Abstract
A rapid and simple turbulent flow liquid chromatography (TFC–LC) method implementing positive heated electrospray ionization (HESI) for the accurate and precise determination of methotrexate (MTX), 7-hydroxy methotrexate (7-OH MTX), and 4-amino-4-deoxy-N10-methylpteroic acid (DAMPA) concentrations in serum was developed. MTX was isolated from serum samples (100 μL) after protein precipitation with methanol containing formic acid and internal standard (MTX-D3) followed by centrifugation. The supernatant was injected into the turbulent flow liquid chromatography which is followed by electrospray positive ionization tandem mass spectrometry (TFC–LC–MS/MS) and quantified using a six-point calibration curve. For MTX and DAMPA the assays were linear from 10 to 1000 nmol/L and for 7-OH MTX from 20 to 2000 nmol/L. Dilutions of 10, 100 and 1000-fold were validated giving a clinically reportable range of 10 nmol/L to 5 × 105 nmol/L. Within-day and between-day precisions at concentrations spanning the analytical measurement ranges were less than 10% for all three analytes. MTX, DAMPA and 7-OH MTX were sufficiently stable under all relevant analytical conditions. No significant matrix effect was observed during the method validation. The TFC–LC-MS/MS MTX method was also compared with three other clinically validated MTX assays: a dihydrofolate reductase (DHFR) inhibition assay, an immunoassay based on fluorescence polarization and a previously developed LC–MS/MS assay.
Keywords: Methotrexate, 7-Hydroxy methotrexate, DAMPA, Carboxypeptidase-G2, Therapeutic drug monitoring, Mass spectrometry, Turbulent flow liquid chromatography, Method correlation, Dihydrofolate reductase inhibition assay, Fluorescence polarization immunoassay
1. Introduction
Methotrexate (MTX) is a folic acid antagonist that is widely used as an immunosuppressant and chemotherapeutic agent. MTX exerts its cytotoxic effects by competitively inhibiting dihydrofolate reductase (DHFR) which is the enzyme responsible for converting folates to tetrahydrofolates. Tetrahydrofolates are the folate carrier that functions in the transfer of carbon units. A normal dividing cell uses large amounts of reduced folates to maintain ongoing purine and thymidine synthesis and the demand is even greater for rapidly dividing malignant cells [1].
High-dose MTX is mainly used for the treatment of some leukemias and osteosarcoma. Intermediate and lower dose MTX regimens are used to treat malignant gestational trophoblastic disease, breast and bladder cancer, ALL, and acute promyelocytic leukemia [1,2]. In addition to its antiproliferative activity, MTX also has anti-inflammatory and immunomodulating properties and thus is a first-line treatment for a growing number of autoimmune rheumatologic, dermatologic and gastroenterologic conditions [1,2]. After high dose administration of MTX, serum levels must be monitored to determine when to administer leucovorin, a folic acid analog that bypasses the enzyme inhibition caused by MTX and reverses its toxicity [3,4]. 7-Hydroxy methotrexate (7-OH-MTX) is less soluble than MTX in the acidic environment of the proximal renal tubule where it can precipitate and cause renal damage [5]. The excessive concentration of 7-OH MTX may also influence the pharmacokinetics of MTX due to competition for transport systems across cell membranes, therefore monitoring its concentration maybe warranted [5,6].
Patients in renal failure who are given high-dose MTX are sometimes given carboxypeptidase-G2 (CPDG2) to reverse the effects of MTX [3,4,7,8]. CPDG2 is an enzyme that converts MTX into glutamate and 4-amino-4-deoxy-N10-methylpteroic acid (DAMPA) which is much less toxic and readily excreted by the kidney. DAMPA cross-reacts considerably in immunoassays for MTX, rendering them unsuitable for monitoring patients who have been given CPDG2 therapy [9] (Fig. 1).
Fig. 1.
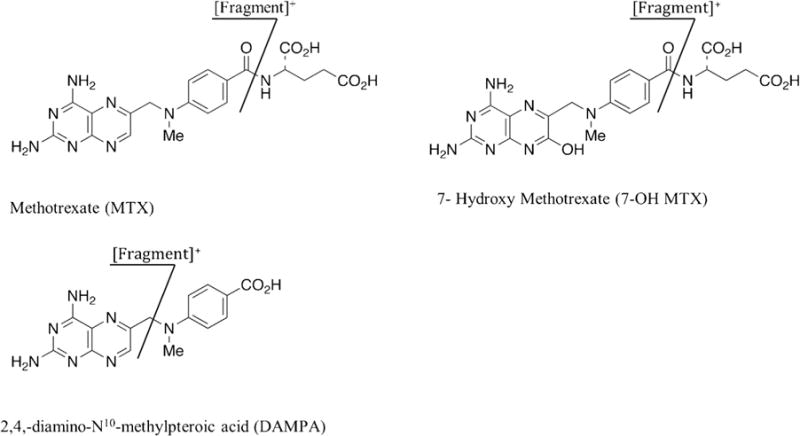
Molecular structures and fragmentation patterns of MTX, 7-OH MTX and DAMPA.
