Abstract
In spite of massive progress in the control of African malaria since the turn of the century, there is a clear and recognized need for additional tools beyond long-lasting insecticide-treated bed nets (LLINs) and indoor residual spraying (IRS) of insecticides, to progress towards elimination. Moreover, widespread and intensifying insecticide resistance requires alternative control agents and delivery systems to enable development of effective insecticide resistance management strategies. This series of articles presents a novel concept for malaria vector control, the ‘eave tube’, which may fulfil these important criteria. From its conceptualization to laboratory and semi-field testing, to demonstration of potential for implementation, the stepwise development of this new vector control approach is described. These studies suggest eave tubes (which comprise a novel way of delivering insecticides plus screening to make the house more ‘mosquito proof’) could be a viable, cost-effective, and acceptable control tool for endophilic and endophagic anophelines, and possibly other (nuisance) mosquitoes. The approach could be applicable in a wide variety of housing in sub-Saharan Africa, and possibly beyond, for vectors that use the eave as their primary house entry point. The results presented in these articles were generated during an EU-FP7 funded project, the mosquito contamination device (MCD) project, which ran between 2012 and 2015. This was a collaborative project undertaken by vector biologists, product developers, modellers, materials scientists, and entrepreneurs from five different countries.
Keywords: Vector control, Resistance, Eave tube, House modification, Africa, Malaria elimination
Background
Virtually all countries that signed up for the Millennium Development Goals in 2000 have shown dramatic advances in reducing malaria morbidity and mortality (as part of MDG6) over the last 15 years. Global malaria incidence has dropped by an estimated 37 % and mortality by 58 % [1]. Close to one billion insecticide-treated bed nets were distributed in sub-Saharan Africa and have been pinpointed as the primary contributor (68 %) to the observed reduction in Plasmodium falciparum prevalence in children 2–10 years of age, which dropped from 33 to 16 % between 2000 and 2015 [2]. Combined with indoor residual spraying, these two vector control interventions made up for 78 % of the estimated 663 million malaria cases averted since the turn of the Millennium. This dramatic and highly encouraging progress has fuelled the belief that global eradication of malaria is feasible, and maps to show how its distribution will shrink to zero by 2040 have been drawn up [3], backed by equally optimistic reports [4].
Considering the fact that vector control has played such a prominent role in these successes, there are two critical issues to consider with regards to its future role in eradication efforts. First, it is now widely accepted that the current two major tools for vector control, LLINs and IRS, both highly effective alone or in combination, will not reduce malaria incidence to zero in high transmission settings [5]. Second, insecticide resistance in the major African malaria vectors, in some countries against several classes of public health insecticides recommended by the World Health Organization, is already widespread and increasing in intensity [6, 7]. Without novel public health insecticides [8] and strategies to manage insecticide resistance [9, 10], it will be difficult to sustain the gains of the last decade [11]. Beyond new actives there is also a dire need for novel delivery tools that can be integrated with current methods, or combined with alternative approaches like larval source management [12] as part of integrated vector management campaigns [13].
Although house improvement for malaria control has a long history [14, 15] and contributed significantly to malaria elimination in Europe and the USA [16], its role in developing countries remains surprisingly small. A recent systematic review and meta-analysis, however, showed that housing is an important risk factor for malaria. It was concluded that, although only one housing intervention study has produced positive clinical outcomes to date [17], further studies on house improvement are warranted [18].
This series of articles introduces a novel house-based malaria vector control intervention called the ‘eave tube’, which combines modifications to make a house more ‘mosquito-proof’ with an innovative way of delivering insecticidal active ingredients. The articles highlight key advances in technology development to date, including initial proof of concept studies, exploration of mode of action, model evaluations, and feasibility for scale-up.
