Abstract
Background
Enterotoxigenic Escherichia coli (ETEC) causes diarrheal disease. Antigenic and structural heterogeneity among ETEC colonization factors has complicated vaccine development efforts. Identifying and characterizing conserved ETEC antigens that induce protective immunity is therefore of interest. We previously characterized three proteins (MipA, Skp, and ETEC_2479) that protected mice in an intranasal ETEC challenge model after vaccination. However, these proteins are conserved not only in multiple ETEC isolates, but also in commensal bacteria. While the impact of inactivated viral vaccines and live-attenuated bacterial vaccines on the host microbiota have been examined, the potential impact of using subunit vaccines consisting of antigens that are also encoded by commensal organisms has not been investigated.
Findings
We addressed this issue by characterizing changes to mouse intestinal microbiomes as a function of vaccination. We failed to observe significant changes to mouse health, to mouse weight gain as a function of time, or to the diversity or richness of mouse intestinal microbiomes, as measured by analyzing alpha- and beta-diversity, as well as overall community structure, before and after vaccination.
Conclusions
We conclude that despite the conservation of MipA, Skp, and ETEC_2479 among Gram-negative bacteria, vaccination with these antigens fails to alter significantly the host intestinal microbiome.
Background
Enterotoxigenic Escherichia coli (ETEC) causes hundreds of millions of cases of diarrhea annually, particularly in developing countries [1]. In addition to their acute impact on human health, repeated infections also contribute to delayed growth and malnutrition [1]. Many vaccine strategies have focused on ETEC colonization factors (CFs). These heterogeneous surface structures function in attachment by binding to host glycoprotein conjugates [2]. Numerous CFs have been described, but many ETEC strains do not produce a recognizable CF [3]. A need therefore exists to identify new vaccine targets that are independent of strain-specific CFs.
We previously characterized three ETEC H10407 proteins as protective antigens in a mouse model involving intranasal bacterial challenge [4]. Antisera raised against the ETEC MipA, Skp, and ETEC_2479 proteins protected HCT-8 cells from attachment by multiple ETEC strains [4]. Immunization with these antigens also protected mice from an otherwise lethal challenge with intranasally administered ETEC H10407 [4]. Skp is a molecular chaperone that rescues misdirected outer membrane proteins [5]. MipA is an immunoreactive protein [6] that belongs to a family of proteins involved in remodeling peptidoglycan. ETEC_2479 is predicted to function as an outer membrane porin involved in long chain fatty acid transport [7]. The intranasal challenge model [8, 9] is a useful alternative for ETEC vaccine studies because mice do not naturally develop diarrheal disease after oral ETEC challenge [10]. This model also permits the quantification of mouse survival, bacterial clearance, and host immune responses, and stimulates mucosal immune responses, especially secretory IgA (sIgA) responses that are important to blocking bacterial adherence to mucosal surfaces.
Identifying broadly conserved, protective antigens is important to vaccine development. However, MipA, Skp, and ETEC_2479 are conserved not only among pathogenic ETEC strains, but also among the commensal Proteobacteria. This may be an important issue because it is known that alteration of commensals can influence susceptibility to gastrointestinal disease [11] and vaccine efficacy [12]. Several previous studies have begun to address this issue. Rotavirus vaccination of humans did not have a major impact to infant microbiomes [13]. A challenge of cynomolgus macaques with an oral-live attenuated Shigella strain found a possible protective role for the microbiota and highlighted the importance of considering host genetics in vaccine studies [14]. Oral immunization with the live-attenuated typhoid vaccine strain Ty21a did not cause significant perturbation of the fecal microbiota related to vaccine administration [15]. Thus, while the impact of inactivated viral vaccines and live-attenuated bacterial vaccines on the host microbiota have been examined, the potential impact of using subunit vaccines consisting of antigens that are also encoded by commensal organisms has not been investigated.
We hypothesized that using the conserved antigens MipA, Skp, and ETEC_2479 as subunit vaccine candidates could negatively impact the health of the host by affecting the intestinal microbiota. Herein, we tested and subsequently refuted this hypothesis.
