Summary
Nitric oxide (NO) is a signalling molecule involved in many biological processes in bacteria, plants and mammals. However, little is known about the role and biosynthesis of NO in fungi. Here we show that NO production is increased at the early stages of the transition from vegetative growth to development in A spergillus nidulans. Full NO production requires a functional nitrate reductase (NR) gene (nia D) that is upregulated upon induction of conidiation, even under N‐repressing conditions in the presence of ammonium. At this stage, NO homeostasis is achieved by balancing biosynthesis (NR) and catabolism (flavohaemoglobins). nia D and flavohaemoglobin fhb A are transiently upregulated upon induction of conidiation, and both regulators AreA and NirA are necessary for this transcriptional response. The second flavohaemoglobin gene fhb B shows a different expression profile being moderately expressed during the early stages of the transition phase from vegetative growth to conidiation, but it is strongly induced 24 h later. NO levels influence the balance between conidiation and sexual reproduction because artificial strong elevation of NO levels reduced conidiation and induced the formation of cleistothecia. The nitrate‐independent and nitrogen metabolite repression‐insensitive transcriptional upregulation of nia D during conidiation suggests a novel role for NR in linking metabolism and development.
Introduction
Reproduction is an essential part of the biological cycle of fungi and a mode of dispersion in the environment. Among fungi, Aspergillus nidulans has been used as a model organism to study reproduction for decades. A. nidulans displays two modes of reproduction: sexual or asexual. The asexual development program (conidiation) is initiated when superficial hyphae are exposed to an air interphase. The asexual reproductive structure of A. nidulans is the conidiophore, which forms green‐pigmented conidiospores approximately 24 h after induction of development (Adams et al., 1998; Etxebeste et al., 2010; Yu, 2010). A central regulatory pathway encompassing BrlA, AbaA and WetA controls conidiation (see reviews by Adams et al., 1998; Etxebeste et al., 2010; Yu, 2010, and references therein). The first component in the regulatory cascade, BrlA, is essential to drive conidiation (Adams et al., 1988). brlA is not expressed during vegetative growth, but its induction during development is controlled by a number of genes, e.g. the fluffy genes (Adams et al., 1998; Yu et al., 2006). These fluffy genes are expressed in vegetative mycelium and are able to respond to stimuli to induce the co‐ordinated activation of the master regulator brlA (Etxebeste et al., 2010).
The sexual program is promoted by signals that can be grouped in four categories: absence of light, nutrient availability, temperature and atmospheric gases. Under laboratory conditions, plate sealing limits the exchange of gases and results in lowering the oxygen levels and increasing the CO2 levels, which promotes sexual reproduction (Dyer and O'Gorman, 2012). A. nidulans is homothallic, i.e. each strain harbours both mating type genes, MAT1/matB and MAT2/matA (Paoletti et al., 2007). During this program, A. nidulans develops spherical fruiting bodies called cleistothecia, which contain meiospores called ascospores (Dyer and O'Gorman, 2012). Genes involved in the regulation of cleistothecia formation were mainly identified through A. nidulans mutants defective at distinct stages of sexual development: acleistothecial strains such as ΔnsdD and ΔstuA (Wu and Miller, 1997; Han et al., 2001), mutations that stop the propagation program after production of Hülle cells such as ΔsteA and ΔmedA (Clutterbuck, 1969; Vallim et al., 2000) and strains that are blocked in the maturation of cleistothecia (Eckert et al., 2000; Hoffmann et al., 2000; Busch et al., 2001).
Early work in H. Ninnemann's laboratory connected nitrate reductase activity to blue light‐promoted conidiation in Neurospora crassa (Klemm and Ninnemann, 1979; Ninnemann, 1991). The pathway for the assimilation of nitrate has been extensively studied in A. nidulans for several decades. Nitrate assimilation requires the action of two enzymes: nitrate reductase (NR, encoded by niaD), mediating the reduction of nitrate to nitrite; and the nitrite reductase (NiR, encoded by niiA) involved in the conversion of nitrite to ammonium. Nitrate assimilation is generally repressed in fungi by the presence of the preferred nitrogen sources ammonium or glutamine and, in A. nidulans, is activated by nitrate or nitrite through the synergistic action of the pathway specific regulator NirA and the general regulator of nitrogen metabolism AreA (Arst and Cove, 1973; Caddick et al., 1986; Burger et al., 1991; Marzluf, 1997; Strauss et al., 1998; Muro‐Pastor et al., 1999; Todd et al., 2005; Berger et al., 2006; 2008; Bernreiter et al., 2007; Schinko et al., 2010).
Recently, the regulation of nitrate assimilation was connected to nitric oxide (NO) metabolism in A. nidulans (Schinko et al., 2010). NO is widely recognised for its role as signalling compound from bacteria to mammals (Rosselli et al., 1998; He et al., 2004; Kwon et al., 2012; Seth et al., 2012). This diatomic molecule is a short‐lived radical with a half‐live of a few seconds. It plays essential regulatory roles in a variety of biological processes but can also cause nitrosative stress to cells. Flavohaemoglobins are known to function in NO detoxification, converting the reactive NO radical into nitrate and consequently protecting against nitrosative stress derived from either exogenous or endogenous sources (Gardner et al., 1998a; Foster et al., 2009). Different routes for cellular NO biosynthesis have been described in nature, including both oxidative and reductive pathways. The oxidative synthesis has been reported in bacteria, plants and mammals involving the conversion of L‐arginine and O2 into citrulline and NO by the nitric oxide synthase (Alderton et al., 2001; Lamotte et al., 2005; Gorren and Mayer, 2007). The reductive NO synthesis was reported as first in legumes (Dean and Harper, 1988; Yamasaki, 2000), but later was shown to be widespread in plants, and it involves the action of the nitrate reductase at saturating nitrite concentrations under reductive conditions (excess of NAPDH) (Yamasaki, 2000; Yamasaki and Sakihama, 2000; Rockel et al., 2002; Lamotte et al., 2005). In mammals alternative reductive pathways for the conversion of nitrite to NO have been identified involving different enzymes such as cytochrome c, P450 or deoxyhaemoglobin in the heart and wall vessels or by non‐enzymatic mechanisms (Zweier et al., 2010). Notably, fungal genomes are devoid of NO synthase homologues (Gorren and Mayer, 2007; Samalova et al., 2013) and the physiological and genetic mechanisms that determine changes in the level of NO in fungi are poorly understood in these organisms. Addition of NO chemical donors induces asexual spore development in the ascomycete N. crassa (Ninnemann and Maier, 1996), sporangiophore development in the zygomycete Phycomyces blakesleeanus (Maier et al., 2001), conidiation in Coniothyrium minitans (Gong et al., 2007) and sexual fruiting bodies in the ascomycete A. nidulans (Baidya et al., 2011) and in the basidiomycete Flammulina velutipes (Song et al., 2000). Magnaporthe oryzae synthesised NO during germination and early development; however, the deletion of candidate genes in both oxidative and reductive routes did not impair NO biosynthesis in this fungus (Samalova et al., 2013). In A. nidulans the two flavohaemoglobins FhbA and FhbB were shown to be involved in the metabolism of NO to nitrate (Gardner et al., 1998b; Poole and Hughes, 2000). In addition, they are essential to protect nitrate reductase and nitrite reductase enzymes against nitrosative damage elicited by growth on nitrite at low environmental pH conditions (Schinko et al., 2010; 2013).
In this work, we provide data indicating the existence of a nitrate reductase‐dependent pathway for the synthesis of NO in A. nidulans and found that NO levels increase immediately after switching from vegetative growth to conidiation. Both flavohaemoglobins are involved in the balance of NO during the developmental switch.
Results
Aspergillus produces NO
Several techniques have been employed to detect and quantify NO production (Yamasaki and Sakihama, 2000; Maier et al., 2001; Planchet and Kaiser, 2006; Nagano, 2009; Samalova et al., 2013). We selected the NO‐sensitive fluorescent probes developed by Nagano and collaborators (Kojima et al., 1998a, 1998b, 1999). This approach was previously reported to be useful in fungi (Carmona et al., 2012; Samalova et al., 2013). DAF‐FM is very sensitive to NO, with a detection limit of 3 nM, whereas DAF‐FM diacetate (DAF‐FM DA) is cell‐permeable and passively diffuses across cellular membranes. Once inside the cells it is deacetylated to DAF‐FM by esterases (Kojima et al., 1999). To test whether they could also be employed in Aspergillus, fungal cells were grown in liquid ammonium minimal medium. Either DAF‐FM or DAF‐FM DA was added to the cultures. After cells were loaded with the dyes for 20 min, the excess of both dyes was washed out in half of the samples, and all of them were transferred to a 96‐well plate for monitoring fluorescence. As shown in Fig. 1A–B, there was an increase in the fluorescence over time when the excess of dye were not washed off the cells. However, when the cells were washed, the fluorescence recorded was drastically lower than in non‐washed cells or in control medium. To analyse whether the monitored fluorescence was the result of the reaction with the NO‐sensitive probe inside or outside the cells, fluorescence was imaged under a fluorescent microscope (Fig. 1C). When the cells were not washed, fluorescence was observed both outside and inside the cells regardless of the dye (DAF‐FM or DAF‐FM DA). After the excess of dye was washed out, fluorescence was observed preferentially inside the cells. To confirm the biological origin of the signal, Aspergillus cells grown for 16 h in liquid ammonium minimal medium were autoclaved. DAF‐FM DA was added to live cells, dead cells and the minimal medium and fluorescence was recorded (Fig. 1D). While fluorescence in live cell samples increased continuously with time, the fluorescence generated in the samples containing heat‐killed fungi remained constant over time, suggesting that NO production was mediated by live cells.
Figure 1.