Rationale
For several years it has been argued that the development of novel tools for anopheline vector control should be guided by a thorough understanding of their ecology and life history behaviours [19]. Current strategies (LLINs and IRS) focus on the fact that in many parts of Africa, during the host-seeking process, female mosquitoes will enter the house at night to gain access to a human host. LLINs target these mosquitoes prior to feeding, IRS afterwards. Both methods have clearly been proven to have public health benefits [20, 21].
The house and the peri-domiciliary domain are closely linked to disease, where up to seventy per cent of infectious disease transmission occurs [22]. This focal transmission results from the shift from nomadic to more sedentary lifestyles and the development of agricultural practices, leading to adaptation of vectors to changed landscapes as well as an increased affinity with human blood. Anthropophagy and utilisation of man-made environments (both indoors and outdoors) thus created an ideal setting for efficient transmission of vector-borne pathogens. It is, therefore, not surprising that the two most commonly used malaria vector control tools in Africa (i.e. LLINs and IRS) are house-based, since mosquitoes are predominantly nocturnal and commonly feed indoors. Although, due to intensified use of LLINs, it has been observed that certain vectors are evading fatal exposure to insecticides indoors by feeding outdoors [23, 24], it has also been determined that outdoor feeding is almost always preceded by attempts to feed indoors [25]. This behaviour indicates that effective house-based killing methods should still result in effective control, even for species in which plasticity in endophagy is observed.
Housing in Africa is currently undergoing design changes at an unprecedented rate. Traditional materials are being replaced with more modern ones that combine features of lower cost and/or durability. Walls that consisted of mud or clay are being replaced with walls consisting of (burnt) bricks or concrete blocks. The same applies to roofing where, across the continent, traditional grass thatch roofs are being replaced with corrugated metal sheet roofing (Fig. 1). Not only are these alternative materials more durable and cheaper over time, they also modify the dynamics of malaria. Given the poikilothermic nature of mosquitoes, changes in indoor climate will affect their survival, blood meal digestion and egg development, as well as parasite development [26, 27]. The dampening effect of a thick grass thatched roof, which keeps the house cooler during the day and warmer at night, is completely lost with metal sheets, resulting in more extreme fluctuations of both temperature and humidity, which combined, influence comfort for house occupants. Moreover, when these house modifications are taking place, many house owners seal the eaves of the house in order to reduce indoor mosquito biting. Apparently, given that it is much more difficult to seal the eaves when the roof is made out of grass thatch, sealing it to prevent mosquito entry is preferred over cooling of the house through air passage through the open eaves. This results in a reduction in indoor comfort, which may result in reduced use of LLINs (these being too hot to sleep under [28]) and an increase in smoke-induced ailments when cooking is done indoors [29]. Besides house modification, large numbers of new houses will be constructed in Africa in the coming decades; estimates indicate some 144 million new structures in rural parts of Africa by 2050 [30]. Africa’s economic growth, with an average expected GDP increase of 6 % per annum over the next decade [30], will result in wealth creation that can be deployed to improve housing so that it becomes less prone to invasion by vectors (Fig. 2).
Fig. 1.
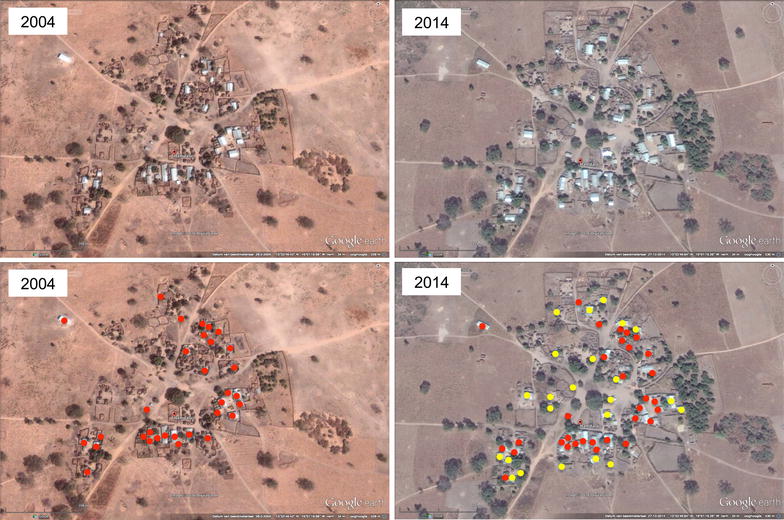
Changes in house design, Hamdalai village, The Gambia. Using Google earth imagery it can clearly be seen that the proportion of houses with corrugated iron sheet roofing in 2004 (red dots) has increased over the last decade both within the village and at its periphery (2014; yellow dots). This phenomenon is prevalent across Africa
Fig. 2.