Methods
Ethics statement
Animal experiments were performed according to Kansas State University Institutional Animal Care and Use Committee-approved protocols (IACUC #3648). This institution complies with all applicable provisions of the Animal Welfare Act and other Federal statutes and regulations relating to animals.
Antigen purification
Escherichia coli BL21(DE3) strains expressing individual antigens were grown overnight as described [4]. After purification, proteins were dialyzed into glycerol in Pierce Slide-A-Lyzer dialysis cassettes. Protein concentrations were quantified by using the Precision Red Advanced Protein Assay (Cytoskeleton, Inc.).
Polyclonal antisera production
Female BALB/c mice of matched age (6 weeks at initial vaccination) were obtained from the Jackson Laboratory (Bar Harbor, Maine) and handled as described previously [4]. Mice were housed (5 per group) in microisolator cages (1 cage per group) and provided with food and water ad libitum. Antigens (20 µg/dose) were mixed with 2.5 µg of cholera toxin in 25 µl phosphate-buffered saline (PBS) and then administered intranasally to the external nares of mice that had been anesthetized with isoflurane [4]. Two booster doses were administered, at 2- and 4-weeks after the initial vaccination. Mice were euthanized 2 weeks after the final immunization and exsanguinated. The blood was processed into serum using centrifugation for 2 min at 2500g in a BD microcontainer serum separator tube. Control serum samples were also obtained from mice immunized with PBS or with EHEC EspB.
Immunoassays
Enzyme-linked immunosorbent assays (ELISAs) were performed as previously described [4], using serial dilutions of mouse serum samples and polystyrene 96-well, flat bottom plates (Whatman) coated with purified antigens or with bovine serum albumin (BSA; 0.5 µg/ml). Plates were developed with 1-StepTM Ultra TMB-ELISA (Thermo) and quenched with 3 N H2SO4. Absorbance was read at 450 nm.
Fecal DNA extraction
A fecal pellet from each mouse was collected weekly, with the initial collection prior to the first vaccination and the final collection 2 weeks after the final vaccination. After collection, the fecal pellets were stored at −80 °C until DNA extraction could be performed. Prior to DNA extraction using QIAamp DNA Stool Mini Kits (Qiagen), 1.4 ml of Buffer ASL was added to fecal samples on ice. Fecal pellets were vortexed until completely resuspended and DNA was extracted by following the manufacturer’s protocol.
Library construction and sequencing
Library construction and sequencing was performed essentially as described [16]. Prior to PCR, DNA concentrations were determined via fluorometry (Qubit dsDNA BR assay, Life Technologies, Carlsbad, CA) and normalized to a standard concentration. Bacterial Microbial 16S rRNA amplicons were generated via amplification of the V4 hypervariable region of the 16S rRNA gene using single-indexed universal primers as described previously [U515F/806R; 17] flanked by Illumina standard adapter sequences. Primer sequences are available at proBase [18; http://www.microbial-ecology.net/probebase/]. PCR was performed using the following parameters: 98 °C(3:00) + [98 °C(0:15) + 50 °C(0:30) + 72 °C(0:30)] × 25 cycles +72 °C(7:00). Following PCR, amplicons were pooled for sequencing using the Illumina MiSeq platform and V2 chemistry with 2 × 250 bp paired-end reads.
Informatics analysis
Informatics analysis was performed essentially as described [16]. FLASH software was used to assemble contiguous DNA sequences [18]. Sequences were culled if determined to be short after trimming for a base quality less than 31. Reference-based and de novo chimera detection and removal was conducted using Qiime v1.8 software [19]. Remaining contiguous sequences were assigned to operational taxonomic units (OTUs) via de novo clustering with a criterion of 97 % nucleotide identity as described [16]. Annotation of selected OTUs was performed using BLAST [20] against the Greengenes database [21] of 16S rRNA sequences and taxonomy. Principal component analysis was performed using ¼ root-transformed OTU relative abundance data via a non-linear iterative partial least squares (NIPALS) algorithm, implemented using an open access Excel macro available from the Riken Institute (http://prime.psc.riken.jp/Metabolomics_Software/StatisticalAnalysisOnMicrosoftExcel/index.html). Sequence data were deposited in the NCBI Sequence Read Archive (SRA) under the BioProjectID PRJNA320839.
