Abstract
8E5/LAV cells harbor a single HIV provirus, and are used frequently to generate standards for HIV genome quantification. Using flow cytometry-based in situ mRNA hybridization validated by qPCR, we find that different batches of 8E5 cells contain varying numbers of cells lacking viral mRNA and/or viral genomes. These findings raise concerns for studies employing 8E5 cells for quantitation, and highlight the value of mRNA FISH and flow cytometry in the detection and enumeration of HIV-positive cells.
Keywords: 8E5, Reservoir quantitation, FISH:FLOW, FISH:FACS, HIV cell-associated RNA, HIV flow cytometry assay, HIV proviral silencing, HIV proviral loss, HIV RNA in situ hybridization
Main text
A significant challenge in the search for a cure for HIV is the quantification of latently infected reservoir cells. The rarity of these cells in the blood of ART-suppressed patients renders assessment of the size of the latent reservoir extremely challenging [1]. Current methods to estimate the number of cell-associated viral genomes usually involve PCR-based amplification of integrated proviral DNA or viral RNA and their comparison with cell-based standards. The human lymphoblastic leukemia cell clone 8E5/LAV (8E5) is widely employed for such studies [2, 3], and harbors a single integrated HIV provirus that drives production of defective virions [3, 4]. These cells are routinely mixed with HIV-uninfected lymphoblasts in known proportions to generate standard curves, to facilitate relative quantification of viral genomes using the number of cycles of PCR amplification.
We recently developed a method to detect HIV RNA-positive cells in patient samples by fluorescence in situ hybridization and flow cytometry (FISH:FLOW) [5]. Short (25nt), tagged ssDNA oligo probes were generated to cover ~600–1400 bp of the HIV gag or nef open reading frames from ADA (nef/LTR, 8794-9407; gag, 823-2240 LGC Biosearch). These probes are hybridized to RNA in PFA-fixed and ethanol-permeabilized cells providing HIV RNA readout with cellular resolution via flow cytometry. To test the limits of this assay, we generated a dilution series of 8E5/CEM cell standards and analyzed them by FISH:FLOW. Surprisingly, we found the 8E5 population was heterogeneous with only 4.5 % of cells positive for HIV nef/LTR and 2.9 % for gag transcripts detected by RNA FISH (Fig. 1a and data not shown). Dilutions of the 8E5 cells yielded linear standard plots (not shown), as might also be expected from PCR analysis of comparable dilution series. However, the absolute number of viral genomes inferred by this method would underestimate the true values obtained using standards that assumed 100 % of 8E5 cells in the starting population were HIV-infected.
Fig. 1.
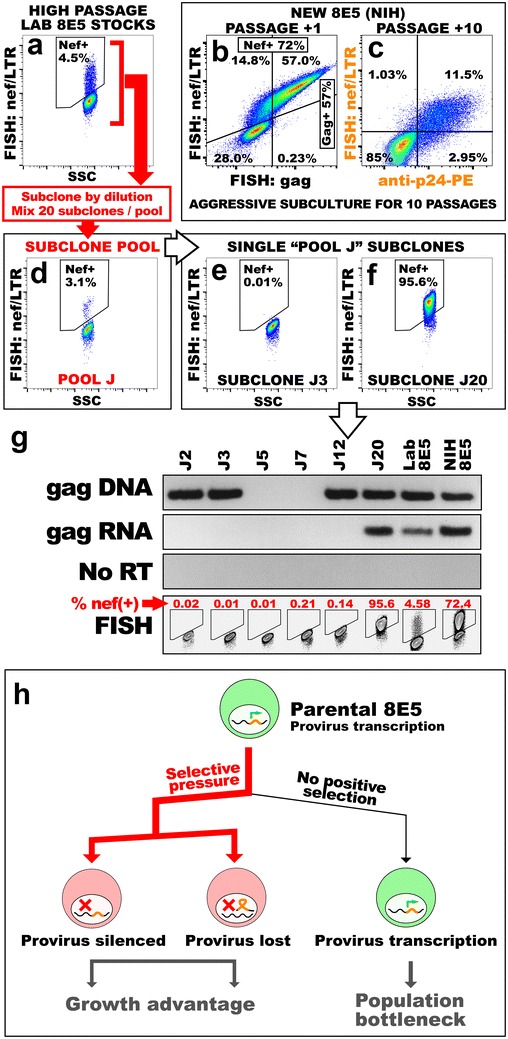
Variable infection of HIV in 8E5 cells. a–c FISH probes detect nef/LTR RNA in the indicated subpopulations (Nef+) of 8E5 cells either from archival laboratory stocks (a, originally purchased from ATCC) or newly acquired (b, c) through the NIH AIDS Reagent Program. FISH:FLOW dot plots show that HIV nef/LTR probe signal correlates in the same cells with HIV gag mRNA (b) and p24 antibody staining (c). Cells in (c) were obtained by repeated high-ratio subculture of the new cell stock from (b). d 8E5 subclones were generated and combined into pools of 20; these pools were screened for HIV-transcribing subclones; Pool J is shown, harboring likely a single clone of 100 % HIV penetrance (<5 % of total cells in the pool are HIV nef/LTR positive). e–g Analyses of HIV mRNA and proviral DNA