Abstract
Background
SLC22 protein family is a member of the SLC (Solute carriers) superfamily of polyspecific membrane transporters responsible for uptake of a wide range of organic anions and cations, including numerous endo- and xenobiotics. Due to the lack of knowledge on zebrafish Slc22 family, we performed initial characterization of these transporters using a detailed phylogenetic and conserved synteny analysis followed by the tissue specific expression profiling of slc22 transcripts.
Results
We identified 20 zebrafish slc22 genes which are organized in the same functional subgroups as human SLC22 members. Orthologies and syntenic relations between zebrafish and other vertebrates revealed consequences of the teleost-specific whole genome duplication as shown through one-to-many orthologies for certain zebrafish slc22 genes. Tissue expression profiles of slc22 transcripts were analyzed using qRT-PCR determinations in nine zebrafish tissues: liver, kidney, intestine, gills, brain, skeletal muscle, eye, heart, and gonads. Our analysis revealed high expression of oct1 in kidney, especially in females, followed by oat3 and oat2c in females, oat2e in males and orctl4 in females. oct1 was also dominant in male liver. oat2d showed the highest expression in intestine with less noticeable gender differences. All slc22 genes showed low expression in gills, and moderate expression in heart and skeletal muscle. Dominant genes in brain were oat1 in females and oct1 in males, while the highest gender differences were determined in gonads, with dominant expression of almost all slc22 genes in testes and the highest expression of oat2a.
Conclusions
Our study offers the first insight into the orthology relationships, gene expression and potential role of Slc22 membrane transporters in zebrafish. Clear orthological relationships of zebrafish slc22 and other vertebrate slc22 genes were established. slc22 members are mostly highly conserved, suggesting their physiological and toxicological importance. One-to-many orthologies and differences in tissue expression patterns of zebrafish slc22 genes in comparison to human orthologs were observed. Our expression data point to partial similarity of zebrafish versus human Slc22 members, with possible compensatory roles of certain zebrafish transporters, whereas higher number of some orthologs implies potentially more diverse and specific roles of these proteins in zebrafish.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-016-2981-y) contains supplementary material, which is available to authorized users.
Keywords: SLC22, Zebrafish, Organic anion transporters, Organic cation transporters, Phylogeny, Synteny, Tissue expression
Background
Human SLC22 protein family belongs to the large Solute Carrier (SLC) superfamily of plasma membrane proteins which is a part of the Major Facilitator Superfamily (MFS) clan of proteins [1]. It is a highly diverse group of transport proteins comprising uniporters, symporters, and antiporters which facilitate the transport of a large number of substrates including ions, drugs, neurotransmitters, nucleosides, amino acids, and peptides across biological membranes. Furthermore, SLC22 transporters are important mediators of the ADME (absorption, distribution, metabolism and excretion) processes, thus maintaining homeostasis of various organic anions, cations and zwitterions, with an important role in toxicological response of the organism to numerous deleterious endo- and xenobiotics. Due to the described role, SLC22 transporters are dominantly expressed in barrier epithelia of liver, kidney and intestine, as well as in the brain and blood–brain barrier where they maintain homeostasis of organic ions [2].
SLC22 family consists of 13 functionally characterized transporters divided into several subfamilies. The classification is based on their specific functions, ranging from organic cation and anion transporters to organic zwitterion/cation transporters [3]. There are 23 SLC22 genes in humans, whereas the overall number of identified slc22 genes in other vertebrate species is variable and species-specific. The length of SLC22 proteins is 541 to 594 amino acids, with molecular masses ranging from 50 to 70 kDa, excluding posttranslational modifications. Most of them have twelve transmembrane α-helices which form the same number of transmembrane domains (TMD). The order and localization of TMDs structurally define the polyspecific active region of the SLC22 transporters [4].
There are three major groups of functionally characterized transporters within human SLC22 family. Their classification is based on functional properties and substrate specificities, and includes (1) organic cation transporters (OCTs, genes SLC22A1-3), (2) organic anion transporters (OATs, genes SLC22A6-20) and (3) organic cation/carnitine transporters (OCTNs, genes SLC22A4-5) [3]. Three members of human organic cation transporters (OCT1-3) are dominantly expressed in basolateral membranes of kidney and liver where they transport various organic cations. OCT substrates are type I organic cations (relative molecular mass below 400 gmol−1) and are variable in structure [5]. OCT/Oct co-orthologs broadly overlap in their substrate/inhibitor specificities. Typical human OCT substrates include model cations: 1-methyl-4-phenylpyridinium (MPP+), tetraethylammonium (TEA), 4-[4-(dimethylamino)-styryl]-N-methylpyridinium (ASP+), and 40,6-diamidino-2-phenylindol (DAPI) [4]; endogenous compounds such as acetylcholine, choline, putrescine, dopamine, epinephrine, norepinephrine, serotonin, histamine and agmatine [4, 6]; numerous xenobiotics such as metformin, acyclovir, berberine, cimetidine [3, 7, 8]; and toxins like aflatoxin B1 and ethidium bromide [9].
Organic cation/carnitine transporters (OCTNs) transport organic cations and zwitterions in a sodium-dependent and/or sodium-independent manner [4, 10], and are ubiquitously expressed in various tissues such as liver, kidney, ileum, colon [11], adipocytes, skeletal muscle cells [12] and blood–retinal barrier [4, 13]. Some of OCTN1 substrates are tetraethylammonium (TEA), acetylcholine [14], quinidine, pyrilamine, verapamil [15], and the anticancer drugs mitoxantrone and doxorubicin [4, 16]. OCTN2 is a major transporter of L-carnitine and plays a role in intestinal absorption and renal reabsorption of L-carnitine. Organic anion transporters (OAT) are ubiquitously expressed in almost all barrier epithelia throughout the organism. OAT1, 3, 5, 8 and 10 are dominant on the basolateral membranes, and OAT4 and URAT1 on apical membranes of renal proximal tubules where they excrete endogenous compounds such as prostaglandin E2 and F2, urate and acidic neurotransmitter metabolites [17, 18], medium chain fatty acids, α-ketoglutarate, citrulline, cyclic nucleotides [19, 20], and numerous small molecule xenobiotics [21]. OAT2, 7 and 9 are mainly expressed on basolateral membranes of hepatocytes [22–24] where they mediate hepatic excretion of endogenous substrates such as estrone sulfate, dehydroepiandrosterone sulfate and butyrate, as well as antiviral drugs and ochratoxin A [25].
However, despite their polyspecificity and physiological and/or toxicological significance well-recognized in mammals, the knowledge on Slc22 transporters in non-mammalian species is truly modest. Among invertebrate Slc22 transporters, Octs have been identified on the genome level in fruit fly (Drosophila melanogaster) and Caenorhabditis elegans [26]. In vertebrates, apart from being identified on genomic level trough zebrafish genome project [27], there has been a limited number of studies on Slc22 transporters, or uptake proteins in general. Several studies on zebrafish Slc21 family were reported by our group, with a comprehensive identification and phylogenetic and expressional characterization [28] followed by more detailed functional characterizations of zebrafish Oatp1d1 [29, 30]. Members from the Oat subfamily in winter flounder (Pseudopleuronectes americanus) have been partly characterized by Ashlamkan et al. [31] and Wolff et al. [32]. They also reported one Oat gene sequence in zebrafish and one gene in pufferfish (Takifugu rubripes) that are similar to the winter flounder Oat. Additionally, Smith et al. [33] and Chatsudthipong and Dantzler [34] partially characterized sodium-coupled transport of organic anions in Cancer borealis and garter snake (Thamnophis spp.).
Therefore, due to evident lack of knowledge on Slc22 transporters in non-mammalian species, in this study we performed the first comprehensive identification and elucidation of orthological relationship among SLC22/Slc22 proteins of fish and other higher vertebrates. In order to do so we have used zebrafish (Danio rerio) as a highly important vertebrate model species in biomedical and environmental research. Our approach was based on detailed phylogenetic and conserved synteny analysis, followed by tissue specific expression profiling of Slc22 transcripts. In addition, the obtained data allowed the first insight into possible roles of these membrane transporters in zebrafish.
