Summary
Pathogenic isolates of Fusarium oxysporum, distinguished as formae speciales (f. spp.) on the basis of their host specificity, cause crown rots, root rots and vascular wilts on many important crops worldwide. Fusarium oxysporum f. sp. cepae (FOC) is particularly problematic to onion growers worldwide and is increasing in prevalence in the UK. We characterized 31 F. oxysporum isolates collected from UK onions using pathogenicity tests, sequencing of housekeeping genes and identification of effectors. In onion seedling and bulb tests, 21 isolates were pathogenic and 10 were non‐pathogenic. The molecular characterization of these isolates, and 21 additional isolates comprising other f. spp. and different Fusarium species, was carried out by sequencing three housekeeping genes. A concatenated tree separated the F. oxysporum isolates into six clades, but did not distinguish between pathogenic and non‐pathogenic isolates. Ten putative effectors were identified within FOC, including seven Secreted In Xylem (SIX) genes first reported in F. oxysporum f. sp. lycopersici. Two highly homologous proteins with signal peptides and RxLR motifs (CRX1/CRX2) and a gene with no previously characterized domains (C5) were also identified. The presence/absence of nine of these genes was strongly related to pathogenicity against onion and all were shown to be expressed in planta. Different SIX gene complements were identified in other f. spp., but none were identified in three other Fusarium species from onion. Although the FOC SIX genes had a high level of homology with other f. spp., there were clear differences in sequences which were unique to FOC, whereas CRX1 and C5 genes appear to be largely FOC specific.
Keywords: effector genes, Fusarium basal rot, Fusarium oxysporum f. sp. cepae, onion, pathogenicity, Secreted In Xylem (SIX)
Introduction
Fusarium oxysporum is a major pathogen of many important crops worldwide, causing crown and root rots as well as vascular wilts (Leslie and Summerell, 2006). The soil‐borne fungus affects a very wide range of crop hosts, including onion, leek, lettuce, tomato, brassicas, asparagus, cucurbits, peppers, coriander, spinach, basil, beans, peas, strawberry, watermelon and banana, and also important non‐food crops, such as carnation and narcissus (Leslie and Summerell, 2006; Michielse and Rep, 2009). Its wide host range, as well as its economic and scientific impact, means that F. oxysporum was recently identified as the fifth most important plant‐pathogenic fungus (Dean et al., 2012). Fusarium oxysporum is a species complex and includes both non‐pathogenic and pathogenic isolates. Non‐pathogenic F. oxysporum isolates commonly occur in the soil as saprophytes, while some have been identified as biocontrol agents and endophytes (Alabouvette et al., 2009). Pathogenic F. oxysporum isolates are distinguished as formae speciales (f. spp.) on the basis of their host specificity (Leslie and Summerell, 2006), and more than 120 have been identified (Michielse and Rep, 2009). Recent advances in the understanding of the pathogenicity in F. oxysporum have been made following publication of the genome of F. oxysporum f. sp. lycopersici (FOL), which infects tomato (Ma et al., 2010). This led to the discovery of lineage‐specific mobile pathogenicity chromosomes which contain pathogenicity‐related genes. These include Secreted In Xylem (SIX) genes, the products of which are small effector proteins secreted by FOL during the colonization of tomato plants (Ma et al., 2010). So far, 14 SIX genes have been identified in FOL, which show no homology with each other or with any other sequenced gene (Houterman et al., 2007; Schmidt et al., 2013), except for SIX6 which has homologues in Colletotrichum (Gawehns et al., 2014). SIX1 (also known as Avr3), SIX3 (Avr2), SIX4 (Avr1) and SIX5 are recognized by resistance genes which have been introgressed into tomato (Houterman et al., 2008, 2009; Ma et al., 2015; Rep et al., 2004; Takken and Rep, 2010), whereas gene knock‐outs have demonstrated that SIX1, SIX3, SIX5 and SIX6 contribute directly to virulence (Gawehns et al., 2014; Houterman et al., 2009; Ma et al., 2015; Rep, 2005; Takken and Rep, 2010). SIX genes have also been found in other f. spp. of F. oxysporum (Fraser‐Smith et al., 2014; Sasaki et al., 2015b), notably SIX1, SIX4, SIX8 and SIX9 in an F. oxysporum isolate infecting Arabidopsis and Brassica (Thatcher et al., 2012), SIX6 in F. oxysporum f. sp. vasinfectum (Chakrabarti et al., 2011), SIX1 and SIX6 in f. sp. betae (Covey et al., 2014), SIX1, SIX7 and SIX10 in f. spp. canariensis and lini (Laurence et al., 2015), SIX1, SIX7 and SIX8 in f. sp. cubense (Meldrum et al., 2012) and SIX3, SIX5 and SIX7 in f. sp. cepae (Sasaki et al., 2015b). In addition, SIX4 has been shown to play a role in the virulence of F. oxysporum f. sp. conglutinans, the cause of cabbage yellows (Kashiwa et al., 2013).
The genetically heterogeneous nature and lack of reliable morphological characters in the F. oxysporum complex have meant that distinguishing between pathogenic and non‐pathogenic isolates and between different f. spp. has been challenging, and has previously relied on pathogenicity tests on different host plants. Molecular methods based on standard approaches, such as DNA fingerprinting and multilocus genotyping using housekeeping genes, have failed to reliably distinguish between different f. spp. (O'Donnell et al., 1998), but the presence/absence of certain SIX genes or sequence differences in these genes may form the basis for more reliable detection and identification. SIX genes have been used to identify and distinguish races in FOL, where race 2 and 3 isolates, which lack the SIX4 gene found in race 1, are identified on the basis of variation in SIX3 sequence (Lievens et al., 2009). Sequence differences in SIX8 have also been used to identify and distinguish races of F. oxysporum f. sp. cubense, including tropical race 4 (Fraser‐Smith et al., 2014).
Bulb onion (Allium cepa L.) is an important crop globally with a total production of 83 million tonnes (FAOSTAT, 2012). Production is often affected by Fusarium basal rot (FBR) caused by F. oxysporum f. sp. cepae (FOC), which is increasing in prevalence, particularly in the UK (Taylor et al., 2013). FOC infects the roots and basal plates of onions, causing symptoms at all stages of plant development, ranging from damping off and delayed seedling emergence to bulb rot at pre‐ and post‐harvest stages (Entwistle, 1990). Infection is favoured by warm temperatures (28–32 °C optimum) and disease incidence is predicted to increase as a result of climate change (Abawi and Lorbeer, 1972; Cramer, 2000; Kehr et al., 1962). FOC also produces chlamydospores which can survive for many years in the soil, making disease management very challenging (Brayford, 1996; Cramer, 2000). Other Fusarium species have also been associated with root rots of onions or other alliums, including F. proliferatum, F. redolens and F. avenaceum, but are generally less common than FOC (Bayraktar and Dolar, 2011; Du Toit et al., 2003; Galván et al., 2008; Ghanbarzadeh et al., 2013; Shinmura, 2002; Stankovic et al., 2007; Yamazaki et al., 2013), particularly in the UK (Vágány, 2012).
Few studies have attempted the molecular characterization of FOC isolates specifically, but phylogenetic analyses based on translation elongation factor 1α (EF‐1α) sequencing and amplified fragment length polymorphism (AFLP) markers have suggested that F. oxysporum can be divided into three distinct clades, with FOC isolates appearing in two of these (Galván et al., 2008; O'Donnell et al., 1998; Sasaki et al., 2015b; Taylor et al., 2013). A more recent study using the intergenic spacer region (IGS) identified eight clades among FOC isolates from bulb onion and Allium fistulosum (Sasaki et al., 2015b). However, few molecular studies with FOC, or indeed other f. spp., have associated pathogenicity tests which has confused identification, although a partial association was observed between pathogenicity on Welsh onion and IGS sequence (Dissanayake et al., 2009a; Sasaki et al., 2015b). Studies have also shown that there is genetic diversity amongst FOC isolates based on AFLP markers, inter‐simple sequence repeat (ISSR) markers, random amplified polymorphic DNA (RAPD) markers, rRNA, EF‐1α or IGS sequencing (Bayraktar and Dolar, 2011; Bayraktar et al., 2010; Dissanayake et al., 2009ba, b; Galván et al., 2008; Sasaki et al., 2015b; Southwood et al., 2012a, 2012b; Vágány, 2012). More recently, homologues of SIX3, SIX5 and SIX7 have been identified in FOC and their presence has been associated with pathogenicity on Welsh onion seedlings (Sasaki et al., 2015b). To date, this is the only record of any putative effector genes in FOC.
The aim of this study was to characterize F. oxysporum isolates from onion through the sequencing of housekeeping and pathogenicity‐related genes with a particular emphasis on SIX genes. The presence/absence of pathogenicity genes was then compared with the ability of the isolates to cause disease in both onion seedlings and bulbs. Isolates of F. oxysporum f. spp. pisi (pea), dianthi (carnation), narcissi (daffodil), cubense (banana), lycopersici (tomato) and other Fusarium species isolated from onion/leek (F. avenaceum, F. proliferatum and F. redolens) were also included in the molecular characterization for comparison.
Results
Pathogenicity testing
In the onion seedling tests, significant differences were observed in the pathogenicity of the 32 F. oxysporum isolates (Table 1) for the two experiments using Napoleon and HZS onion cultivars (Fig. 1, P < 0.001). The pathogenicity of each F. oxysporum isolate was highly correlated between the two cultivars (r = 0.97, P < 0.001). Across both onion cultivars, 18 of the 32 isolates resulted in a significant reduction in seedling survival compared with the uninoculated control and were classed as pathogenic, whereas the remaining 14 isolates (including Fo47) were non‐pathogenic and had little or no effect (Fig. 1). Two isolates (A1_2 and 55) caused significant seedling mortality on cv. Napoleon, but not on HZS. Over all the isolates, Napoleon was more susceptible than HZS to F. oxysporum.
Table 1.