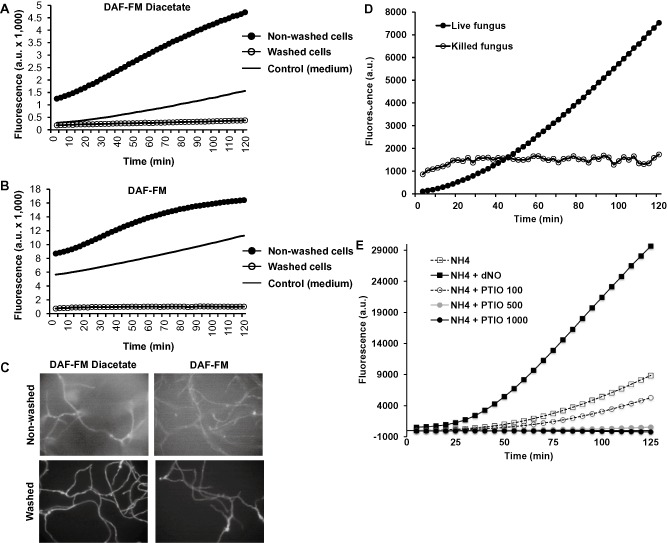
Quantification of NO produced by A spergillus. A . nidulans was grown in liquid ammonium minimal medium. DAF‐FM DA (A) or DAF‐FM (B) was added to the cultures and incubated for 20 min in the dark. After cell loading of the dyes, one sample containing each dye was washed three times, whereas control samples were not washed. Fresh medium was used as an additional control to detect the background signal. Fluorescence was monitored by fluorometry (A and B) or cells were imaged by fluorescent microscopy (C). (D) A . nidulans was grown in liquid ammonium minimal medium for 16 h. One sample was kept as a living control, whereas the rest of the cells were killed by autoclaving. DAF‐FM DA was added to the samples and the fluorescence was recorded by fluorometry. Background signal obtained with fresh medium was subtracted from signals obtained with both cell samples. (E) A spergillus cells were grown in the presence or in the absence of the NO‐releasing compound dNO (1.5 mM) or different concentrations of the NO‐scavenger PTIO (100–1000 μM). DAF‐FM DA was added to the samples and fluorescence was recorded by fluorometry. Under all these conditions, fungal growth was similar. In all cases, one representative experiment is shown.
It has been previously reported that the DAF family of NO‐sensitive dyes can also react with ascorbic acid and dehydroascorbic acid (Zhang et al., 2002a, 2002b). Although this possibility was already disregarded by Samalova and collaborators in Magnaporthe (Samalova et al., 2013), we tested this possibility in Aspergillus by employing the NO‐scavenger 4,4,5,5‐tetramethylimidazoline‐l‐oxyl3‐oxide (PTIO). Addition of different concentrations of PTIO resulted in a dose‐dependent decrease of fluorescence compared with the control sample in the absence of the NO scavenger (Fig. 1E). Addition of the NO‐releasing compound detaNONOATE (dNO) produced a high increase of fluorescence, as expected. Collectively, it shows that DAF‐FM and DAF‐FM DA were able to detect the NO produced by Aspergillus, both intracellular and extracellularly. We selected DAF‐FM DA for the rest of the study for having a better signal ratio between samples containing cells and control media in our 96‐well plate assays (3.3 with DAF‐FM DA versus 1.5‐fold with DAF‐FM).
NO synthesis during growth in liquid media partially depends on niaD
It has been known for a long time that fungi can produce NO (Ninnemann and Maier, 1996); however, the mechanisms of NO synthesis have remained obscure so far. In order to study a possible role of the nitrate assimilation pathway in the biosynthesis of NO, our experimental set up was as follows: the A. nidulans wild type strain was grown in liquid media containing nitrate, nitrite or ammonium as sole nitrogen source for 16 h; then NO levels were followed for up to 200 min by addition of the NO‐sensitive fluorescent dye DAF‐FM DA directly to the growth medium and by subsequent fluorometric quantification (Fig. 2A). The level of fluorescence intensity increased in the samples containing fungal cells over time, to a higher extent than in control samples (which contain only the culture media). Subtraction of the background signal derived from the growth media confirmed that, interestingly, NO levels were higher in ammonium than in nitrite or nitrate‐containing media (Fig. 2B, wild type strain).
Figure 2.

NO quantification in mycelium in liquid medium.
A. A . nidulans wild type was inoculated in media containing the indicated compounds as sole nitrogen sources. DAF‐FM DA was added to the cultures and non‐inoculated media (control samples). Media, which was not inoculated, was employed as control to determine the biological origin of the NO produced. Fluorescence was recorded over time. One representative experiment is shown.
B. A . nidulans wild type (light grey bars) and Δnia D (dark grey bars) strains were grown in liquid media with the indicated nitrogen sources. The Δnia D strain could not grow in nitrate, and consequently, NO production was not tested in this sample (n.d.). Data shown correspond to one time point after addition of DAF‐FM DA (100 min) and are referred to the wild‐type strain grown in ammonium media (100%). Data shown are the mean and the standard error of the mean of at least three independent biological replicates. N.d.: not determined.
C. A . nidulans wild type and Δnia D strains were grown in ammonium liquid medium for 12 h. The mycelia were filtered, washed and transferred to ammonium (control) and ammonium + 10 mM nitrate. The cultures were grown for 6 additional hours. DAF‐FM DA was added to quantify NO production, and fluorescence was recorded for 60 min. Data shown are the mean and the standard error of the mean of two independent biological replicates.
D. A. nidulans Δfhb A Δfhb B and Δfhb A Δfhb B Δnia D strains were grown in ammonium liquid medium for 12 h. The mycelia were filtered, washed and transferred to ammonium (control), ammonium + 10 mM nitrate, and ammonium + 100 mM nitrate. The cultures were grown for 6 additional hours. DAF‐FM DA was added to quantify NO production, and fluorescence was recorded for 60 min. Data shown are the mean and the standard error of the mean of two independent biological replicates.
We then investigated whether a nitrate reductase‐dependent route for the synthesis of NO is operational in A. nidulans, similar to the situation described in plants (Dean and Harper, 1988; Yamasaki and Sakihama, 2000; Rockel et al., 2002). We employed a ΔniaD mutant lacking nitrate reductase. Obviously this mutant cannot grow on nitrate as sole nitrogen source, but it can grow on nitrite, which is, in plants, the substrate for the nitrate reductase to produce NO. No major differences were found in the NO levels between the wild type and the mutant strain on ammonium (Fig. 2B). However, when both strains were grown in nitrite, the ΔniaD mutant produced 30% less NO than the wild type, indicating that this enzyme participates in the production of NO and is responsible for around 30% of this metabolite synthesis when nitrite served as N‐source. On nitrate medium, the wild type strain showed the smallest amount of NO compared with the other two types of nitrogen (Fig. 2B). In order to test also NO production of ΔniaD on nitrate, the strains were pre‐grown on ammonium for 12 h and then transferred to nitrate‐containing ammonium liquid medium or back to sole ammonium medium as control. After 6 h of growth under these conditions, DAF‐FM DA was added to the cultures, and fluorescence was quantified (Fig. 2C). The wild‐type strain showed higher NO levels than the mutant when the medium was supplemented with nitrate indicating again that NR is involved in the production of NO through the nitrate assimilation route.
Our data suggested a putative role for niaD in mediating the synthesis of NO. Previous data reasoned that the flavohaemoglobin genes could be involved in the catabolism and thus detoxification of NO (Schinko et al., 2010). The deletion of both flavohaemoglobin genes, fhbA and fhbB, did not affect NO levels in ammonium medium (Fig. 2C–D, compare NO levels with the wild type strain), but there was a drastic increase in NO levels much higher than in the wild type (up to 3.5‐fold increase) when nitrate was present in the medium (Fig. 2D). Importantly, even in the presence of the repressing nitrogen source ammonium, nitrate was an obvious source for NO production. This is in agreement with findings by Schinko et al. (2010), who showed that the transcription of fhbA is independent from AreA function and that full induction of this gene also occurs under induced‐repressed conditions (i.e. ammonium plus nitrate). When the nitrate reductase niaD was deleted in the flavohaemoglobin deletion mutant (ΔniaD ΔfhbA ΔfhbB), the increase of NO in the presence of nitrate was not observed, confirming that a nitrate pathway operates to synthesise NO in A. nidulans and that this pathway requires a functional niaD gene.
Deletion of niaD results in drastic reduction of NO production during growth on solid media
Previous transcriptomic data obtained in our laboratory suggested that the nitrate utilisation pathway was expressed during the induction of conidiation (Canovas et al., 2014), which allowed us to hypothesise that, in addition to vegetative growth, NO could also be produced through the nitrate pathway during development. For this reason, we were interested in investigating whether NR was also relevant for NO production on solid medium. We compared the NO levels in the wild type and the ΔniaD strain. Both strains were grown on solid medium containing different nitrogen sources (ammonium, proline or proline supplemented with nitrate). Proline allows the growth of the wild type and also the ΔniaD strain, and it is a neutral (non‐repressing, non‐inducing) nitrogen source for the nitrate assimilation system. Surprisingly, we found that NO levels of the ΔniaD mutant on ammonium solid medium was only 41% of the wild type (Fig. 3A), whereas in liquid ammonium medium both exhibited similar levels (Fig. 2). On proline solid medium, the ΔniaD mutant produced less NO compared with the wild type. When nitrate was added to the proline solid media, the wild type increased NO production (P < 0.05; Student's t‐test), whereas in the ΔniaD strain reduced levels were found: 30% less relative to proline media (P < 0.05; Student's t‐test), and 55% less NO than the wild type in proline plus nitrate (P < 0.01; Student's t‐test). In this case, we observed that the NO levels in the nitrite reductase defective mutant (niiA4) were similar to the wild‐type levels (Fig. 3A). Therefore, all these data suggest that there is a niaD‐dependent pathway for the synthesis of NO both in liquid and on solid media and that additional pathway(s) for the synthesis of NO must operate in A. nidulans.
Figure 3.
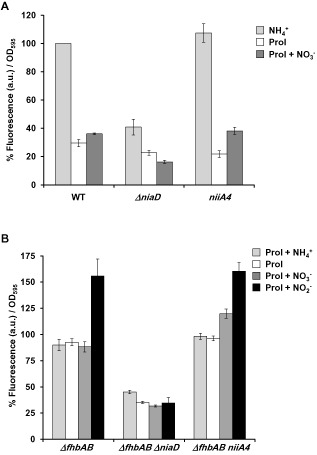
NO quantification on solid medium.