The objective of this study was to develop a MTX assay performed by Turbulent flow chromatography coupled with tandem mass spectrometry (TFC-LC-MS/MS) with the following characteristics: (a) analytically sensitive with a clinically useful dynamic range, (b) good specificity with no interference from metabolites or other compounds, (c) suitable analytical transferability for a high volume clinical laboratory, and (d) the ability to accurately measure 7-OH MTX and DAMPA to support clinical trials utilizing CPDG2 and related compounds.
TFC-LC-MS/MS is a technique that has been shown to eliminate much time-consuming sample processing and increase productivity and throughput [10]. TFC uses high mobile phase flow rates over large porous particles (30–60 μM). Small molecules are driven into the pores of the sorbent and larger molecules and matrix constituents are excluded and diverted into waste. The trapped analytes are desorbed from the TFC column by back-flushing with an organic solvent, and the eluent can be transferred onto the analytical LC-MS/MS system for further purification and detection.
Several MTX assays using mass spectrometry have been recently described and validated in human serum [11–15], however this is the first method to utilize TFC and to provide for the accurate and precise determination of MTX, 7-OH MTX, and DAMPA concentrations in a single specimen.
2. Experimental
2.1. Materials and reagents
MTX and folinic acid calcium salt hydrate were from Sigma-Aldrich (Saint-Louis, MO, USA) and 7-OH MTX was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). DAMPA, 4-amino-10-methylpteroyl-di-γ-L-glutamic acid ammonium salt and 4-amino-10-methylpteroyl-tetra-γ-L-glutamic acid ammonium salt were from Schircks Laboratories (Jona, Switzerland). Deuterated methotrexate (MTX-D3) was from Cerilliant (Round Rock, TX, USA). All compounds had purity greater than 99%. LCMS (Optima) grade water, methanol, acetonitrile, 2-propanol, acetone, formic acid, and ammonium hydroxide were from Fisher Scientific (Fair Lawn, NJ, USA). Blank human serum and MTX controls were from UTAK Laboratories (Valencia, CA, USA).
2.2. Instrument and analytical conditions
A Thermo Scientific Aria TLX-2 turbulent flow chromatography system (Franklin, MA, USA) comprised of a CTC analytics PAL autosampler, a low-pressure mixing quaternary pump (loading pump), a high-pressure mixing binary pump (eluting pump) and a three-valve switching device unit (VIM) containing six-port valves were operated in accordance with manufacturer recommendations. Fig. 2 is a schematic representation of the loading step in an Aria TLX-2 system. A sample was introduced into the system by an autosampler and a loading pump. The system also involved a solvent holding loop connected to valve A. This loop contained a solvent mixture strong enough to elute the analytes from the TFC into the HPLC column. An eluting pump delivered a mixture of solvents and enabled a normal chromagraphic separation and detection [10].
Fig. 2.
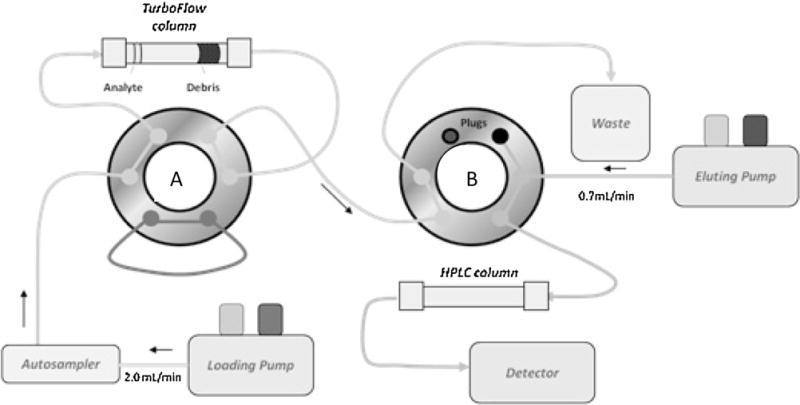
Aria TLX-2 valve in loading configuration.
The entire system was controlled with Aria software, version 1.6.2. The mobile phases consisted of ammonium formate buffer in either water or methanol. The TurboFlow column used was a Cyclone-P and the HPLC column a Hypersil Gold C8. The temperature of the analytical column was maintained using a column heater.
The triple quadrupole mass spectrometer was a Thermo Scientific TSQ Quantum Ultra (San Jose, CA, USA) equipped with a heated electrospray ionization probe that was maintained at 380 °C. All analyses were performed in the positive ionization mode with a spray voltage set at 4500 V. Nitrogen was used as the sheath, auxiliary and ion sweep gas at 60,15 and 2 arbitrary units, respectively. The system was operated in the selected reaction monitoring (SRM) mode with argon as the collision gas at a pressure of 1.5 mTorr. The ion transfer tube was maintained at 235 °C.