Methods
Phylogenetic analysis
Designation of gene and protein names used throughout the text is based on the Zebrafish Nomenclature Guidelines: (https://wiki.zfin.org/display/general/ZFIN+Zebrafish+Nomenclature+Guidelines); e.g., fish: shh/Shh, human: SHH/SHH. Gene and protein sequences were retrieved from NCBI [35] and ENSEMBL [36] databases using blastx algorithm. Following species were included in the phylogenetic analysis: mammals – human (Homo sapiens) and mouse (Mus musculus); bird – chicken (Gallus gallus); reptile – anole lizard (Anolis carolinensis); amphibian – frog (Xenopus laevis); actinopterygian or ray-finned fishes – zebrafish (Danio rerio), pufferfishes Japanese pufferfish (Takifugu rubripes) and green spotted pufferfish (Tetraodon nigroviridis), Atlantic cod (Gadus morhua), stickleback (Gasterosteus aculeatus), medaka (Oryzias latipes) and rainbow trout (Oncorhynchus mykiss); sarcopterygian or lobe-finned fish – West Indian Ocean coelacanth (Latimeria chalumnae); and a tunicate – sea squirt (Ciona intestinalis). MUSCLE algorithm [37] was used for alignment of SLC22/slc22 sequences and phylogenetic tree was constructed using Maximum Likelihood method in PhyML 3.0.1 software [38]. Confidence of nodes was estimated by approximate likelihood ratio test (aLRT) [39]. Based on the phylogenetic relationships, all previously unclassified genes were provisionally annotated. Names were given in accordance with the new nomenclature adopted by the HUGO Gene Nomenclature Committee [40].
Orthology predictions using syntenic relationships between zebrafish and human genes of interest were made using Genomicus [41], a conserved synteny browser synchronized with genomes from the Ensembl database [42].
Analysis of transcription factor binding motifs was made using AliBaba 2.1, a web-based server for prediction of transcription factor binding sites by constructing matrices on the fly from TRANSFAC 4.0 sites. Analysis was conducted using default parameters. Amino acid sequence motif comparison was performed using the Multiple Expectation-maximum for Motif Elicitation (MEME) suite (http://alternate.meme-suite.org/tools/meme) [43]. Detection of motifs was performed for 41 selected zebrafish and human Slc22/SLC22 sequences with a threshold of 16 motifs and amino acid length of 6–10 using normal discovery mode.
Tissue-specific gene expression analysis: RNA isolation, reverse transcription and qRT-PCR
Adult, approximately nine month old female and male zebrafish of the AB strain were purchased from a certified local supplier. All zebrafish specimens that were sacrificed for tissue/RNA isolations were anesthetized with overdose of tricaine methane sulfonate (MS222, 200 mg/L) via prolonged immersion, followed by one of the established confirmatory methods [44]. In order to collect sufficient amount of kidney tissue for RNA extraction we pooled kidneys form 15 individual fish. The rest of isolated tissues (brain, gills, liver, intestine, heart, skeletal muscle, eye and gonads) were pooled in three independent pools of five individual fish. Tissues were stored in RNA later (Qiagen, Hilden, Germany), and afterwards homogenized for 20 s using rotor-stator homogenizer (Ultra-turrax T25, IKA, Germany) at 10,000 rpm. Total RNA isolation was carried out using Rneasy Mini Kit (Qiagen, Hilden, Germany). For the purpose of RNA extraction we used 20 mg of each tissue. Genomic DNA digestion was carried out using Rnase-free DNase Set (Qiagen, Hilden, Germany). RNA was quantified, and 260/280 and 260/230 nm ratios were analyzed using BioSpec nano micro-volume spectrophotometer (Shimadzu, Kyoto, Japan). RNA integrity was checked visually by gel electrophoresis. cDNA was produced from 1 μg of total RNA using High Capacity cDNA Reverse Transcription Kit with Rnase Inhibitor (Applied Biosystems, Foster City, CA, USA).
For the purpose of quantitative Real time PCR (qRT-PCR), specific primers were designed in the Primer express 3.0 Software (Applied Biosystems, CA, USA), adjusted manually if necessary and purchased from Invitrogen (Carlsbad, CA, USA) (Table 1). Target amplicons of 90–100 bp were cloned using pGEM-T Vector System I (Promega, Madison, WI, USA). Plasmids were purified by QIAprep Spin Miniprep Kit (Qiagen, Hilden, Germany) and amplicons were verified by sequencing at the Rudjer Boskovic Institute DNA Service (Zagreb, Croatia). Primer efficiencies were determined using the recombinant pGEM-T as a template for each primer pair. Primer concentrations were optimized combining three primer concentrations: 300, 600 and 900 nM. Primer concentrations resulting in the highest fluorescence signal at the lowest Ct number were chosen as optimal. Primer sequences, optimal concentrations and primer efficiency of target gene sequences are given in Table 1, while accession numbers of target genes are given in the Additional file 1: Table S1. For the purposes of the inter-gene comparison throughout one tissue, relative quantification was used as method of choice. Target genes were normalized to the housekeeping gene (HKG) using Q-Gene application (http://www.qgene.org/) for the processing of qRT-PCR data (described in detail by Muller et al. [45] and Simon [46], according to the equation (1):
| 1 |
where MNE stands for mean normalized expression; Eref is housekeeping gene efficiency; Etarget is target gene efficiency; Ctref, mean is mean Ct value for the housekeeping gene; and Cttarget, mean stands for mean Ct value of the target gene. Data are presented as gene of interest expression relative to the housekeeping gene expression multiplied by the factor of 10,000. Elongation factor (EF1α) was chosen as a housekeeping gene given the fact that its expression was similar across all analyzed tissues. Expression was considered to be very high for MNE > 8000*105 (Ct < 19), high for MNE 800*105 - 8000*105 (Ct 20–23), moderate for MNE 30*105 - 800*105 (Ct = 23–26), and low for MNE < 30*105 (Ct >27).
Table 1.
Primers used in the quantitative Real time PCR (qRT-PCR)
| Protein name | Primer sequence 5′ -> 3′ | Ta | Final conc. (nM) | Efficiency (%) |
|---|---|---|---|---|
| Oatl | F TGCTGTTCTGATCTTGGACGA | 62 | 300 | 92 |
| R TGCT ATTAAACCAGCGAT GAC | 60 | 300 | ||
| Oat3 | F GGGTCAGCATTTACCTCATCCA | 60 | 300 | 105 |
| R GATGGCCGTCGTCCTAACAT | 58 | 300 | ||
| Oat2a | F TCGCCATTGCAAGAACCTTAT | 58 | 300 | 92 |
| R AAGGTGCGATGCTTAACATCTG | 58 | 300 | ||
| Oat2b | F GATTGTAAGTGTTCCAGCACAAGAA | 58 | 300 | 101 |
| R TGAGCTGCTGGACGAGTTTATC | 58 | 300 | ||
| Oat2c | F GCACTTTGATAACAGCACCTTCAT | 58 | 300 | 95 |
| R GAAGAAGATGGTGGTTGTCAATTTC | 59 | 300 | ||
| Oat2d | F ACAGTATGGCATGGGCTGTT | 60 | 300 | 100 |
| R AAGGTGAAGTGACAGCCACT | 60 | 300 | ||
| Oat2e | F GGTGTTATGAtCAGTTTGGATT | 60 | 300 | 95 |
| R TTGGAGCAGTTACTGTGAGG | 58 | 300 | ||
| Octl | F GAGTCACAGGGATTCTGGT | 58 | 300 | 99 |
| R ACCATCCAACCGCCCTTCA | 60 | 300 | ||
| Oct2 | F TGGCCTTGGAGTCTCTGG | 60 | 300 | 99 |
| R TGGCGGTCCATGCTCCTTT | 60 | 300 | ||
| Octnl | F GCTCTGGAATCGGGCAGAT | 60 | 300 | 100 |
| R GGCCAGTGAGGGCGTTTG | 60 | 300 | ||
| Octn2 | F CACCGCCTCACTGGCCAACC | 60 | 300 | 112 |
| R ATCTCTTCCTGGAAAGCTT | 60 | 300 | ||
| Oct6 | F GCTGTAGGGAGTGGTAGTAT | 60 | 300 | 96 |
| R CGATGAGCTGCGGCAGATA | 60 | 900 | ||
| Orctl3 | F ACCTCGTGTCCCAGTTCATT | 60 | 300 | 100 |
| R TGCATATGATCTCTGGGCGA | 60 | 300 | ||
| Orctl4 | F TCACCGCCTTCCACATGTT | 59 | 300 | 96 |
| R TTGGAGCCTGTCGGAGGAT | 59 | 300 | ||
| Eflα | F CCTGGGAGTGAAACAGCTGATC | 60 | 300 | 96 |
| R GCTGACTTCCTTGGTGATTTCC | 60 | 300 |
qRT-PCR was performed using the ABI PRISM 7000 Sequence Detection System using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The reaction mix was prepared to a final volume of 10 μl containing: 5 μl of SYBER Green master mix, 0.5 μl of each primer (of adequate concentration), 1 μl of template (10 ng/well) and 3 μl of Ultrapure Dnase/Rnase free distilled water (Molecular Bioproducts, San Diego, CA, USA). After the denaturation at 95 °C for 10 min, 40 cycles of amplification were carried out with denaturation at 95 °C for 15 s, annealing and elongation at 60 °C for 1 min altogether followed by the melting curve analysis. Data were analyzed with ABI PRISM Sequence Detection Software 1.4 (Applied Biosystems, Foster City, CA, USA) and GraphPad Prism Software version 5.00.