Changes in house design, Kilombero Valley, southern Tanzania. Traditional mud wall and grass thatch houses (a) are being replaced by grass thatch and (burnt) brick wall houses (b) and subsequently corrugated iron sheet roofing is installed (c)
These alterations in house design present opportunities for the development of novel vector control tools [31]. Eaves, which constitute the gap between the roof and the walls of houses, have long been incriminated as the primary entry point into houses for malaria vectors [32–36], and have formed the basis for malaria control interventions, notably through the use of eave curtains [37, 38] and, more recently, push–pull interventions [39]. Pyrethroid-treated eave curtains have been shown to reduce malaria morbidity and mortality [20, 40], which clearly demonstrates that the eave is an effective place to target host-seeking mosquitoes when they try to enter [41].
Conceptualization
In late 2012 a diverse group of researchers put together a project called the ‘mosquito contamination device (MCD) project’. The project, supported by the European Union Seventh Framework Programme, aimed to develop a number of novel malaria mosquito control tools. The motivation was to develop technologies that could be made operational in as short a time frame as possible. As such, the project was not structured as a conventional research project and this was reflected in the diverse nature of the project partners, which included vector biologists, product developers, modellers, materials scientists, and entrepreneurs from five different countries.
When rationalizing a novel control tool the following eight criteria were considered essential:
Human contact with insecticides should be minimized;
Application of novel insecticides (including resistance-breaking actives like entomopathogenic fungi) beyond the currently approved ones should be feasible; possibly even combinations of different classes of insecticides should be considered;
The amount of insecticide applied per house should be reduced as much as possible to save costs and reduce possible impact on the environment and non-target organisms;
The approach should not compromise indoor comfort of house occupants or have any other health impacts;
The technology should preferably operate passively without any active engagement of house occupants;
The approach should become widely accepted and adopted in affected communities and possibly form part of income-generating activities;
The tool should be cost-competitive with LLINs and/or IRS, easy to mass-produce, and easy to install and maintain/service;
The technology should be able to operate without depending on electricity or addition of mosquito attractants, notably carbon dioxide.
During the MCD project-planning workshop that was held in Ifakara, Tanzania, in February 2013, the authors visited several villages in the Kilombero Valley and took note of the changes in house design. The observation that house owners were regularly closing the eaves when they installed corrugated metal sheet roofing triggered the idea for eave tubes for three primary reasons. First, the closure of the eaves by house owners resulted in the obstruction of airflow and thus possible changes to indoor comfort. Partial opening of the eaves (through the installation of tubes) would potentially reinstate the airflow and thus could improve comfort. Second, by installing tubes and reinstating airflow, anopheline mosquitoes would be able to perform their natural behaviour whilst host-seeking, i.e. they would respond to host odours [42] emanating from the tubes (at eave height) and fly into these. By installing a physical barrier inside the tube (i.e. netting), mosquitoes could be prevented from entering the house and at the same time be exposed to insecticidal agents that were applied to the netting. In doing so, mosquitoes would not simply be presented with a mosquito-proof house and be diverted to other houses in the vicinity but have a significant chance of becoming exposed to insecticide during any of the 3–4 host-feeding cycles before becoming infectious and contribute to malaria transmission. The third reason combined several possible advantages in that (a) it would be possible to use bioactivities in a safe place beyond the reach of children and other house occupants, (b) provide an opportunity to use novel (bio) pesticides or combinations thereof, and (c) use much less insecticide, so creating opportunities for utilizing products that might be cost prohibitive in conventional applications such as IRS. A secondary reason for focusing on tubes was the reduced requirements for netting compared to eave curtains, and since one of the partners (In2Care BV) had developed a novel electrostatic coating for application on netting that is pyrethroid-resistance breaking [43], the authors wanted to use only minimal quantities of this special netting for treatment with powder formulations of insecticides.