Statistical analyses
Statistical analysis was performed using Sigma Plot 12.3 (Systat Software Inc., Carlsbad, CA). Interactions and differences between treatment groups and time-points in Chao1 indices were determined using 2-way analysis of variance (ANOVA). Analysis of molecular variance (AMOVA) was implemented in a general linear model using SPSS software, version 23 (IBM, Armonk, NY). Results were considered statistically significant for p values ≤0.05.
Results
Mice were vaccinated intranasally with purified, recombinant forms of ETEC MipA, Skp, and ETEC_2479 [4], as well as with a purified, recombinant form of E. coli O157:H7 EDL933 EspB [22]. EspB was used as an external control because it is immunogenic but is not expressed by either ETEC or by commensal bacteria [23]. After 3 immunizations, mice were sacrificed and their serum was used in ELISAs to quantify antibody titers. All mice (5/group) produced detectable IgG titers (Fig. 1a). Mouse health and weight gain were monitored during the vaccination regimen. Neither obvious changes to mouse health or behavior, nor changes in the rate of weight gain were observed. The weights of the mice after vaccination were 21.1 ± 1.0 g for the PBS control group, 20.7 ± 1.0 g for EspB, 21.2 ± 0.9 g for MipA, 21.2 ± 2.0 g for Skp, and 21.3 ± 0.9 g for ETEC_2479 (p > 0.05). Fecal IgA responses for mice immunized with either MipA, Skp, or ETEC_2479 ranged from a 13.2 ± 2.2, 13.0 ± 3.4, and 26.8 ± 4.6 fold-increase as compared with control groups, respectively [4].
Fig. 1.
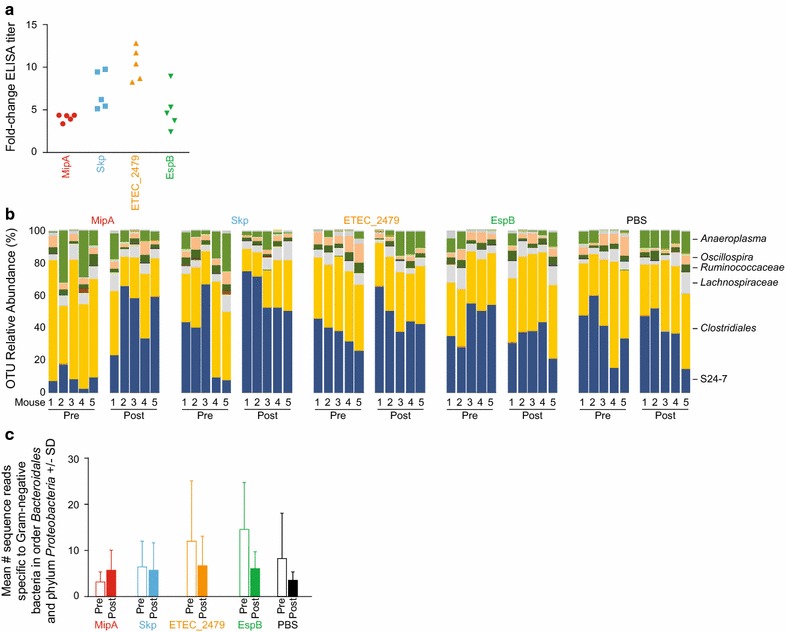
Vaccination with ETEC MipA, Skp, and ETEC_2479. a Serum IgG responses in mice. Data are plotted as the fold-change in serum IgG after immunization with the indicated antigens, n = 5/group. b Bar chart showing relative abundance of all operational taxonomic units (OTUs) detected in the feces of mice prior to (pre) and 6 weeks after (post) vaccination with the indicated antigens, as detected using 16S rRNA amplicon sequencing. The identities of dominant taxa are shown at the right. c Mean number + standard deviation (SD) of sequence reads that were specific to Bacteriodales or Proteobacteria in indicated treatment groups
To determine whether vaccination affected the diversity and overall composition of the mouse intestinal microbiota, 16S rRNA amplicon sequencing was performed using DNA extracted from feces collected prior to vaccination, from the mid-point of the vaccination regimen (after the second vaccination), and two weeks after the final vaccination, as template. Following vaccination, there were no apparent differences in the microbial profiles when resolved