Fusarium isolates used for pathogenicity testing and/or molecular characterization in this study.
| Fusarium species | Isolate code | Location | Origin | Source* | Year isolated |
|---|---|---|---|---|---|
| Isolates used for pathogenicity testing and/or molecular characterization | |||||
| F. oxysporum | A13 | Bedfordshire, UK, site 1 | Onion bulb | V. Vagany, WCC | 2009 |
| F. oxysporum | A23 | Bedfordshire, UK, site 2 | Onion bulb | V. Vagany, WCC | 2009 |
| F. oxysporum | A28 | Bedfordshire, UK, site 2 | Onion bulb | V. Vagany, WCC | 2009 |
| F. oxysporum | A35 | Bedfordshire, UK, site 3 | Onion bulb | V. Vagany, WCC | 2009 |
| F. oxysporum | F1 | Bedfordshire, UK, site 4 | Onion bulb | V. Vagany, WCC | 2010 |
| F. oxysporum | 195 | Suffolk, UK, site 1 | Onion bulb | C. Handy, WCC | 2012 |
| F. oxysporum | 224 | Suffolk, UK, site 1 | Onion bulb | C. Handy, WCC | 2012 |
| F. oxysporum | 244 | Suffolk, UK, site 1 | Onion bulb | C. Handy, WCC | 2012 |
| F. oxysporum | A21 | Suffolk, UK, site 2 | Onion bulb | V. Vagany, WCC | 2009 |
| F. oxysporum | R3 | Suffolk, UK, site 3 | Onion bulb | V. Vagany, WCC | 2009 |
| F. oxysporum | M1 | Suffolk, UK, site 4 | Onion bulb | V. Vagany, WCC | 2010 |
| F. oxysporum | M9 | Suffolk, UK, site 4 | Onion bulb | V. Vagany, WCC | 2010 |
| F. oxysporum | G12 | Suffolk, UK, site 5 | Onion bulb | V. Vagany, WCC | 2009 |
| F. oxysporum | K3b | Suffolk, UK, site 6 | Onion bulb | V. Vagany, WCC | 2009 |
| F. oxysporum | S1B | Essex, UK, site 1 | Onion bulb | V. Vagany, WCC | 2009 |
| F. oxysporum | A14 | Essex, UK, site 2 | Onion bulb | V. Vagany, WCC | 2009 |
| F. oxysporum | A19 | Essex, UK, site 2 | Onion bulb | V. Vagany, WCC | 2009 |
| F. oxysporum | NL70/7 | Essex, UK, site 3 | Onion bulb | V. Vagany, WCC | 2010 |
| F. oxysporum | A1_2 | Warwickshire, UK | Onion bulb | V. Vagany, WCC | 2008 |
| F. oxysporum f. sp. cepae | FUS2 | Lincolnshire, UK | Onion bulb | R. Noble, East Malling Research | Unknown |
| F. oxysporum | 55 | Lincolnshire, UK, site 1 | Onion bulb | C. Handy, WCC | 2012 |
| F. oxysporum | 84 | Lincolnshire, UK, site 1 | Onion bulb | C. Handy, WCC | 2012 |
| F. oxysporum | 125 | Lincolnshire, UK, site 1 | Onion bulb | C. Handy, WCC | 2012 |
| F. oxysporum | RO2 | Lincolnshire, UK, site 2 | Onion bulb | V. Vagany, WCC | 2010 |
| F. oxysporum | FUS1 | Nottinghamshire, UK | Onion bulb | R. Noble, East Malling Research | Unknown |
| F. oxysporum | FUS3 | Nottinghamshire, UK | Onion bulb | R. Noble, East Malling Research | Unknown |
| F. oxysporum | PG | Cambridgeshire, UK | Onion bulb | T. O'Neill, ADAS | Unknown |
| F. oxysporum | CB3 | UK | Onion set | C. Handy, WCC | 2012 |
| F. oxysporum | HB17 | UK | Onion set | C. Handy, WCC | 2012 |
| F. oxysporum | HB6 | UK | Onion set | C. Handy, WCC | 2012 |
| F. oxysporum | JB4 | UK | Onion set | C. Handy, WCC | 2012 |
| F. oxysporum | NRRL 54002 (FO47) | France | Soil | ARS collection | Unknown |
| F. oxysporum | HAZ | USA | Onion bulb | H. van den Biggelaar, Hazera seeds | Unknown |
| F. oxysporum | L2‐1 | UK, site 1 | Leek | A. Taylor, WCC | 2011 |
| F. oxysporum | L9‐1 | UK, site 2 | Leek | A. Taylor, WCC | 2011 |
| F. oxysporum | ATCC90245 | Colorado, USA | Pinto bean | ATCC collection | 1990 |
| F. oxysporum f. sp. pisi race 1 | FOP1 | UK | Pea | C. Linfield, WCC | Unknown |
| F. oxysporum f. sp. pisi race 2 | FOP2 | UK | Pea | C. Linfield, WCC | Unknown |
| F. oxysporum f. sp. pisi race 5 | FOP5 | UK | Pea | C. Linfield, WCC | Unknown |
| F. oxysporum f. sp. pisi | NRRL36311 | The Netherlands | Pea | ARS collection | Unknown |
| F. oxysporum f. sp. lini | FOLIN | UK | Linseed | C. Linfield, WCC | 2010 |
| F. oxysporum f. sp. dianthi | R207 | UK | Carnation | C. Linfield, WCC | Unknown |
| F. oxysporum f. sp. narcissi | FOXN7 | UK | Daffodil | C. Handy, WCC | 2013 |
| F. oxysporum f. sp. narcissi | FOXN139 | UK | Daffodil | C. Handy, WCC | 2013 |
| F. oxysporum f. sp. freesia | NRRL26990 | The Netherlands | Freesia | ARS collection | Unknown |
| F. oxysporum f. sp. freesia | NRRL26988 | The Netherlands | Freesia | ARS collection | Unknown |
| F. oxysporum f. sp. cubense | E421A3 | UK | Banana | C. Nellist, WCC | Unknown |
| F. avanaceum | L5 | UK, site 1 | Leek | A. Taylor, WCC | 2011 |
| F. proliferatum | A8 | Bedfordshire, UK, site 3 | Onion bulb | V. Vagany, WCC | 2009 |
| F. proliferatum | A40 | Bedfordshire, UK, site 3 | Onion bulb | V. Vagany, WCC | 2009 |
| F. proliferatum | SP1‐2 | Spain | Onion bulb | V. Vagany, WCC | 2010 |
| F. redolens | NL96 | Essex, UK, site 3 | Onion bulb | V. Vagany, WCC | 2010 |
| F. oxysporum f. sp. lycopersici race 3 | NRRL54003 (MN25) | USA | Tomato | ARS Collection | Unknown |
| Genome sequenced isolates used for comparison in molecular characterization | |||||
| F. oxysporum f. sp. pisi | NRRL37622 (HDV247) | Unknown | Pea | ARS Collection | Unknown |
| F. oxysporum | NRRL32931 (FOSC 3‐a) | USA | Human | ARS Collection | Unknown |
| F. oxysporum f. sp. conglutinans | NRRL54008 (PHW808) | USA | Brassica | ARS Collection | Unknown |
| F. oxysporum f. sp. raphani | NRRL54005 (PHW815) | France | Radish | ARS Collection | Unknown |
| F. oxysporum f. sp. radicis‐lycopersici | NRRL26381 (CL57) | USA | Tomato | ARS Collection | Unknown |
| F. oxysporum f. sp. cubense | NRRL54006 (II5) | Indonesia | Banana | ARS Collection | Unknown |
| F. oxysporum f. sp. melonis | NRRL26406 | USA | Melon | ARS Collection | Unknown |
| F. oxysporum f. sp. vasinfectum | NRRL25433 | China | Cotton | ARS Collection | Unknown |
| F. oxysporum f. sp. lycopersici race 2 | NRRL34936 (FOL4287) | USA? | Tomato | ARS Collection | Unknown |
| F. oxysporum | Fo5176 | Australia | Brassica oleracea | ARS Collection | Unknown |
*ATCC, American Type Culture Collection, USA; ARS, Agricultural Research Service culture collection, USA; WCC, Warwick Crop Centre, University of Warwick, UK.
Figure 1.
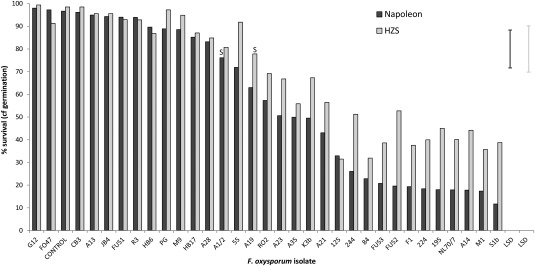
Pathogenicity of 32 Fusarium oxysporum isolates on onion seedlings (cv. Napoleon and Hazera Seeds standard susceptible line, HZS). Data shown are the percentage survival values relative to germination after 42 days in a glasshouse. Error bars represent the least significant difference (LSD) (5%) level for each onion cultivar. An ‘S’ indicates the value below which there is a significant difference from control plants.
In the onion bulb test (cv. Napoleon), significant differences were observed in disease levels amongst the 32 F. oxysporum isolates (P < 0.001, Fig. 2), with 21 pathogenic isolates resulting in 8.7%–58.6% bulb area affected compared with the control (0%), and 11 non‐pathogenic isolates (including Fo47) having no significant effect (0%–5.0% bulb area affected). Of the 21 pathogenic isolates, A1_2 (8.7%) and HB6 (19.1%) showed lower levels of pathogenicity, whereas isolate 55 showed an intermediate level of pathogenicity (29.1%). Highly significant correlations were observed between F. oxysporum isolate pathogenicity in the bulb test and the seedling tests with cv. Napoleon (r = −0.91, P = 0.001) and cv. HZS (r = −0.88, P = 0.001). Pathogenic isolates resulting in significantly greater disease levels compared with the uninoculated controls in onion seedling or bulb tests were considered to be FOC.
Figure 2.

Pathogenicity of 32 Fusarium oxysporum isolates on onion bulbs (cv. Napoleon). Data shown are the percentage bulb areas diseased on bisected bulbs after 9 weeks at 20 °C. Error bar represents the least significant difference (LSD) (5%) level. An ‘S’ indicates the value above which there is a significant difference from the uninoculated control bulbs.
Molecular characterization: housekeeping genes
The concatenated tree for EF‐1α, RNA polymerase II second largest subunit (RPB2) and β‐tubulin (TUB2) sequences resulted in the majority of the 53 F. oxysporum isolates being separated into six clades (Fig. 3). Isolates from onion were represented in clades 1, 3 and 5, and clade 1 contained all those that showed some level of pathogenicity in either the onion seedling or bulb tests (or both), with the exception of A1_2. Clade 1 also included isolate L2‐1 from infected leeks, which has also been shown to be pathogenic on onion bulbs (A. Taylor, A. Jackson & J. P. Clarkson, unpublished data). However, clade 1 also included the non‐pathogenic isolate HB17 (from onion sets) and isolates of F. oxysporum f. sp. pisi race 5 (FOP5), f. sp. freesia (NRRL26990) and f. sp. pisi (NRRL36311). Two non‐pathogenic onion isolates, A28 and CB3, showed high similarity to Fo47 in clade 3, whereas four other non‐pathogenic onion isolates (A13, M9, R3 and JB4) were placed in clade 5, together with several other f. spp. Fusarium oxysporum f. sp. cubense isolates were all placed in a separate and distinct clade (clade 6) and the other Fusarium species (F. proliferatum, F. redolens and F. avenaceum) formed distinct outgroups. Additional trees for each of the housekeeping genes were constructed using neighbour‐joining, minimum evolution and UPGMA (Unweighted Pair Group Method with Arithmetic Mean) methods, and similar topography was observed (data not shown).
Figure 3.