2.3. Preparation of stock solutions, calibration standards and quality control sample
Stock solutions of MTX, 7-OH MTX and DAMPA were prepared in methanol containing 0.1 N sodium hydroxide at a concentration of 1 mg/mL and stored at −20 °C. The internal standard working solution (65.6 nmol/L) was prepared bi-weekly, in methanol containing 0.1% formic acid from the Cerilliant stock solution.
To prepare calibration standards a working solution containing 2000 nmol/L of 7-OH MTX and 1000 nmol/L of MTX and DAMPA was prepared in drug-free human serum. The working solution was then diluted serially to obtain concentration ranges of 15.6 to 2000 nmol/L and 7.8 to 1000 nmol/L for 7-OH MTX and MTX and DAMPA, respectively. 7-OH and DAMPA controls were prepared by adding the standard stock solution into drug-free human serum at final concentrations of 500, 1000, and 1500 nmol/L for 7-OH MTX and 250, 500, and 750 nmol/L for DAMPA. MTX controls were obtained from UTAK Laboratories. Assay imprecision was assessed by analyzing each control in duplicate over a period of twenty days. Calibrators and QC samples were stored at −80 °C.
2.4. Sample preparation
Samples were prepared for analysis by transferring 100 μL of calibrator, control, or unknown to a 1.5 mL microcentrifuge tube containing 200 μL of internal standard solution. Samples were then vortex-mixed briefly to precipitate serum proteins. The samples were then centrifuged at 13,000 RPM for ten minutes. Supernatants were transferred to vials with inserts and 50 μL was injected into the system and analyzed by TFC-LC-MS/MS.
2.5. Method Validation
The method validation was adopted from guidelines from the U.S. Food and Drug Administration (FDA) [16], CLSI [17], and CLIA. The assay was fully validated for linearity, accuracy, precision, selectivity, carryover, recovery, matrix effect and stability.
2.5.1. Specificity
The bioanalytical method should be selective and specific for an analyte and not affected by interfering or co-eluting compounds in the biological matrix. The selection of a specific precursor ion followed by the formation and detection of a unique product ion renders quantitative TFC-LC-MS/MS highly specific. Specificity was investigated by comparing chromatograms of six drug-free serum specimens spiked with MTX, 7-OH MTX and DAMPA including the internal standard. The chromagraphic peaks of analytes were identified based on their SRM responses and retention times.
2.5.2. Linearity
The linearity of this method for all three analytes was evaluated by calibration curves as described in Section 2.3. The standard curves are based on linear regression analysis at six different levels spanning the analytical measure range (AMR) for MTX, 7-OH MTX and DAMPA. Weighted linear regression models with weights inversely proportional to the X values were used. The analysis compared the ratio of I.S. peak area to sample peak area (y-axis) versus analyte concentration (x-axis) using the appropriate quantifying ions. The LLOQ was defined as the lowest concentration of analyte where the coefficient of variation (CV) was below 20% as stated in the FDA guideline of bioanalytical method validation [16]. In addition, the signal-to-noise ratio (S/N) of analyte at the LLOQs exceeded 100 in all cases.
2.5.3. Accuracy and precision
Between-day assay imprecision was evaluated over a twenty-day period with a minimum of three levels of controls extracted twice daily. The average concentration and standard deviation were determined over the validation period for all three analytes. The CV was not to exceed 15% for controls over the duration of the imprecision study [16]. The accuracy of the assay was evaluated by performing a recovery experiment for MTX, 7-OH MTX, and DAMPA. Analytes were added to blank serum to produce five concentrations that span the analytical measurement range (AMR) and were extracted in triplicate.
In addition, the accuracy of the MTX TFC-LC-MS/MS assay was evaluated by comparison with established assays for MTX that have been validated according to CLIA guidelines and used routinely in clinical laboratories. Permission to use residual human patient specimens was granted from the Memorial Sloan Kettering Cancer Center Institutional Review Board. The samples were collected by venipuncture in serum separator tubes and the serum was separated by centrifugation of blood as 3000 × g for 10 min. Specimens from sixty patients receiving MTX therapy were analyzed by TFC-LC-MS/MS, a DHFR enzymatic inhibition assay [18], the Abbott TDx fluorescence polarization immunoassay (Abbott Laboratories, Abbott Park, IL), and an LC-MS/MS assay performed at a national reference laboratory that had the following characteristics: an AMR of 4.0–2000 ng/mL and a CV <10% at various concentrations spanning the AMR. The slopes of the linear regression curves comparing the four assays were examined. 7-OH MTX and DAMPA could not be evaluated in this manner as no commercially available tests exist for these analytes.
Since MTX concentrations can exceed the AMR, a dilution protocol was validated at 1:10, 1:100, and 1:1000 fold dilutions. The dilution protocol was as follows: to 900 μL of blank serum 100 μL of patient sample was added and vortex mixed briefly. With each successive dilution, a 100 μL was taken from the prior dilution and added to 900 μL of the drug-free serum.