A. A . nidulans wild‐type, Δnia D and nii A 4 strains were grown on solid media containing the indicated nitrogen sources at 10 mM (ammonium or nitrate) or 3 mM (proline) at 37°C for 18 h. DAF‐FM DA was added to quantify NO production, and fluorescence was recorded for 100 min. Data shown are referred to the wild‐type strain grown on ammonium media (100%). Data shown are the mean and the standard error of the mean of at least three independent biological replicates.
B. A . nidulans Δfhb A Δfhb B , Δfhb A Δfhb B Δnia D and Δfhb A Δfhb B nii A 4 strains were grown on proline solid media for 18 h. DAF‐FM DA was added to quantify NO production. Then, the indicated nitrogen sources were added, and fluorescence was recorded for 60 min. Data shown are referred to the control strain (Δfhb A Δfhb B) grown on proline without the addition of any other nitrogen source. Error bars represent the standard error of the mean.
With the aim to confirm the source of NO, the flavohaemoglobin mutants were used again to reduce NO catabolism and thus to increase the overall levels of this metabolite. The three strains were grown on solid media containing proline as a sole nitrogen source; the fluorescence recording was started by addition of DAF‐FM, and then different nitrogen sources were individually added to the assay as the recording continued. As shown in Fig. 3B, addition of nitrate did not have major effects on ΔfhbA ΔfhbB. However, addition of nitrite elicited an increase in the fluorescence. The nitrite reductase mutant (ΔfhbA ΔfhbB niiA4), on the other hand, exhibited an increase in the NO levels after the addition of either nitrate or nitrite, but not ammonium, demonstrating that the nitrite reductase (NiR) was not responsible for the synthesis of NO. Addition of either nitrate or nitrite to the ΔfhbA ΔfhbB ΔniaD mutant did not increase the NO levels over the control sample; furthermore, this strain showed reduced NO levels in the control conditions (proline) compared with the wild type (ΔfhbA ΔfhbB) and the ΔfhbA ΔfhbB niiA4 strains (Fig. 3B). Addition of ammonium to any of the strains did not change the NO levels significantly over the control condition (proline), as expected. Therefore, taken together, our data demonstrate that the nitrate reductase is partially responsible for the synthesis of NO employing nitrite as substrate.
NO levels are higher on solid medium than in liquid medium
During the previous experiments, we observed a higher production of NO during growth on solid media than in liquid media. In order to compare the NO levels in both type of media, the A. nidulans wild‐type strain was grown in liquid or solid media containing ammonium (control) or ammonium supplemented with 10 or 100 mM nitrate for 18 h. DAF‐FM was added to the samples, and fluorescence was recorded for 200 min. Interestingly, production of NO was much higher when A. nidulans was grown on solid media than in submerged culture under these experimental conditions (Fig. 4A). Additionally, the increment in the production of NO in the presence of nitrate was also significantly higher on solid media.
Figure 4.
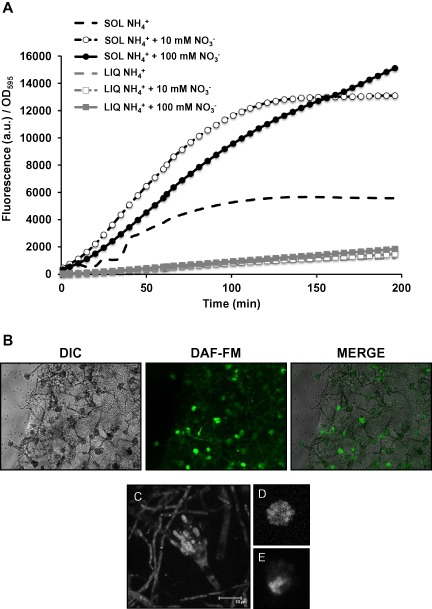
NO production is higher on solid medium than in liquid medium.
A. A . nidulans wild‐type strain was grown in liquid or solid media for 18 h. In liquid medium, A . nidulans was grown in ammonium liquid medium for 12 h. The mycelia were filtered, washed and transferred to ammonium (LIQ NH4+), ammonium + 10 mM nitrate (LIQ NH4+ + 10 mM NO3−) and ammonium + 100 mM nitrate (LIQ NH4+ + 100 mM NO3−). The cultures were grown for 6 additional hours (total growth time was 18 h). On solid media spores were inoculated on solid media containing ammonium (SOL NH4+), ammonium + 10 mM nitrate (SOL NH4+ + 10 mM NO3−) and ammonium + 100 mM nitrate (SOL NH4+ + 100 mM NO3−), and the plates were incubated at 37°C for 18 h. DAF‐FM DA was added to all cultures to quantify NO production, and fluorescence was recorded for 200 min. The plot shown is the average of two replicate samples of a representative experiment.
B–E. A spergillus wild‐type strain was grown on solid ammonium medium for 24 h. Agar cubes containing fungal biomass were submerged in a DAF‐FM DA solution for 20 min and washed three times. Conidiophores were imaged by fluorescence microscopy (B) or confocal microscopy (maximal projection in C, and single planes in D–E).
In order to find out whether NO was produced or accumulated in hyphae or conidiophores, Aspergillus was grown on solid medium. DAF‐FM DA was added and allowed to permeate the cells for 20 min before washing out the excess of the dye. Visualisation under the fluorescent microscope revealed that both hyphae and conidiophores were stained; however, the signal was stronger in the conidiophores than in the hyphae (Fig. 4B). Under this low magnification and using a fluorescent microscope, the signal appeared to be greater in sterigmata cells (metulae and phialides) than in the stalk cells. Confocal microscopy confirmed that the fluorescent signal could be detected in the sterigmata cells (Fig. 4C–E), but also in the vesicle and stalk cells, and in hyphae (Fig. 4C).
Fungal growth mediates nitrite and nitrate synthesis in ammonium media
The high levels of NO observed on ammonium were intriguing, but the fact that the ΔniaD strain produced less NO than the wild type on ammonium solid medium (Fig. 3A) posed two interesting questions. The first one is: can NO be synthesised from nitrate in a medium containing ammonium as sole nitrogen source? This suggests that nitrate could be present in this medium. To test this hypothesis, we employed the A. nidulans ΔniaD strain, which does not metabolise nitrate allowing its accumulation, and quantified both nitrate and nitrite during growth in the media. The ΔniaD strain was grown on the surface of a petri dish containing liquid media supplemented with ammonium as sole nitrogen source, as previously described (Ruger‐Herreros et al., 2011). Under these conditions, A. nidulans conidiation proceeded as on solid media. Neither nitrate nor nitrite could be detected in the culture media before the inoculation with the fungal spores. But surprisingly, both nitrate and nitrite were found in the culture media after 19, 24 and 48 h of fungal growth (Fig. 5). When the experiment was performed with a wild‐type strain, nitrate and nitrite were also found in the media but at lower levels, due to the metabolism of nitrate by an active nitrate reductase (Supplementary Fig. S1).
Figure 5.
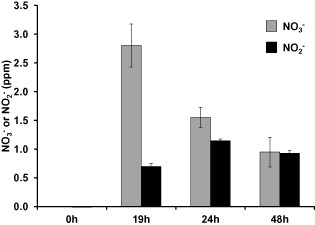
Quantification of nitrate and nitrite in the culture media. A . nidulans Δnia D strain was grown on the surface of a petri dish containing liquid media for the indicated time points. Nitrate (light grey bars) and nitrite (dark grey bars) were quantified with the Griess reagent. Neither nitrate nor nitrite was detected in the media before fungal growth.
niaD is induced during conidiation
The second question is: if according to the theory of nitrogen regulation, the nitrate reductase gene niaD must be repressed in a media containing the repressing nitrogen source ammonium (Arst and Cove, 1973), how is the nitrate reductase active in these conditions? Transcriptomics data obtained previously suggested that the nitrate utilisation pathway was expressed during induction of development on ammonium nitrate medium (Canovas et al., 2014). To further confirm this, we first obtained wild‐type vegetative mycelia grown in ammonium liquid medium for 18 h, and then, conidiation was induced by transferring the mycelia to solid media containing ammonium or nitrate as a sole nitrogen source (Fig. 6). Two hours after the induction of conidiation, niaD mRNA levels were roughly 70‐fold higher on nitrate solid media than in vegetative mycelia and, strikingly, niaD was also transcribed on ammonium solid media. However, the nitrate‐induced level was 8.6‐fold higher compared with ammonium. Longer induction of conidiation on nitrate solid media did not result in much further increase of niaD expression (from 70‐ to 92‐fold induction compared with vegetative mycelia). However, longer induction of conidiation on ammonium media increased the induction of niaD from 8.5‐ to 23‐fold relative to vegetative mycelia.
Figure 6.
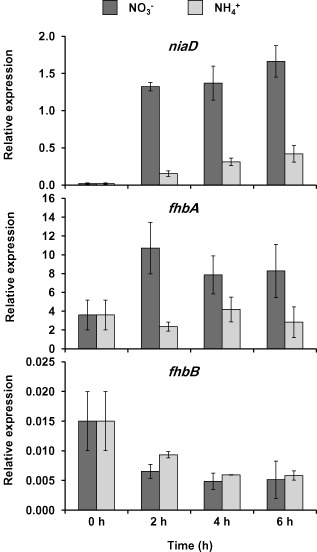
Expression of the flavohaemoglobins and nitrate reductase genes in media containing nitrate or ammonium as sole nitrogen source early during conidiation. A . nidulans wild‐type strain was grown vegetatively in ammonium liquid minimal medium for 18 h and then transferred to nitrate (dark grey bars) or ammonium (light grey bars) solid medium to induce conidiation. Samples were taken at the indicated time points for RNA isolation. fhb A , fhb B and nia D expression was quantified by real time RT‐PCR. Data were normalised against the expression of the ß‐tubuline gene (ben A). The plots show the average and standard error of the mean of the relative expression values in at least three independent experiments.