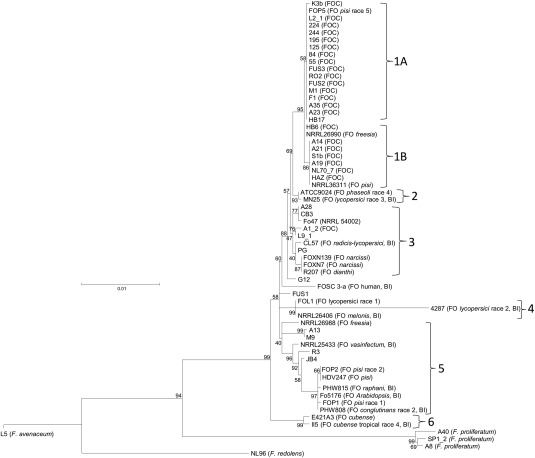
Maximum likelihood tree of Fusarium isolates from onion and other hosts based on a concatenated alignment of translation elongation factor 1α (EF‐1α) (GenBank accession numbers KP964857–KP964909), RNA polymerase II second largest subunit (RPB2) (GenBank accession numbers KP964804–KP964856) and β‐tubulin (TUB2) (GenBank accession numbers KP964910–KP964962) genes. Numbers represent bootstrap values from 1000 replicates. Scale bar indicates 0.01 substitutions per site. The tree is rooted through L5 (F. avenaceum) and this branch has been collapsed because of its distance from F. oxysporum. BI refers to a sequence derived from the genomes on the Broad Institute Fusarium database (Broad Institute/MIT, 2007).
Molecular characterization: SIX genes
Fusarium oxysporum isolates from onion
Using primers based on the FOL or FOC genomes, homologues of SIX3, SIX5, SIX7, SIX10, SIX12 and SIX9, SIX14, respectively were identified within the 31 F. oxysporum isolates from UK onions (Table 2) and USA isolate HAZ. All seven of these SIX genes were present in the 18 highly pathogenic isolates identified from the onion seedling and bulb tests (Table 2). In contrast, all SIX genes were absent in the 11 non‐pathogenic isolates, with the exception of isolate PG which contained SIX9 (Table 2). Isolate 55, which had an intermediate level of pathogenicity on onion bulbs, and was mildly pathogenic on seedlings (cv. Napoleon), contained only SIX9 and SIX14, whereas isolates HB6 and A1_2, which were weakly pathogenic on Napoleon bulbs (and on seedlings for A1_2), contained no SIX genes. All seven of the SIX genes identified were associated with a predicted signal peptide (Table 3).
Table 2.
Presence/absence of Secreted In Xylem (SIX)1–14 and three putative novel effectors in Fusarium oxysporum and other selected species.
| Fusarium species | Host | Isolate code | Pathogenicity* | SIX genes† | C5 ‡ | CRX1 § | CRX2 ¶ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |||||||
| F. oxysporum (FOC) | Onion | A23 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | A19 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | − |
| F. oxysporum (FOC) | Onion | RO2 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | A14 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | − |
| F. oxysporum (FOC) | Onion | K3B | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | 195 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | FUS2 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | 125 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | FUS3 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | NL70/7 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | − |
| F. oxysporum (FOC) | Onion | 84 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | M1 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | 224 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | F1 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | S1B | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | − |
| F. oxysporum (FOC) | Onion | A35 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | A21 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | − |
| F. oxysporum (FOC) | Onion | 244 | B/S1/S2 | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | 55 | B/S1 | − | − | − | − | − | − | − | − | + | − | − | − | − | + | + | + | 1 |
| F. oxysporum (FOC) | Onion | HB6 | B | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. oxysporum (FOC) | Onion | A1_2 | B/S1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. oxysporum | Onion | G12 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 2 |
| F. oxysporum | Onion | CB3 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. oxysporum | Onion | PG | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | 3 |
| F. oxysporum | Onion | R3 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. oxysporum | Onion | A13 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. oxysporum | Onion | FUS1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 1 |
| F. oxysporum | Onion | M9 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. oxysporum | Onion | JB4 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. oxysporum | Onion | HB17 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. oxysporum | Onion | A28 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | 2 |
| F. oxysporum | – | FO47 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. oxysporum | Onion | HAZ | (+) | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | + |
| F. oxysporum | Leek | L2‐1 | (+) | − | − | + | − | + | − | + | − | + | + | − | + | − | + | + | + | + |
| F. oxysporum | Leek | L9‐1 | (−) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. oxysporum f. sp. lycopersici | Tomato (race 3) | NRRL54003 (MN25) | NT | + | + | + | − | + | + | + | + | + | + | + | + | + | + | − | − | − |
| F. oxysporum f. sp. lycopersici | Tomato (race 1) | FOL1 | NT | NT | NT | NT | + | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | − | − | − |
| F. oxysporum f. sp. phaseoli | Pinto bean (race 4) | ATCC90245 | NT | − | − | − | − | − | + | − | + | − | − | + | − | − | − | − | − | − |
| F. oxysporum f. sp. pisi | Pea (race 1) | FOP1 | NT | − | − | − | − | − | − | + | − | − | + | + | + | − | + | − | − | − |
| F. oxysporum f. sp. pisi | Pea (race 2) | FOP2 | NT | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − | + |
| F. oxysporum f. sp. pisi | Pea (race 5) | FOP5 | NT | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − |
| F. oxysporum f. sp. pisi | Pea | NRRL36311 | NT | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − |
| F. oxysporum f. sp. lini | Linseed | FOLIN | NT | − | − | − | − | − | − | + | − | − | + | − | + | + | − | − | − | − |
| F. oxysporum f. sp. dianthi | Carnation | R207 | NT | − | − | − | − | − | − | + | − | + | + | − | + | − | − | − | − | − |
| F. oxysporum f. sp. narcissi | Daffodil | FOXN7 | NT | − | − | − | − | − | − | + | − | + | + | − | + | − | − | − | − | − |
| F. oxysporum f. sp. narcissi | Daffodil | FOXN139 | NT | − | − | − | − | − | − | + | − | + | + | − | + | − | − | − | − | − |
| F. oxysporum f. sp. freesia | Freesia | NRRL26990 | NT | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. oxysporum f. sp. freesia | Freesia | NRRL26988 | NT | − | − | − | − | − | − | + | − | − | + | − | + | + | + | − | − | − |
| F. oxysporum f. sp. cubense | Banana | E421A | NT | + | − | − | − | − | − | − | + | − | − | − | − | + | − | − | − | − |
| F. proliferatum | Onion | A8 | (+) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| F. proliferatum | Onion | A40 | (+) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| F. proliferatum | Onion | SP1‐2 | (+) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| F. avenaceum | Leek | L5 | NT | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| F. redolens | Onion | NL96 | (−) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
*Isolate pathogenicity: B, pathogenic on onion bulbs; S1, pathogenic on onion seedlings cv. Napoleon; S2, pathogenic on onion seedlings cv. HZS; –, non‐pathogenic; NT, not tested. Symbols in parentheses refer to preliminary, unpublished pathogenicity data on onion bulbs and/or seedlings.
†GenBank accession numbers KP964963–KP965006.
‡GenBank accession number KP965007.
§GenBank accession number KP965011.
¶GenBank accession numbers KP965008–KP965010 and KP965012–KP965017. Numbers indicate sequence type (Fusarium isolates from onion only, Fig. 6); +, presence of CRX2; −, absence of CRX2.
Table 3.
Putative effector genes in Fusarium oxysporum f. sp. cepae with associated nucleotide and protein percentage identities using blast (Boratyn et al., 2013) in comparison with F. oxysporum f. sp. lycopersici unless otherwise stated.
| Gene | Nucleotide ID | Protein ID | Signal peptide |
|---|---|---|---|
| SIX3 | 91 | 86 | Yes |
| SIX5 | 90 | 73 | Yes |
| SIX7 | 91 | 82 | Yes |
| SIX9 | 90* | 82* | Yes |
| SIX10 | 96† | 91† | Yes |
| SIX12 | 95 | 94 | Yes |
| SIX14 | 62 | 78 | Yes |
| C5 | No homology | No homology | No |
| CRX1 | 89‡ | 80‡ | Yes |
| CRX2 | 100‡ | 93‡ | Yes |
*Percentage identity to SIX9a in an Arabidopsis‐infecting F. oxysporum isolate (HQ260603).
†Percentage identity does not include an intron which is present in F. oxysporum f. sp. cepae, but not in F. oxysporum f. sp. lycopersici.
‡Closest match F. oxysporum CL57: FOCG_17596.1: hypothetical protein.
The SIX3, SIX5, SIX7, SIX10 and SIX12 sequences from FOC all had a high level of homology with the corresponding FOL SIX genes, ranging from 85% to 96% nucleotide identity, whereas the FOC SIX9 and SIX14 homologues were more divergent (73% and 62% nucleotide identity, respectively; Table 3). The FOC SIX9 gene also showed very high homology (90% nucleotide identity) with SIX9a identified in the F. oxysporum isolate infecting Arabidopsis and brassica (Thatcher et al., 2012). Sequences for SIX3, SIX7, SIX10, SIX12 and SIX14 were identical across all the FOC isolates (where present), including isolate HAZ from the USA. In addition, the FOC SIX3 gene identified in our study had a 100% match to the SIX3 sequence from a Japanese FOC isolate (Genbank accession number BAP74165). No other SIX gene homologues were identified in the FUS2 genome, confirming the negative polymerase chain reaction (PCR) results.
Fusarium oxysporum from different hosts and other Fusarium species from onion/leek
Two F. oxysporum isolates from leek were included in this study, one of which was pathogenic on both onion and leek (L2‐1; A. Taylor, A. Jackson and J. P. Clarkson, unpublished data). This isolate had an identical SIX gene profile to the isolates which were pathogenic on onion and identical sequences. Five SIX genes were identified in F. oxysporum f. spp. pisi race 1 (FOP1; SIX7, SIX10, SIX11, SIX12 and SIX14) and freesia (NRRL26988; SIX7, SIX10, SIX12, SIX13 and SIX14). However, a second F. oxysporum f. sp. freesia isolate (NRRL26990) had no SIX genes at all. The SIX1 gene was only identified in F. oxysporum f. sp. cubense and FOL isolates, whereas SIX2 and SIX4 were unique to FOL. SIX3, SIX5 and SIX12 were only found in FOC and FOL. SIX6 was only detected in FOL and f. sp. phaseoli, whereas SIX7 and SIX10 were found in FOC, FOL and f. spp. pisi race 1, dianthi, narcissi and freesia. SIX8 was detected in FOL and F. oxysporum f. sp. cubense and f. sp. phaseoli, whereas SIX9 was detected in FOL using the FOL primers, and in FOC, F. oxysporum f. sp. dianthi and f. sp. narcissi using the FOC primers. SIX11 was found in F. oxysporum f. sp. phaseoli, f. sp. pisi race 1 and FOL. SIX13 was detected in F. oxysporum f. sp. pisi races 2 and 5, f. sp. dianthi, f. sp. narcissi, f. sp. freesia and FOL. SIX14 was detected in FOC, FOL, F. oxysporum f. sp. pisi and f. sp. freesia. None of the other Fusarium species tested contained any of the SIX genes.
SIX gene sequence variation was observed across the different F. oxysporum f. spp., and phylogenetic trees showed that FOC isolates were clearly separated from the other f. spp. based on SIX7, SIX10 and SIX12, but not SIX9 (Fig. 4). The FOL SIX9 gene formed part of a separate clade to the SIX9a and SIX9b genes identified in the Arabidopsis/brassica‐infecting isolate (Fo5176), whereas the SIX9 sequence from FOC was in the same clade as SIX9a/SIX9b and was closer to SIX9a than SIX9b. The separation of FOC isolates from other F. oxysporum f. spp. was less clear for SIX9 as the sequence was very similar to that from both F. oxysporum f. spp. narcissi and dianthi. Fusarium oxysporum isolates from carnation and Narcissus could not be distinguished on the basis of any of the SIX gene sequences. For SIX5, six FOC isolates (S1b, A14, A19, A21, NL70/7 and HAZ) had a single base change at position 316 (G to A, Fig. S1, see Supporting Information) causing a single amino acid change from R to K. All of these isolates were in clade 1b in the housekeeping gene tree (Fig. 3). For SIX9, two onion isolates, A21 (pathogenic) and PG (non‐pathogenic), had a different sequence type, differing by a single base pair (T instead of A), resulting in an amino acid change from D to V.