2.5.4. Matrix interference and ion suppression
To evaluate matrix effects, a tee-fitting was placed between the outlet of the HPLC column and the infusion pump on the front of the MS. A standard mix solution containing all analytes was infused into the eluent stream and each SRM transition was monitored. After a steady baseline was achieved, a blank serum extract was injected and processed by the TFC-LC-MS/MS system. Any eluting compound that interferes with the ionization of the target analyte leads to an elevation or depression of the baseline which reflects the matrix effects. Detector response due to matrix variability should not exceed 15% [18].
In order to rule out interferences from structurally similar compounds, folinic acid (200 ng/mL) as well as the MTX metabolites, 4-amino-10-methylpteroyl-di-γ-L-glutamic acid and 4-amino-10-methylpteroyl-tetra-γ-L-glutamic acid (500nmol/L) were added into drug free serum. The analytes of interest (MTX, 7-OH MTX and DAMPA) were compared in the presence and absence of the structurally similar analogs.
2.5.5. Quantitation of sample carryover
Sample carryover was evaluated by running a serum blank after the highest calibrator on all calibration curves during the validation period. The percent carryover was required to be <25% of the LLOQ for all compounds [17].
2.5.6. Stability
To ensure the integrity of the compounds, a stability assay comprising of short-term and long-term stability, freeze-thaw and post-extraction stability were performed. In this protocol, sample stability was tested by analyzing samples after short-term (24 h) storage at room temperature and long-term storage at −80 °C for 30 days. Additionally, freeze-thaw stability was determined by testing samples after three cycles from −80 °C to room temperature. Post-preparation stability was monitored by analyzing samples in the autosampler (10°C) after a period of 24 h.
3. Results and discussion
3.1. Chromatographic conditions
The turbulent flow chromatography parameters were optimized to maximize sensitivity for all analytes. It was determined that the combination of a Cyclone-P (50 × 0.5 mm) and Hypersil Gold C8 columns (2.1 × 50 mm, 5 μm) produced adequate retention of the compounds (Fig. 3). Various mobile phase compositions, flow rates and profiles were evaluated. The desired sensitivity was achieved by using 10mM ammonium formate in water and methanol as the aqueous and organic solvents, respectively. The analytes were loaded on the TFC in 100% mobile phase A and transferred to the HPLC column with 80% mobile phase B using a 200 μL transfer loop. The loading and eluting mobile phase composition for the HPLC column was identical to that of the TFC (Table 1).The injection-to-injection run time (5.25 min) is slightly greater than the individual steps in Table 1 due to the initial step of the autosampler method that causes a slight delay. The optimal injection volume was 50 μL and the analytical column was maintained at 70 °C. Mobile phase A and B consisted of 10mM ammonium formate in water and methanol, respectively. Mobile phase C was a mixture (6:3:1 v/v) of acetonitrile:2-propanol:acetone.The integration parameters for all four analytes were similar with a baseline window of 20, area noise factor of 5, peak noise factor of 10 and an integration window of 15 s.
Fig. 3.
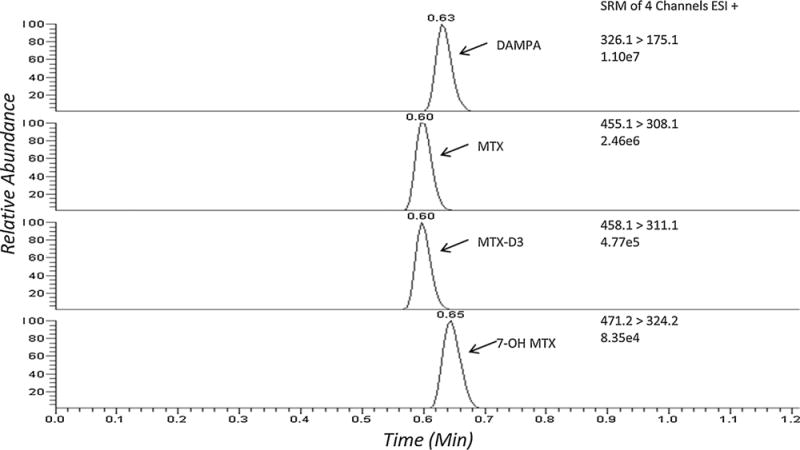
TFC–LC–MS/MS ion chromatograms of DAMPA, MTX, MTX-D3 (I.S.), and 7-OH MTX product ions in a serum sample.
Table 1.
Gradient used on the loading and eluting pump for the TFC–LC–MS/MS analysis of methotrexate and its metabolites.
| Step | Time (s) | TFC–LC parameters
|
HPLC parameters
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Flow (mL/min) | A (%) | B (%) | C (%) | Flow (mL/Min) | Grad. | A (%) | B (%) | ||
| 1 | 30 | 2.00 | 100 | – | – | 0.7 | Step | 100 | – |
| 2a | 45 | 0.15 | 100 | – | – | 0.7 | Step | 100 | – |
| 3 | 15 | 2.00 | – | – | 100 | 0.7 | Ramp | 20 | 80 |
| 4b | 45 | 2.00 | – | – | 100 | 0.7 | Step | 20 | 80 |
| 5 | 45 | 2.00 | 20 | 80 | – | 0.7 | Step | 20 | 80 |
| 6 | 45 | 2.00 | 20 | 80 | – | 0.7 | Step | – | 100 |
| 7 | 75 | 2.00 | 100 | – | – | 0.7 | Step | 100 | – |
During this step valve “A” and valve “B” are in line and the analyte is transferred from the TurboFlow column to the analytical column.