Eave tube technology does not only consist of eave tubes but also window screening (with untreated netting) as well as sealing of cracks and gaps in walls and (whenever possible) improvement of the door (Fig. 3). These are commonly used strategies to keep mosquitoes out of the house and will not be reported on separately here. However, whenever mention of ‘eave tubes’ is made, it should be clear that this represents a suite of modifications, i.e. closure of the eaves, installation of the tubes with insecticide-treated netting, rendering all windows mosquito proof through the installation of (untreated) window screening, door repairs to reduce the possibility for mosquito entry, and general closure of openings and cracks, with the same goal.
Fig. 3.
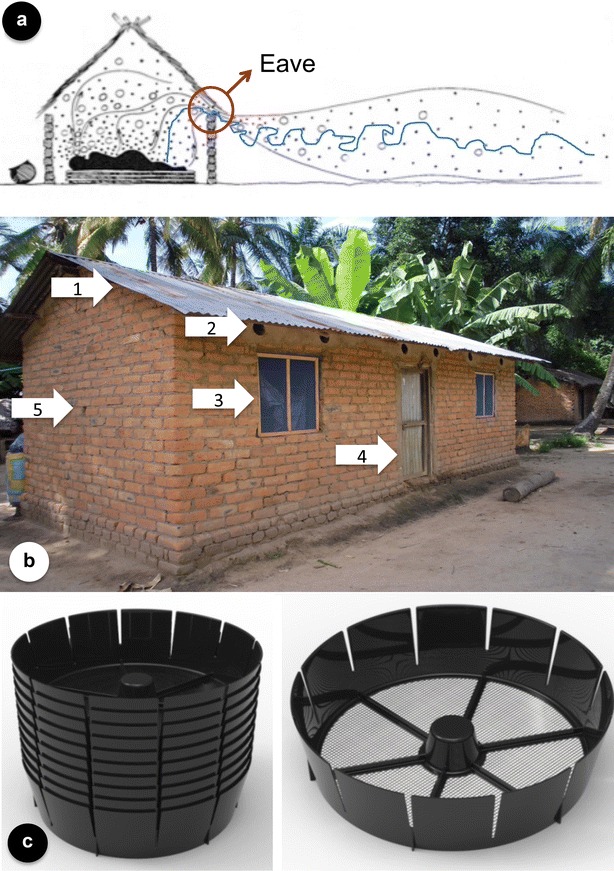
The eave tube concept. a African anophelines fly upwind in host odour plumes (blue line) and enter houses through the gap between the roof and the walls, the eave (red circle); drawing modified after [44]. b A house in southern Tanzania fitted with eave tubes and rendered mosquito-proof through fitting of window screening and sealing of the eaves. The house modification comprises: 1 Closing the eaves, 2 Installation of eave tubes, 3 Fitting of window screening, 4 Repairs of the door (where necessary), and 5 Closing of cracks and holes. c Eave tube inserts (stack on the left), fitted with insecticide-treated netting that fit inside PVC pipes. Development of this insert is described in Snetselaar et al. [pers. comm.]