Figure 4.
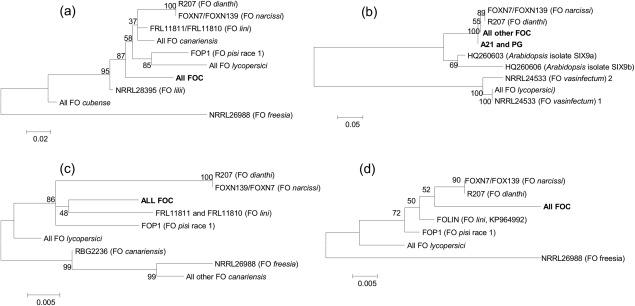
Maximum likelihood trees of Fusarium isolates from onion and other hosts based on (a) SIX 7, (b) SIX9, (c) SIX10, and (d) SIX12 gene sequences. Numbers represent bootstrap values from 1000 replicates. Scale bars indicate the number of substitutions per site. All FO lycopersici refers to the genome sequenced isolates listed in Table 1 as well as additional identical sequences obtained from a BLAST search. All FO cubense refers to the genome sequenced isolate II5 as well as identical BLAST hits. Sequences of other NRRL isolates were extracted from genome sequences (Broad Institute/MIT, 2007). All FO canariensis and FO lini isolates (with the exception of FOLIN, SIX12) are as described by Laurence et al. (2015).
Molecular characterization: putative novel effectors
Two putative novel effectors, C5 and CRX1, were detected in all of the 18 FOC isolates that were highly pathogenic across both seedling and bulb assays (Table 2), although CRX1 was also found in isolate A28 which was non‐pathogenic. The presence of a third putative effector (CRX2) was partially associated with pathogenicity, being detected in 13 of the 18 highly pathogenic isolates (Table 2) and in four non‐pathogenic isolates (A28, FUS1, G12 and PG). FOC isolate 55, which demonstrated ‘intermediate’ pathogenicity on onion bulbs, contained C5, CRX1 and CRX2 (as well as SIX9 and SIX14), whereas FOC isolate A1_2, which was weakly pathogenic, had no putative effectors. C5 and CRX1 were not present in any of the other F. oxysporum f. spp., whereas CRX2 was detected in f. sp. pisi race 2, F. redolens and all the F. proliferatum isolates. Genes with homology to CRX1/CRX2 were also found in the genome sequences of F. oxysporum f. sp. pisi (HDV247: FOVG_18080) and f. sp. radicis‐lycopersici (CL57: FOCG_17596 and FOCG_16735) (Broad Institute/MIT, 2007). C5 was not present in any of the published Fusarium genomes (Table 1) and there are no sequence matches at the National Center for Biotechnology Information (NCBI).
The putative effectors CRX1 and CRX2 in F. oxysporum contained RxLR domains close to the N‐terminus (at amino acid positions 37–40, Fig. 5), whereas F. proliferatum isolates had a modified (RQVR) sequence in this position. The RxLR domains were flanked by modified dEER domains, defined by the presence of >10% D or E residues (Jiang et al., 2008). All C5 and CRX1 sequences were identical across the F. oxysporum isolates and, based on the partial coding DNA sequence, a phylogenetic tree clearly separated CRX1 and CRX2 (Fig. 6). The majority of the 13 (pathogenic) FOC isolates that contained CRX2 had identical sequences, although the non‐pathogenic isolate FUS1 also had the same sequence (Fig. 6). Three non‐pathogenic isolates from onion (A28, G12 and PG) had slightly different CRX2 sequence types and, in the case of isolates PG and G12, this resulted in a stop codon in the middle of the coding region (data not shown). CRX2 sequences from F. oxysporum f. sp. radicis‐lycopersici (FOCG_17596) and F. redolens (NL96) were very similar to the predominant FOC sequence, whereas the three F. proliferatum isolates, which also contained CRX2, formed a distinct clade.
Figure 5.

Amino acid alignment of putative RxLR effectors from Fusarium oxysporum and F. proliferatum. The signal peptide (as predicted by SignalP) is shaded in light grey, whereas the RxLR domain is shown in bold (bold and italics for an incomplete RxLR domain). Amino acids that often occur after an RxLR domain (dEER) are underlined. Sequences from HDV247 and CL57 were obtained from the Broad Institute Fusarium database (Broad Institute/MIT, 2007).
Figure 6.
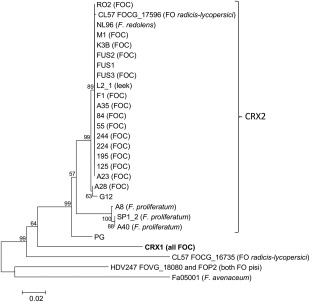
Neighbour‐joining tree of Fusarium oxysporum CRX1 and CRX2 genes and their homologues. Numbers represent bootstrap values from 1000 replicates. The scale bar indicates 0.02 substitutions per site. Sequences from HDV247 and CL57 were obtained from the Broad Institute Fusarium database (Broad Institute/MIT, 2007). The sequence from Fa05001 was obtained from an assembled genome (GenBank accession number GCA_000769215).
Expression of putative effectors
Using real‐time reverse transcription‐polymerase chain reaction (RT‐PCR), the seven SIX genes and the three putative effectors C5, CRX1, CRX2 were all shown to be expressed in planta across the time course following inoculation with FOC isolate FUS2. SIX3, SIX5, SIX7, SIX9, SIX10, SIX12 and CRX1 showed significant increases in expression levels at 36–72 h post‐inoculation (hpi) compared with the first time point at 8 h (Fig. 7), whereas SIX14 only showed a significant increase at 72 hpi. There was no increase in C5 expression for any of the time points compared with 8 hpi, although a significant increase was detected between 16 and 72 hpi. CRX2 expression levels did not change significantly across the time course.
Figure 7.
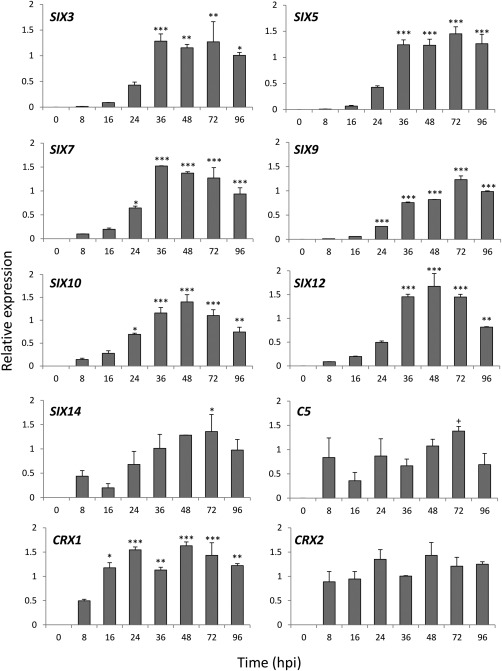
Quantitative expression of a set of putative effector genes in onion roots following inoculation with Fusarium oxysporum f. sp. cepae (FOC) isolate FUS2. Expression was calculated relative to translation elongation factor 1α (EF‐1α) and β‐tubulin (TUB2). Error bars show the standard error of the mean (SEM) of three replicates; hpi, hours post‐inoculation. Asterisks indicate expression levels significantly different from 8 hpi based on analysis of variance (ANOVA) followed by Tukey's test (*P < 0.05, **P < 0.01, ***P < 0.001). + indicates that the expression of C5 was significantly higher at 72 hpi relative to 16 hpi (P < 0.05).
Discussion
In this study, we have demonstrated, for the first time, a clear association between the presence of seven SIX genes and two other putative effectors (C5, CRX1) in FOC and pathogenicity on onion. A third putative effector (CRX2) showed a partial association with pathogenicity. Pathogenicity tests on onion seedlings and bulbs were consistent across all the F. oxysporum isolates, and a strong correlation between pathogenicity results using two different onion cultivars in the seedling tests supports the suggestion that there is no cultivar × isolate interaction (Taylor et al., 2013).
Three of the seven SIX genes identified in FOC (SIX3, SIX5 and SIX7) had been identified previously, but their presence was only related to pathogenicity on onion seedlings (Sasaki et al., 2015b), and not in a bulb test, which is more appropriate to disease expression in the field. In addition, this study did not provide evidence of expression in planta. The remaining four SIX genes (SIX9, SIX10, SIX12 and SIX14) are reported here for the first time in FOC. The presence of SIX genes has similarly been associated with the pathogenicity of F. oxysporum isolates on tomato (SIX1–7; Lievens et al., 2009), cotton (SIX6; Chakrabarti et al., 2011) and banana (SIX1, SIX7 and SIX8; Meldrum et al., 2012). The function of the 14 SIX genes detected so far in F. oxysporum is unclear as they show little or no homology with any known proteins (Fraser‐Smith et al., 2014). However, SIX1, SIX3, SIX4, SIX5 and SIX6 have all been shown to make a direct contribution to pathogenicity (Gawehns et al., 2014; Houterman et al., 2009; Ma et al., 2015; Rep, 2005; Takken and Rep, 2010; Thatcher et al., 2012).
One F. oxysporum isolate from leek (L2‐1) also shared the same effector gene profile as the pathogenic onion isolates. Preliminary work (Taylor A, Jackson A.C, Clarkson J.P, unpublished.) has demonstrated that this leek isolate is also pathogenic on onion bulbs, whereas another leek isolate (L9‐1), which was non‐pathogenic, lacked any of the effector genes. FOC has also been shown to cause basal rot in Welsh onion (A. fistulosum; Dissanayake et al., 2009a, 2009b; Sasaki et al., 2015b) and garlic (A. sativum; Rout et al., 2014), suggesting that FOC might be more appropriately named F. oxysporum f. sp. allii. However, it should be noted that, although FOC isolates from A. fistulosum can be pathogenic on A. cepa seedlings, they lack SIX3, SIX5 and SIX7 (Sasaki et al., 2015b), and the pathogenicity of these isolates on onion bulbs has yet to be tested.
It is likely that the FOC SIX genes, and possibly the other putative effectors, are located on dispensable/supernumerary chromosomes, as has been reported for FOL (Ma et al., 2010). FOC SIX3 has been shown to be located on a small (4‐Mb) chromosome, which may be equivalent to a FOL supernumerary chromosome (Sasaki et al., 2015b). It has also been shown recently that SIX3 and SIX5 share the same promoter and appear to act as a gene pair, which is recognized by the I‐2 resistance gene (Houterman et al., 2009; Ma et al., 2015). It has been suggested that this is unique to FOL, but our data and those of Sasaki et al. (2015a) confirm that these genes are also present in FOC. For all of the seven SIX genes in FOC, sequences were mostly identical and, similarly, there was also a high level of sequence conservation with those reported in FOL, suggesting a conservation of function. Indeed, five of the seven FOC SIX genes (SIX3, SIX7, SIX10, SIX12 and SIX14) showed no intraspecific sequence differences within isolates originating from the UK, USA and Japan. This supports the suggestion that SIX genes in FOC have been acquired by horizontal gene transfer of the supernumerary chromosomes, as proposed for other F. oxysporum f. spp. (Fraser‐Smith et al., 2014; Laurence et al., 2015; Ma et al., 2010). A naturally occurring isolate of F. oxysporum from onion (isolate 55) identified in this study had only two of the seven SIX genes (SIX9 and SIX14) and a corresponding intermediate level of pathogenicity. This phenomenon has not been observed so far in other f. spp., and may suggest a missing or mutated copy of a supernumerary chromosome.