Results
Gene identification and phylogenetic analysis
Detailed identification of slc22 genes in representative vertebrate species and C. intestinalis have revealed total of 20 zebrafish slc22 genes (Fig. 1, Additional file 1: Figure S1, Additional file 2). Two of them, slc22a1 and slc22a2, belong to the subgroup of organic cation transporters (OCTs). In higher vertebrates (reptiles to humans), three OCT/Oct orthologs are present, while in lower vertebrates (i.e., fish and amphibians) there are two Oct orthologs. The only exception is amphibian X. laevis with one Oct ortholog (Fig. 1). Phylogenetic tree revealed clear clustering of higher vertebrates OCTs/Octs separately from fish orthologs, with the exception of zebrafish Oct2 and coelacanth Oct3a and Oct3b (Fig. 1). Amino acid sequence identities within vertebrate OCT/Oct subfamilies are 42–53 %, whereas among fish Oct proteins identities are 43–72 %.
Fig. 1.
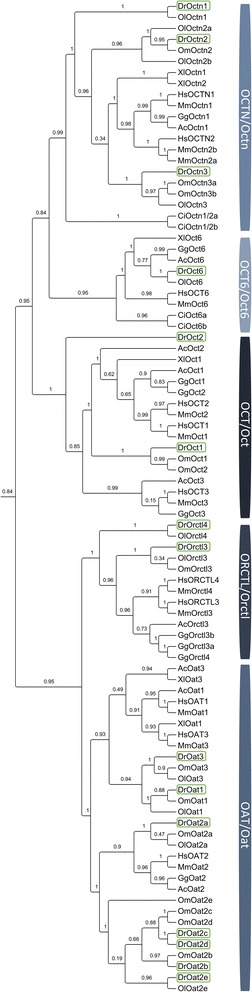
Phylogenetic tree of SLC22/Slc22 protein family in vertebrates. Species abbreviations: Hs, Homo sapiens; Gg, Gallus gallus; Ac, Anolis carolinensis; Xl, Xenopus leavis; Dr, Danio rerio; Om, Oncorhynchus mykiss. Zebrafish genes are framed in green
OCTN/Octn group is well conserved from fish to mammals with two members in each tetrapod species: OCTN1/Octn1 and OCTN2/Octn2. Two co-orthologs of these human genes are found in all vertebrates including zebrafish. Additionally, slc22a21 gene subcluster is positioned within the Octn cluster, indicating that these genes might be novel orthologs of Octn subfamily (Fig. 1). OCTN/Octn subfamily shares 44–52 % amino acid sequence identity within vertebrates, whereas OCT6/Oct6 orthologs share 39–59 %, and 70–74 % identity among vertebrates and among teleost species, respectively.
Within the subgroup of organic anion transporters (OATs), 7 genes were found. Within the OAT1/OAT3 cluster, two genes are present in each tetrapod species, as well as in each analyzed teleost species. However, teleost subcluster is distinct from the tetrapod cluster and direct orthologs of human OAT1 and OAT3 in fish species cannot be distinguished among each other. That is the reason why all teleost transporters are provisionally annotated as Oat1 and/or Oat3 in the order of appearance within the phylogenetic tree (Fig. 1). Phylogenetic analysis of OAT2/Oat2 showed one-to-many orthology between human and zebrafish genes, revealing five zebrafish Oat2 (a-e) co-orthologs (Fig. 1). The third cluster of OATs includes OAT4-7 and URAT1 genes. These genes are found only in mammals and do not have orthologs in other vertebrates (Fig. 1). Relatively high amino acid sequence identity was revealed between vertebrate OAT1/Oat1 and OAT3/Oat3 subfamily (46-56 %) which is the same identity percentage as within each separate subfamily. Oppositely, OAT2/Oat2 subfamily showed lower amino acid identity with OAT1/OAT3 cluster (28-30 %), with 40-54 % identity within vertebrate OAT2/Oat2 subfamily.
SLC22A13, also known as organic cation transporter-like 3 (ORCTL3) or OAT10, and SLC22A14, known as organic cation transporter-like 4 (ORCTL4), together with their co-orthologs in other vertebrates form a distinct cluster that is closer to the OAT than to OCT or OCTN group (Fig. 1).
Within human SLC22 family, there are several subfamilies of orphan genes which are also present in zebrafish, namely slc22a15 (FLIPT1), slc22a17 (BOIT), slc22a23 and slc22a31, whose functions are still unknown. Phylogenetic analysis revealed their specific clustering and clear one-to-one orthology relationships to corresponding genes in other vertebrate species (Fig. 1).
Accession codes and annotations of protein sequences for all chordate SLC22/Slc22 family members are given in the Additional file 1 (Table S1).
Conserved synteny analysis
Human OCT (SLC22A1-3) genes are located within one cluster on chromosome 6, between 160.12 and 160.35 megabase pair (Mbp), with forward orientation of OCT1 and OCT3 and reverse orientation of OCT2 (Fig. 2). Interestingly, there are other SLC22 members present on human chromosome 6, such as OAT2, OCT6 and SLC22A23. Syntenic comparison revealed conserved synteny of zebrafish oct1 (chromosome 20) with human OCT cluster (Fig. 2). Neighboring genes of zebrafish oct1 matched the immediate gene environment of the human ortholog cluster. igf2r gene on zebrafish chromosome 20 is located upstream at 42.59 Mbp, next to oct1 gene, in the same forward orientation as human IGF2R. plg and snx9 genes, which are located downstream of oct1, showed opposite reverse orientation in comparison with human orthologs, with snx9 located downstream of oct1, whereas the human ortholog is located upstream of OCT cluster (Fig. 2). Another zebrafish ortholog, oct2, is located on chromosome 17. Synteny analysis of oct2 showed conserved syntenic relationship with human OCT cluster which was confirmed by analysis of four neighboring genes, txlnbb, fuca2, gje1 and hivep2, all located downstream of oct2 with the same orientations as human orthologs (Fig. 2).
Fig. 2.
