertility in males treated for Hodgkins disease with COPP/ABV hybrid. Pediatr Blood Cancer. 2005;44:193–196. doi: 10.1002/pbc.20172. [DOI] [PubMed] [Google Scholar]
- 52.Sieniawski M, Reineke T, Josting A, et al. Assessment of male fertility in patients with Hodgkin’s lymphoma treated in the German Hodgkin Study Group (GHSG) clinical trials. Ann Oncol. 2008;19:1795–1801. doi: 10.1093/annonc/mdn376. [DOI] [PubMed] [Google Scholar]
- 53.Green DM, Sklar CA, Boice JD, Jr, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: Results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2374–2381. doi: 10.1200/JCO.2008.21.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94:525–533. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 55.Shankar A, Hall GW, Gorde-Grosjean S, et al. Treatment outcome after low intensity chemotherapy [CVP] in children and adolescents with early stage nodular lymphocyte predominant Hodgkin’s lymphoma - an Anglo-French collaborative report. Eur J Cancer. 2012;48:1700–1706. doi: 10.1016/j.ejca.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Hodgson DC, Dieckmann K, Terezakis S, et al. Implementation of contemporary radiation therapy planning concepts for pediatric Hodgkin lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Pract Radiat Oncol. 2015;5:85–92. doi: 10.1016/j.prro.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Hartmann S, Eichenauer DA, Plütschow A, et al. The prognostic impact of variant histology in nodular lymphocyte-predominant Hodgkin lymphoma: A report from the German Hodgkin Study Group (GHSG) Blood. 2013;122:4246–4252. doi: 10.1182/blood-2013-07-515825. quiz 4292. [DOI] [PubMed] [Google Scholar]
- 58.Shankar AG, Kirkwood AA, Hall GW, et al. Childhood and adolescent nodular lymphocyte predominant Hodgkin lymphoma - A review of clinical outcome based on the histological variants. Br J Haematol. 2015 doi: 10.1111/bjh.13540. [DOI] [PubMed] [Google Scholar]
- 59.Saini KS, Azim HA, Jr, Cocorocchio E, et al. Rituximab in Hodgkin lymphoma: Is the target always a hit? Cancer Treat Rev. 2011;37:385–390. doi: 10.1016/j.ctrv.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Mocikova H, Pytlik R, Stepankova P, et al. Can rituximab improve the outcome of patients with nodular lymphocyte-predominant Hodgkin’s lymphoma? Acta Haematol. 2015;134:187–192. doi: 10.1159/000381327. [DOI] [PubMed] [Google Scholar]
- 61.Advani RH, Horning SJ, Hoppe RT, et al. Mature results of a phase II study of rituximab therapy for nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol. 2014;32:912–918. doi: 10.1200/JCO.2013.53.2069. [DOI] [PubMed] [Google Scholar]