In this study, we also report, for the first time, three novel putative effectors in FOC (C5, CRX1 and CRX2), and the presence of C5 and CRX1 had an almost complete correlation with pathogenicity. C5 has no homology to any sequenced gene, suggesting that it may be FOC specific, but both CRX1 and CRX2 contained RxLR domains, a motif found in many diverse oomycete candidate effector genes which, in some reports, has been shown to facilitate entry into host cells (Kale, 2012; Kale et al., 2010; Rehmany et al., 2005). Putative RxLR effectors have also been reported in FOL (Ma et al., 2013), but this is the first report of putative RxLR effectors in FOC. CRX2 only had a partial association with pathogenicity in FOC, and also has very close homologues in F. oxysporum f. sp. radicis‐lycopersici, F. proliferatum and F. redolens. We also demonstrated, for the first time, that the SIX genes and the putative FOC‐specific effector CRX1 are up‐regulated during the infection process, with levels of expression in planta increasing over a time course, further supporting the hypothesis that these genes play a role in pathogenicity. In addition, with the exception of C5, all other putative effectors and SIX genes had an associated signal peptide, supporting the hypothesis that they are secreted. However, SIX12 in FOL, which is also reported to have no associated signal peptide, was found in the xylem sap of infected tomato plants, leading to the conclusion that it was secreted by a different mechanism (Schmidt et al., 2013). Additional functional analyses are now required to reveal the potential roles of the new putative effectors in pathogen virulence.
The characterization of all the F. oxysporum isolates using the three housekeeping genes EF‐1α, RPB2 and TUB2 showed that there was considerable genetic variation between isolates from onion, as reported previously using AFLP markers, EF‐1α and IGS sequencing (Galván et al., 2008; Sasaki et al., 2015b). Although all pathogenic isolates, with the exception of A1_2 (weakly pathogenic), were placed in clade 1, the same clade also contained a small number of non‐pathogenic isolates and some other f. spp., indicating that housekeeping genes are not useful for distinguishing between different F. oxysporum f. spp. or between pathogenic and non‐pathogenic isolates. However, one study found a partial association between IGS sequence and pathogenicity (Sasaki et al., 2015b), but it was not clear whether other F. oxysporum f. spp. share this sequence type. The non‐pathogenic F. oxysporum isolates from onion were generally scattered throughout the phylogenetic tree, suggesting that they are more diverse than the pathogenic isolates. Although all F. oxysporum isolates in this study were originally from diseased onion tissue, the isolation of non‐pathogenic isolates is common and they are likely to be either endophytes or saprophytes, which are secondary colonizers of infected roots or bulbs (Alabouvette et al., 2009).
Our main reason for selecting the additional F. oxysporum f. spp. for characterization in this study was to expand the knowledge concerning the distribution of SIX genes. A study of SIX1–SIX7 in a wider range of F. oxysporum f. spp. suggested that only SIX6 (in f. spp. melonis and radicis‐cucumerinum) and SIX7 (in f. sp. lilii) were present in f. spp. other than FOL (Lievens et al., 2009). However, more recent work (and the release of whole genome sequences for selected F. oxysporum f. spp.) has shown that all of the 14 SIX genes are present in various complements in other f. spp. (Broad Institute/MIT, 2007; Fraser‐Smith et al., 2014; Laurence et al., 2015; Sasaki et al., 2015b). FOC isolates in this study contained seven of the 14 SIX genes, whereas five were identified for F. oxysporum f. sp. freesia (NRRL26988) and f. sp. pisi race 1. Interestingly, a second freesia isolate (NRRL26990) examined here contained no SIX genes at all, suggesting that it may not be pathogenic or may have been misidentified. We also found a range of SIX genes present in isolates of F. oxysporum f. sp. pisi and there appeared to be distinct variation between races in terms of SIX gene complement. The only previous literature on SIX genes in F. oxysporum f. sp. pisi showed an absence of SIX6 in races 1, 2, 5 and 6 (Chakrabarti et al., 2011), which is in agreement with our findings.
In this study, an F. oxysporum f. sp. dianthi isolate (R207) contained SIX7, SIX9, SIX10 and SIX12, which is in contrast with previous findings that reported an absence of SIX1–7 in f. sp. dianthi isolates from the USA and the Netherlands (Lievens et al., 2009). Overall, our results, in combination with other reports, suggest that SIX gene complements and/or sequences can be used to separate F. oxysporum f. spp. without the need for pathogenicity testing. Recently, a quantitative PCR assay was developed for the detection of FOC in planta based on SIX3 (Sasaki et al., 2015a). Although this test requires more rigorous validation against a range of F. oxysporum and other soil‐borne fungi, it could potentially be useful as a soil or plant test for FOC.
Finally, the other Fusarium species F. proliferatum, F. avenaceum and F. redolens, which were also isolated from diseased onions and leeks in this study, did not contain any SIX genes. This confirms the results for the isolates of F. proliferatum and F. avenaceum from sugar beet, where SIX1 and SIX6 were absent (Covey et al., 2014). This was also the case for F. graminearum, F. solani and F. javanicum, which lacked SIX1–7 (Lievens et al., 2009). Whole genome sequences have recently become available for F. avenaceum isolates from barley and wheat (Lysøe et al., 2014), and these genomes were examined for the presence of all putative effectors. The only hit was for CRX2 (80% identity), which was only present in an isolate from barley (Fa05001, Fig. 6). The F. proliferatum and F. avenaceum isolates used in our study were also shown to be pathogenic on onion (A. Taylor, A. C. Jackson, and J. P. Clarkson, unpublished data) and must therefore possess a different mechanism of infection compared with FOC. Overall, our findings provide a greater understanding of pathogenicity in FOC, and could potentially improve the diagnosis and control of Fusarium basal rot of onion in the future.
Experimental Procedures
Fusarium isolates
Fusarium isolates were obtained from diseased onion roots and bulbs from different locations in the UK collected between 2008 and 2012 (Table 1), as described by Taylor et al. (2013). Thirty‐one F. oxysporum isolates were selected for subsequent molecular characterization and pathogenicity tests based on sample location, colony morphology, preliminary pathogenicity data (where available) and EF‐1α sequences of a few isolates (Taylor et al., 2013; Vágány, 2012), as well as the genome sequenced non‐pathogenic biocontrol agent Fo47 (NRRL54002). In addition, a genome‐sequenced isolate of FOL race 3 (MN25, NRRL54003), a FOC isolate from the USA (HAZ), two F. oxysporum isolates from diseased UK leeks (L2‐1, L9‐1) and 17 isolates of F. oxysporum f. spp. pisi, dianthi, narcissi, cubense and lycopersici, as well as three other Fusarium species from diseased onions/leeks (F. avenaceum, F. proliferatum and F. redolens), were also obtained from various researchers and culture collections for comparison in the molecular characterization studies (Table 1).
Pathogenicity testing: seed inoculation
The 31 F. oxysporum isolates from UK onions and the non‐pathogenic isolate Fo47 were assessed for their pathogenicity on onion seedlings as described by Taylor et al. (2013). Two experiments were carried out: one using cv. Napoleon (Syngenta, Cambridge, Cambridgeshire UK) and the second using cv. HZS (a standard FOC susceptible line from Hazera Seeds, Made, The Netherlands). For each experiment, there were four independent replicates over time, each consisting of a tray of 28 onion seeds per isolate, which were positioned in a glasshouse using an alpha design (Genstat v.12, VSN International, Hemel Hempstead, Hertfordshire, UK). Three trays of uninoculated control treatments (seeds soaked in sterile distilled water (SDW)) were included in each replicate experiment. The number of surviving seedlings was recorded after 6 weeks and the percentage survival was calculated relative to germination to allow for any variation in germination between replicates. Significant differences between treatments (isolates) for these data were assessed using residual (or restricted) maximum likelihood (REML) analysis (Welham & Thompson, 1997) in GenStat. The Pearson product moment correlation coefficient was also calculated to determine the correlation between the two cultivars.
Pathogenicity testing: bulb inoculation
The same 32 F. oxysporum isolates used in the seedling tests were assessed for pathogenicity on healthy, stored onion bulbs (cv. Napoleon). The outer scales of the bulbs were removed to leave a single brown layer of skin, after which the basal plate of each onion was cut off and the bulb surface was sterilized with 70% ethanol. A potato dextrose agar (PDA) plug (8 mm) taken from the edge of an actively growing colony of each F. oxysporum isolate (grown for 7 days at 20 °C) was then positioned on the basal plate of each bulb. Control bulbs were inoculated with a sterile plug of PDA. Bulbs were placed on moist tissue in a plastic box (four per box) inside a sealed plastic bag to maintain high humidity and incubated at 20 °C in the dark. Boxes were randomized in trays following an alpha design. After 48 h, each bulb was wrapped with cling‐film to ensure that the agar plug did not dry out. After 9 weeks, each bulb was bisected longitudinally and a digital image was taken (including a 10‐cm scale bar). Images were then analysed using ImageJ software (Schneider et al., 2012) to quantify the area of infection as a percentage of the total bulb area. Three independent replicates (four onion bulbs per replicate) were set up for each F. oxysporum isolate and significant differences between isolate disease area data were analysed using REML in GenStat. Pearson product moment correlation coefficients were calculated to determine correlations between disease data from seedling and bulb tests.
Molecular characterization of Fusarium isolates: housekeeping genes
Molecular characterization through sequencing of housekeeping genes was carried out for the 32 F. oxysporum isolates used in the pathogenicity tests, as well as 21 other f. spp. of F. oxysporum and Fusarium species (Table 1). Each isolate was grown on PDA for 4–7 days at 25 °C, and three agar plugs (5 mm) taken from the leading edge were placed in a 50‐mL tube containing 25 mL of sterile 50% potato dextrose broth (PDB). After incubation at 25 °C for 5 days and centrifugation at 3000 × g for 5 min, excess liquid was removed and the mycelium was rinsed twice with SDW. Finally, excess water was removed and the mycelium was flash frozen in liquid nitrogen before lyophilizing for 24 h. DNA was then extracted from approximately 20 mg of mycelium using a DNeasy plant mini kit (Qiagen, Hilden, Germany) with minor modifications, whereby the mycelium was first homogenized in a lysing matrix A tube (MP Biomedicals, Santa Ana, CA, USA) placed in a FastPrep‐24™ machine (MP Biomedicals) set at 6 m/s for 40 s. The manufacturer's protocol was then followed with the addition of an extra centrifugation step (12 500 × g for 5 min) after the cell lysis stage. DNA integrity was confirmed by gel electrophoresis.