Data Acquisition window: 1.75–3.25 min.
The HPLC conditions used in this assay are illustrated in Table 1.
3.2. MS/MS detection
The TFC-LC-MS/MS analysis was carried out using the conditions stated in Section 2.2. The optimization of the SRM parameters was performed by direct infusion of the standards using positive electrospray ionization. The transitions monitored in SRM mode were 455.1 >308.1, 471.2 >324.2, 326.1 > 175.1, and 458.1 > 311.1 for MTX, 7-OH MTX, DAMPA and MTX-D3, respectively. Collision-induced dissociation (CID) mass spectra were recorded for each analyte. The optimum collision energies (CE) and tube lens values were determined for the selected ion transitions. It was found that the optimal CE and tube lens values for all compounds were 18 V and 105, respectively. The skimmer offset was determined in MS mode while the chrom filter peak width was optimized based on chromatogram review and was concluded to be ideal at 10 V and 5 s for all compounds, respectively. The scan time (s) and width (m/z) were both 0.05 for MTX and DAMPA while the scan time was 0.1 s for 7-OH MTX. Data was processed using LCquan software version 2.6.
3.3. Method validation
As previously stated the method validation was adopted from guidelines from the U.S. Food and Drug Administration (FDA) [16], CLSI [17], and CLIA. The assay was fully validated for linearity, accuracy, precision, selectivity, carryover, recovery, matrix effect and stability.
3.3.1. Specificity
Fig. 3 a typical SRM chromatogram with all four compounds present shows each at their appropriate retention time. The retentions times of MTX, 7-OH MTX, DAMPA, and MTX-D3 are 0.60, 0.65, 0.63, and 0.60 min, respectively. There were no interferences observed from six individual blank specimens.
3.3.2. Linearity
A linear relationship was found between analyte concentrations and peak area ratios within the AMR of each compound. The coefficient of correlations (r) as determined by a six point calibration curve for each analyte are greater than >0.996 for each analyte. The LLOQs were determined to be 10 nmol/L for MTX and DAMPA and 20 nmol/L for 7-OH MTX. The lowest signal-to-noise ratio (S/N) of all analytes at the LLOQs was 7-OH MTX which had a value greater than a 100; more than five times the minimum S/N requirement.
3.3.3. Accuracy and precision
The CV was <10% for all controls over the duration of the twenty day imprecision study (Table 2). The accuracy of the assay was evaluated by performing a recovery experiment for MTX, 7-OH MTX, and DAMPA. Eight concentrations were spiked into blank serum and extracted three separate times, yielding results (Table 3) ranging from 92.4% to 107.4% recovery for all compounds. This indicates that the LC–MS/MS method is both accurate and precise for the quantitation of MTX, 7-OH MTX and DAMPA in human serum.
Table 2.
Within and between-day precision of analytes in human serum.
| Sample | Nominal value (nmol/L) | Mean (nmol/L) within-day | Within-day CV (%) n = 10 | Mean (nmol/L) Between-day | Between-day CV (%) n = 20 |
|---|---|---|---|---|---|
| MTX, LLOQ | 10 | 10.8 | 7.9 | 10.2 | 12.2 |
| MTX, Level 1 | 50 | 46.8 | 3.7 | 49.3 | 9.9 |
| MTX, Level 2 | 75 | 72.2 | 3.7 | 70.2 | 8.8 |
| MTX, Level 3 | 500 | 564.3 | 4.1 | 499.2 | 9.3 |
| MTX, Level 4 | 750 | 762.4 | 3.1 | 734.1 | 8.9 |
| DAMPA, LLOQ | 10 | 10.1 | 2.9 | 11.0 | 11.9 |
| DAMPA, Level 1 | 250 | 241.3 | 5.3 | 239.8 | 6.0 |
| DAMPA, Level 2 | 500 | 452.6 | 4.4 | 478.4 | 5.2 |
| DAMPA, Level 3 | 750 | 650.0 | 3.8 | 727.9 | 4.8 |
| 7-OH MTX, LLOQ | 20 | 26.6 | 6.2 | 23.1 | 19.9 |
| 7-OH MTX, Level 1 | 500 | 505.6 | 5.7 | 509.1 | 8.5 |
| 7-OH MTX, Level 2 | 1000 | 967.0 | 4.0 | 1020.6 | 6.8 |
| 7-OH MTX, Level 3 | 1500 | 1397.6 | 2.8 | 1593.1 | 6.0 |
Table 3.