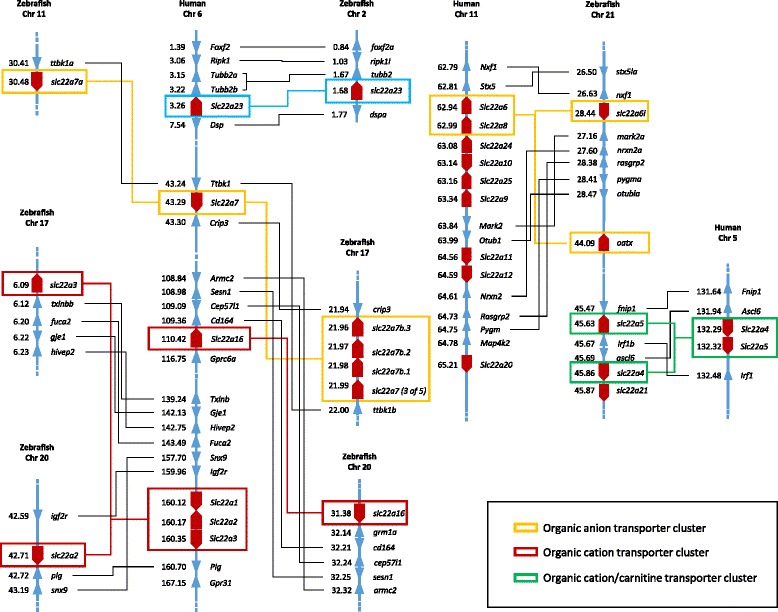
Conserved synteny analysis of human and zebrafish SLC22/slc22 genes. Numbers next to the gene names represent megabase pair (Mbp) of particular gene location on the chromosome
Phylogenetic and synteny analysis revealed five zebrafish oat2 co-orthologs, separated on two chromosomes (chr 11 and chr 17) (Fig. 2). Zebrafish oat2a (slc22a7a) is positioned on chromosome 11 with only one neighboring gene ttbk1a in the same arrangement and orientation as human ortholog OAT2 (SLC22A7) (Fig. 2). The other four zebrafish orthologs (oat2b-e, slc22atb.3, slc22a7b.2, slc22a7b.1, slc22a7 (3 of 5)) are located in a cluster on chromosome 17. Analysis revealed syntenic relationship between zebrafish oat2b-e cluster and human OAT2 based on two neighboring genes, crip3 and ttbk1b, located on opposite sides and with opposite orientation than human orthologs (Fig. 2).
Another SLC22 gene on human chromosome 6 is OCT6 (SLC22A16) at 110.42 Mbp, located between OAT2 and OCT cluster (Fig. 2). Human OCT6 showed syntenic relationship with single zebrafish ortholog on chromosome 20 at 31.38 Mbp as was determined based on five orthologous neighboring genes. Finally, the last of SLC22 genes on human chromosome 6 is SLC22A23 in the beginning of the chromosome, at 3.26 Mbp, which is syntenic to zebrafish slc22a23 as was based on four neighboring genes (Fig. 2).
Most of human SLC22 genes are located on chromosome 11: nine SLC22 genes are organized in one big cluster of six genes (SLC22A6, 8, 9, 19, 24 and 25), one small cluster of two genes (SLC22A11 and 12) and one isolated gene (SLC22A20) (Fig. 2). Majority of these genes are specific for mammals and there are no orthologs in zebrafish. Only two genes showed phylogenetic and syntenic relationship with zebrafish orthologs, SLC22A6 and 8. They showed syntenic relationship with zebrafish oat1 (oatx) and oat3 (slc22a6l) on chromosome 21 which was confirmed with seven neighboring genes (Fig. 2). However, although neighboring genes remained close to oat1 gene their distribution and orientation drastically changed in comparison to human orthologs. Interestingly, zebrafish oat3, localized far form oat1 at 44.09 Mbp, showed no syntenic relationship with human ortholog OAT3 (SLC22A8) (Fig. 2). There are three more zebrafish slc22 genes on chromosome 21 close to oat3: octn1 (slc22a4), octn2 (slc22a5) and slc22a21. octn genes showed syntenic relationship with human orthologs OCTN1 and OCTN2 on chromosome 5 (confirmed with three neighboring genes) (Fig. 2).
Two representatives of human ORCTL subfamilies, SLC22A13 and SLC22A14, are located in a cluster on chromosome 3 and are syntenic to zebrafish orthologs on chromosomes 24 and 17, respectively (Figure S2). There is one more slc22 gene on zebrafish chromosome 17, slc22a15 at 19.48 Mbp. However, it did not show any conserved genetic neighborhood with human ortholog on chromosome 1 (Figure S2). Zebrafish gene slc22a17 on chromosome 24 at 41.09 Mbp showed weak syntenic relationship with human ortholog on chromosome 14, which was confirmed with only one neighboring gene, MYH6 (Figure S2).
Finally, zebrafish genes slc22a18 and slc22a31 are both located on chromosome 7 at 39.27 and 56.13 Mbp, respectively. They showed syntenic relationship with human orthologs, with neighboring genes that remained in the same orientation (Additional file 1: Figure S2).
Gene regulation and specific sequence motif analysis
In silico prediction of transcription factor binding sites revealed several motifs for hepatocyte nuclear factor 1, 3 and 4α (HNF-1, 3, 4α), activating transcription factor 1 (ATF1) and cAMP responsive element binding protein 1 (CREB1) (Additional file 3). Analysis also revealed presence of motifs for steroid hormone receptors such as corticoid receptors (GR), progesterone receptor (PR), androgen receptor (AR), estrogen receptor (ER) and thyroid hormone receptor (T3R).
Multiple Expectation-maximum for Motif Elicitation (MEME) revealed 16 Slc22/SLC22 specific motifs (Additional file 4). All zebrafish and human Slc22/SLC22 transporters have 13 amino acid long, major facilitator superfamily signature (MFS), G(RKPATY)L(GAS)(DN)(RK)(FY)GR(RK)(RKP)(LIVGST)(LIM) between the second and the third transmembrane domain (TMD), together with conserved amphiphilic solute facilitator (ASF) domain located before TMD2. Analysis also revealed 14 amino acid long Oat/OAT – specific motif in the large loop between TMD1 and 2 (Additional files 4 and 5).
Tissue expression profiles
In zebrafish liver we observed high expression of oct1 transcript in males, followed by moderate expression oct1 in females and oct2 in both sexes (Fig. 3, Additional file 1: Table S2). More diverse slc22 expression pattern was found in kidney. Very high expression was observed for oct1 in both sexes and oat3 in females. oat2c, oat2e, octn2 and ortcl4 showed high expression in both sexes, and oct2 in females. Moderate expression was observed for oct2 in males, oct6 in females and oat2d in both sexes (Fig. 3). Most pronounced gender differences in slc22 expression in kidney were observed for oct6 and oat3 which are 22- and 8-fold more expressed in females, respectively (Fig. 3).
Fig. 3.
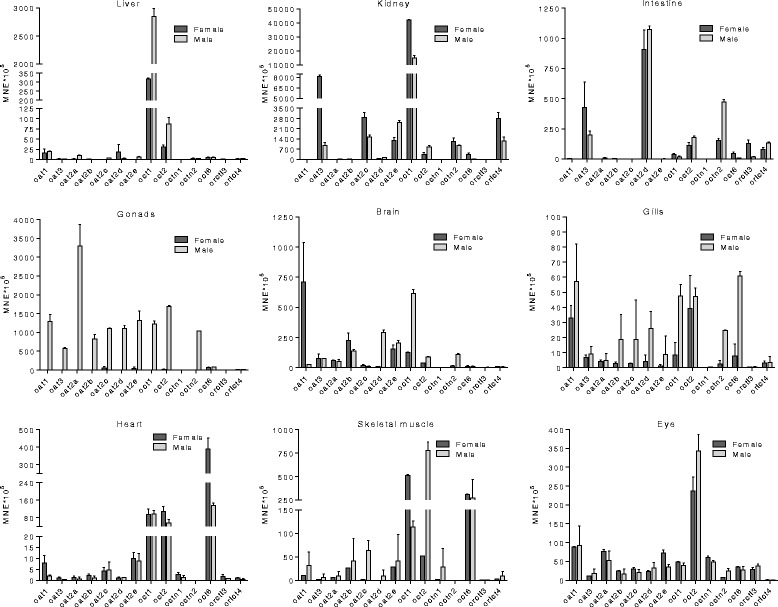
Tissue expression profile of major zebrafish slc22 genes. Results of three independent measurements (three pools) are given, except in the case of kidney (one pool of 15 individuals). Tissue expression results are presented as mean values ± SEM from 3 to 5 pools. MNE stands for mean normalized expression normalized to the housekeeping gene elongation factor 1α (EF1α)
In the intestine, the levels of expression of slc22 genes are not as high as in liver and kidney (Fig. 3, Additional file 1: Table S2). Dominant gene found in intestine are oat2d, present at high expression levels in both sexes, followed by moderate expression of oat3, octn2, oct2, and orctl4 in both sexes. Most pronounced gender expression difference was observed for orctl3 that is 7 times higher expressed in females.