We also quantified the levels of the flavohaemoglobin mRNAs after the induction of conidiation. As previously reported, fhbA was induced by nitrate (Schinko et al., 2010), and there was no significant variation in the expression levels in ammonium during the first hours of conidiation (Fig. 6). On the other hand, the fhbB gene was slightly repressed immediately after induction of asexual development regardless of the nitrogen source and dropped threefold after 2 h (Fig. 6). Downregulation of fhbB was similar in both nitrogen sources ammonium and nitrate, suggesting that fhbB is not constitutive, but rather developmentally regulated.
NO levels are increased during the first hours of conidiation
The higher levels of NO produced during growth on solid media compared with liquid media suggests that there might be a connection between NO and development. Indeed the expression pattern of fhbB points to an early regulation of the NO levels during the transition from vegetative growth to conidiation. In order to study this possible connection between NO and asexual development, we first quantified the NO levels at the early stages of the transition from vegetative growth to conidiation. The wild‐type strain and the mutant lacking both flavohaemoglobins (ΔfhbA ΔfhbB), which cannot detoxify NO, were grown in ammonium liquid medium, and then transferred to solid media to induce conidiation as above. Samples were taken at 1 h intervals, and NO was quantified with DAF‐FM. As can be seen in Fig. 7A–B, NO increased after 1 h of induction compared with vegetative growth in all the conditions. In fact, we observed this increase after induction of conidiation for 1 h, and it remained elevated at least for 7 h (data not shown). There were no major differences in the NO levels between ammonium and nitrate growth conditions during the early induction of conidiation.
Figure 7.
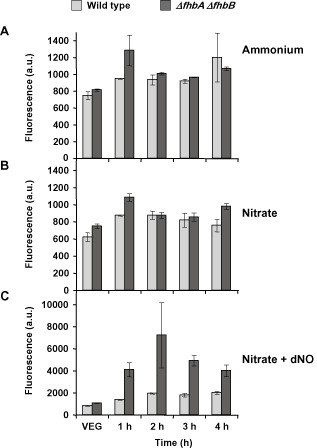
NO quantification after induction of development. A . nidulans wild‐type strain (light grey bars) and a Δfhb A Δfhb B mutant (dark grey bars) were grown vegetatively in ammonium liquid medium for 18 h (VEG) and then transferred to solid media containing different nitrogen sources to induce development: ammonium tartrate as control nitrogen source (A), sodium nitrate (B) and sodium nitrate supplemented with NO‐releasing chemical compound dNO (C). Samples were taken at different times, and the fluorescence produced by the reaction of NO and DAF‐FM DA was quantified. Please note the different graph scales in A–C.
We were interested in creating a higher range of NO levels for the following experiments. Therefore, we added the NO‐releasing chemical compound detaNONOate (dNO), which was previously tested for increased levels of NO (Fig. 1E), and checked for an additional increase of NO levels (Fig. 7C). In this case, we found a drastic increase of NO in both strains, as expected, and NO levels were threefold higher in the mutant than in the wild type.
Conidiation (asexual development) is repressed by elevated NO levels
In order to get insight into the effects of NO during reproduction, we first tested the effects of different NO levels on conidiation using the wild‐type strain and the mutant lacking both flavohaemoglobins (ΔfhbA ΔfhbB). Conidiation was induced as described above by transferring vegetative mycelia of both strains to solid media with or without dNO. Based on the results shown above, we expected that these conditions and strains generated a gradient of NO. As NO levels quickly increased after induction of conidiation, we quantified the levels under these conditions and observed the expected gradient of increasing NO levels (Fig. 8A). After 3 days of incubation, the number of conidia was counted. Conidiation was slightly reduced in the double mutant compared with the wild type in the control nitrate media (Fig. 8B). Addition of the NO‐donor dNO to the media resulted in a drastic increase of NO levels, especially in the mutant strain, and a further reduction of conidiation (ca. 60%) both in the wild type (P < 0.1; Student's t‐test) and in the mutant strain (P < 0.05; Student's t‐test). Observation of the fungal growth on the solid media with a stereoscopic microscope revealed a reduction in the density of conidiophores (Fig. S2). These results suggest that an increase of NO correlates with a decrease in conidiation.
Figure 8.
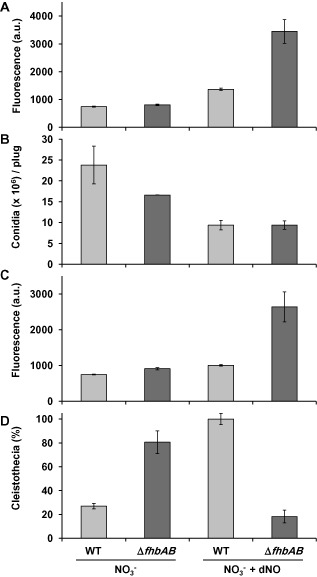
Effects of NO on asexual (conidiation) and sexual reproduction.
A and B. Wild type and Δfhb A Δfhb B strains were grown in minimal media for 18 h and then transferred to nitrate solid medium with or without 1.5 mM dNO. The plates were incubated at 37°C for 1 h for NO quantifications (A) or for 72 h for conidial counting (B). Data show the average of at least three independent experiments and standard error of the mean.
C and D. Wild type and Δfhb A Δfhb B strains were grown in minimal media for 18 h and then transferred to nitrate solid medium with or without 1.5 mM dNO. The plates were sealed and incubated at 37°C in the dark for 1 h for NO quantifications (C) or for 15 days for the quantification of the number of cleistothecia. Data are given as the percentage of WT grown in nitrate + dNO (D). Data show the average of at least three independent experiments and standard error of the mean.
Sexual development is induced by NO
Conidiation and sexual development are normally balanced in A. nidulans, meaning that when one of them increases, the other one decreases (Adams et al., 1998; Rodriguez‐Romero et al., 2010; Dyer and O'Gorman, 2012). To study the effects of NO in sexual development of A. nidulans, we followed a similar strategy as described above but growing strains under conditions to induce sexual development (i.e., sealed plates in the dark). Again, this strategy also allowed creating the NO gradient under the conditions to induce sexual development (Fig. 8C). Opposite to the effects observed in conidiation, there was an increase in the number of cleistothecia (the sexual reproductive structures of A. nidulans) in the mutant strain compared with the wild type in the control medium (Fig. 8D). When the NO‐releasing compound dNO was added to the media, the number of cleistothecia even increased in the parental strain compared with the control media (Fig. 8D). Similar results were obtained by Baidya et al. (2011). This increase in the number of cleistothecia correlated with the increase of NO. However, addition of dNO to the double ΔfhbAΔfhbB mutant resulted in the lowest number of cleistothecia, which was the opposite to what could be expected based on the other samples. We reasoned that this may be due to toxicity from the excessive amounts of NO in our experimental set up, which were more than twofold compared with the amounts of NO produced by the wild‐type under the same conditions (Fig. 8C). Baidya and collaborators found different results in their experiments using the strains and conditions developed in our laboratories: the number of cleistothecia was not reduced upon addition of dNO to the flavohaemoglobins mutant. However, they did not quantify the NO levels of the corresponding samples (Baidya et al., 2011).
In order to monitor the toxic effect of the NO radicals, we quantified the radial growth rate on solid nitrate media of the wild type and the double mutant (Supplementary Fig. S3). Addition of dNO resulted in a slight decrease of the wild‐type growth rate and in a drastic decrease of the mutant (56% of the wild‐type growth rate under the same conditions). This suggests that dNO was indeed exerting a toxic effect under our experimental conditions, particularly in the case of the flavohaemoglobin mutant, which lacks the NO detoxification enzymes.
Different NO levels affect the expression of the regulator of sexual development nsdD and the regulator of conidiation brlA
Using the same conditions as above, the expression of the master regulator of conidiation brlA was quantified at different time points after induction of conidiation (Fig. 9A). The expression pattern of brlA was similar in all the conditions tested, i.e. we did not observe that the expression levels of brlA correlated with the number of conidia. In fact, the highest value of brlA expression was found at 10 h in the wild type grown with dNO, which corresponds to one of the conditions with the lowest number of conidia. This suggests that the effects of NO on conidiation were not mediated through the control of the brlA expression levels.
Figure 9.
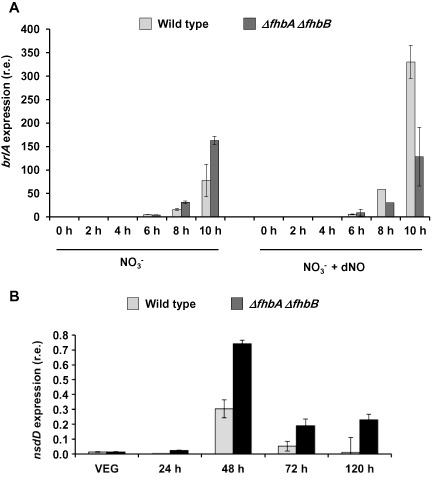
Expression of developmental regulators during asexual or sexual reproduction at different NO levels.
A. brl A expression during conidiation. A . nidulans wild type and Δfhb A Δfhb B strains were grown in liquid minimal medium for 18 h and then transferred to nitrate solid medium with or without dNO to induce conidiation.
B. nsd D expression during sexual development. A . nidulans wild type and Δfhb A Δfhb B strains were grown in liquid minimal medium for 18 h and then transferred to nitrate solid medium with or without dNO. Plates were sealed and incubated in the dark to induce sexual development. Samples were taken at the indicated time points for RNA isolation. brl A and nsd D expression was quantified by real‐time RT‐PCR. Data were normalised against the expression of the ß‐tubuline gene (ben A). The plot shows the average and standard error of the mean of the relative expression values in at least three independent experiments.