PCR amplification and sequencing of EF‐1α, RPB2 and TUB2 was carried out for all Fusarium isolates using published primers (Table 4) with reactions set up using REDTaq® ReadyMix® (Sigma‐Aldrich Gillingham, Dorset, UK) in 20‐µL volumes containing approximately 50 ng of DNA and a final concentration of 0.5 µm of each primer. For EF‐1α, the thermocycling conditions were as follows: one cycle of 5 min at 94 °C; 40 cycles of 45 s at 94 °C, 30 s at 64 °C and 2 min at 72 °C, followed by one cycle of 10 min at 72 °C. For RPB2, the conditions were as follows: one cycle of 1.5 min at 94 °C; 40 cycles of 30 s at 94 °C, 1.5 min at 60 °C and 2 min at 68 °C, followed by one cycle of 10 min at 68 °C. For TUB2, the conditions were as follows: one cycle of 3 min at 95 °C; 35 cycles of 1 min at 94 °C, 30 s at 60 °C and 1 min at 72 °C, followed by one cycle of 10 min at 72 °C. All PCR amplicons were purified using a QIAquick PCR Purification Kit (Qiagen), sequenced using forward and reverse primers, and contigs were constructed using the SeqBuilder package of DNASTAR® Lasergene® version 10 (DNASTAR Inc., Madison, WI, USA). Sequences were aligned (clustalw method, Thompson et al., 1994), concatenated using mega version 5.1 (Tamura et al., 2011) and a maximum likelihood tree was constructed using the calculated best model, Kimura‐2‐parameter plus gamma (Kimura, 1980). Bootstrap consensus trees were inferred from 1000 replicates (Felsenstein, 1985). Sequences from published F. oxysporum genomes (Broad Institute/MIT, 2007) were also included in this analysis and were identified using a Broad Institute (BI) label.
Table 4.
Primer pairs used for molecular characterization of Fusarium isolates with product size, annealing temperature and relevant publications.
| Gene* | Primers | Sequence 5′–3′ (forward primer/reverse primer) | Product size | Annealing temperature (ºC) | Publication |
|---|---|---|---|---|---|
| TUB2 | T1/T22 | AACATGCGTGAGATTGTAAGT/TCTGGATGTTGTTGGGAATCC | ∼1500 | 60 | O'Donnell and Cigelnik (1997) |
| RPB2 | 7cF/11aR | ATGGGYAARCAAGCYATGGG/GCRTGGATCTTRTCRTCSACC | 881 | 57 | O'Donnell et al. (2007) |
| EF‐1α | exTEF‐F/FUexTEF‐R | ACCCGGTTCAAGCATCCGATCTGCGA/AGCTTGCCRGACTTGATCTCACGCTC | 1269 | 64 | Vágány (2012) |
| SIX1 | SIX1 | GTATCCCTCCGGATTTTGAGC/AATAGAGCCTGCAAAGCATG | 992 | 59 | Lievens et al. (2009) |
| SIX2 | SIX2 | CAACGCCGTTTGAATAAGCA/TCTATCCGCTTTCTTCTCTC | 749 | 59 | Lievens et al. (2009) |
| SIX3 | SIX3 | CCAGCCAGAAGGCCAGTTT/GGCAATTAACCACTCTGCC | 608 | 59 | Lievens et al. (2009) |
| SIX4 | SIX4 | TCAGGCTTCACTTAGCATAC/GCCGACCGAAAAACCCTAA | 967 | 59 | Lievens et al. (2009) |
| SIX5 | SIX5 | ACACGCTCTACTACTCTTCA/GAAAACCTCAACGCGGCAAA | 667 | 59 | Lievens et al. (2009) |
| SIX6 | SIX6 | CTCTCCTGAACCATCAACTT/CAAGACCAGGTGTAGGCATT | 793 | 59 | Lievens et al. (2009) |
| SIX7 | SIX7 | CATCTTTTCGCCGACTTGGT/CTTAGCACCCTTGAGTAACT | 862 | 59 | Lievens et al. (2009) |
| SIX8 | SIX8 | TCGCCTGCATAACAGGTGCCG/TTGTGTAGAAACTGGACAGTCGATGC | 250 | 59 | Meldrum et al. (2012) |
| SIX9 | FOL SIX9 | GGGTGGACCATATCACGATGTTCG/GAATACCTGAGTGGAGTTGTGTCTTG | 458 | 69 | This study |
| SIX9 | FOC SIX9 | GGCCCAGCCCTAGTCTAACTCC/AACTTAACATGCTGGCCGTCAATCG | 347 | 67 | This study |
| SIX10 | SIX10 | GTTAGCAACTGCGAGACACTAGAA/AGCAACTTCCTTCCTCTTACTAGC | 636 | 65 | This study |
| SIX11 | SIX11 | ATTCCGGCTTCGGGTCTCGTTTAC/GAGAGCCTTTTTGGTTGATTGTAT | 559 | 61 | This study |
| SIX12 | SIX12 | CTAACGAAGTGAAAAGAAGTCCTC/GCCTCGCTGGCAAGTATTTGTT | 449 | 61 | This study |
| SIX13 | SIX13 | CCTTCATCATCGACAGTACAACG/ATCAAACCCGTAACTCAGCTCC | 1027 | 61 | This study |
| SIX14 | FOL SIX14 | ATAAAGTGCGACTGGACTTCTGCC/ACCCCCATCCACATTCCTAAGCGA | 422 | 67 | This study |
| SIX14 | FOL SIX14 nest | GATCCCAATGGGGGCTGTGT/GCTGGTGGCTAGAATCTCTTTGGA | 232 | 59 | This study |
| SIX14 | FOC SIX14 | ACAACACCGCGACGCTAAAAAT/GCACACTCAGTGCGACAAGTTC | 438 | 61 | This study |
| C5 | C5 | AGAGTGTGAAGTGAGGACGAGGGA/CTACGTTCGCCTCACTCATTGCCT | 1064 | 63 | This study |
| CRX1 | CRX1 | CACCATCTGTCTACATAAGGCCGCCC/AAAGTTCAAGGACCGGACCGCCG | 1654 | 69 | This study |
| CRX2 | CRX2 | TTAGTCGCACATCTACCATCACTG/GGAGTCGATCTAACTTCAGG | 856 | 58 | This study |
| CRX2 | CRX2 FP | CCAGTGCATTGGTTTGAGACGTT/ATGCGCTCGCTTTCTATGTATCTG | 902 | 63 | This study |
| Primers used for real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) | |||||
| SIX3 | QSIX3 | GGCCGTCTTCTACTTCATTTAC/GGGAGAATGTTCTAGCATAACC | 69 | 63 | This study |
| SIX5 | QSIX5 | TGGGCTCGAAAAGTCCAGCAT/TGTTTCGCCGTCAATGTCGCC | 114 | 63 | This study |
| SIX7 | QSIX7 | TCGATCTCTTTCCAAGACAAGGGCA/GTGGACGCGGCGTTGGTGAAC | 130 | 63 | This study |
| SIX9 | QSIX9 | GCCGACCCAGACCTACGCTTT/GCTGGTTTTGGAAGCCCAGTTGT | 129 | 63 | This study |
| SIX10 | QSIX10 | CCCGGAAAGCCTGCATCGACTA/AGAACAAACGTCGGTGGGACCA | 53 | 63 | This study |
| SIX12 | QSIX12 | TGCTGCTCCAAGTACAAACTACCTT/GCTGATACCTTTGGGTCCAACGC | 71 | 63 | This study |
| SIX14 | QSIX14 | ATGTCGTATGCCGGACGGGAA/TTATCTCGTAGACGCCTTCCT | 109 | 60 | This study |
| C5 | QC5 | GCCTATGGCAGGACTTGTTGAC/CCACAGCTTCTTGGACTATCTCC | 126 | 63 | This study |
| CRX1 | QCRX1 | AACTCAGGTACCACATCGGGA/CAGGTCGTCCTAGCGTCAGT | 89 | 60 | This study |
| CRX2 | QCRX2 | CAATCAGAAACCACGACGGAA/GGAGTCGATCTAACTTCAGG | 89 | 60 | This study |
| EF‐1α | QTEF | GGTCAGGTCGGTGCTGGTTACG/TGGATCTCGGCGAACTTGCAGG | 77 | 63 | This study |
| TUB2 | QTUB | TTCTGCTGTCATGTCCGGTGT/TCAGAGGAGCAAAGCCAACCA | 134 | 63 | This study |
*EF‐1α, translation elongation factor 1α; RPB2, RNA polymerase II second largest subunit ; SIX, Secreted In Xylem; TUB2, β‐tubulin.
Molecular characterization of Fusarium isolates: putative effectors
Molecular characterization through the detection of the presence/absence and sequencing of putative effector genes was carried out for all Fusarium isolates in Table 1. Isolates were assessed for the presence/absence of SIX1–SIX14 using published primers where available (for SIX1–SIX8) (Lievens et al., 2009; Meldrum et al., 2012), whereas new primers were designed for SIX9–14 based on the published genome sequences of FOL (isolates MN25/4287, Table 4). Nested primers (FOL SIX14 nest) were used for SIX14 because of the very short sequence reads and poor quality sequence data, and PCR was carried out as described below, using 1 µL of purified PCR product. FOL isolate MN25 was used as a positive control for all SIX genes, with the exception of SIX4, where isolate FOL1 was used, as MN25 does not contain SIX4 (Broad Institute/MIT, 2007). All primer sequences were checked against target SIX sequences using a preliminary assembly of the FOC genome (Vágány, 2012) and, following this, new primers were designed for SIX9 (FOC SIX9) and SIX14 (FOC SIX14), because of the large sequence differences between FOC and FOL (Table 4). All PCRs for the SIX genes were set up as described for the housekeeping genes with standard thermocycling conditions as follows: one cycle of 2 min at 94 °C; 30 cycles of 45 s at 94 °C, 30 s annealing (see Table 4 for temperatures) and 1 min at 72 °C, followed by one cycle of 5 min at 72 °C. A de novo assembly of 70‐bp paired‐end reads (Illumina, San Diego, CA, USA, GAIIx sequencing) was carried out using Velvet to obtain a preliminary FOC genome for isolate FUS2 (Vágány, 2012; Zerbino and Birney, 2008), and resulted in 1511 contigs with an N50 of 184 kb (raw 70‐bp paired‐end Illumina reads submitted to the NCBI Sequence Read Archive under BioProject PRJNA287483). This assembly was investigated for putative new effectors, including SIX genes. Proteins predicted by Augustus (Keller et al., 2011; Stanke and Morgenstern, 2005), which were located on the same contig as any of the SIX genes and had at least two of the three main characteristics of effector proteins [short length, presence of a signal peptide as predicted by SignalP version 4 (Petersen et al., 2011), no homology to any other known protein] were considered. This led to the discovery of several putative effectors, including gene C5. Then, any predicted protein in the rest of the assembly that met these criteria was considered. This list was refined by screening for the presence of published motifs related to pathogenicity, including RxLR‐dEER (Rehmany et al., 2005), which led to the discovery of two very closely related genes, CRX1 and CRX2. Primers were then designed for CRX1, CRX2 and C5 (Table 4), and PCR was carried out using the thermocycling conditions described for the SIX genes. Additional primers were also designed (CRX2 FP) to amplify CRX2 from F. proliferatum (forward primer based on F. oxysporum because of a lack of sequence for F. proliferatum; reverse primer based on F. proliferatum using sequence from initial PCR with CRX2 primers) in order to assess the presence/absence of the RxLR domain. All PCR amplicons were purified as described previously, and sequencing was carried out using forward primers or both primers for longer amplicons. Where both primers were used, contigs were constructed as described previously. For F. proliferatum (isolates A8, A40 and SP1_2), contigs were constructed from sequences of both PCRs. Sequences were aligned and maximum likelihood trees were constructed for SIX7, SIX9, SIX10 and SIX12, as described previously. The models used were Kimura‐2‐parameter, gamma distributed for SIX7 and with invariant sites for SIX9 (Kimura, 1980), Jukes–Cantor for SIX10 and Jukes‐Cantor, gamma distributed for SIX12 (Jukes and Cantor, 1969). All available homologous sequences (NCBI) were included and sequences were extracted from all available Fusarium genome data following blast searches (Broad Institute/MIT, 2007). Trees were not constructed for any of the other SIX genes because of the limited number of sequences.