Research and development
In the research and development phase that followed the initial conceptualisation, research focused primarily on semi-field studies that facilitated quick evaluations of key characteristics of the technology. These studies were undertaken both in Tanzania (at the Ifakara Health Institute in Ifakara; [Sternberg et al. pers. comm.]) and at the Thomas Odhiambo campus of ICIPE at Mbita Point, Kenya [Snetselaar et al. pers. comm.], so that different strains and anopheline species could be tested. The data generated from these studies formed the basis for a parameterized modelling exercise to gain further insight on the potential of the technology in terms of reducing transmission alone or in combination with LLINs or IRS [Waite et al. pers. comm.]. Both semi-field and field studies were undertaken to study the behaviour of mosquitoes when entering the eave tubes. Videographic studies were conducted to quantify behaviours inside the eave tubes and measure exposure durations upon contact with different active ingredients [Sperling et al. pers. comm.]. Finally, studies were undertaken in the village of Mngeta (Tanzania) to assess community acceptance of eave tube technology and undertake an operational feasibility study that incorporated some 1900 houses. This enabled us also to gain insight in the economics of this approach when compared with LLINs or IRS [Knols et al. pers. comm.].
This series of six articles provides an overview of the research that was undertaken over the past 3 years that has led up to a Phase III randomized controlled trial that is currently being prepared in Côte d’Ivoire.
Authors’ contributions
BGJK served as the principal investigator for the MCD project and drafted the manuscript. It was subsequently reviewed and commented on by all authors that also read and approved the final submitted version. All authors contributed to the conceptualization, research and development, and appropriation of eave tube technology. All authors read and approved the final manuscript.
Acknowledgements
This article is the prelude to a series of articles on eave tubes as a novel approach for malaria vector control. This work was part of the mosquito contamination device (MCD) project (www.mcdproject.org) that was supported by European Union Seventh Framework Programme Grant 306105, FP7-HEALTH-2012-INNOVATION-1. Much of the development of eave tubes was guided by close interaction with the project’s External Advisory Board that consisted of Greg Devine (Chair, Australia), Leonard Mboera (Tanzania), Steve Lindsay (UK), Michelle Helinski (Netherlands), Cor van der Weele (Netherlands), Marcel Verweij (Netherlands), Owen Jones (UK), David Malone (UK), and Rickard Ignell (Sweden). We are strongly indebted to this group for their expertise, advice and generous support. We also acknowledge the excellent guidance, encouragement and support from the EU Project’s scientific officer, Inmaculada Peñas Jimenez.
Competing interests
BGJK, MF, RAS, and AJO hold shares in In2Care BV. In2Care BV has one or more patents or patent applications related to the subject of this paper.
Funding
This work was part of the mosquito contamination device (MCD) project (www.mcdproject.org) that was supported by European Union Seventh Framework Programme Grant 306105, FP7-HEALTH-2012-INNOVATION-1.
Abbreviations
- IRS
indoor residual spraying
- LLIN
long-lasting insecticide-treated net
- MCD
mosquito contamination device
- MDG
Millennium Development Goal
Contributor Information
Bart G. J. Knols, Email: bart@in2care.org
Marit Farenhorst, Email: marit@in2care.org.
Rob Andriessen, Email: rob@in2care.org.
Janneke Snetselaar, Email: janneke@in2care.org.
Remco A. Suer, Email: remco@in2care.org
Anne J. Osinga, Email: anne@in2care.org
Johan M. H. Knols, Email: johan@in2care.org
Johan Deschietere, Email: jdeschietere@ctf2000.com.
Kija R. Ng’habi, Email: kija@ihi.or.tz
Issa N. Lyimo, Email: ilyimo@ihi.or.tz
Stella T. Kessy, Email: skessy@ihi.or.tz
Valeriana S. Mayagaya, Email: vmayagaya@ihi.or.tz
Sergej Sperling, Email: sergej.sperling@biogents.com.
Michael Cordel, Email: Michael.Cordel@biogents.com.
Eleanore D. Sternberg, Email: eds16@psu.edu
Patrick Hartmann, Email: phartmann@ctf2000.com.