in single J-pool subclones. Clone J3 (e) harbors no nef/LTR transcript detected by RNA FISH, while J20 (f) is uniformly nef/LTR-positive. g (Top 3 panels) Proviral gag DNA qPCR and corresponding gag mRNA qRT-PCR with no reverse transcriptase cDNA controls. g (Bottom panel) FISH:FLOW contour plots for nef/LTR RNA from selected J-pool subclones. Frequencies of nef/LTR-positive cells indicated in red. h Model for 8E5 cell population dynamics. Parental 8E5 cells are under strong selective pressure to exclude or silence the HIV provirus. Cells that achieve these outcomes (red) acquire significant growth or survival advantages. During long-term culture, this advantage will shift the 8E5 population, leading to a bottleneck of HIV-expressing cells (green) in the culture
HIV transcription may not proceed in all 8E5 cells at all points in the cell cycle, a scenario that could explain a subpopulation of HIV RNA-negative cells in 8E5 cultures. However, given the observed low fraction of positive cells, we reasoned that proviral loss or durable silencing [6, 7] would more likely account for a majority of cells negative for HIV nef/LTR or gag mRNA. To assess the maintenance of the HIV proviral genome in the 8E5 cells we generated 200 subclones by limiting dilution, and expanded these cells for analyses by RNA FISH. We first combined the subclones into pools of 20, and screened for HIV positive pools. To our surprise, only one subclone pool (Pool J) showed signal for HIV nef/LTR at a frequency suggesting that only a single clone in the pool was positive (Fig. 1d). We then analyzed subclones individually and found that most clones were entirely negative for nef/LTR RNA (representative clone J3, Fig. 1e). By contrast, clone J20 was homogeneously positive for HIV nef/LTR RNA by FISH:FLOW analyses (Fig. 1f). The segregation of positive and negative clones is clearly visible in the FISH contour plots (Fig. 1g, bottom panel). FISH:FLOW analysis with HIV gag RNA probes yielded similar results (data not shown). Interestingly, the lower than expected frequency of HIV-transcribing clones (1/200 vs. ~4.5/100 expected) suggests that cells containing active HIV proviral genomes are at a survival or clonogenic disadvantage compared to those that have silenced or lost the provirus.
Absence of HIV transcription could result from proviral silencing or loss of proviral genomic DNA. Either scenario could be the product of negative selective pressure experienced by HIV-infected lymphoblasts in long-term culture. Transcriptionally silent or HIV-negative subclones within the 8E5 population would have a growth advantage, and would rapidly outcompete the HIV-expressing population. To address this experimentally we compared relative frequencies of proviral DNA (Qiagen QIamp) and HIV gag mRNA (Trizol) by qPCR and qRT-PCR (BioRad iTAQ). Two independent regions of gag were amplified and normalized to GAPDH genomic DNA or cDNA from the same sample (Fig. 1g, GAPDH data not shown). Intriguingly, some subclones lacking HIV nef/LTR transcripts still harbored gag proviral DNA, while in other subclones the HIV provirus was undetectable. Possible genomic DNA contamination was ruled out using controls lacking reverse transcriptase (no RT, Fig. 1g). These data indicate that both proviral genome silencing and genome deletion are occurring in 8E5 cells maintained in culture. Interestingly, the LAV provirus in 8E5 is integrated at chromosome 13q14-q21 [8], a site containing common fragile sites that would render this clone susceptible to proviral loss by genomic instability.