Expression of putative effectors
For the examination of expression in planta, onion seedlings were infected with isolate FUS2 as described in Methods S1 (see Supporting Information). RNA was extracted from the pooled root systems of five seedlings using Trizol® reagent (Life Technologies, Paisley, UK), any DNA was removed using DNase I (Sigma‐Aldrich) and first‐strand cDNA was synthesized using Superscript II reverse transcriptase (Life Technologies) following the manufacturer's guidelines. Real‐time RT‐PCR was performed in a Roche Lightcycler 480 using the Lightcycler 480 SYBR Green I Master mix (Roche, Burgess Hill, Sussex, UK), following the manufacturer's instructions. Primers were used at a final concentration of 0.4 µm with the annealing temperatures in Table 4. The cycling conditions were as follows: one cycle of 95 °C for 5 min, followed by 45 cycles of 95 °C for 10 s, 60/63 °C for 10 s and 72 °C for 10 s. Melt curve analyses were used to confirm a single PCR product. All samples were run in triplicate, standard curves were plotted for each gene and data were expressed as the quantity of the target gene relative to the geometric mean of EF‐1α and TUB2.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's website:
Fig. S1 Partial nucleotide alignment of the SIX5 gene from Fusarium oxysporum f. spp. cepae and lycopersici (isolate MN25, Broad Institute Fusarium database). Shaded bases differ from the predominant sequence type.
Methods S1 Protocol for analysing expression of putative effector genes in planta.
Acknowledgements
This research was funded through the Biotechnology and Biological Sciences Research Council's Horticulture and Potato Initiative (Project BB/K020870/1), an Agriculture and Horticulture Development Board Fellowship (CP113, to AT) and an Erasmus Fellowship (to AR), and we thank these funding agencies for support. The approaches and preliminary data used in this research (including original F. oxysporum f. sp. cepae genome sequence) were inspired and developed from the PhD of V. Vagany (as cited) which was funded by the Department for Environment, Food and Rural Affairs (Defra) and originally supervised by the late Dr Dez Barbara (University of Warwick, UK) and Dr Surapareddy (Prasad) Sreenivasaprasad (University of Bedfordshire, UK), with additional support from the Food and Environment Research Agency (FERA). We also thank Jeanette Selby for technical support with genome sequencing, the Warwick Crop Centre Horticultural Services team for their assistance with the glasshouse seedling assays, Reinout de Heer (Hazera Seeds) and Syngenta for providing onion seed, Andy Richardson (Allium and Brassica Centre, Boston, Lincs, UK) for providing onion bulbs and Patrick/Ruth Schäfer (University of Warwick, UK) for advice on growing onions in the sterile system. The authors have no conflicts of interest to declare.
References
- Abawi, G.S. and Lorbeer, J.W. (1972) Several aspects of the ecology and pathology of Fusarium oxysporum f. sp. cepae . Phytopathology, 62, 870–876. [Google Scholar]
- Alabouvette, C. , Olivain, C. , Migheli, Q. and Steinberg, C. (2009) Microbiological control of soil‐borne phytopathogenic fungi with special emphasis on wilt‐inducing Fusarium oxysporum . New Phytol. 184, 529–544. [DOI] [PubMed] [Google Scholar]
- Bayraktar, H. and Dolar, F.S. (2011) Molecular identification and genetic diversity of Fusarium species associated with onion fields in Turkey. J. Phytopathol. 159, 28–34. [Google Scholar]
- Bayraktar, H. , Türkkan, M. and Dolar, F.S. (2010) Characterization of Fusarium oxysporum f.sp. cepae from onion in Turkey based on vegetative compatibility and rDNA RFLP analysis. J. Phytopathol. 158, 691–697. [Google Scholar]
- Boratyn, G.M. , Camacho, C. , Cooper, P.S. , Coulouris, G. , Fong, A. , Ma, N. , Madden, T.L. , Matten, W.T. , McGinnis, S.D. , Merezhuk, Y. , Raytselis, Y. , Sayers, E.W. , Tao, T. , Ye, J. and Zaretskaya, I. (2013) BLAST: a more efficient report with usability improvements. Nucleic Acids Res. 41, W29–W33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayford, D. (1996) IMI descriptions of fungi and bacteria set 127. Mycopathologia, 133, 35–63. [DOI] [PubMed] [Google Scholar]
- Broad Institute/MIT. (2007) Fusarium Comparative Sequencing Project. Available at: http://www.broadinstitute.org/. Accessed March 2015.
- Chakrabarti, A. , Rep, M. , Wang, B. , Ashton, A. , Dodds, P. and Ellis, J. (2011) Variation in potential effector genes distinguishing Australian and non‐Australian isolates of the cotton wilt pathogen Fusarium oxysporum f.sp. vasinfectum . Plant Pathol. 60, 232–243. [Google Scholar]
- Covey, P.A. , Kuwitzky, B. , Hanson, M. and Webb, K.M. (2014) Multilocus analysis using putative fungal effectors to describe a population of Fusarium oxysporum from sugar beet. Phytopathology, 104, 886–896. [DOI] [PubMed] [Google Scholar]
- Cramer, C. (2000) Breeding and genetics of Fusarium basal rot resistance in onion. Euphytica, 115, 159–166. [Google Scholar]
- Dean, R. , Van Kan, J.A. , Pretorius, Z.A. , Hammond‐Kosack, K.E. , Di Pietro, A. , Spanu, P.D. , Rudd, J.J. , Dickman, M. , Kahmann, R. , Ellis, J. and Foster, G.D. (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake, M. , Kashima, R. , Tanaka, S. and Ito, S.‐I. (2009a) Genetic diversity and pathogenicity of Fusarium oxysporum isolated from wilted Welsh onion in Japan. J. Gen. Plant Pathol. 75, 125–130. [Google Scholar]
- Dissanayake, M. , Kashima, R. , Tanaka, S. and Ito, S.‐I. (2009b) Pathogenic variation and molecular characterization of Fusarium species isolated from wilted Welsh onion in Japan. J. Gen. Plant Pathol. 75, 37–45. [Google Scholar]
- du Toit, L.J. , Inglis, D.A. and Pelter, G.Q. (2003) Fusarium proliferatum pathogenic on onion bulbs in Washington. Plant Dis. 87, 750–750. [DOI] [PubMed] [Google Scholar]
- Entwistle, A.R. (1990) Root diseases In: Onions and Allied Crops (Brewster J.L., ed.), pp. 103–154. Boca Raton, FL: CRC Press. [Google Scholar]
- FAOSTAT. (2012) Food and Agricultural Organization of the United Nations – Production Statistics. Available at: http://faostat3.fao.org/home/E. Accessed March 2015.
- Felsenstein, J. (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39, 783–791. [DOI] [PubMed] [Google Scholar]
- Fraser‐Smith, S. , Czislowski, E. , Meldrum, R.A. , Zander, M. , O'Neill, W. , Balali, G.R. and Aitken, E.A.B. (2014) Sequence variation in the putative effector gene SIX8 facilitates molecular differentiation of Fusarium oxysporum f. sp. cubense . Plant Pathol. 63, 1044–1052. [Google Scholar]
- Galván, G. , Koning‐Boucoiran, C. , Koopman, W. , Burger‐Meijer, K. , González, P. , Waalwijk, C. , Kik, C. and Scholten, O. (2008) Genetic variation among Fusarium isolates from onion, and resistance to Fusarium basal rot in related Allium species. Eur. J. Plant Pathol. 121, 499–512. [Google Scholar]
- Gawehns, F. , Houterman, P.M. , Ichou, F.A. , Michielse, C.B. , Hijdra, M. , Cornelissen, B.J. , Rep, M. and Takken, F.L. (2014) The Fusarium oxysporum effector Six6 contributes to virulence and suppresses I‐2‐mediated cell death. Mol. Plant–Microbe Interact. 27, 336–348. [DOI] [PubMed] [Google Scholar]
- Ghanbarzadeh, B. , Mohammadi Goltapeh, E. and Safaie, N. (2013) Identification of Fusarium species causing basal rot of onion in East Azarbaijan province, Iran and evaluation of their virulence on onion bulbs and seedlings. Arch. Phytopathol. Plant Protect. 47, 1050–1062. [Google Scholar]
- Houterman, P.M. , Speijer, D. , Dekker, H.L. , De Koster, C.G. , Cornelissen, B.J.C. and Rep, M. (2007) The mixed xylem sap proteome of Fusarium oxysporum‐infected tomato plants. Mol. Plant Pathol. 8, 215–221. [DOI] [PubMed] [Google Scholar]
- Houterman, P.M. , Cornelissen, B.J.C. and Rep, M. (2008) Suppression of plant resistance gene‐based immunity by a fungal effector. PLoS Pathog. 4, e1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houterman, P.M. , Ma, L. , Van Ooijen, G. , De Vroomen, M.J. , Cornelissen, B.J.C. , Takken, F.L.W. and Rep, M. (2009) The effector protein Avr2 of the xylem‐colonizing fungus Fusarium oxysporum activates the tomato resistance protein I‐2 intracellularly. Plant J. 58, 970–978. [DOI] [PubMed] [Google Scholar]
- Jiang, R.H.Y. , Tripathy, S. , Govers, F. and Tyler, B.M. (2008) RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving superfamily with more than 700 members, Proc. Natl. Acad. Sci. USA 105, 4874–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes, T.H. and Cantor, C.R. (1969) Evolution of protein molecules In: Mammalian Protein Metabolism (Munro H.N., ed.), pp. 21–132. New York: Academic Press. [Google Scholar]
- Kale, S.D. (2012) Oomycete and fungal effector entry, a microbial Trojan horse. New Phytol. 193, 874–881. [DOI] [PubMed] [Google Scholar]
- Kale, S.D. , Gu, B. , Capelluto, D.G.S. , Dou, D. , Feldman, E. , Rumore, A. , Arredondo, F.D. , Hanlon, R. , Fudal, I. , Rouxel, T. , Lawrence, C.B. , Shan, W. and Tyler, B.M. (2010) External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell, 142, 284–295. [DOI] [PubMed] [Google Scholar]
- Kashiwa, T. , Inami, K. , Fujinaga, M. , Ogiso, H. , Yoshida, T. , Teraoka, T. and Arie, T. (2013) An avirulence gene homologue in the tomato wilt fungus Fusarium oxysporum f. sp. lycopersici race 1 functions as a virulence gene in the cabbage yellows fungus F. oxysporum f. sp. conglutinans . J. Gen. Plant Pathol. 79, 412–421. [Google Scholar]
