Abstract
Background:
Treatment of anterior choroidal artery (AChA) aneurysms with endovascular coiling or surgical clipping may increase the risk of ischemic complications owing to the critical territory supplied by the AChA. We analyzed the surgical results of endovascular coiling and surgical clipping for AChA aneurysms performed in a single institution, as well as the role of indocyanine green-videoangiography (ICG-VAG) and motor-evoked potential (MEP).
Methods:
We analyzed 50 patients (51 aneurysms; 21 men, 29 women; mean age: 58 years) including 25 with subarachnoid hemorrhage treated with endovascular coiling or surgical clipping between April 1990 and October 2013. The complication rates and clinical outcomes of the coil group (mean follow-up: 61 months) and the clip group (mean follow-up: 121 months) were analyzed with a modified Rankin scale.
Results:
The overall clinical outcome of the coil group (95%) was better than that of the clip group (85%). Especially, the outcomes in the coil group were better in the first investigated period (1990–2007) (P < 0.05). However, after the introduction of ICG-VAG and MEP, the outcomes in the clip group improved significantly (P = 0.005), and treatment-related complications decreased from 20 to 4.7%. Eleven aneurysms (coil group: 8, clip group: 3) showed small neck remnants but no remarkable regrowth, except for 1 case during the mean follow-up period of 91 months.
Conclusions:
Surgical clipping of AChA aneurysms has become safer because of ICG-VAG and MEP monitoring. Coiling and clipping of AChA aneurysms showed good and comparable outcomes with these monitoring methods.
Keywords: Anterior choroidal artery aneurysm, endovascular coiling, surgical clipping
INTRODUCTION
Anterior choroidal artery (AChA) aneurysms are rare, representing 2–5% of all intracranial aneurysms. Furthermore, few literature reports have focused on microneurosurgical treatment.[6,7,17,23,24,25] Surgical clipping as a treatment method for AChA aneurysms may increase the risk of debilitating ischemic complications because of the critical territory supplied by the AChA. In the available literature, the mortality and treatment-related permanent morbidity rates of surgical clipping range from 6 to 33% and from 10 to 28.6%, respectively.[5,6,7,11,16,17,23,24,25] In contrast, endovascular treatment of AChA aneurysms is associated with lower mortality and morbidity rates (0–5.5%).[9,11,18] Accordingly, some recent reports have suggested that endovascular treatment of these aneurysms yields favorable clinical outcomes and a lower incidence of ischemic complication than those obtained with surgical clipping.[9,11] The same result—i.e., endovascular coiling leads to better clinical outcomes because of the risk of AChA occlusion with clipping—was documented before 1990. However, the more recent introduction of indocyanine green-videoangiography (ICG-VAG)[19] and motor-evoked potential (MEP)[22] have demonstrated favorable surgical results. In this study, we analyzed the clinical results of both endovascular coiling and surgical clipping in the era of ICG-VAG and MEP.
MATERIALS AND METHODS
Patients
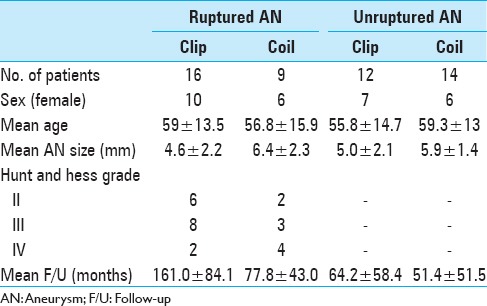
We performed a retrospective analysis of 50 patients (51 AChA aneurysms) who were treated with endovascular coiling or surgical clipping at the Kurume University School of Medicine between April 1990 and October 2013. Of these patients, 21 were men and 29 were women, with a mean age of 58.0 years. The average aneurysm size was 5.5 mm (range, 2–10.1 mm). The study participants included 25 patients with subarachnoid hemorrhage (SAH). In these patients, the distribution of Hunt and Hess grades II, III, and IV was 8, 11, and 6, respectively [Table 1].
Table 1.