Ladslaus L. Mnyone, Email: llaurent@ihi.or.tz
Andreas Rose, Email: andreas.rose@biogents.com.
Matthew B. Thomas, Email: mbt13@psu.edu
References
- 1.United Nations. The Millennium Development Goals Report 2015. New York. http://www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%20%28July%201%29.pdf.
- 2.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UCSF Global Health Group. http://www.shrinkingthemalariamap.org/. Accessed 1 May 2016.
- 4.http://www.endmalaria2040.org/. Accessed 1 May 2016.
- 5.Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330. doi: 10.1186/1475-2875-13-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toé KH, N’Falé S, Dabiré RK, Ranson H, Jones CM. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genom. 2015;16:146. doi: 10.1186/s12864-015-1342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemingway J. The role of vector control in stopping the transmission of malaria: threats and opportunities. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130431. doi: 10.1098/rstb.2013.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. The innovative vector control consortium: improved control of mosquito-borne diseases. Trends Parasitol. 2006;22:308–312. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Global plan for insecticide resistance management in malaria vectors. Geneva: World Health Organization; 2012.
- 10.Hemingway J, Vontas J, Poupardin R, Raman J, Lines J, Schwabe C, et al. Country-level operational implementation of the global plan for insecticide resistance management. Proc Natl Acad Sci USA. 2013;110:9397–9402. doi: 10.1073/pnas.1307656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet. 2016;387:1785–1788. doi: 10.1016/S0140-6736(15)00417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Larval source management: a supplementary measure for malaria vector control: an operational manual. Geneva: World Health Organization; 2013.
- 13.WHO. Handbook for integrated vector management. Geneva: World Health Organization; 2012.
- 14.Celli A. The new prophylaxis against malaria in Lazio. Lancet. 1900;156:1603–1606. doi: 10.1016/S0140-6736(01)89058-9. [DOI] [Google Scholar]
- 15.Manson P. Experimental proof of the mosquito-malaria theory. BMJ. 1900;75:949–951. doi: 10.1136/bmj.2.2074.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson L, Simpson D, Stephens M. Durable housing improvements to fight malaria transmission: can we learn new strategies from past experience? Habitat for Humanity International Global Programs Department, Atlanta; 2014.
- 17.Kirby M, Ameh D, Bottomley C, Green C, Jawara M, Milligan P, et al. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet. 2009;374:998–1009. doi: 10.1016/S0140-6736(09)60871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tusting LS, Ippolito MM, Willey BA, Kleinschmidt I, Dorsey G, Gosling RD, et al. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malar J. 2015;14:209. doi: 10.1186/s12936-015-0724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mulla MS, et al. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;4:006657. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. Vector control: methods for use by individuals and communities. Geneva: World Health Organization, Switzerland; 1997.
- 23.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sougoufara S, Diédhiou SM, Doucouré S, Diagne N, Sembène PM, Harry M, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13:125. doi: 10.1186/1475-2875-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Killeen GF, Govella NJ, Lwetoijera DW, Okumu FO. Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malar J. 2016;15:225. doi: 10.1186/s12936-016-1280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paaijmans KP, Thomas MB. The influence of mosquito resting behaviour and associated microclimate for malaria risk. Malar J. 2011;10:183. doi: 10.1186/1475-2875-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanford JI, Blanford S, Crane RG, Mann ME, Paaijmans KP, Schreiber KV, et al. Implications of temperature variation for malaria parasite development across Africa. Sci Rep. 2013;3:1300. doi: 10.1038/srep01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Seidlein L, Ikonomidis K, Bruun R, Jawara M, Pinder M, Knols BG, et al. Airflow attenuation and bed net utilization: observations from Africa and Asia. Malar J. 2012;11:200. doi: 10.1186/1475-2875-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon SB, Bruce NG, Grigg J, Hibberd PL, Kurmi OP, Lam KB, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2:823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.http://www.economist.com/news/specialreport/21572377africanliveshavealreadygreatlyimprovedoverpastdecadesaysoliveraugust. Accessed 1 May 2016.