In zebrafish gills, slc22 genes are generally lower expressed in comparison to other tissues (Fig. 3, Additional file 1: Table S2). oct6 showed moderate expression in males, followed by oct1, whereas oat1 and oct2 showed similar moderate expression in both genders (Fig. 3). In the brain, most of investigated genes showed moderate expressions with high expression difference among genders observed for oat1, which is 30 times higher expressed in females, as well as for oat2d and oct1 which showed 25 times higher expression in males (Fig. 3). In zebrafish heart, organic cation transporters (oct1, 2 and 6) showed expression in moderate range, whereas other slc22 genes showed low expression (Fig. 3, Additional file 1: Table S2). Similar expression pattern with dominant expression of organic cation transporters in moderate range was shown in skeletal muscle (Fig. 3, Additional file 1: Table S2). In zebrafish eye, oct2 is moderately expressed, followed by oat1, oat2a and octn1 (Fig. 3, Additional file 1: Table S2).
In the gonads, expression pattern slc22 genes differs the most among genders (Fig. 3, Additional file 1: Table S2). In testes, most of the genes show moderate to high expression, with only three low expressed genes: octn1, orctl3 and 4 (Fig. 3). The dominant slc22 gene in testes is oat2a (high expression), followed by high expression of oat1, oat2b-e, oct1, oct2 and octn2 and moderate expression of oat3 and oct6 (Fig. 3). On the other hand, only oat2c, oat2e and oct6 showed moderate expression levels in ovaries, whereas low expression was determined for all the other genes (Fig. 3).
Discussion
Previous research on human and other mammalian SLC22/Slc22 proteins showed the presence of 23 human SLC22 genes out of which 13 were functionally characterized [47]. Data obtained in this study offer the first insight into the zebrafish Slc22 membrane transporters, and our phylogenetic and synteny analysis revealed orthological relationships of fish Slc22 with related transporters in other vertebrate species.
Two identified members of zebrafish organic cation transporters (Octs) showed three-to-two gene orthology comparing with human OCTs (Fig. 1). All vertebrates, starting from fish to amphibians, have two oct members, whereas reptiles and other higher vertebrates have three OCT/Oct genes (Fig. 1). Our synteny analysis allowed better insight into the orthological relationship between human and zebrafish OCTs/octs, revealing that human OCT cluster located on chromosome 6 shows clear syntenic relationship with zebrafish oct genes separated on chromosomes 17 and 20 (Fig. 2). This separation of usually clustered slc22 genes may suggest different evolutionary pathways which could have lead towards different functional roles of zebrafish Octs [48, 49]. That possibility is additionally emphasized with significantly different tissue expression profiles of oct1 and oct2, especially in comparison with human OCTs [9]. Very high expression of oct1 in kidney followed by high expression in liver and moderate expression in brain is similar expression pattern to the human OCT1 and 2. It points to the possibility that Oct1 in zebrafish could play similar roles as human OCT1 and 2 [50]. On the contrary, moderate expression of oct2 throughout all examined tissues, apart from testes and kidney, suggests a more specific physiological role and possible involvement in the elimination of endogenous cations and drugs through kidneys (Fig. 3). Accordingly, zebrafish oct2 expression pattern is different than human OCT2, which is primarily expressed in kidney, followed by brain, intestine, placenta, lungs and inner ear [51, 52]. Furthermore, considering that zebrafish do not have an OCT3 ortholog, it is possible that Oct1 and 2 transporters in zebrafish compensate for the function of Oct3, especially considering extensive substrate overlap among mammalian OCT1, 2 and 3 [4, 51, 53]. This assumption is additionally supported by the highest expression of oct2 in comparison to other examined slc22 transcripts in zebrafish eye, which is similar to expression of human and murine OCT3/Oct3 in the eye, where it plays an important role in absorption, distribution and clearance of various xenobiotic substrates [54, 55]. Organic cation transporters also showed gender dependent differences in tissue expression. The observed difference might be consequence of differential gene regulation with steroid hormones which can occur not only in gonads but also in other tissues [56]. Identified steroid – dependent regulatory elements in the slc22 promoter regions also support the observed expressional differences.
OCTN/Octn transporters have an important physiological role in vertebrates, primarily in the transport of L-carnitine, an essential compound in the fatty acid metabolism [57]. We have found that OCTN/Octn transporters are highly evolutionary conserved and are present in all analyzed vertebrate species, ranging from primitive chordate Ciona intestinalis to human (Fig. 1). Apart from OCTN1 and 2 orthologs, we have identified the third octn gene in zebrafish, slc22a21, which is present only in fish species (Fig. 1). The expression pattern of zebrafish octn1 and octn2 differs in comparison to their human orthologs. Zebrafish octn1 showed only moderate expression in zebrafish eye, and its expression in other examined tissues was low, whereas human OCTN1 is ubiquitously expressed including kidney, intestine, brain, testes, lung and heart [11, 50, 58–60]. Zebrafish octn2 is highly expressed in kidney and testes and moderately expressed in intestine, a pattern that in part corresponds to the expression of human and rodent OCTN2/Octn2. OCTN2/Octn2 is expressed in the kidney and intestine but not in the testes of mammals [3, 11]. Taking into account high renal expression of zebrafish octn2, we hypothesize it might be responsible for the secretion and reabsorption of organic cations in zebrafish kidney, similar to its mammalian ortholog OCTN2 [15]. Absence of octn transcript in zebrafish heart and skeletal muscle could be explained by higher expression of oct6 in these tissues which indicate that Oct6 transporter could play compensatory role in carnitine transport.
Similar to octn, we found that OCT6/oct6 is highly conserved SLC22/slc22 gene within the vertebrate phyla. It is present in all investigated vertebrates from C. intestinalis to human, with clear one-to-one orthology. (Fig. 1). However, there is a partial divergence in tissue expression pattern in comparison to human OCT6. Human OCT6 is primarily expressed in testes, where it may be involved in regulation of L-carnitine and spermidine concentrations in spermatozoa [3]. Human and rodent OCT6/Oct6 is also present in kidney, liver, brain, heart and other organs, although its function in these organs is still unknown [61–64]. Zebrafish oct6 showed moderate expression in fibrous tissues like skeletal muscle and heart, and is moderately expressed in female kidney, gonads, male gills and female intestine (Fig. 3). Its moderate expression in testes and clear orthology relationship to OCT6 may suggest its potential role in transport of L-carnitine and spermidine in testes, while the role in other organs remains to be addressed in future studies.
We have identified seven organic anion transporter genes in zebrafish. One-to-one orthology of oat1 and oat3 to human OAT1 and OAT3 (Fig. 1) points to high degree of conservation of OAT1 and OAT3 function in the physiology of vertebrates. However, tissue expression data partially differ among zebrafish and human orthologs, especially in the case of oat1. Zebrafish oat1 is primarily expressed in testes and female brain (high expression), while human OAT1 and mouse Oat1 are primarily expressed in kidney and brain [65]. Zebrafish oat3 showed more ubiquitous tissue expression than oat1, with dominance in kidney, especially in females, followed by moderate expression in testes, intestine and brain. In human and mouse OAT3/Oat3 is present in kidney and liver where it is responsible for transport of various xenobiotics [21, 65]. Considering high expression of zebrafish oat3 in kidney, it could have partially overlapping function with its human ortholog OAT3 in the transport and subsequent elimination of xenobiotics. Potential functional similarities of zebrafish and human organic anion transporters are also supported by the identified Oat/OAT – specific amino acid sequence motif, long with more conserved MFS and ASF domains (Additional file 4). These evolutionary conserved regions could be the structural basis of the Slc22 family, whereas class – specific motif may have functional features, allowing the flexibility for polyspecific substrate transport [66].