- Kehr, A.E. , O'Brien, J. and Davis, E.W. (1962) Pathogenicity of Fusarium oxysporum f. sp. cepae and its interaction with Pyrenochaeta terrestris on onion. Euphytica, 11, 197–208. [Google Scholar]
- Keller, O. , Kollmar, M. , Stanke, M. and Waack, S. (2011) A novel hybrid gene prediction method employing protein multiple sequence alignments. Bioinformatics, 27, 757–763. [DOI] [PubMed] [Google Scholar]
- Kimura, M. (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120. [DOI] [PubMed] [Google Scholar]
- Laurence, M.H. , Summerell, B.A. and Liew, E.C.Y. (2015) Fusarium oxysporum f. sp. canariensis: evidence for horizontal gene transfer of putative pathogenicity genes. Plant Pathol. 64, 1068–1075. [Google Scholar]
- Leslie, J.F. and Summerell, B.A. (2006) The Fusarium Laboratory Manual. Oxford: Blackwell Publishing. [Google Scholar]
- Lievens, B. , Houterman, P.M. and Rep, M. (2009) Effector gene screening allows unambiguous identification of Fusarium oxysporum f. sp. lycopersici races and discrimination from other formae speciales. FEMS Microbiol. Lett. 300, 201–215. [DOI] [PubMed] [Google Scholar]
- Lysøe, E. , Harris, L.J. , Walkowiak, S. , Subramaniam, R. , Divon, H.H. , Riiser, E.S. , Llorens, C. , Gabaldon, T. , Kistler, H.C. , Jonkers, W. , Kolseth, A.K. , Nielsen, K.F. , Thrane, U. and Frandsen, R.J. (2014) The genome of the generalist plant pathogen Fusarium avenaceum is enriched with genes involved in redox, signaling and secondary metabolism. PLos One, 9, e112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L.‐J. , van der Does, H.C. , Borkovich, K.A. , Coleman, J.J. , Daboussi, M.‐J. , Di Pietro, A. , Dufresne, M. , Freitag, M. , Grabherr, M. , Henrissat, B. , Houterman, P.M. , Kang, S. , Shim, W‐B. , Woloshuk, C. , Xie, X. , Xu, J‐R. , Antoniw, J. , Baker, S.E. , Bluhm, B.H. , Breakspear, A. , Brown, D.W. , Butchko, R.A.E. , Chapman, S. , Coulson, R. , Coutinho, P.M. , Danchin, E.G.J. , Diener, A. , Gale, L.R. , Gardiner, D.M. , Goff, S. , Hammond‐Kosack, K.E. , Hilburn, K. , Hua‐Van, A. , Jonkers, W. , Kazan, K. , Kodira, C.D. , Koehrsen, M. , Kumar, L. , Lee, Y‐H. , Li, L. , Manners, J.M. , Miranda‐Saavedra, D. , Mukherjee, M. , Park, G. , Park, J. , Park, S‐Y. , Proctor, R.H. , Regev, A. , Ruiz‐Roldan, M.C. , Sain, D. , Sakthikumar, S. , Sykes, S. , Schwartz, D.C. , Turgeon, B.G. , Wapinski, I. , Yoder, O. , Young, S. , Zeng, Q. , Zhou, S. , Galagan, J. , Cuomo, C.A. , Kistler, H.C. and Rep, M. (2010) Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium . Nature, 464, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Cornelissen, B.J.C. and Takken, F.L.W. (2013) A nuclear localization for Avr2 from Fusarium oxysporum is required to activate the tomato resistance protein I‐2. Front. Plant Sci. 4, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L , Houterman, P.M , Gawehns, F. , Cao, L. , Sillo, F , Richter, H. , Clavijo‐Ortiz, M.J. , Schmidt, S.M. , Boeren, S. , Vervoort, J. , Cornelissen, B.J. , Rep, M. and Takken, F.L. (2015) The AVR2‐SIX5 gene pair is required to activate I‐2‐mediated immunity in tomato. New Phytol. 208, 507–518. [DOI] [PubMed] [Google Scholar]
- Meldrum, R.A. , Fraser‐Smith, S. , Tran‐Nguyen, L.T.T. , Daly, A.M. and Aitken, E.A.B. (2012) Presence of putative pathogenicity genes in isolates of Fusarium oxysporum f. sp. cubense from Australia. Australas. Plant Pathol. 41, 551–557. [Google Scholar]
- Michielse, C.B. and Rep, M. (2009) Pathogen profile update: Fusarium oxysporum . Mol. Plant Pathol. 10, 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, K. , Kistler, H.C. , Cigelnik, E. and Ploetz, R.C. (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA, 95, 2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, K. and Cigelnik, E. (1997) Two divergent dntragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7, 103–16. [DOI] [PubMed] [Google Scholar]
- O'Donnell, K. , Sarver, B.A. , Brandt, M. , Chang, D.C. , Noble‐Wang, J. , Park, B.J. , Sutton, D.A. , Benjamin, L. , Lindsley, M. , Padhye, A. , Geiser, D.M. and Ward, T.J. (2007) Phylogenetic diversity and microsphere array‐based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens‐associated U.S. keratitis outbreaks of 2005 and 2006. J Clin Microbiol 45, 2235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, T.N. , Brunak, S. , von Heijne, G. and Nielsen, H. (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods, 8, 785–786. [DOI] [PubMed] [Google Scholar]
- Rehmany, A.P. , Gordon, A. , Rose, L.E. , Allen, R.L. , Armstrong, M.R. , Whisson, S.C. , Kamoun, S. , Tyler, B.M. , Birch, P.R.J. and Beynon, J.L. (2005) Differential recognition of highly divergent downy mildew avirulence gene alleles by RPP1 resistance genes from two Arabidopsis lines. Plant Cell, 17, 1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rep, M. (2005) Small proteins of plant‐pathogenic fungi secreted during host colonization. FEMS Microbiol. Lett. 253, 19–27. [DOI] [PubMed] [Google Scholar]
- Rep, M. , Van Der Does, H.C. , Meijer, M. , Van Wijk, R. , Houterman, P.M. , Dekker, H.L. , De Koster, C.G. and Cornelissen, B.J.C. (2004) A small, cysteine‐rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I‐3‐mediated resistance in tomato. Mol. Microbiol. 53, 1373–1383. [DOI] [PubMed] [Google Scholar]
- Rout, E. , Nanda, S. , Nayak, S. and Joshi, R.K. (2014) Molecular characterization of NBS encoding resistance genes and induction analysis of a putative candidate gene linked to Fusarium basal rot resistance in Allium sativum . Physiol. Mol. Plant Pathol. 85, 15–24. [Google Scholar]
- Sasaki, K. , Nakahara, K. , Shigyo, M. , Tanaka, S. and Ito, S.‐i. (2015a) Detection and quantification of onion isolates of Fusarium oxysporum f. sp. cepae in onion plant. J. Gen. Plant Pathol. 81, 232–236. [Google Scholar]
- Sasaki, K. , Nakahara, K. , Tanaka, S. , Shigyo, M. and Ito, S.I. (2015b) Genetic and pathogenic variability of Fusarium oxysporum f. sp. cepae isolated from onion and Welsh onion in Japan. Phytopathology, 105, 525–532 [DOI] [PubMed] [Google Scholar]
- Schmidt, S.M. , Houterman, P.M. , Schreiver, I. , Ma, L. , Amyotte, S. , Chellappan, B. , Boeren, S. , Takken, F.L. and Rep, M. (2013) MITEs in the promoters of effector genes allow prediction of novel virulence genes in Fusarium oxysporum . BMC Genomics, 14, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, C.A. , Rasband, W.S. and Eliceiri, K.W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura, A. (2002) Studies on the ecology and control of Welsh onion root rot caused by Fusarium redolens . J. Gen. Plant Pathol. 68, 265. [Google Scholar]
- Southwood, M.J. , Viljoen, A. , Mostert, G. and McLeod, A. (2012a) Molecular identification of two vegetative compatibility groups of Fusarium oxysporum f. sp. cepae . Phytopathology, 102, 204–213. [DOI] [PubMed] [Google Scholar]
- Southwood, M.J. , Viljoen, A. , Mostert, L. , Rose, L.J. and McLeod, A. (2012b) Phylogenetic and biological characterization of Fusarium oxysporum isolates associated with onion in South Africa. Plant Dis. 96, 1250–1261. [DOI] [PubMed] [Google Scholar]
- Stanke, M. and Morgenstern, B. (2005) AUGUSTUS: a web server for gene prediction in eukaryotes that allows user‐defined constraints. Nucleic Acids Res. 33, W465–W467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic, S. , Levic, J. , Petrovic, T. , Logrieco, A. and Moretti, A. (2007) Pathogenicity and mycotoxin production by Fusarium proliferatum isolated from onion and garlic in Serbia. Eur. J. Plant Pathol. 118, 165–172. [Google Scholar]
- Takken, F. and Rep, M. (2010) The arms race between tomato and Fusarium oxysporum . Mol. Plant Pathol. 11, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , Peterson, D. , Peterson, N. , Stecher, G. , Nei, M. and Kumar, S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol . Biol. Evol. 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, A. , Vagany, V. , Barbara, D.J. , Thomas, B. , Pink, D.A.C. , Jones, J.E. and Clarkson, J.P. (2013) Identification of differential resistance to six Fusarium oxysporum f. sp. cepae isolates in commercial onion cultivars through the development of a rapid seedling assay. Plant Pathol. 62, 103–111. [Google Scholar]
- Thatcher, L.F. , Gardiner, D.M. , Kazan, K. and Manners, J.M. (2012) A highly conserved effector in Fusarium oxysporum is required for full virulence on Arabidopsis . Mol. Plant–Microbe Interact. 25, 180–190. [DOI] [PubMed] [Google Scholar]
- Thompson, J.D. , Higgins, D.G. and Gibson, T.J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position‐specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vágány, V. (2012) Characterisation of Fusarium pathogens in the UK. PhD Thesis. University of Warwick, Warwick: School of Life Sciences.
- Welham, S.J. and Thompson, R. (1997) Likelihood Ratio Tests for Fixed Model Terms using Residual Maximum Likelihood. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 59, 701–14. [Google Scholar]
- Yamazaki, M. , Morita, Y. , Kashiwa, T. , Teraoka, T. and Arie, T. (2013) Fusarium proliferatum, an additional bulb rot pathogen of Chinese chive. J. Gen. Plant Pathol. 79, 431–434. [Google Scholar]
- Zerbino, D.R. and Birney, E. (2008) Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18, 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's website:
Fig. S1 Partial nucleotide alignment of the SIX5 gene from Fusarium oxysporum f. spp. cepae and lycopersici (isolate MN25, Broad Institute Fusarium database). Shaded bases differ from the predominant sequence type.
Methods S1 Protocol for analysing expression of putative effector genes in planta.