- 31.Roll back malaria. Housing and malaria: consensus statement. Vector Control Working Group. Geneva; 2015.
- 32.Lindsay SW, Snow RW. The trouble with eaves; house entry by vectors of malaria. Trans R Soc Trop Med Hyg. 1988;82:645–646. doi: 10.1016/0035-9203(88)90546-9. [DOI] [PubMed] [Google Scholar]
- 33.Lindsay SW, Jawara M, Paine K, Pinder M, Walraven GE, Emerson PM. Changes in house design reduce exposure to malaria mosquitoes. Trop Med Int Health. 2003;8:512–517. doi: 10.1046/j.1365-3156.2003.01059.x. [DOI] [PubMed] [Google Scholar]
- 34.Kirby MJ, Green C, Milligan PM, Sismanidis C, Jasseh M, Conway DJ, et al. Risk factors for house-entry by malaria vectors in a rural town and satellite villages in The Gambia. Malar J. 2008;7:2. doi: 10.1186/1475-2875-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Njie M, Dilger E, Lindsay SW, Kirby MJ. Importance of eaves to house entry by anopheline, but not culicine, mosquitoes. J Med Entomol. 2009;46:505–510. doi: 10.1603/033.046.0314. [DOI] [PubMed] [Google Scholar]
- 36.Wanzirah H, Tusting LS, Arinaitwe E, Katureebe A, Maxwell K, Rek J, et al. Mind the gap: house construction and the risk of malaria in Ugandan children. PLoS ONE. 2015;10:e0117396. doi: 10.1371/journal.pone.0117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diallo DA, Habluetzel A, Cuzin-Ouattara N, Nebié I, Sanogo E, Cousens SN, et al. Widespread distribution of insecticide-impregnated curtains reduces child mortality, prevalence and intensity of malaria infection, and malaria transmission in rural Burkina Faso. Parassitologia. 1999;41:377–381. [PubMed] [Google Scholar]
- 38.Cuzin-Ouattara N, Van den Broek AH, Habluetzel A, Diabaté A, Sanogo-Ilboudo E, Diallo DA, et al. Wide-scale installation of insecticide-treated curtains confers high levels of protection against malaria transmission in a hyperendemic area of Burkina Faso. Trans R Soc Trop Med Hyg. 1999;93:473–479. doi: 10.1016/S0035-9203(99)90343-7. [DOI] [PubMed] [Google Scholar]
- 39.Menger DJ, Omusula P, Wouters K, Oketch C, Carreira AS, Durka M, et al. Eave screening and push-pull tactics to reduce house entry by vectors of malaria. Am J Trop Med Hyg. 2016;94(4):868–878. doi: 10.4269/ajtmh.15-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley J, Rehman AM, Schwabe C, Vargas D, Monti F, Ela C, et al. Reduced prevalence of malaria infection in children living in houses with window screening or closed eaves on Bioko Island, Equatorial Guinea. PLoS ONE. 2013;8:e80626. doi: 10.1371/journal.pone.0080626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spitzen J, Koelewijn T, Mukabana WR, Takken W. Visualization of house-entry behaviour of malaria mosquitoes. Malar J. 2016;15:233. doi: 10.1186/s12936-016-1293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takken W, Knols BGJ. Odor-mediated behavior of afrotropical malaria mosquitoes. Annu Rev Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- 43.Andriessen R, Snetselaar J, Suer RA, Osinga AJ, Deschietere J, Lyimo IN, et al. Electrostatic coating enhances bioavailability of insecticides and breaks pyrethroid resistance in mosquitoes. Proc Natl Acad Sci USA. 2015;112:12081–12086. doi: 10.1073/pnas.1510801112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wernsdorfer WH, McGregor I. Malaria. Principles and practice of malariology. 2. Edinburgh: Churchill Livingstone; 1988. [Google Scholar]