To further study the correlation between NO levels and number of cleistothecia, (Fig. 8C–D) sexual development was induced in the wild‐type strain, and the accumulation of mRNA of nsdD was quantified. NsdD is a GATA‐transcription factor necessary to activate sexual development (Han et al., 2001). As shown in Fig. 9B, nsdD mRNA accumulation increased up to 48 h and then decreased. nsdD expression was higher in the flavohaemoglobin mutant than in the wild‐type strain in all the time points tested, and it still remained relatively high until 120 h, suggesting that NO could play a role in the induction of the transcription of nsdD. Interestingly, opposite to the conidiation regulator brlA, the effect of NO in the increased expression of the sexual developmental regulator nsdD correlated with the amount of cleistothecia. However, whether the modulation of expression of these regulators by NO is direct or indirect still remains to be addressed.
fhbA and fhbB are regulated differently during conidiation
As the NO levels were regulated during conidiation, we studied the expression of the NO‐metabolising flavohaemoglobin genes fhbA and fhbB for a longer period after induction of conidiation. The wild‐type strain was grown in ammonium liquid medium and then transferred to nitrate solid medium. Samples were again taken at different hours after induction of conidiation, and the mRNA levels of both genes fhbA and fhbB were quantified (Fig. 10). Both flavohaemoglobins were expressed during conidiation, but they showed distinct patterns of expression. In particular, fhbA expression was induced in the first hours and then gradually decreased (Fig. 10A), whereas fhbB expression was low during the first hours, and then was induced from 24 h until 120 h (Fig. 10B). These results suggest that both flavohaemoglobins are regulated differently during NO metabolism, which may reflect different biological roles in the control of the homeostasis of the regulatory molecule NO.
Figure 10.
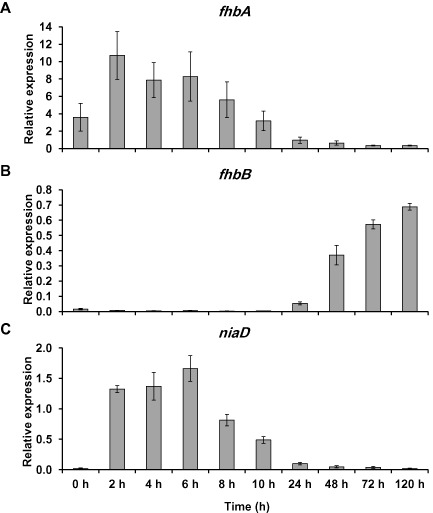
Expression of the flavohaemoglobin and nitrate reductase genes during conidiation. A . nidulans wild‐type strain was grown in ammonium liquid minimal medium for 18 h and then transferred to nitrate solid medium to induce conidiation. Samples were taken at the indicated time points for RNA isolation. fhb A (A), fhb B (B) and nia D (C) expression was quantified by real‐time RT‐PCR. Data were normalised against the expression of the ß‐tubuline gene (ben A) and it is shown as relative to vegetative growth. The plots show the average and standard error of the mean of the relative expression values in at least three independent experiments.
Furthermore, it was also interesting to find that the expression of niaD was induced during the early stages reaching a maximum at 6 h after the transition to conidiation (91‐fold induction with respect to vegetative growth) and then it started to decrease, showing an expression pattern very similar to the nitrogen regulated fhbA gene (Fig. 10C).
areA and nirA regulate the expression of niaD and fhbA during conidiation
We questioned whether the general regulator of nitrogen metabolism, areA, and the nitrate pathway specific regulator, nirA, were required for the induction of niaD and fhbA during the induction of conidiation. Wild‐type, areA600 and ΔnirA, strains were grown in ammonium liquid media and then transferred to nitrate or ammonium solid media. In the areA600 loss‐of‐function mutant, the niaD mRNA levels were three‐ to fourfold lower than in the wild type after induction of conidiation on nitrate (Fig. 11A). Expression on ammonium was also reduced, particularly at 6 h. However, in both media induction was not completely abolished suggesting that other factors might also contribute to the induction. Deletion of the pathway specific regulator nirA also resulted in a strong decrease of the expression levels in both media.
Figure 11.
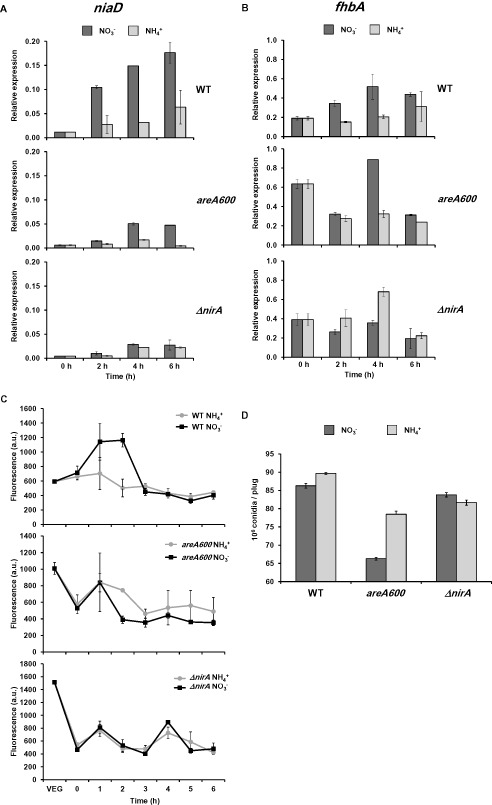
Induction of nia D and fhb A during conidiation requires functional are A and nir A.
A and B. Conditions are the same as stated in Fig. 5. Wild type, are A 600 and Δnir A strains were transferred to nitrate (dark grey bars) or ammonium (light grey bars) solid media to induce conidiation. The expression of nia D (A) and fhb A (B) was quantified by real‐time RT‐PCR.
C. NO levels were quantified as in Fig. 6 in the wild‐type strain and are A 600 and Δnir A mutants on nitrate (dark grey squares) or ammonium (light grey circles). VEG indicates samples that were always maintained in submerged cultures, and time 0 indicates samples that were employed to quantify NO levels immediately after transferring to a solid surface.
D. Conidia were counted in the three strains 72 h after the transfer to a solid surface. In all cases, data shown is the average of three independent samples and the standard error of the mean.
We found an increased expression of fhbA during vegetative growth in both mutants (Fig. 11B). This result, although it might be surprising, was also reported by Schinko et al. (2010), and it might result from an areA and nirA‐mediated repression on ammonium. In both areA600 and ΔnirA mutants, there was no induction of fhbA after the transfer to solid media, suggesting that under these experimental conditions both regulators are required for induction of fhbA.
The opposing activities (synthesis and metabolism of NO) were regulated in a similar way in the wild type, which made us wonder whether this would result in higher or lower levels of NO. As expected, the wild‐type strain showed an immediate increase in the NO levels upon transfer from liquid to solid nitrate media. Unlike the wild‐type strain, neither the areA nor the nirA mutants were capable of producing increased NO levels upon transfer to solid media regardless of the nitrogen source (Fig. 11C). Significantly, the NO levels were higher during vegetative growth in liquid media in the mutants, which may lead to greater levels of fhbA expression (through an unknown mechanism). Surprisingly, this reduction of the NO levels was not accompanied by an increase in the conidiation levels, but rather by a slight reduction of 9–13% on ammonium (Fig. 11D). The difference was higher on nitrate media in the case of the areA mutant (23% decrease).
Discussion
While the pathways for the synthesis of NO are clear and well studied in mammals and plants (Alderton et al., 2001; Gorren and Mayer, 2007; Gupta et al., 2011), in fungi, they have remained elusive so far. A recent and very thorough study in Magnaporthe could not provide any evidence of the putative routes and genes for the biosynthesis of NO (Samalova et al., 2013). In this work, we provide evidence for the existence of a nitrate‐reductase dependent route involved in the biosynthesis of NO in A. nidulans. Although this route is functional during growth in both liquid and solid media, it is more active on solid media where reproductive development occurs.
The expression pattern of niaD and fhbA after induction of development in nitrate media is similar, probably as a consequence of the nitrate‐dependent regulation of both genes that required functional areA and nirA genes. However, they showed a different pattern in ammonium media, where niaD but not fhbA was induced. These differences in the regulation between both genes allow the fungus to produce similar NO levels in both media during the induction of conidiation. Importantly, we have shown that the niaD route is operative during conidiation even in the presence of the repressing nitrogen source ammonium. It is well known from studies in liquid media that nitrogen metabolite repression (e.g. in the presence of ammonium) leads to loss of niaD expression. This regulation is mediated by inactivation of the GATA‐type zinc finger protein AreA, which normally acts as co‐activator for many genes, under nitrogen metabolite repression (Arst and Cove, 1973; Caddick et al., 1986; Burger et al., 1991; Marzluf, 1997; Todd et al., 2005; Berger et al., 2006). The question is how the ammonium‐repression is overcome to induce niaD. For example, the glucose‐repressed acuD gene, which is involved in the utilisation of alternative two‐carbon molecules (such as acetate or ethanol), is regulated during development by the conidiation regulators BrlA and AbaA in Penicillium marneffei (Canovas and Andrianopoulos, 2006). However, no consensus sequences for the binding sites of either AbaA or BrlA could be found in the promoter region of niaD. Actually, niaD gene expression during conidiation required functional areA and nirA genes. Induction of fhbA was also abolished in both mutants, although fhbA was reported to be regulated only by nirA, and not by areA (Schinko et al., 2010). However, there are four AreA consensus binding sites in the promoter of fhbA, which points to an AreA‐dependent regulation of fhbA only during induction of conidiation and not during vegetative growth. This unique mechanism would require additional players to act selectively during this specific stage of the fungal life cycle (for example, six GATA sites were found in that region) or specific post‐transcriptional modifications of AreA, which allow it to override the repression imposed by the presence of ammonium in the media to induce niaD expression in the early stages of conidiation. As the expression levels of areA are rather constant during the entire life cycle, post‐transcriptional modifications are the most feasible explanation. It could be argued whether this induction requires developmental or endogenous signals. In principle to propose an endogenous induction (i.e. by intracellular accumulation of nitrate), in addition to de‐repression, expression of niaD would also require nitrate or nitrite. Indeed, when A. nidulans was grown in ammonium media, both nitrate and nitrite were found in the media after culturing the fungus. Nitrate could originate from the oxidation of NO (Schinko et al., 2013). An alternative option would involve heterotrophic nitrification pathways. This process has not been studied in detail, and we could only find two reports in the literature demonstrating the formation of nitrate and nitrite through unknown mechanisms by Aspergillus flavus in media containing ammonium in a pH‐dependent manner (Hirsch et al., 1961) or in media containing certain amino acids (Hatcher and Schmidt, 1971). The data presented here suggest that a considerable amount of NO produced in ammonium media is niaD‐dependent and, therefore, it is produced through the nitrate route. As not all the NO was produced by the nitrate reduction pathway, it implies that there must be additional routes for the synthesis of NO in fungi. The most plausible alternative would be from arginine by an alleged nitric oxide synthase. However, work published in Magnaporthe could not prove this hypothesis, despite knocking out all putative genes (Samalova et al., 2013) and consequently the additional routes and sources of NO still remain unknown in fungi.