to the level of operational taxonomic unit (OTU), and the same six OTUs (families S24-7, Lachnospiraceae, Ruminococcaceae, order Clostridiales, Oscillospira sp., and Anaeroplasma sp.) dominated the pre- and post-vaccination microbiota of all groups (Fig. 1b). Regarding the effect of vaccination on other Gram-negative taxa that potentially express the targeted antigens, i.e., microbes in the order Bacteroidales or phylum Proteobacteria, no differences were detected between pre- and post-vaccination samples in the relative abundance of these bacteria (Fig. 1c). The Chao1 index, a measure of α-diversity (i.e., within samples), was also compared between groups to determine if vaccination affected the richness or distribution of microbes. Two-way ANOVA detected no significant differences between groups, or between pre- and post-vaccination samples (Fig. 2a). Samples obtained from the mid-point of the vaccination regimen (after the second vaccination) were also analyzed and were not significantly different from pre-or post-vaccination samples (Fig. 2a). Similarly, neither the Shannon diversity index, nor the raw number of unique sequences detected in each group, were significantly different among groups (Fig. 2b, c).
Fig. 2.
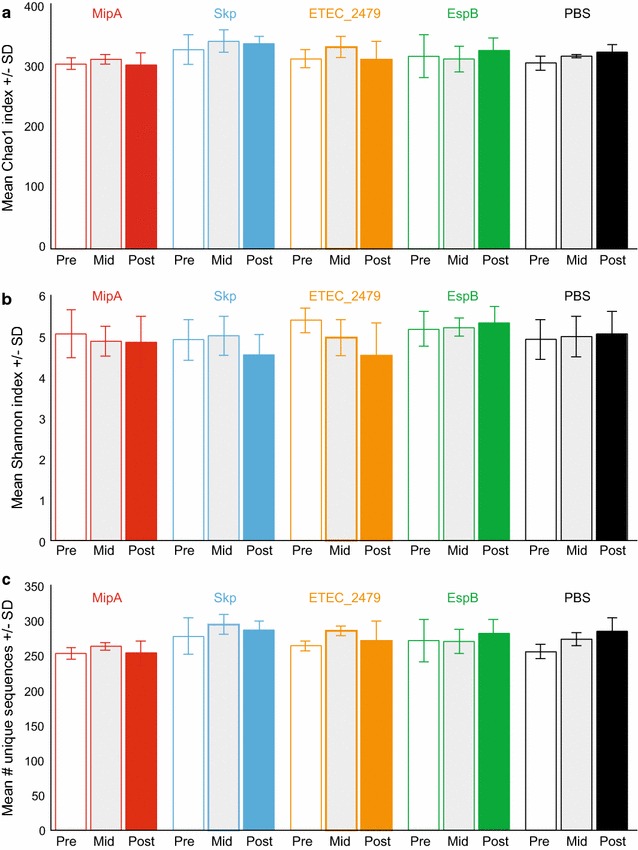
Sequence diversity among treatment groups. a Bar chart showing mean + standard deviation (SD) Chao1 a-diversity index of fecal microbiota in mice pre- and post-vaccination with the indicated antigens, as detected using 16S rRNA amplicon sequencing. b Mean Shannon diversity indices. c Mean number of unique sequences in treatment groups
Principal component analysis (PCA) was performed both to assess β-diversity (i.e., between samples) and to determine if vaccination induced changes in the overall composition of the microbiota. No clustering of post-vaccination samples was observed in plots of principal component 1 (PC1) against PC2 or PC3, which accounted collectively for over 60 % of the variability between samples, suggesting that there were negligible shifts in the bacterial community composition of any treatment group (Fig. 3a). A comparison of the mean intragroup unweighted UniFrac distance between pre- and post-vaccination samples detected no greater dissimilarity between the two samples in treated groups as compared to the control group, as determined by performing ANOVA with post hoc Dunnett’s tests (Fig. 3b). Supporting our prior analysis, AMOVA detected no significant effect of vaccination treatment (p = 0.053; F value = 3.302), and also no significant effect of time-point (p = 0.359; F value = 4.517), using the first and last samples as time-points.