Clinical characteristics in 51 anterior choroidal artery aneurysms
Selection of treatment method: Endovascular coiling or surgical clipping
The fundamental treatment strategy of our department for AChA aneurysms has changed over time. During the first examined period (1990–1997), surgical clipping was the preferred method. However, during the second period (1998–2007), endovascular coiling became the first choice owing to the incidence of AChA occlusion after clipping and the subsequent development of an endovascular coiling device. During the third period (2008–present), with the introduction of ICG-VAG and MEP, the treatment method of choice has been based on the circumstances of each case; endovascular coiling has become the first choice for patients with severe SAH (Hunt and Hess grades III and IV and/or small neck aneurysm) with an appropriate neck-to-dome ratio (<1.5). However, other cases have required surgical clipping with ICG-VAG and MEP monitoring.
In this study, we evaluated the clinical outcomes of patients treated within the first and second periods combined, and those treated during the third period [Figure 1].
Figure 1.
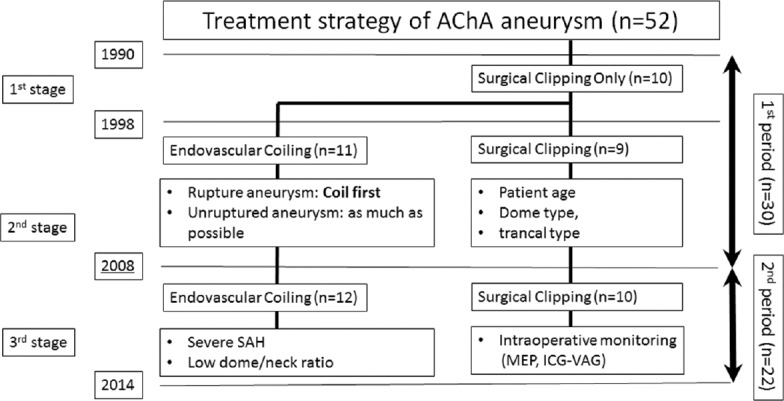
Treatment strategy of AChA aneurysm according to their stage and period in this study
Surgical complications and clinical outcomes
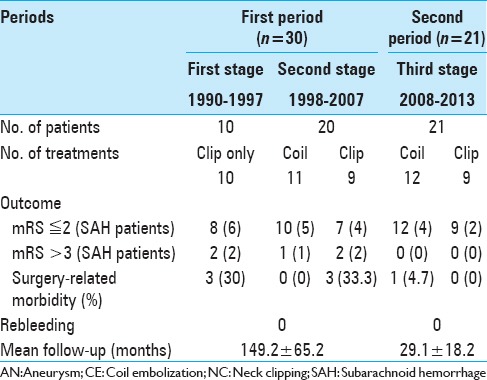
In this study, surgical complications were defined as new neurological deficits lasting 24 h or longer—after treatment with coil embolization or surgical clipping—that were associated with a new infarction or hemorrhage on magnetic resonance imaging or computed tomography (excluding vasospasms on SAH). The clinical outcomes were evaluated with a modified Rankin scale (mRS) during the last follow-up [Table 2].
Table 2.
Clinical outcomes of modified Rankin scale and surgery-related morbidity
Classification of anterior choroidal artery aneurysms in this study
We have devised a new classification—representing a modification of the one used in previous reports[5,7,10,12,15,16]—that takes into consideration both the branching site and the number of AChAs [Figure 2]. Type A (artery type) AChAs arise directly from the internal carotid artery; type B (neck type) AChAs arise from the aneurysmal neck; type C (dome type) AChAs arise from the aneurysmal dome; and type D (truncal type) aneurysms originate in a part of the AChA itself. Furthermore, we observed, in this study, some cases of duplicated AChAs, prompting us to establish 2 subtypes—B-2 and C-2—in which the aneurysm is located between duplicated AChAs within each aneurysmal type.
Figure 2.