Although, first, it puzzled us to observe that the NO levels were lower when the strains were grown in nitrate compared with ammonium, the nitrate‐dependent induction of fhbA can explain this reduction. Indeed, addition of nitrate to repressing ammonium or non‐repressing proline media only provoked modest increases in the NO levels. fhbA is expressed in nitrate media even in the presence of repressing nitrogen sources, such as ammonium (Schinko et al., 2010). Consequently, deletion of fhbA allowed drastic increases of the NO levels upon addition of nitrate to the media. Other authors have observed that both ΔfhbA and ΔfhbA ΔfhbB mutants display the same phenotype with a promoting effect of sexual development, but the ΔfhbB mutant does not (Baidya et al., 2011). This agrees with our data that fhbB but not fhbA is developmentally repressed upon induction of development, and consequently, deletion of fhbA would allow a higher increase of the NO levels at the beginning of development. On the other hand, deletion of fhbB cannot have a major impact in the early decisions, as this gene is repressed immediately after induction of conidiation, but rather in steady state levels of NO as it was expressed 24 h after induction of conidiation. The two fungal flavohaemoglobins are localised in different subcellular compartments (te Biesebeke et al., 2010), which also support the idea of playing different biological roles during the life cycle of the fungus. Collectively, the levels of NO could be explained by a co‐ordinated action of the flavohaemoglobins during development, acting first the cytoplasmic fhbA, and then the mitochondrial fhbB in late development. The role of the flavohaemoglobins in NO metabolism was previously reported in A. nidulans (Schinko et al., 2010), and here we further confirmed with additional new data their role in the fine tuning of the NO metabolism and concentration during development. Another factor that might influence an increase levels of NO during sexual development over conidiation is the lowering of oxygen availability, which would decrease the rate of NO oxidation (i.e. conversion back to nitrate), both spontaneous and/or mediated by the haem‐containing flavohaemoglobins. Two additional genes have recently been implicated in the response to nitrosative stress in A. nidulans. The porphobilinogen deaminase hemC acts by promoting the activity of the flavohaemoglobins through an unknown mechanism, whereas the NO‐inducible nitrosothionein ntpA scavenges NO through S‐nitrosylation (Zhou et al., 2012; 2013). However, the possible role of these two genes during conidiation and sexual reproduction still remains to be studied.
At 24 h, the mitochondrial flavohaemoglobin fhbB expression is induced threefold and at 48 h this induction boosts over 20‐fold, suggesting that it might contribute to control the NO levels. This time coincides with the maximal expression of the sexual regulator nsdD and could contribute to the balance between conidiation and sexual development. Indeed, dissimilatory nitrate reduction, in which nitrate is employed as a terminal acceptor of electrons, and production of NO have been found in Aspergillus terreus under anoxic conditions (Stief et al., 2014). This dissimilatory reduction of nitrate producing ammonium was found to be cytosolic, and involves the assimilatory nitrate and nitrite reductases (niaD and niiA) coupled to ethanol oxidation under anoxic conditions in A. nidulans (Takasaki et al., 2004). Taken altogether, it suggests that the assimilatory NR is responsible for the synthesis of NO working as a dissimilatory NR under anoxic conditions. If this hypothesis is true, the dissimilatory nitrate reductase activity must be restricted to a secluded area of the mycelial mat in close contact with the solid surface, but isolated from the air interface.
The levels of the signalling molecule NO also increased immediately after the induction of conidiation suggesting that it could be one of the earliest signals appearing during the transition from vegetative mycelia to the reproduction modes. This increase in the NO levels could be explained with the repression of the expression of the flavohaemoglobin fhbB and the induction of the nitrate reductase niaD. The increase of NO seemed to favour the sexual reproduction program while reducing the conidiation one. In the case of the sexual reproductive mode, increasing the levels of NO provoked the increased expression of the sexual regulator nsdD and the number of cleistothecial structures. Such correlation was not found in the case of the number of conidia and brlA expression. However, mutation of areA or nirA abolished the induction of niaD expression and the subsequent increased in NO levels. Blocking this increase of NO levels did not result in the expected increase in conidiation. One hypothesis that agrees with these data is that the induction of the reproduction programmes under laboratory conditions is a several‐step process, in which the first step is the commitment for the transition between a vegetative growth style to the reproduction program, and then the fungus regulates the balance between asexual and sexual programmes depending on the environmental factors. Considering the greater signal observed in conidiophores by fluorescence microscopy, NO could play different roles at the different stages.
In conclusion, here we report that the nitrate reductase is involved in the synthesis of NO from nitrite in Aspergillus. niaD and fhbA are expressed even in the presence of the repressing nitrogen source ammonium during conidiation in a NirA‐ and AreA‐dependent manner, which contributes together with a temporal repression of fhbB to a transient increase in the NO levels upon induction of conidiation.
Experimental procedures
Strains, media and culture conditions
Strains used in this study are listed in Table S1. Strains were grown in complete or minimal media containing the appropriate supplements at 37°C (Cove, 1966). One per cent glucose was used as carbon source. Ammonium, nitrate, nitrite and/or proline were used as nitrogen sources as indicated. The NO chemical donor 3,3‐bis(aminoethyl)‐1‐hydroxy‐2‐oxo‐1‐triazene, also called detaNONOate (dNO) and the NO‐scavenger 4,4,5,5‐tetramethylimidazoline‐l‐oxyl3‐oxide (PTIO) were purchased from Sigma.
RNA isolation and real‐time RT‐PCR
Isolation of RNA and quantification of mRNA was performed as previously described (Ruger‐Herreros et al., 2011). Briefly, Aspergillus mycelia (100–200 mg) were disrupted in 1 ml of TRI reagent (Sigma) with 1.5 g of zirconium beads using a cell homogeniser (FastPrep‐24, MP Biomedicals). Cell debris was removed by centrifugation. Supernatants were extracted with chloroform, and RNA was precipitated with isopropanol. RNA samples were further purified using the NucleoSpin RNA II Nucleic Acid and Protein Purification Kit (Macherey‐Nagel).
The primers employed for real‐time RT‐PCR are detailed in Table S2. Real‐time RT‐PCR experiments were performed in a LightCycler 480 II (Roche) using the One Step SYBR® PrimeScript™ RT‐PCR Kit (Takara Bio). The fluorescent signal obtained for each gene was normalised to the corresponding fluorescent signal obtained with the ß‐tubulin gene benA to correct for sampling errors. Expression data are the average of at least three independent replicates.
Developmental biology experiments
Strains were grown in liquid medium for 18 h at 37°C and then transferred to solid media. Plates were incubated under light for conidiation conditions, or alternatively they were sealed and incubated in the dark for sexual developmental conditions. Plugs were cut out from the plate 72 h after induction of development. Conidia in the plugs were resuspended in Tween 0.1% buffer and counted. For counting cleistothecia, plates incubated for 15 days were first sprayed with 70% ethanol to facilitate the visualisation of the sexual structures and then photographed. Data shown are the average of at least four independent experiments.
NO quantification
Nitric oxide was quantified in samples by using the NO‐sensitive fluorescent dye DAF‐FM DA (Invitrogen) following the manufacturer's instructions except in Fig. 1, in which DAF‐FM (Sigma) was also employed. In Fig. 1, fungal cells were grown in liquid ammonium minimal medium for 16 h, and 50 μl of 5 μM DAF‐FM DA or DAF‐FM were added to 100 μl of each sample. Cells were loaded with the dye for 20 min and washed three times with a 0.05% tween solution (when indicated). Samples were transferred to 96‐well plates, and fluorescence was recorded in a Synergy HT Multi‐mode Microplate Reader (Biotek) equipped with FITC filter sets (λEm 488 nm, λEx 520 nm). In Fig. 2A and B, conidia were inoculated into 100 μl of minimal media at a final concentration of 105 conidia ml−1 in 96‐well plates, and the plates were incubated with shaking at 37°C for 16 h to allow aeration of the culture. After incubation, DAF‐FM DA was added to the cultures to a final concentration of 2.5 μM, and fluorescence was recorded in POLARstar Omega fluorometer (MG Labtech) equipped with FITC filter sets (λEm 488 nm, λEx 520 nm) for up to 200 min. Data were analysed with Omega Control (version 1.10) software. For the rest of the experiments, the strains were grown in ammonium liquid medium for 12 h, then transferred to liquid media containing the appropriate nitrogen sources and incubation continued for 6 additional hours. Cells were loaded with the dye for 20 min and transferred to 96‐well plates. Alternatively, the strains were grown in ammonium liquid medium for 18 h and then transferred to solid media for the indicated time points. Plates were incubated under the conditions described above for conidiation or sexual development. Samples were taken from the plates at the indicated time points, submerged in a solution of 5 μM DAF‐FM DA for 1 h. Samples were centrifuged, and the supernatant was transferred to a 96‐well plate for fluorescence quantification. For the other experiments performed on solid media in Figs 3 and 4, 105 conidia were inoculated on 96‐well plates containing solid media containing the indicated nitrogen sources and incubated for 18 h before quantification of NO. In Fig. 3B, fungal cells were grown in proline solid media for 18 h, fluorescence quantification started after addition of DAF‐FM DA and then the indicated nitrogen sources were added and fluorescence quantification continued for 2 additional hours. In all cases 2.5 μM DAF‐FM DA was added to the samples. Fluorescence was detected and quantified in a Synergy HT Multi‐mode Microplate Reader (Biotek) equipped with GFP filter sets. Data were analysed with Gen5TM Data Analysis Software. In all experiments, data were normalised to dry weight or OD595 as indicated. Experiments were repeated at least two times and performed in duplicates or triplicates, depending on the experiment.