Fig. 3.
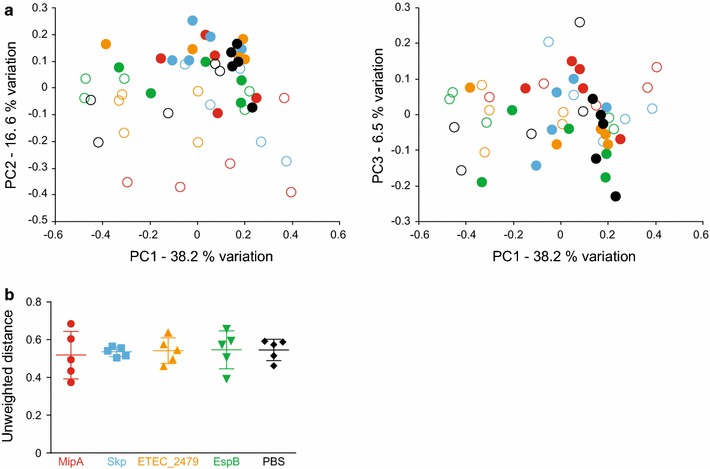
Principal component analyses and UniFrac distances. a Unweighted principal component analyses showing β-diversity of fecal microbiota in mice pre- and post-vaccination with the indicated antigens, as detected via 16S rRNA amplicon sequencing. Principal component 1 (PC1) versus PC2 (left) and PC1 versus PC3 (right) are shown. Color-coding is identical to Fig. 2, with open symbols representing pre-vaccination and closed symbols representing post-vaccination samples. b Mean intragroup unweighted UniFrac distances between pre- and post-vaccination samples
Discussion
The discovery and characterization of broadly conserved ETEC vaccine antigens that are independent of strain-specific CFs is of emerging interest. However, an important consideration is whether targeting antigens also expressed by commensal flora will negatively impact host health or vaccine efficacy. We have characterized the ETEC proteins MipA, Skp, and ETEC_2479 for their protective efficacy in an intranasal challenge model. These proteins are highly conserved not only in diverse ETEC isolates, but also in commensal Proteobacteria and other E. coli and Shigella strains, sharing ~99 % identity with the corresponding Shigella proteins. Because altering commensal abundance and diversity may affect host health and vaccine efficacy, we were therefore interested in determining whether using these antigens in a subunit vaccine would affect the mouse microbiota. Commensal E. coli strains may contribute to colonization resistance against pathogens [24]. For example, E. coli Nissle 1917 has been extensively characterized as a probiotic agent [25] and could potentially function by competing for nutrients that are required by pathogens [24]. They also play important, though incompletely defined roles in maintaining intestinal homeostasis [26].
We did not observe changes to mouse health, behavior, or rate of weight gain following intranasal vaccination with either MipA, Skp, or ETEC_2479. We also observed no significant differences among the microbial profiles when resolved to the level of operational taxonomic unit (OTU), as determined by performing 16S rRNA amplicon sequencing. Analysis of the Chao1 index, the Shannon diversity index, and the raw number of unique sequences detected in each treatment group, also failed to reveal any significant differences. PCA analysis and comparison of UniFrac distances also suggested negligible shifts in the bacterial community composition of any treatment group.