Five members of oat2 (a - e) subfamily were identified in zebrafish (Fig. 1). Interestingly, OAT2/oat2 genes of higher tetrapods showed one-to-one orthologies, whereas all investigated fish species showed one-to-many orthologies with human genes. This may be a consequence of the independent whole genome duplication (WGD) in teleost fish, an additional WGD in salmonids (e.g., rainbow trout) and additional individual gene or gene cluster duplications [27, 67]. The presence of only one OAT2/oat2 ortholog in all other examined tetrapods suggests that the second round of genome duplication might have been a trigger for diversification of oat2 genes [68]. Our conserved synteny analysis confirmed multiple gene duplication of zebrafish genes. It showed double conserved synteny of five zebrafish oat2 genes on chromosomes 11 (oat2a) and 17 (oat2b-e) with human OAT2 on chromosome 6 (Fig. 2). None of the zebrafish oat2 co-orthologs corresponds to the human OAT2 in terms of expression profiles. Human OAT2 is dominant in liver, moderately expressed in kidney, and low expressed in testes, intestine and uterus [22], whereas none of the zebrafish oat2 genes is dominant in liver (Fig. 3). Similarity in expression profiles is found for oat2c and oat2e which are highly expressed in kidney (Fig. 3), like human OAT2 [18, 22]. Unlike human and rodent OAT2/Oat2, all zebrafish Oat2 members (Oat2a - e) were highly expressed in testes and moderately expressed in brain. The divergence in terms of orthology relationships and expression profiles point to potentially different roles of zebrafish Oat2 transporters in comparison to mammalian OAT2/Oat2. The only overlap is present in respect to the renal expression of oat2c and oat2e, suggesting their functional similarities with human OAT2, which plays important roles in renal-handling of creatinine, secretion of uric acid and other numerous xenobiotics [64, 69].
Human ORCTL4 transporter has been identified only on the genome level, without known function, whereas ORCTL3 (OAT10) [3] was found to be responsible for the uptake of vitamin B3 (nicotinate) in the intestine and urate in the kidney [70]. Our results on tissue distribution of zebrafish ortcl3 correspond to the human ORCTL3 expression (high expression in kidneys, moderate in intestine) (Fig. 3), which would suggest similar physiological role of ORCTL3/Ortcl3 from zebrafish to mammals. Considering clear one-to-one orthologies of OAT10 within the whole vertebrate subphylum, as well as overlapping expression profiles between zebrafish and human Oat10/OAT10 (high expression in kidneys, moderate in intestine) (Fig. 3), we suggest a conserved role of Oat10.
Conclusions
Data from this study provided new insights into orthology relationships of SLC22/Slc22 transporters among fish and other vertebrates, and offered the first integral evidence on Slc22 transporters in zebrafish as a highly relevant vertebrate model species. Phylogenetic and synteny analysis of SLC22/slc22 genes point to certain similarities among human (and mammalian in general) and zebrafish Slc22 transporters. Moreover, clear one-to-one orthology, conserved synteny and gene conservation within the whole vertebrate subphylum imply physiologically important roles for the majority of Slc22 transporters. Those similarities can be most directly observed within the Octn group, indicating possible important role of zebrafish Octn2 in maintaining renal organic cation homeostasis. Octn2 may be involved in transport of L-carnitine as a major physiological substrate, Oct6 could have evolutionary conserved physiological function in transport of L-carnitine and spermidine, and finally, due to its dominant expression in liver and kidney zebrafish Oct1 may have similar function as human OCT1 and OCT2. Other zebrafish Slc22 members showed less conserved similarities with human and other vertebrate SLC22/Slc22 transporters, possibly due to the gene divergence following the teleost specific WGD. This specific evolutionary event resulted in emergence of novel slc22 genes in zebrafish, which were changing, multiplying or disappearing during the course of evolution. This in turn resulted in higher diversity of slc22 genes which is especially evident in the group of organic anion transporters. Consequently, due to occurrence of novel functional changes during the evolution of zebrafish slc22 genes their expression profiles are partly different in comparison to human orthologs. However, taking into account the described high expression of certain slc22 genes (e.g., oct1, oat3, oat2c, oat2d, oat2e, octn2 and orctl4) in toxicologically important organs/tissues, as well as polyspecific substrate preferences of their human orthologs, we hypothesize that these transporters probably have important roles in defense against various deleterious endo- and xenobiotic substances, making the Slc22 transporters crucial elements and determinants in ADME/Tox processes in zebrafish. Based on data from this study, functional role(s) of zebrafish Slc22 transporters remains to be addressed in further studies directed to detailed functional characterization of single transporters in suitable expression systems.
Abbreviations
ADME, absorption, distribution, metabolism and excretion; ASP+, 4-[4-(dimethylamino)-styryl]-N-methylpyridinium; DAPI, 4,6-diamidino-2-phenylindol; LP1, Large extracellular loop 1; Mbp, Megabase pair; MNE, Mean normalized expression; MPP+, 1-methyl-4-phenylpyridinium; MS222, Tricaine methane sulfonate; MSF, Major Facilitator Superfamily; OAT, Organic anion transporter; OCT, Organic cation transporter; OCTN, Organic cation/carnitine transporter; ORCTL, Organic Cation Transporter-Like; SLC, solute carrier; TEA, Tetraethylammonium; TMD, transmembrane domain
Acknowledgements
The authors thank Dr. Ivan Sabolić and Dr. Davorka Breljak from the Institute for Medical Research and Occupational Health, Zagreb, Croatia, for kindly providing murine Slc22 antibodies.
Funding
This work was supported by the Croatian National Science Foundation (Project No. 4806) and the SCOPES programme joint research project granted by the Swiss National Science Foundation (SNSF) (Grant No. SCOPES - IZ73ZO_152274/1).
Availability of data and materials
All datasets on which the conclusions of the manuscript rely are presented and publicly available in the main paper and/or additional supporting files. In addition, the phylogenetic data are uploaded and publicly available in the Treebase repository (available online at http://purl.org/phylo/treebase/phylows/study/TB2:S19597).
Authors’ contributions
All authors contributed extensively to the work presented in this paper. IM and MP designed and performed experiments and wrote the manuscript; TS supervised the work, helped in evaluation and interpretation of data and edited the manuscript.
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All experimental procedures involving live fish were carried out in accordance with the directions given in the EU Guide for the Care and Use of Laboratory Animals, Council Directive (86/609/EEC) and the Croatian Federal Act on the Protection of Animals (NN 135/2006). The protocol used in the study was approved by the Bioethics Committee of the Ruđer Bošković Institute, Zagreb, Croatia (Permit Number: BP-1504/2-2011).
Additional files
Extended phylogenetic tree of Slc22/slc22 protein family in vertebrates; Figure S2: Conserved synteny analysis of less characterized human and zebrafish Slc22/slc22 genes; Figure S3: Tissue expression profile of major zebrafish slc22 genes organized by individual genes; Table S1: Protein annotations with accession numbers of all analysed species; Table S2: Heatmap of zebrafish Slc22 gene tissue expression results. (XLSX 1109 kb)
Gene structure analysis of zebrafish and human slc22/Slc22. Structure of coding exons, exons and introns together with gene lengths. (XLSX 539 kb)
Transcription factor binding motif analysis of zebrafish and human slc22/Slc22 genes. Identification of potential sequence motifs and regulatory elements of human and zebrafish Slc22/slc22 genes. (XLSX 55 kb)
Amino acid sequence motif analysis of zebrafish and human Slc22/SLC22. Identification of characteristic Slc22 amino acid sequences with identified major facilitator superfamily (MFS) motif, amphiphilic solute facilitator (ASF) domain and OAT-specific motif. (XLSX 518 kb)
Analysis of zebrafish and human Slc22/SLC22 protein topologies. Secondary structures of Slc22/SLC22 proteins showing transmembrane domains and extracellular and intracellular loops. (XLSX 563 kb)