Microscopy
Aspergillus was grown either on liquid ammonium medium for 14–16 h or solid ammonium medium for 24 h. A solution of 5 μM DAF‐FM DA (solid and liquid samples) or DAF‐FM (liquid samples) was added to the mycelium. After cell loading of the dye for 20 min, the excess of dye was washed out with a 0.05% Tween 80 solution. Images were recorded in an Olympus IX2‐UCB inverted fluorescence microscope equipped with a FITC filter set (λEm 488 nm; λEx 520 nm). Images in Fig. 1C were taken using identical settings for comparative purposes. Images in Fig. 4B were processed and merged using Adobe ImageReady CS2 (Adobe Systems Incorporated, CA, USA). Confocal images were obtained in a ZEISS LSM 7 DUO confocal microscope with an excitation at 488 nm using an OPSS laser beam. Confocal images were processed using ZEN Lite 2012 (Zeiss, Germany).
Nitrate and nitrite measurements in the culture supernatant
Nitrate and nitrite were quantified in the culture media using the Griess reagent as previously described (Schinko et al., 2010).
Supporting information
Supporting information
Acknowledgements
We would like to thank Michael Hynes for the niiA4 strain, Thorsten Schinko for helpful discussions, Modesto Carballo for help with the fluorometric assays (Servicio de Biología, Centro de Investigación Tecnología e Innovación, Universidad de Sevilla) and Juan Luis Ribas for help with the confocal microscopy (Centro de Investigación Tecnología e Innovación, Universidad de Sevilla). Work in Valencia was supported by grant number BIO2012‐34381 from the MINECO to JFM, and in Vienna was supported by grant number LS12‐009 from the n(f + b) Lower Austria Science Fund and M01693‐B22 from the FWF. We would like to thank the anonymous referees for helpful suggestions to improve this manuscript.
References
- Adams, T.H. , Boylan, M.T. , and Timberlake, W.E. (1988) brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans . Cell 54 : 353–362. [DOI] [PubMed] [Google Scholar]
- Adams, T.H. , Wieser, J.K. , and Yu, J.H. (1998) Asexual sporulation in Aspergillus nidulans . Microbiol Mol Biol Rev 62 : 35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderton, W.K. , Cooper, C.E. , and Knowles, R.G. (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357 : 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arst, H.N., Jr , and Cove, D.J. (1973) Nitrogen metabolite repression in Aspergillus nidulans . Mol Gen Genet 126 : 111–141. [DOI] [PubMed] [Google Scholar]
- Baidya, S. , Cary, J.W. , Grayburn, W.S. , and Calvo, A.M. (2011) Role of nitric oxide and flavohemoglobin homolog genes in Aspergillus nidulans sexual development and mycotoxin production. Appl Environ Microbiol 77 : 5524–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, H. , Pachlinger, R. , Morozov, I. , Goller, S. , Narendja, F. , Caddick, M. , and Strauss, J. (2006) The GATA factor AreA regulates localization and in vivo binding site occupancy of the nitrate activator NirA. Mol Microbiol 59 : 433–446. [DOI] [PubMed] [Google Scholar]
- Berger, H. , Basheer, A. , Bock, S. , Reyes‐Dominguez, Y. , Dalik, T. , Altmann, F. , and Strauss, J. (2008) Dissecting individual steps of nitrogen transcription factor cooperation in the Aspergillus nidulans nitrate cluster. Mol Microbiol 69 : 1385–1398. [DOI] [PubMed] [Google Scholar]
- Bernreiter, A. , Ramon, A. , Fernandez‐Martinez, J. , Berger, H. , Araujo‐Bazan, L. , Espeso, E.A. , et al (2007) Nuclear export of the transcription factor NirA is a regulatory checkpoint for nitrate induction in Aspergillus nidulans . Mol Cell Biol 27 : 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, G. , Strauss, J. , Scazzocchio, C. , and Lang, B.F. (1991) nirA, the pathway‐specific regulatory gene of nitrate assimilation in Aspergillus nidulans, encodes a putative GAL4‐type zinc finger protein and contains four introns in highly conserved regions. Mol Cell Biol 11 : 5746–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch, S. , Hoffmann, B. , Valerius, O. , Starke, K. , Duvel, K. , and Braus, G.H. (2001) Regulation of the Aspergillus nidulans hisB gene by histidine starvation. Curr Genet 38 : 314–322. [DOI] [PubMed] [Google Scholar]
- Caddick, M.X. , Arst, H.N., Jr , Taylor, L.H. , Johnson, R.I. , and Brownlee, A.G. (1986) Cloning of the regulatory gene areA mediating nitrogen metabolite repression in Aspergillus nidulans . EMBO J 5 : 1087–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canovas, D. , and Andrianopoulos, A. (2006) Developmental regulation of the glyoxylate cycle in the human pathogen Penicillium marneffei . Mol Microbiol 62 : 1725–1738. [DOI] [PubMed] [Google Scholar]
- Canovas, D. , Marcos, A.T. , Gacek, A. , Ramos, M.S. , Gutierrez, G. , Reyes‐Dominguez, Y. , and Strauss, J. (2014) The histone acetyltransferase GcnE (GCN5) plays a central role in the regulation of Aspergillus asexual development. Genetics 197 : 1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona, L. , Gandia, M. , Lopez‐Garcia, B. , and Marcos, J.F. (2012) Sensitivity of Saccharomyces cerevisiae to the cell‐penetrating antifungal peptide PAF26 correlates with endogenous nitric oxide (NO) production. Biochem Biophys Res Commun 417 : 56–61. [DOI] [PubMed] [Google Scholar]
- Clutterbuck, A.J. (1969) A mutational analysis of conidial development in Aspergillus nidulans . Genetics 63 : 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove, D.J. (1966) The induction and repression of nitrate reductase in the fungus Aspergillus nidulans . Biochim Biophys Acta 113 : 51–56. [DOI] [PubMed] [Google Scholar]
- Dean, J.V. , and Harper, J.E. (1988) The conversion of nitrite to nitrogen oxide(s) by the constitutive NAD(P)H‐nitrate reductase enzyme from soybean. Plant Physiol 88 : 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer, P.S. , and O'Gorman, C.M. (2012) Sexual development and cryptic sexuality in fungi: insights from Aspergillus species. FEMS Microbiol Rev 36 : 165–192. [DOI] [PubMed] [Google Scholar]
- Eckert, S.E. , Kubler, E. , Hoffmann, B. , and Braus, G.H. (2000) The tryptophan synthase‐encoding trpB gene of Aspergillus nidulans is regulated by the cross‐pathway control system. Mol Gen Genet 263 : 867–876. [DOI] [PubMed] [Google Scholar]
- Etxebeste, O. , Garzia, A. , Espeso, E.A. , and Ugalde, U. (2010) Aspergillus nidulans asexual development: making the most of cellular modules. Trends Microbiol 18 : 569–576. [DOI] [PubMed] [Google Scholar]
- Foster, M.W. , Liu, L. , Zeng, M. , Hess, D.T. , and Stamler, J.S. (2009) A genetic analysis of nitrosative stress. Biochemistry 48 : 792–799. [DOI] [PubMed] [Google Scholar]
- Gardner, P.R. , Costantino, G. , and Salzman, A.L. (1998a) Constitutive and adaptive detoxification of nitric oxide in Escherichia coli. Role of nitric‐oxide dioxygenase in the protection of aconitase. J Biol Chem 273 : 26528–26533. [DOI] [PubMed] [Google Scholar]
- Gardner, P.R. , Gardner, A.M. , Martin, L.A. , and Salzman, A.L. (1998b) Nitric oxide dioxygenase: an enzymic function for flavohemoglobin. Proc Natl Acad Sci USA 95 : 10378–10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, X. , Fu, Y. , Jiang, D. , Li, G. , Yi, X. , and Peng, Y. (2007) L‐arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans . Fungal Genet Biol 44 : 1368–1379. [DOI] [PubMed] [Google Scholar]
- Gorren, A.C. , and Mayer, B. (2007) Nitric‐oxide synthase: a cytochrome P450 family foster child. Biochim Biophys Acta 1770 : 432–445. [DOI] [PubMed] [Google Scholar]
- Gupta, K.J. , Igamberdiev, A.U. , Manjunatha, G. , Segu, S. , Moran, J.F. , Neelawarne, B. , et al (2011) The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Sci 181 : 520–526. [DOI] [PubMed] [Google Scholar]
- Han, K.H. , Han, K.Y. , Yu, J.H. , Chae, K.S. , Jahng, K.Y. , and Han, D.M. (2001) The nsdD gene encodes a putative GATA‐type transcription factor necessary for sexual development of Aspergillus nidulans . Mol Microbiol 41 : 299–309. [DOI] [PubMed] [Google Scholar]
- Hatcher, H.J. , and Schmidt, E.L. (1971) Nitrification of aspartate by Aspergillus flavus . Appl Microbiol 21 : 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Tang, R.H. , Hao, Y. , Stevens, R.D. , Cook, C.W. , Ahn, S.M. , et al (2004) Nitric oxide represses the Arabidopsis floral transition. Science 305 : 1968–1971. [DOI] [PubMed] [Google Scholar]
- Hirsch, P. , Overrein, L. , and Alexander, M. (1961) Formation of nitrite and nitrate by actinomycetes and fungi. J Bacteriol 82 : 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, B. , Wanke, C. , Lapaglia, S.K. , and Braus, G.H. (2000) c‐Jun and RACK1 homologues regulate a control point for sexual development in Aspergillus nidulans . Mol Microbiol 37 : 28–41. [DOI] [PubMed] [Google Scholar]
- Klemm, E. , and Ninnemann, H. (1979) Nitrate reductase – a key enzyme in blue light‐promoted conidiation and absorbance change of Neurospora . Photochem Photobiol 29 : 629–632. [DOI] [PubMed] [Google Scholar]