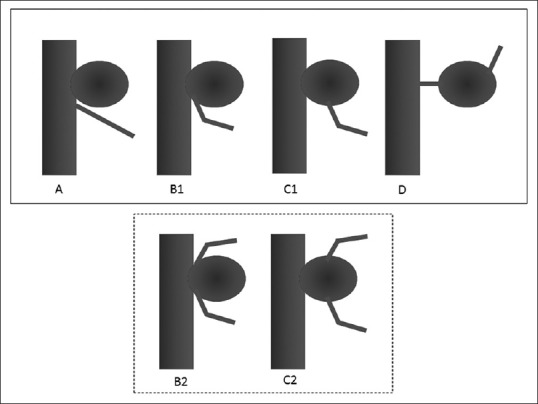
Classification of anterior choroidal artery (AChA) aneurysm types. A, Artery type—AChA arises from the internal carotid artery. B1, Neck type—AChA arises from the aneurysmal neck. B2, Neck subtype—Aneurysm is located between the duplicated AChAs. C1, Dome type—AChA arises from the aneurysmal dome. C2, Dome subtype—Duplicated AChA arises from the aneurysmal dome. D, Truncal type—Aneurysm originates in a portion of the AChA itself
RESULTS
Overall outcomes
The overall outcomes are listed in Table 2. The average follow-up period was 91 months (61.4 months in the coil group, 121.6 months in the clip group; P < 0.05). No rebleeding was observed in either group during the follow-up period. The average aneurysm size was significantly larger in the coil group than in the clip group (6.1 mm vs. 4.7 mm; P < 0.05). The clinical outcomes of patients (evaluated with the mRS) in the coil group in the first stage of the examined period were better than those of patients in the clip group (P < 0.05); however, the distributions of both the Hunt and Hess grades system and the Fisher group grades did not differ significantly. In the second examined period, the outcomes in the clip group significantly improved compared with those in the first period (P = 0.005). In addition, the treatment-related complication rate decreased from 20 to 4.7% in the second examined period.
According to our aneurysm type classification [Figure 2], there were 8 cases of type A (15.6%), 41 of type B (80.3%), 2 of type C (4%), and none of type D (0%) [Table 3]. Types B and C had 5 and 1 duplication subtypes, respectively.
Table 3.
Incidence of treatment-related complication according to the aneurysmal type
Clinical outcomes in the coil group
Twenty-two patients in the coil group (95.6%) had favorable outcomes (mRS ≤ 2) including 9 patients with SAH [Table 2]. Transient ischemic symptoms were found in 1 case, and small neck remnant was found in 8 cases (1 type A and 7 type B) [Table 3]. Five patients developed aneurysmal regrowth, however, only 1 patient underwent an additional clipping operation. Faint regrowth of the residual neck was found in 4 cases, but it was not accompanied by dome filling [Figure 3]. Other surgical complications included 1 intraoperative rupture, 2 asymptomatic radiological small cerebral infarctions, and 1 femoral artery hemorrhage [Table 3].
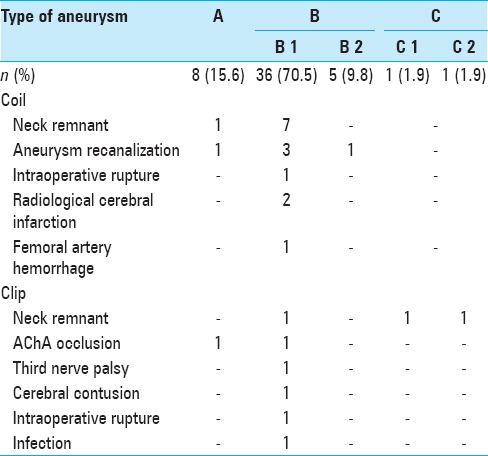
Figure 3.
Findings in a 79-year-old woman (a) Preoperative angiogram shows the right anterior choroidal artery (AChA) with a ruptured aneurysm. The AChA arises from the aneurysmal neck (classified as type B1). (b) Endovascular coil embolization is performed with small neck remnant. (c) Two years postoperatively, follow-up magnetic resonance angiogram (Volume Rendering Image) indicates that the aneurysmal dome has disappeared without remarkable regrowth
Clinical outcomes in the clip group
Within the first examined period, 79% of patients in the clip group showed positive clinical outcomes. Two patients (7.1%) experienced permanent AChA occlusion as a surgical complication. An additional 4 patients showed poor prognosis (mRS ≥ 3), including 2 with AChA syndrome because of AChA occlusion, 1 with cerebral contusion during surgery [Table 3], and 1 with vasospasm due to SAH. However, the clinical outcomes of 9 patients, including 2 patients with SAH, in this group, in the second examined period were all excellent (mRS = 0). No ischemic complications were reported. During the second period, no significant difference in clinical outcomes was apparent between the coil and clip groups.