While no consistent shifts in the composition of the fecal microbiota were detected following vaccination against the three conserved candidate antigens, a closer examination of the effects of vaccination on the relative abundance of taxa expected to express these antigens, i.e., bacteria within order Bacteroidales and phylum Proteobacteria, was performed. While no vaccination-dependent differences were detected in the relative abundance of these groups, we recognize that both Bacteroidales and Proteobacteria are rare taxa in the current samples. This is not however unique to this cohort of mice, as we have demonstrated very comparable levels of these taxa in multiple genetic backgrounds of mice purchased from the same vendor and the overwhelming majority of gut bacteria in most research mice are Gram-positive [27]. Regardless, the low numbers of Gram-negative bacteria present in the present cohorts were not affected by vaccination. It will be important in future studies to validate these studies using diarrheal disease models for ETEC in either mice or piglets.
Overall, despite the conservation of MipA, Skp, and ETEC_2479 among Gram-negative bacteria, vaccination with these antigens fails to alter significantly the host intestinal microbiota and suggests that their inclusion in future ETEC vaccine preparations may be efficacious.
Authors' contributions
MPH, ACE, and YY performed the experiments. ACE and PRH designed the study. ACE and PRH wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The project described was supported in part by grant number AI092266 from the National Institute of Allergy and Infectious Diseases (NIAID). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID. We thank William Spollen for assistance with depositing the sequence data in the NCBI Sequence Read Archive (SRA).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Sequence data were deposited in the NCBI Sequence Read Archive (SRA) under the BioProjectID PRJNA320839.
Consent to publish
This manuscript does not describe any details, images, or videos relating to individual participants, and thus did not require written informed consent for publication.
Abbreviations
- AMOVA
analysis of molecular variance
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- ELISA
enzyme-linked immunosorbent assay
- ETEC
enterotoxigenic Escherichia coli
- OTU
operational taxonomic unit
- PBS
phosphate-buffered saline
- PCA
principal component analysis
- sIgA
secretory IgA
Contributor Information
Michael P. Hays, Email: hays@vet.k-state.edu
Aaron C. Ericsson, Email: ericssona@missouri.edu
Yang Yang, Email: sheepyang@k-state.edu.
Philip R. Hardwidge, Phone: 785-532-2506, Email: hardwidg@vet.k-state.edu
References
- 1.Fleckenstein JM, Hardwidge PR, Munson GP, Rasko DA, Sommerfelt H, Steinsland H. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 2010;12(2):89–98. doi: 10.1016/j.micinf.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knutton S, Lloyd DR, Candy DC, McNeish AS. Adhesion of enterotoxigenic Escherichia coli to human small intestinal enterocytes. Infect Immun. 1985;48(3):824–831. doi: 10.1128/iai.48.3.824-831.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleckenstein J, Sheikh A, Qadri F. Novel antigens for enterotoxigenic Escherichia coli vaccines. Exp Rev Vacc. 2014;13(5):631–639. doi: 10.1586/14760584.2014.905745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Hays M, Lim F, Foster LJ, Zhou M, Zhu G, et al. Protective enterotoxigenic Escherichia coli antigens in a murine intranasal challenge model. PLoS Negl Trop Dis. 2015;9(8):e0003924. doi: 10.1371/journal.pntd.0003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sklar JG, Wu T, Kahne D, Silhavy TJ. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev. 2007;21(19):2473–2484. doi: 10.1101/gad.1581007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy K, Bartels S, Qadri F, Fleckenstein JM. Enterotoxigenic Escherichia coli elicits immune responses to multiple surface proteins. Infect Immun. 2010;78(7):3027–3035. doi: 10.1128/IAI.00264-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar GB, Black PN. Linker mutagenesis of a bacterial fatty acid transport protein. Identification of domains with functional importance. J Biol Chem. 1991;266(2):1348–1353. [PubMed] [Google Scholar]