- Kojima, H. , Nakatsubo, N. , Kikuchi, K. , Kawahara, S. , Kirino, Y. , Nagoshi, H. , et al (1998a) Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70 : 2446–2453. [DOI] [PubMed] [Google Scholar]
- Kojima, H. , Sakurai, K. , Kikuchi, K. , Kawahara, S. , Kirino, Y. , Nagoshi, H. , et al (1998b) Development of a fluorescent indicator for nitric oxide based on the fluorescein chromophore. Chem Pharm Bull (Tokyo) 46 : 373–375. [DOI] [PubMed] [Google Scholar]
- Kojima, H. , Urano, Y. , Kikuchi, K. , Higuchi, T. , Hirata, Y. , and Nagano, T. (1999) Fluorescent indicators for imaging nitric oxide production. Angew Chem Int Ed Engl 38 : 3209–3212. [DOI] [PubMed] [Google Scholar]
- Kwon, E. , Feechan, A. , Yun, B.W. , Hwang, B.H. , Pallas, J.A. , Kang, J.G. , and Loake, G.J. (2012) AtGSNOR1 function is required for multiple developmental programs in Arabidopsis . Planta 236 : 887–900. [DOI] [PubMed] [Google Scholar]
- Lamotte, O. , Courtois, C. , Barnavon, L. , Pugin, A. , and Wendehenne, D. (2005) Nitric oxide in plants: the biosynthesis and cell signalling properties of a fascinating molecule. Planta 221 : 1–4. [DOI] [PubMed] [Google Scholar]
- Maier, J. , Hecker, R. , Rockel, P. , and Ninnemann, H. (2001) Role of nitric oxide synthase in the light‐induced development of sporangiophores in Phycomyces blakesleeanus . Plant Physiol 126 : 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluf, G.A. (1997) Genetic regulation of nitrogen metabolism in the fungi. Microbiol Mol Biol Rev 61 : 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro‐Pastor, M.I. , Gonzalez, R. , Strauss, J. , Narendja, F. , and Scazzocchio, C. (1999) The GATA factor AreA is essential for chromatin remodelling in a eukaryotic bidirectional promoter. EMBO J 18 : 1584–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano, T. (2009) Bioimaging probes for reactive oxygen species and reactive nitrogen species. J Clin Biochem Nutr 45 : 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninnemann, H. (1991) Photostimulation of conidiation in mutants of Neurospora crassa . J Photochem Photobiol B 9 : 189–199. [DOI] [PubMed] [Google Scholar]
- Ninnemann, H. , and Maier, J. (1996) Indications for the occurrence of nitric oxide synthases in fungi and plants and the involvement in photoconidiation of Neurospora crassa . Photochem Photobiol 64 : 393–398. [DOI] [PubMed] [Google Scholar]
- Paoletti, M. , Seymour, F.A. , Alcocer, M.J. , Kaur, N. , Calvo, A.M. , Archer, D.B. , and Dyer, P.S. (2007) Mating type and the genetic basis of self‐fertility in the model fungus Aspergillus nidulans . Curr Biol 17 : 1384–1389. [DOI] [PubMed] [Google Scholar]
- Planchet, E. , and Kaiser, W.M. (2006) Nitric oxide (NO) detection by DAF fluorescence and chemiluminescence: a comparison using abiotic and biotic NO sources. J Exp Bot 57 : 3043–3055. [DOI] [PubMed] [Google Scholar]
- Poole, R.K. , and Hughes, M.N. (2000) New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol Microbiol 36 : 775–783. [DOI] [PubMed] [Google Scholar]
- Rockel, P. , Strube, F. , Rockel, A. , Wildt, J. , and Kaiser, W.M. (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53 : 103–110. [PubMed] [Google Scholar]
- Rodriguez‐Romero, J. , Hedtke, M. , Kastner, C. , Muller, S. , and Fischer, R. (2010) Fungi, hidden in soil or up in the air: light makes a difference. Annu Rev Microbiol 64 : 585–610. [DOI] [PubMed] [Google Scholar]
- Rosselli, M. , Keller, P.J. , and Dubey, R.K. (1998) Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum Reprod Update 4 : 3–24. [DOI] [PubMed] [Google Scholar]
- Ruger‐Herreros, C. , Rodriguez‐Romero, J. , Fernandez‐Barranco, R. , Olmedo, M. , Fischer, R. , Corrochano, L.M. , and Canovas, D. (2011) Regulation of conidiation by light in Aspergillus nidulans . Genetics 188 : 809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samalova, M. , Johnson, J. , Illes, M. , Kelly, S. , Fricker, M. , and Gurr, S. (2013) Nitric oxide generated by the rice blast fungus Magnaporthe oryzae drives plant infection. New Phytol 197 : 207–222. [DOI] [PubMed] [Google Scholar]
- Schinko, T. , Berger, H. , Lee, W. , Gallmetzer, A. , Pirker, K. , Pachlinger, R. , et al (2010) Transcriptome analysis of nitrate assimilation in Aspergillus nidulans reveals connections to nitric oxide metabolism. Mol Microbiol 78 : 720–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinko, T. , Gallmetzer, A. , Amillis, S. , and Strauss, J. (2013) Pseudo‐constitutivity of nitrate‐responsive genes in nitrate reductase mutants. Fungal Genet Biol 54 : 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth, D. , Hausladen, A. , Wang, Y.J. , and Stamler, J.S. (2012) Endogenous protein S‐Nitrosylation in E. coli: regulation by OxyR. Science 336 : 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, N.‐K. , Jeong, C.‐S. , and Choi, H.‐S. (2000) Identification of nitric oxide synthase in Flammulina velutipes . Mycologia 92 : 1027–1032. [Google Scholar]
- Stief, P. , Fuchs‐Ocklenburg, S. , Kamp, A. , Manohar, C.S. , Houbraken, J. , Boekhout, T. , et al (2014) Dissimilatory nitrate reduction by Aspergillus terreus isolated from the seasonal oxygen minimum zone in the Arabian Sea. BMC Microbiol 14 : 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, J. , Muro‐Pastor, M.I. , and Scazzocchio, C. (1998) The regulator of nitrate assimilation in ascomycetes is a dimer which binds a nonrepeated, asymmetrical sequence. Mol Cell Biol 18 : 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Biesebeke, R. , Levasseur, A. , Boussier, A. , Record, E. , van den Hondel, C.A. , and Punt, P.J. (2010) Phylogeny of fungal hemoglobins and expression analysis of the Aspergillus oryzae flavohemoglobin gene fhbA during hyphal growth. Fungal biology 114 : 135–143. [DOI] [PubMed] [Google Scholar]
- Takasaki, K. , Shoun, H. , Yamaguchi, M. , Takeo, K. , Nakamura, A. , Hoshino, T. , and Takaya, N. (2004) Fungal ammonia fermentation, a novel metabolic mechanism that couples the dissimilatory and assimilatory pathways of both nitrate and ethanol. Role of acetyl CoA synthetase in anaerobic ATP synthesis. J Biol Chem 279 : 12414–12420. [DOI] [PubMed] [Google Scholar]
- Todd, R.B. , Fraser, J.A. , Wong, K.H. , Davis, M.A. , and Hynes, M.J. (2005) Nuclear accumulation of the GATA factor AreA in response to complete nitrogen starvation by regulation of nuclear export. Eukaryot Cell 4 : 1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallim, M.A. , Miller, K.Y. , and Miller, B.L. (2000) Aspergillus SteA (sterile12‐like) is a homeodomain‐C2/H2‐Zn+2 finger transcription factor required for sexual reproduction. Mol Microbiol 36 : 290–301. [DOI] [PubMed] [Google Scholar]
- Wu, J. , and Miller, B.L. (1997) Aspergillus asexual reproduction and sexual reproduction are differentially affected by transcriptional and translational mechanisms regulating stunted gene expression. Mol Cell Biol 17 : 6191–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki, H. (2000) Nitrite‐dependent nitric oxide production pathway: implications for involvement of active nitrogen species in photoinhibition in vivo. Philos Trans R Soc Lond B Biol Sci 355 : 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki, H. , and Sakihama, Y. (2000) Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR‐dependent formation of active nitrogen species. FEBS Lett 468 : 89–92. [DOI] [PubMed] [Google Scholar]
- Yu, J.‐H. (2010) Regulation of development in Aspergillus nidulans and Aspergillus fumigatus . Mycobiology 38 : 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J.H. , Mah, J.H. , and Seo, J.A. (2006) Growth and developmental control in the model and pathogenic aspergilli. Eukaryot Cell 5 : 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Kim, W.S. , Hatcher, N. , Potgieter, K. , Moroz, L.L. , Gillette, R. , and Sweedler, J.V. (2002a) Interfering with nitric oxide measurements. 4,5‐diaminofluorescein reacts with dehydroascorbic acid and ascorbic acid. J Biol Chem 277 : 48472–48478. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Stuart, J.N. , and Sweedler, J.V. (2002b) Capillary electrophoresis with wavelength‐resolved laser‐induced fluorescence detection. Anal Bioanal Chem 373 : 332–343. [DOI] [PubMed] [Google Scholar]
- Zhou, S. , Narukami, T. , Nameki, M. , Ozawa, T. , Kamimura, Y. , Hoshino, T. , and Takaya, N. (2012) Heme‐biosynthetic porphobilinogen deaminase protects Aspergillus nidulans from nitrosative stress. Appl Environ Microbiol 78 : 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S. , Narukami, T. , Masuo, S. , Shimizu, M. , Fujita, T. , Doi, Y. , et al (2013) NO‐inducible nitrosothionein mediates NO removal in tandem with thioredoxin. Nat Chem Biol 9 : 657–663. [DOI] [PubMed] [Google Scholar]
- Zweier, J.L. , Li, H. , Samouilov, A. , and Liu, X. (2010) Mechanisms of nitrite reduction to nitric oxide in the heart and vessel wall. Nitric Oxide 22 : 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