References
- 1.Höglund PJ, Nordström KJV, Schiöth HB, Fredriksson R. The solute carrier families have a remarkably long evolutionary history with the majority of the human families present before divergence of Bilaterian species. Mol Biol Evol. 2011;28:1531–41. doi: 10.1093/molbev/msq350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okura T, Ito R, Ishiguro N, Tamai I, Deguchi Y. Blood–brain barrier transport of pramipexole, a dopamine D2 agonist. Life Sci. 2007;80:1564–71. doi: 10.1016/j.lfs.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 3.Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol Aspects Med. 2013;34:413–35. doi: 10.1016/j.mam.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–51. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 5.Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3) J Pharmacol Exp Ther. 2004;308:2–9. doi: 10.1124/jpet.103.053298. [DOI] [PubMed] [Google Scholar]
- 6.Winter TN, Elmquist WF, Fairbanks CA. OCT2 and MATE1 provide bidirectional agmatine transport. Mol Pharm. 2011;8:133–42. doi: 10.1021/mp100180a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, Brett CM, Giacomini KM. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117:1422–31. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.More SS, Li S, Yee SW, Chen L, Xu Z, Jablons DM, Giacomini KM. Organic cation transporters modulate the uptake and cytotoxicity of picoplatin, a third-generation platinum analogue. Mol Cancer Ther. 2010;9:1058–69. doi: 10.1158/1535-7163.MCT-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011;201:105–67. doi: 10.1007/978-3-642-14541-4_3. [DOI] [PubMed] [Google Scholar]
- 10.Urban TJ, Yang C, Lagpacan LL, Brown C, Castro RA, Taylor TR, Huang CC, Stryke D, Johns SJ, Kawamoto M, Carlson EJ, Ferrin TE, Burchard EG, Giacomini KM. Functional effects of protein sequence polymorphisms in the organic cation/ergothioneine transporter OCTN1 (SLC22A4) Pharmacogenet Genomics. 2007;17:773–82. doi: 10.1097/FPC.0b013e3281c6d08e.. [DOI] [PubMed] [Google Scholar]
- 11.Meier Y, Eloranta JJ, Darimont J, Ismair MG, Hiller C, Fried M, Kullak-Ublick GA, Vavricka SR. Regional distribution of Solute Carrier mRNA expression along the human intestinal tract. Drug Metab Dispos. 2007;35:590–4. doi: 10.1124/dmd.106.013342. [DOI] [PubMed] [Google Scholar]
- 12.Furuichi Y, Sugiura T, Kato Y, Shimada Y, Masuda K. OCTN2 is associated with carnitine transport capacity of rat skeletal muscles. Acta Physiol. 2010;200:57–64. doi: 10.1111/j.1748-1716.2010.02101.x. [DOI] [PubMed] [Google Scholar]
- 13.Tachikawa M, Takeda Y, Tomi M, Hosoya K. Involvement of OCTN2 in the transport of acetyl-L-carnitine across the inner blood-retinal barrier. Invest Ophthalmol Vis Sci. 2010;51:430–6. doi: 10.1167/iovs.09-4080. [DOI] [PubMed] [Google Scholar]
- 14.Pochini L, Scalise M, Galluccio M, Pani G, Siminovitch KA, Indiveri C. The human OCTN1 (SLC22A4) reconstituted in liposomes catalyzes acetylcholine transport which is defective in the mutant L503F associated to the Crohn’s disease. Biochim Biophys Acta. 1818;2012:559–65. doi: 10.1016/j.bbamem.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Tamai I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21) Biopharm Drug Dispos. 2012;34:29–44. doi: 10.1002/bdd.1816. [DOI] [PubMed] [Google Scholar]
- 16.Okabe M, Szakacs G, Reimers MA, Suzuki T, Hall MD, Abe T, Weinstein JN, Gottesman MM. Profiling SLCO and SLC22 genes in the NCI-60 cancer cell lines to identify drug uptake transporters. Mol Cancer Ther. 2008;7:3081–91. doi: 10.1158/1535-7163.MCT-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, Fukatsu A, Ogawa O, Inui K. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002;13:866–74. doi: 10.1681/ASN.V134866. [DOI] [PubMed] [Google Scholar]
- 18.Rizwan AN, Burckhardt G. Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm Res. 2007;24:450–70. doi: 10.1007/s11095-006-9181-4. [DOI] [PubMed] [Google Scholar]
- 19.Nakakariya M, Shima Y, Shirasaka Y, Mitsuoka K, Nakanishi T, Tamai I. Organic anion transporter OAT1 is involved in renal handling of citrulline. Am J Physiol Renal Physiol. 2009;297:F71–9. doi: 10.1152/ajprenal.90662.2008. [DOI] [PubMed] [Google Scholar]
- 20.Cropp CD, Komori T, Shima JE, Urban TJ, Yee SW, More SS, Giacomini KM. Organic Anion Transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol Pharmacol. 2008;73:1151–8. doi: 10.1124/mol.107.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nigam SK, Bush KT, Martovetsky G, Ahn SY, Liu HC, Richard E, Bhatnagar V, Wu W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol Rev. 2015;95:83–123. doi: 10.1152/physrev.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonson GD, Vincent AC, Roberg KJ, Huang Y, Iwanij V. Molecular cloning and characterization of a novel liver-specific transport protein. J Cell Sci. 1994;107:1065–72. doi: 10.1242/jcs.107.4.1065. [DOI] [PubMed] [Google Scholar]
- 23.Sun W, Wu RR, van Poelje PD, Erion MD. Isolation of a family of organic anion transporters from human liver and kidney. Biochem Bioph Res Co. 2001;283:417–22. doi: 10.1006/bbrc.2001.4774. [DOI] [PubMed] [Google Scholar]
- 24.Tsuchida H, Anzai N, Shin HJ, Wempe MF, Jutabha P, Enomoto A, Cha SH, Satoh T, Ishida M, Sakurai H, Endou H. Identification of a Novel Organic Anion Transporter Mediating Carnitine Transport in Mouse Liver and Kidney. Cell Physiol Biochem. 2010;25:511–22. doi: 10.1159/000303060. [DOI] [PubMed] [Google Scholar]
- 25.Jung KY, Takeda M, Kim DK, Tojo A, Narikawa S, Yoo BS, Hosoyamada M, Cha SH, Sekine T, Endou H. Characterization of ochratoxin A transport by human organic anion transporters. Life Sci. 2001;69:2123–35. doi: 10.1016/S0024-3205(01)01296-6. [DOI] [PubMed] [Google Scholar]
- 26.Eraly SA, Monte JC, Nigam SK. Novel slc22 transporter homologs in fly, worm, and human clarify the phylogeny of organic anion and cation transporters. Physiol Genomics. 2004;18:12–24. doi: 10.1152/physiolgenomics.00014.2004. [DOI] [PubMed] [Google Scholar]
- 27.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popovic M, Zaja R, Smital T. Organic anion transporting polypeptides (OATP) in zebrafish (Danio rerio): Phylogenetic analysis and tissue distribution. Comp Biochem Phys A. 2010;155:327–35. doi: 10.1016/j.cbpa.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Popovic M, Zaja R, Fent K, Smital T. Molecular characterization of zebrafish Oatp1d1 (Slco1d1), a novel organic anion-transporting polypeptide. J Biol Chem. 2013;22:33894–911. doi: 10.1074/jbc.M113.518506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popovic M, Zaja R, Fent K, Smital T. Interaction of environmental contaminants with zebrafish organic anion transporting polypeptide, Oatp1d1 (Slco1d1) Toxicol Appl Pharm. 2014;280:149–58. doi: 10.1016/j.taap.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Aslamkhan AG, Thompson DM, Perry JL, Bleasby K, Wolff NA, Barros S, Miller DS, Pritchard JB. The flounder organic anion transporter fOat has sequence, function, and substrate specificity similarity to both mammalian Oat1 and Oat3. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1773–80. doi: 10.1152/ajpregu.00326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff NA, Grünwald B, Friedrich B, Lang F, Godehardt S, Burckhardt G. Cationic amino acids involved in dicarboxylate binding of the flounder renal organic anion transporter. J Am Soc Nephrol. 2001;12:2012–8. doi: 10.1681/ASN.V12102012. [DOI] [PubMed] [Google Scholar]
- 33.Smith PM, Miller DS, Pritchard JB. Sodium-coupled organic anion transport by Cancer borealis urinary bladder. Am J Physiol Reg I. 1990;259:R147–56. doi: 10.1152/ajpregu.1990.259.1.R147. [DOI] [PubMed] [Google Scholar]
- 34.Chatsudthipong V, Dantzler WH. PAH-α-KG countertransport stimulates PAH uptake and net secretion in isolated snake renal tubules. Am J Physiol. 1991;261:F858–67. doi: 10.1152/ajprenal.1991.261.5.F858. [DOI] [PubMed] [Google Scholar]
- 35.http://www.ncbi.nlm.nih.gov/. Accessed 10 Dec 2015.
- 36.http://www.ensembl.org/index.html Accessed 10 Dec 2015.
- 37.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guindon S, Gascuel O. A Simple, Fast, and Accurate Algorithm to Estimate Large Phylogenies by Maximum Likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 39.Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst Biol. 2006;55:539–52. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- 40.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–65. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 41.http://www.genomicus.biologie.ens.fr/genomicus Accessed 10 Dec 2015.