Method accuracy.
| Sample | Nominal value (nmol/L) | Measured value (nmol/L) | Recovery (measured/nominal) (%) |
|---|---|---|---|
| MTX, Level 1 | 50.0 | 47.3 ± 3.4 | 94.6 ± 6.9 |
| MTX, Level 2 | 150.0 | 156.4 ± 2.5 | 104.3 ± 1.7 |
| MTX, Level 3 | 300.0 | 295.0 ± 10.5 | 98.3 ± 3.5 |
| MTX, Level 4 | 600.0 | 597.0 ± 42.2 | 99.5 ± 7.0 |
| MTX, Level 5 | 1000.0 | 970.2 ± 42.9 | 97.0 ± 4.3 |
| MTX, Level 6a | 5000.0 | 509.8 ± 25.7 | 102.0 ± 5.1 |
| MTX, Level 7a | 50,000.0 | 496.4 ± 15.5 | 99.3 ± 3.1 |
| MTX, Level 8a | 500,000.0 | 483.0 ± 22.0 | 96.6 ± 4.4 |
| DAMPA, Level 1 | 50.0 | 51.4 ± 1.7 | 102.8 ± 3.4 |
| DAMPA, Level 2 | 150.0 | 155.4 ± 3.6 | 103.6 ± 2.4 |
| DAMPA, Level 3 | 300.0 | 315.2 ± 6.9 | 105.1 ± 2.3 |
| DAMPA, Level 4 | 600.0 | 596.6 ± 3.6 | 99.4 ± 0.6 |
| DAMPA, Level 5 | 1000.0 | 923.9 ± 57.5 | 92.4 ± 5.8 |
| DAMPA, Level 6a | 5000.0 | 495.8 ± 13.5 | 99.2 ± 2.7 |
| DAMPA, Level 7a | 50,000.0 | 513.2 ± 8.5 | 102.6 ± 1.7 |
| DAMPA, Level 8a | 500,000.0 | 480.0 ± 22.4 | 96.0 ± 4.5 |
| 7-OH MTX, Level 1 | 100.0 | 98.8 ± 3.6 | 98.8 ± 3.6 |
| 7-OH MTX, Level 2 | 300.0 | 295.3 ± 13.8 | 98.4 ± 4.6 |
| 7-OH MTX, Level 3 | 600.0 | 644.5 ± 27.3 | 107.4 ± 4.6 |
| 7-OH MTX, Level 4 | 1200.0 | 1248.7 ± 32.8 | 104.1 ± 2.7 |
| 7-OH MTX, Level 5 | 2000.0 | 1888.5 ± 216.7 | 94.4 ± 10.8 |
| 7-OH MTX, Level 6a | 10,000.0 | 966.9 ± 89.5 | 96.7 ± 9.0 |
| 7-OH MTX, Level 7a | 100,00.0 | 983.4 ± 11.9 | 98.3 ± 1.2 |
| 7-OH MTX, Level 8a | 1,000,000.0 | 971.2 ± 31.2 | 97.1 ± 3.1 |
Samples above the AMR were diluted following the dilution protocol stated in Section 2.5.3
In order to evaluate our dilution protocol, blank serum was added at three concentrations for all analytes. The concentrations were as follows: 5000, 50,000, and 500,000 nmol/L for MTX and DAMPA while 7-OH MTX required concentrations twice as elevated. Samples containing all analytes were diluted down into the AMR. All samples were extracted in triplicate and each dilution scheme yielded a percent difference within 4% of the expected concentration.
3.3.4. Matrix interference and ion suppression
The results of the tee-infusion experiment indicated there was no ion suppression or enhancement. This demonstrated that the extraction matrix had little co-eluting endogenous substances that could influence the ionization of the analytes and I.S.
3.3.5. Quantitation of sample carryover
Sample carryover was evaluated by running a serum blank after the highest calibrator on all calibration curves during the validation period. The average carryover was <20% of the calculated response of the LLOQfor all three target compounds. LLOQs were determined to be 10 nmol/L for MTX and DAMPA and 20 nmol/L for 7-OH MTX.
3.3.6. Stability
Quality control samples were subjected to short-term (room temperature and 4 °C) conditions, long-term storage conditions (−80 °C), post-preparative (10 °C) and three freeze-thaw cycles. All of the stability studies were conducted at two concentrations with three determinations each. The stability experiment confirmed that serum samples were stable over 30 days when stored at −80 °C and through three freeze-thaw cycles. Short-term stability was also confirmed at 4 °C for 24 h and 4 h at room temperature. The prepared serum samples were stable 24 h at 10 °C (Table 4).
Table 4.