- 8.Turbyfill KR, Hartman AB, Oaks EV. Isolation and characterization of a Shigella flexneri invasin complex subunit vaccine. Infect Immun. 2000;68(12):6624–6632. doi: 10.1128/IAI.68.12.6624-6632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Verg LL, Mallett CP, Collins HH, Larsen T, Hammack C, Hale TL. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect Immun. 1995;63(5):1947–1954. doi: 10.1128/iai.63.5.1947-1954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy K, Hamilton D, Allen KP, Randolph MP, Fleckenstein JM. The EtpA exoprotein of enterotoxigenic Escherichia coli promotes intestinal colonization and is a protective antigen in an experimental model of murine infection. Infect Immun. 2008;76(5):2106–2112. doi: 10.1128/IAI.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Pan Y, Yan R, Zeng B, Wang H, Zhang X, et al. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat Immunol. 2015;16(9):918–926. doi: 10.1038/ni.3233. [DOI] [PubMed] [Google Scholar]
- 12.Nakaya HI, Bruna-Romero O. Is the gut microbiome key to modulating vaccine efficacy? Exp Rev Vacc. 2015;14(6):777–779. doi: 10.1586/14760584.2015.1040395. [DOI] [PubMed] [Google Scholar]
- 13.Ang L, Arboleya S, Lihua G, Chuihui Y, Nan Q, Suarez M, et al. The establishment of the infant intestinal microbiome is not affected by rotavirus vaccination. Sci Rep. 2014;4:7417. doi: 10.1038/srep07417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seekatz AM, Panda A, Rasko DA, Toapanta FR, Eloe-Fadrosh EA, Khan AQ, et al. Differential response of the cynomolgus macaque gut microbiota to Shigella infection. PloS ONE. 2013;8(6):e64212. doi: 10.1371/journal.pone.0064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eloe-Fadrosh EA, McArthur MA, Seekatz AM, Drabek EF, Rasko DA, Sztein MB, et al. Impact of oral typhoid vaccination on the human gut microbiota and correlations with S. Typhi-specific immunological responses. PloS ONE. 2013;8(4):e62026. doi: 10.1371/journal.pone.0062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart ML, Meyer A, Johnson PJ, Ericsson AC. Comparative evaluation of DNA extraction methods from feces of multiple host species for downstream next-generation sequencing. PloS ONE. 2015;10(11):e0143334. doi: 10.1371/journal.pone.0143334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;15(108 Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczynski J, Stombaugh J, Walters WA, Gonzalez A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr Protoc Bioinformatics. 2011;Chapter 10:Unit 10.7. doi:10.1002/0471250953.bi1007s36. [DOI] [PMC free article] [PubMed]
- 20.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnenberg MS, Yu J, Kaper JB. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175(15):4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tacket CO, Sztein MB, Losonsky G, Abe A, Finlay BB, McNamara BP, et al. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect Immun. 2000;68(6):3689–3695. doi: 10.1128/IAI.68.6.3689-3695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conway T, Cohen PS. Commensal and pathogenic Escherichia coli metabolism in the gut. Microbiol Spectr. 2015;3(3). doi:10.1128/microbiolspec.MBP-0006-2014. [DOI] [PMC free article] [PubMed]
- 25.Grozdanov L, Raasch C, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, et al. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J Bacteriol. 2004;186(16):5432–5441. doi: 10.1128/JB.186.16.5432-5441.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin R, Miquel S, Ulmer J, Kechaou N, Langella P, Bermudez-Humaran LG. Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease. Microb Cell Fact. 2013;12:71. doi: 10.1186/1475-2859-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ericsson AC, Davis JW, Spollen W, Bivens N, Givan S, Hagan CE, et al. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. PloS ONE. 2015;10(2):e0116704. doi: 10.1371/journal.pone.0116704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequence data were deposited in the NCBI Sequence Read Archive (SRA) under the BioProjectID PRJNA320839.