- 42.Louis A, Muffato M, Roest CH. Genomicus: five genome browsers for comparative genomics in eukaryota. Nucleic Acids Res. 2013;41:700–5. doi: 10.1093/nar/gks1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey TL, Baker ME, Elkan CP. An artificial intelligence approach to motif discovery in protein sequences: application to steriod dehydrogenases. J Steroid Biochem Mol Biol. 1997;62:29–44. doi: 10.1016/S0960-0760(97)00013-7. [DOI] [PubMed] [Google Scholar]
- 44.Matthews M, Varga ZM. Anesthesia and euthanasia in zebrafish. ILAR J. 2012;53:192–204. doi: 10.1093/ilar.53.2.192. [DOI] [PubMed] [Google Scholar]
- 45.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Bio Techniques. 2002;32:1372–9. [PubMed] [Google Scholar]
- 46.Simon P. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics. 2003;19:1439–40. doi: 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
- 47.Volk C. OCTs, OATs, and OCTNs: structure and function of the polyspecific organic ion transporters of the SLC22 family. WIREs Membr Transp Signal. 2014;3:1–13. doi: 10.1002/wmts.100. [DOI] [Google Scholar]
- 48.Eraly SA, Hamilton BA, Nigam SK. Organic anion and cation transporters occur in pairs of similar and similarly expressed genes. Biochem Biophys Res Commun. 2003;300:333–42. doi: 10.1016/S0006-291X(02)02853-X. [DOI] [PubMed] [Google Scholar]
- 49.Wu W, Baker ME, Eraly SA, Bush KT, Nigam SK. Analysis of a large cluster of SLC22 transporter genes, including novel USTs, reveals species-specific amplification of subsets of family members. Physiol Genomics. 2009;38:116–24. doi: 10.1152/physiolgenomics.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koepsell H. Substrate recognition and translocation by polyspecific organic cation transporters. Biol Chem. 2011;392:95–101. doi: 10.1515/bc.2011.009. [DOI] [PubMed] [Google Scholar]
- 51.Koepsell H, Schmitt BM, Gorboulev V. Organic cation transporters. Rev Physiol Biochem Pharmacol. 2003;150:36–90. doi: 10.1007/s10254-003-0017-x. [DOI] [PubMed] [Google Scholar]
- 52.Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstädt H, Lanvers-Kaminsky C, am Zehnhoff-Dinnesen A, Schinkel AH. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176:1169–80. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nies AT, Koepsell H, Winter S, Burk O, Klein K, Kerb R, Zanger UM, Keppler D, Schwab M, Schaeffeler E. Expression of Organic Cation Transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) Is Affected by Genetic Factors and Cholestasis in Human Liver. Hepatology. 2009;50:1227–40. doi: 10.1002/hep.23103. [DOI] [PubMed] [Google Scholar]
- 54.Nirmal J, Sinhg SB, Biswas NR, Thavaraj V, Azad RV, Velpandian T. Potential pharmacokinetic role of organic cation transporters in modulating the transcorneal penetration of its substrates administered topically. Eye. 2013;27:1196–203. doi: 10.1038/eye.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang T, Xiang CD, Gale D, Carreiro S, We EY, Zhang EY. Drug transporter and cytochrome P450 mRNA expression in human ocular barriers: implications for ocular drug disposition. Drug Metab Dispos. 2008;36:1300–7. doi: 10.1124/dmd.108.021121. [DOI] [PubMed] [Google Scholar]
- 56.Urikami Y, Okuda M, Saito H, Inui K. Hormonal regulation of organic cation transporter OCT2 expression in rat kidney. FEBS Lett. 2000;473:173–6. doi: 10.1016/S0014-5793(00)01525-8. [DOI] [PubMed] [Google Scholar]
- 57.Tamai I, Yabuuchi H, Nezu J, Sai Y, Oku A, Shimane M, Tsuji A. Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS Lett. 1997;419:107–11. doi: 10.1016/S0014-5793(97)01441-5. [DOI] [PubMed] [Google Scholar]
- 58.Markova NG, Karaman-Jurukovska N, Dong KK, Damaghi N, Smiles KA, Yarosh DB. Skin cells and tissue are capable of using L-ergothioneine as an integral component of their antioxidant defense system. Free Radical Biol Med. 2009;46:1168–76. doi: 10.1016/j.freeradbiomed.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Gilchrist SE, Alcorn J. Lactation stage-dependent expression of transporters in rat whole mammary gland and primary mammary epithelial organoids. Fundam Clin Pharmacol. 2010;24:205–14. doi: 10.1111/j.1472-8206.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- 60.Lamhonwah AM, Mai L, Chung C, Lamhonwah D, Ackerley C, Tein I. Upregulation of mammary gland OCTNs maintains carnitine homeostasis in suckling infants. Biochem Biophys Res Commun. 2011;404:1010–5. doi: 10.1016/j.bbrc.2010.12.100. [DOI] [PubMed] [Google Scholar]
- 61.Enomoto A, Wempe MF, Tsuchida H, Shin HJ, Cha SH, Anzai N, Goto A, Sakamoto A, Niwa T, Kanai Y, Anders MW, Endou H. Molecular identification of a novel carnitine transporter specific to human testis. Insights into the mechanism of carnitine recognition. J Biol Chem. 2002;277:36262–71. doi: 10.1074/jbc.M203883200. [DOI] [PubMed] [Google Scholar]
- 62.Eraly SA, Nigam SK. Novel human cDNAs homologous to Drosophila Orct and mammalian carnitine transporters. Biochem Biophys Res Commun. 2002;297:1159–66. doi: 10.1016/S0006-291X(02)02343-4. [DOI] [PubMed] [Google Scholar]
- 63.Kwok B, Yamauchi A, Rajesan R, Chan L, Dhillon U, Gao W, Xu H, Wang B, Takahashi S, Semple J, Tamai I, Nezu J, Tsuji A, Harper P, Ito S. Carnitine/xenobiotics transporters in the human mammary gland epithelia, MCF12A. Am J Physiol Regul Integr Comp Physiol. 2006;290:R793–802. doi: 10.1152/ajpregu.00087.2005. [DOI] [PubMed] [Google Scholar]
- 64.Sato M, Mamada H, Anzai N, Shirasaka Y, Nakanishi T, Tamai I. Renal Secretion of Uric Acid by Organic Anion Transporter 2 (OAT2/SLC22A7) in Human. Biol Pharm Bull. 2010;33:498–503. doi: 10.1248/bpb.33.498. [DOI] [PubMed] [Google Scholar]
- 65.Lee KL, Jung SM, Kwak JO, Cha SH. Introduction of organic anion transporters (SLC22A) and a regulatory mechanism by caveolins. Electrolyte Blood Press. 2006;4:8–17. doi: 10.5049/EBP.2006.4.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu C, Nigam KB, Date RC, Bush KT, Springer SA, Saler MH, Jr, Wu W, Nigam SK. Evolutionary analysis and classification of OATs, OCTs, OCTNs, and other SLC22 transporters: structure-function implications and analysis of sequence motifs. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0140569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berthelot C, Brunet F, Chalopin D, Juanchich A, Bernard M, Noël B, et al. The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nat Commun. 2014;5:3657. doi: 10.1038/ncomms4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Froschauer A, Braasch I, Volff J. Fish genomes, comparative genomics and vertebrate evolution. Curr Genomics. 2006;7:43–57. doi: 10.2174/138920206776389766. [DOI] [Google Scholar]
- 69.Shen H, Liu T, Morse BL, Zhao Y, Zhang Y, Qiu X, Chen C, Lewin AC, Tang XT, Liu G, Christopher LJ, Marathe P, Lai Y. Characterization of organic anion transporter 2 (SLC22A7): a highly efficient transporter for creatinine and species-dependent renal tubular expression. Drug Metab Dispos. 2015;43:984–93. doi: 10.1124/dmd.114.062364. [DOI] [PubMed] [Google Scholar]
- 70.Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, Krick W, Burckhardt BC, Sabolic I, Burckhardt G. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13) J Biol Chem. 2008;283:16332–41. doi: 10.1074/jbc.M800737200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets on which the conclusions of the manuscript rely are presented and publicly available in the main paper and/or additional supporting files. In addition, the phylogenetic data are uploaded and publicly available in the Treebase repository (available online at http://purl.org/phylo/treebase/phylows/study/TB2:S19597).