Stability of analytes (n = 3).
| MTX
|
DAMPA
|
7-OH MTX
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Nominal Conc. nMol/L |
Accuracy (R.E.%) |
Precision (CV) | Nominal Conc. nMol/L |
Accuracy (R.E.%) |
Precision (CV) | Nominal Conc. nMol/L |
Accuracy (R.E.%) |
Precision (CV) | |
| Room Temp. | 50 | −3.8 | 1.6 | 50 | 5.6 | 1.2 | 100 | −5.6 | 5.0 |
| (4 h) | 500 | −2.3 | 2.9 | 500 | 4.5 | 2.9 | 1000 | −4.5 | 2.8 |
| 4 °C | 50 | 1.1 | 6.6 | 50 | −12.3 | 6.4 | 100 | −8.8 | 5.2 |
| (24 h) | 500 | 4.8 | 1.5 | 500 | 4.6 | 5.1 | 1000 | 5.6 | 5.1 |
| −80 °C | 50 | −1.9 | 5.0 | 50 | 3.4 | 4.2 | 100 | −5.6 | 5.0 |
| (30 Days) | 500 | 1.7 | 6.3 | 500 | −1.4 | 6.6 | 1000 | 7.4 | 2.7 |
| Post-prep 10 °C | 50 | −2.3 | 0.5 | 50 | −11.3 | 3.1 | 100 | −10.0 | 4.4 |
| (24 h) | 500 | −0.1 | 4.0 | 500 | −3.1 | 2.9 | 1000 | −4.9 | 7.4 |
| Freeze–thaw | 50 | 2.3 | 0.4 | 50 | −9.5 | 3.5 | 100 | −3.0 | 6.7 |
| (3 cycles) | 500 | 4.5 | 3.3 | 500 | −2.1 | 5.6 | 1000 | 6.7 | 3.0 |
3.4. Correlation to alternative methods
The accuracy of the MTXTFC-LC-MS/MS assay was further evaluated by comparing results from sixty patient specimens to a DHFR enzymatic inhibition assay, the Abbott TDx fluorescence polarization immunoassay (Abbott Laboratories, Abbott Park, IL), and an alternate LC-MS/MS assay. The Abbott immunoassay is an FDA approved assay while the DHFR inhibition and LC-MS/MS assays are laboratory developed tests that have been validated according to CLIA guidelines and are used in patient management. The slopes of the linear regression curves comparing the four assays were all within 1% and exhibit excellent correlation coefficients as shown in Fig. 4.
Fig. 4.
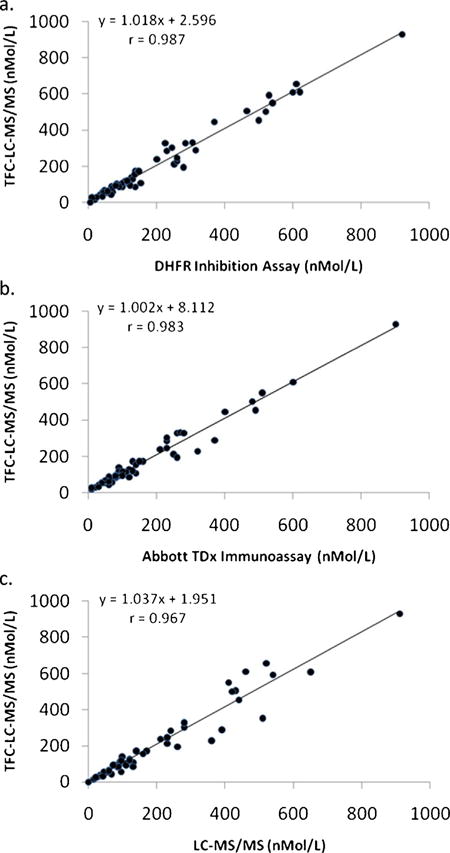
The TFC–LC–MS/MS assay for MTX compared with a (a) DHFR inhibition assay, (b) the Abbott TDx fluorescence polarization immunoassay and (c) a clinically validated LC–MS/MS assay.
4. Conclusion
This communication describes the development and validation of a novel TFC-LC-MS/MS method for the simultaneous quantification of MTX and its metabolites 7-OH MTX and DAMPA in serum. TFC for analyte extraction and purification allows for significantly reduced sample preparation time, high throughput and a small volume of sample (100μL). This method is robust and sensitive and was validated in serum on the basis of specificity, linearity, sensitivity, precision and accuracy, recovery and stability. The method is accurate, with recoveries ranging between 92 and 107% at concentrations spanning the AMR and shows excellent agreement with three alternative clinically-validated MTX assays. Within-day and between-day precisions at concentrations spanning the AMR are less than 10% for all three analytes. For MTX and DAMPA the assays are linear from 10 to 1000nmol/L and for 7-OH MTX from 20 to 2000 nmol/L. There is no matrix interferences observed or interferences from other MTX metabolites. All compounds and extracts are sufficiently stable over the relevant time frames employed in the assay. Unlike immunoassays affected by DAMPA the TFC-LC-MS/MS methodology is suitable for both routine clinical monitoring of MTX and analytical determination of MTX after the administration of carboxypeptidase.
Acknowledgments
This work was supported by the Department of Laboratory Medicine of Memorial Sloan Kettering Cancer Center.