Surgery-related complications occurred in patients with types A, B1, C1, and C2 aneurysms. Three patients experienced incomplete clipping owing to wide neck type (B1) and type C (C1 and C2) aneurysms; however, the residual neck showed neither regrowth nor aneurysmal rupture at follow-up. The presence of AChA duplication appears to be the key issue inherent in surgical clipping. However, in this study, we could not preoperatively detect the AChA duplication in 3 cases (2 of type B2 and 1 of type C2), which were detected only during operation [Figures 4 and 5].
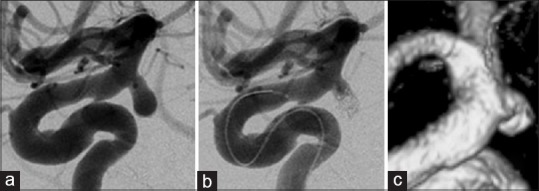
Figure 4.
Findings in a 50-year-old man (a and b) Three-dimensional angiography demonstrates a single anterior choroidal artery (AChA) arising from the neck of the AChA aneurysm (classified as type B1). (c) Intraoperative photograph shows another branch of the AChA arising from the distal aneurysmal neck, undetected on preoperative angiography. This is classified as type B2 (white arrowhead). (d) Intraoperative indocyanine green-videoangiography demonstrates good flow without constriction of the AChA after surgical clipping
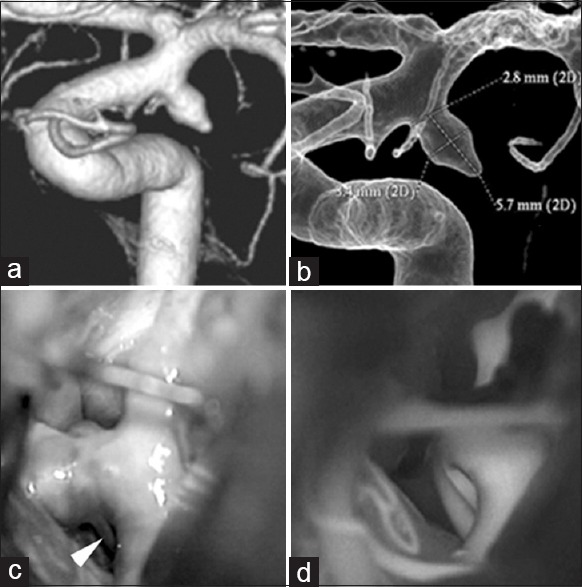
Figure 5.
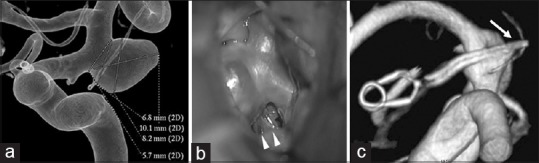
Findings in a 72-year-old woman (a) Preoperative 3-dimentional angiography demonstrates a single anterior choroidal artery (AChA) arising from the aneurysmal dome (classified as type C1). (b) Intraoperative photograph shows another branch of the AChA arising from the aneurysmal dome, undetected on preoperative angiography. This is classified as type C2 (white arrowhead). (c) Postoperative 3-dimensional angiography indicates preservation of the AChA, which is undetectable (white arrow)
DISCUSSION
The importance of anterior choroidal artery preservation
AChA aneurysms are rare, representing only 2–5% of all intracranial aneurysms, with few reports in the literature focusing on microneurosurgical treatment.[6,7,17,23,24,25] Even if the AChA is completely occluded, complications do not necessarily occur in cases wherein the AChA is anastomosed between the AChA and the posterior choroidal artery, and even directly behind the posterior cerebral artery.[13,18] However, historically, the AChA has been considered to be one the most dangerous arteries for surgical or interventional procedures because of the risk of serious complications. In the available literature, the mortality and treatment-related permanent morbidity rates for surgical clipping range from 6% to 33% and from 10% to 28.6%, respectively.[1,5,8,10,14,18,20,24,25] On the other hand, endovascular treatment of AChA aneurysms has yielded lower mortality and morbidity rates (0–5.5%) than surgical clipping.[9,11,18] Consequently, some reports have suggested that endovascular treatment is preferred.[9,11] At our department, we experienced some cases of surgical clipping resulting in unfavorable outcomes before 1990; as a result, endovascular coiling was the preferred treatment method until 2007. However, the subsequent introduction of ICG-VAG[19] and MEP[22] has allowed avoiding the occurrence of accidental AChA occlusion.