We acquired a fresh aliquot of 8E5 cells from the HIV AIDS Reagent Program to determine whether population heterogeneity might be a consistent feature of these cells. These 8E5 cells were tested by nef/LTR and gag RNA FISH within 5 days of their establishment in culture. The nef-positive gate constituted the main population of cells (72 %, Fig. 1b). Importantly, no cells stained positive for gag RNA without also expressing nef/LTR RNA in this multiplex assay (Fig. 1b) as would be expected based on the staged transcription of HIV. This representation highlights the ability of FISH:FLOW to discern among different stages of HIV infection (gag heterogeneity in the nef + population). Subsequent passaging of the newly obtained cells at a 1:10 ratio led, by passage 10, to the reduction of nef/LTR and gag RNA-positive cells below 50 % (not shown). Aggressive subculture by splitting the cells very low (bottleneck founder effect) accelerated the loss of nef/LTR transcription, where only 15 % of 8E5 cells fresh from a public repository transcribed HIV nef/LTR by passage 10 (Fig. 1c); importantly almost all of the nef-positive cells also stained p24-positive (Beckman anti-p24 KC57) (Fig. 1c). These data demonstrate FISH:FLOW is a surrogate assay for standard measures of HIV production and that the HIV transcriptional loss documented in 8E5 subclones is a reproducible characteristic of this cell line. We believe this observation reflects the strong selection of founder subclones that achieve spontaneous loss of HIV proviral DNA.
Together our data support a model (Fig. 1h) where 8E5 cells acquire a selective advantage in continuous cell culture if they extinguish HIV expression, either by transcriptional silencing or by proviral genome loss. We suspect that stressing the cells during culture through delayed passage is likely to exacerbate this behavior, hastening a bottleneck of HIV-positive cells in the population. The loss of HIV from 8E5 cultures is of practical significance considering the widespread utilization of these cells for quantitation of HIV genome abundance in patient samples. Our findings appear consistent with cautions raised in a recent analysis, which found variable numbers of proviral insertions within common latently infected cell lines previously assumed to be homogeneous [9]. Our findings highlight the robust data these cell lines yield and underscore their intrinsic value to the field, but also support recent initiatives to validate common reagents in pursuit of reproducibility. The FISH:FLOW method we present here is a convenient means to rigorously validate the penetrance of HIV in the 8E5 starting population in any laboratory with access to a flow cytometer; without such validation, we suggest that quantification of HIV genomes using 8E5 cells should be restricted to relative comparisons only.
Worldwide, investigators studying persistence and therapeutic reactivation of latent HIV reservoirs are heavily invested in PCR readouts of genome quantitation. The FISH:FLOW method we have developed is one example of the very few tools that allow quantification of HIV infection at the level of the individual cell; importantly this resolution is lost in PCR studies of cell-associated DNA or RNA from bulk populations. We feel strongly that a critical mass of laboratories investing in cell-level tools has not yet been reached, and the simple results presented here using a common cell line highlight the value of such studies as a complement to PCR approaches. Importantly, combining FISH:FLOW with antibody surface phenotyping meets the evermore urgent need to characterize specific cell subsets that harbor HIV in vivo. Multiple probe colors can assay different HIV transcripts in a single cell, allowing one to discern between early and late stage transcription, and increases the confidence that positive signal corresponds to intact provirus. The ability to then FACS-purify infected cells from human patient samples and study their transcriptional profile adds functional genomics to the growing list of possibilities. We feel these are opportunities not to be missed in context of the current challenges facing the field of HIV persistence and eradication.