Abbreviations
- 7-OH MTX
7-hydroxy methotrexate
- ALL
acute lympho-cytic leukemia
- CLIA
Clinical Laboratory Improvement Amendments
- CPDG2
carboxypeptidase-G2
- DAMPA
4-amino-4-deoxy-N10-methylpteroic acid
- DHFR
dihydrofolate reductase
- FPIA
fluorescence polarization immunoassay
- HESI
heated electrospray ionization
- MTX
methotrexate
- MTX-D3
deuterated methotrexate
- TFLC
turbulent flow liquid chromatography
- VIM
valve interface module
References
- 1.LaCasce A. Therapeutic use and toxicity of high-dose methotrexate. In: Post TW, editor. UpToDate. Waltham: 2014. (accessed 1.10.14) [Google Scholar]
- 2.Lennard L. Therapeutic drug monitoring of antimetabolic cytotoxic drugs. Br J Clin Pharmacol. 1999;47:131–143. doi: 10.1046/j.1365-2125.1999.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 4.Fleisher M. Antifolate analogs: mechanism of action analytical methodology, and clinical efficacy. Therapeut Drug Monit. 1993;15(6):521–526. [PubMed] [Google Scholar]
- 5.Klapkova E, Klapkova J, Kotaska K, Suchanska I, Urinovska R, Prusa R. The influence of 7-OH methotrexate metabolite on clinical relevance of methotrexate determination. Clin Lab. 2011;57:599–606. [PubMed] [Google Scholar]
- 6.Lankelma J, Van Der Klein E. The role of 7-hydroxymethotrexate during methotrexate anti-cancer therapy. Cancer Lett. 1980;9:133–142. doi: 10.1016/0304-3835(80)90117-2. [DOI] [PubMed] [Google Scholar]
- 7.DeAngellis LM, Tong WP, Lin S, Fleisher M, Bertino J. Carboxypeptidase G2 rescue after high-dose methotrexate. J Clin Oncol. 1996;14:2145–2149. doi: 10.1200/JCO.1996.14.7.2145. [DOI] [PubMed] [Google Scholar]
- 8.Widemann BC, Sung E, Anderson L, et al. Pharmacokinetics and metabolism of the methotrexate metabolite 2,4-diamino-N-methylpteroic acid. J Pharmacol Exp Therapeut. 2000;294:894–901. [PubMed] [Google Scholar]
- 9.Albertioni F, Rask C, Eksborg S, Poulsen J, Pettersson B, Beck O, Schroeder H, Peterson C. Evaluation of clinical assays for measuring high-dose methotrexate in plasma. Clin Chem. 1996;42:39–44. [PubMed] [Google Scholar]
- 10.Stolker A, Ruud J, Peters B, Zuiderent R, Dibussolo J, Claudia P, Martins B. Fully automated screening of veterinary drugs in milk by turbulent flow chromatography and tandem mass spectrometry. Anal Bioanal Chem. 2010;397(7):2841–2849. doi: 10.1007/s00216-010-3660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V, Law T, Kellogg M. Clinical Applications of Mass Spectrometry. Humana Press; New York: 2010. Liquid chromatography-tandem mass spectrometry (LC–MS–MS) method for monitoring methotrexate in the setting of carboxypeptidase-G2 therapy; pp. 359–363. [DOI] [PubMed] [Google Scholar]
- 12.Guo P, Wang X, Liu L, Belinsky M, Kruh G, Gallo J. Determination of methotrexate and its major metabolite 7-hydroxymethotrexate in mouse plasma and brain tissue by liquid chromatography–tandem mass spectrometry. J Pharmaceut Biomed Anal. 2007;43(5):1789–1795. doi: 10.1016/j.jpba.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair H, Lawrence L, Hoofnagle AN. Liquid chromatography-tandem mass spectrometry work flow for parallel quantification of methotrexate and other immunosuppressants. Clin Chem. 2012;58:943–945. doi: 10.1373/clinchem.2011.175067. [DOI] [PubMed] [Google Scholar]
- 14.Bouquié R, et al. A fast LC–MS/MS assay for methotrexate monitoring in plasma: validation, comparison to FPIA and application in the setting of carboxypeptidase therapy. Anal Methods. 2014;6(1):178–186. [Google Scholar]
- 15.Bouquie R, Deslandes G, Bernaldez B, Renaud C, Dailly E, Jolliet-Evin P. High-dose methotrexate induced renal dysfunction-development of LC–MS/MS monitoring in the setting of carboxypeptidase therapy Fundamental and Clinical Pharmacology. Vol. 27. Wiley-Blackwell; Hoboken: 2013. p. 27. [Google Scholar]
- 16.FDA guidance for industry bioanalytical method validation, US department of health and human service, Food and Drug Administration, Center for drug evaluation and research (CDER), 2001.
- 17.CLSI. CLSI document C62-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. Liquid Chromatography–Mass Spectrometry Methods; Approved Guideline. [Google Scholar]
- 18.Fleisher M, Schwartz M. Measurement of anti-folate analogs. Clin Chem. 1992;38:609–610. [PubMed] [Google Scholar]