Authors’ contributions
DWG conceived the study and designed the experiments. FISH method developed by HCM and DGR, revised by DWG and KMW, with validation and data interpretation by KCJ and SB. KMW and DWG performed the experiments with contributions from SS. All authors interpreted data and refined the questions. DWG and DGR wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Doug Richman, UC San Diego and Guido Poli, Ospedale S. Raffaele, Milano for some insightful comments during the course of this study. This work was supported by the National Institutes of Health awards T32OD011182 to DWG, AI118582 and HL100928 to DGR, and by the Wellcome Trust and the Bill and Melinda Gates Foundation awards 088696/Z/09/Z and OPP1125279 respectively, to HCM.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
Data generated or analysed during this study are included in this published article. Additional information on materials, methods or data detailed in this article are freely available from the corresponding author.
Funding
The authors declare that the funding agencies listed below played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. Generous support for these studies provided by: National Institute of Allergy and Infectious Diseases (AI118582) to Dr. David G. Russell; National Heart, Lung, and Blood Institute (HL100928) to Dr. David G. Russell; Wellcome Trust (088696/Z/09/Z) to Dr. Henry C. Mwandumba; Bill and Melinda Gates Foundation (OPP1125279) to Dr. Henry C. Mwandumba; NIH Office of the Director (T32OD011182) to Mr. David W. Gludish.
Contributor Information
Kaley M. Wilburn, Email: kmw248@cornell.edu
Henry C. Mwandumba, Email: Henry.Mwandumba@lstmed.ac.uk
Kondwani C. Jambo, Email: kcj35@cornell.edu
Saikat Boliar, Email: sb929@cornell.edu.
Sabrina Solouki, Email: ss3457@cornell.edu.
David G. Russell, Email: dgr8@cornell.edu
David W. Gludish, Email: dwg62@cornell.edu
References
- 1.Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol. 2016;14:55–60. doi: 10.1038/nrmicro.2015.5. [DOI] [PubMed] [Google Scholar]
- 2.Desire N, Dehee A, Schneider V, Jacomet C, Goujon C, Girard PM, Rozenbaum W, Nicolas JC. Quantification of human immunodeficiency virus type 1 proviral load by a TaqMan real-time PCR assay. J Clin Microbiol. 2001;39:1303–1310. doi: 10.1128/JCM.39.4.1303-1310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folks TM, Powell D, Lightfoote M, Koenig S, Fauci AS, Benn S, Rabson A, Daugherty D, Gendelman HE, Hoggan MD, et al. Biological and biochemical characterization of a cloned Leu-3-cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986;164:280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quillent C, Dumey N, Dauguet C, Clavel F. Reversion of a polymerase-defective integrated HIV-1 genome. AIDS Res Hum Retroviruses. 1993;9:1031–1037. doi: 10.1089/aid.1993.9.1031. [DOI] [PubMed] [Google Scholar]
- 5.Jambo KC, Banda DH, Kankwatira AM, Sukumar N, Allain TJ, Heyderman RS, Russell DG, Mwandumba HC. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol. 2014;7:1116–1126. doi: 10.1038/mi.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbonye U, Karn J. Transcriptional control of HIV latency: cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology. 2014;454–455:328–339. doi: 10.1016/j.virol.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mok HP, Lever AM. Chromatin, gene silencing and HIV latency. Genome Biol. 2007;8:228. doi: 10.1186/gb-2007-8-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deichmann M, Bentz M, Haas R. Ultra-sensitive FISH is a useful tool for studying chronic HIV-1 infection. J Virol Methods. 1997;65:19–25. doi: 10.1016/S0166-0934(96)02164-7. [DOI] [PubMed] [Google Scholar]
- 9.Symons J, Chopra A, Malantinkova EE, Spiegelaerde W, Leary S, Cooper D, Vandekerckhove L, Mallal S, Lewin SR, Cameron PU: Integration analysis of latently infected cell lines: evidence of ongoing replication. In: CROI 2016, 23rd conference of retroviruses and opportunistic infections, Boston, MA, USA; 2016.