Few reports have discussed surgical outcomes and aneurysmal classification based on the origin of the AChA. AChA aneurysms can be classified into 4 types based on their origin, namely, arising mainly from the internal carotid artery (artery type), arising from the aneurysmal neck (neck type), arising from the aneurysmal dome (dome type), and arising from the AChA itself (truncal type).[5,7,10,12,15,16] Kodama et al. categorized a similar pattern and reported that type B aneurysm is the most common (72.4%).[12] Our results showed a similar prevalence (type B1/B2: 80.3%). In addition, we wish to emphasize the presence of AChA duplication in some cases. We experienced 5 duplication cases of type B and 1 of type C, including 3 cases that remained undetected with preoperative cerebral angiography. Although we cannot determine which AChA is more important, both AChAs should be preserved. Therefore, the existence of AChA duplication should be recognized, preoperative angiograms should be carefully examined, and intraoperative findings should be closely evaluated.
Endovascular coiling
The recent developments in both the device and technique of endovascular treatment are remarkable. The double-catheter technique,[2] balloon-assisted coil embolization,[21] stent-assisted coil embolization,[3] and other techniques are all potential treatment methods for aneurysms of various shapes. The primary problems experienced with endovascular coiling are neck remnants and ischemic complications. Furthermore, long-term follow-up is necessary after endovascular coiling, especially in cases showing neck remnants. In our study, we experienced 2 cases of radiological asymptomatic small infarctions.
Kang et al. analyzed complications after coil embolization and reported symptomatic complications occurring in the neck type (in this study, types B1 and B2).[9] Endovascular coiling is not indicated for types C and D AChA aneurysms or for wide neck aneurysms (dome-to-neck ratio of <1.5). In our study, we found that the 8 cases of small neck remnants could almost be classified as type B in the coil group (7 type B, 1 type A). However, retreatment was performed in only 1 case with neck remnant. To preserve the AChA, incomplete coiling with neck remnant should be performed rather than complete aneurysmal occlusion. Notably, fewer available studies report on the long-term outcomes of AChA aneurysms treated with endovascular coiling than those for cases treated with surgical clipping. Although additional long-term follow-up studies are clearly necessary, only 1 patient in our study showed enlargement of the neck remnant in an average follow-up period of 73.7 months.
Surgical clipping
In cases of treatment with surgical clipping, some complications after AChA aneurysm have been previously reported. Cho et al. reported that the frequency of infarction was the highest in the neck type aneurysms, with a statistically significant difference.[5] Moreover, unruptured small aneurysms <5 mm were found to have a higher prevalence of post-clipping infarction, however, this finding was not deemed to be statistically significant.[5] Bohnstedt et al. reported that ischemic complications stemming from surgical treatment of AChA aneurysms are most closely associated with a higher frequency of temporary clip applications for proximal control.[4] To preserve the AChA and avoid severe hemiparesis, monitoring with ICG-VAG and MEP is very useful. ICG-VAG is a safe and effective method that provides necessary information for modifying clip placement immediately.[19] Furthermore, MEP during surgery provides a measurement of contralateral neurophysiological function in the vascular territory receiving surgical clipping.[22] In fact, in this study, both AChA occlusion and severe hemiparesis were avoided by using ICG-VAG and MEP monitoring. The clinical outcomes of our 9 patients treated with intraoperative monitoring since 2008 are excellent. Kodama et al.[12] reported similar excellent outcomes after intraoperative monitoring. Although ICG-VAG and MEP are clearly beneficial, the type of aneurysm should be evaluated and the number of AChAs (single or double) should be observed in each case. Care must also be taken to avoid the risk of AChA occlusion owing to constriction at the AChA branching site,[14] and temporary clipping should be done safely under MEP monitoring.
To preserve the AChA, type C aneurysms must be treated successfully with dome clipping (neck remnant) and not with coil treatment; these cases require long-term follow-up because of possible aneurysm regrowth, as observed in this study.[1,20]
Furthermore, in this study, we did not observe residual neck regrowth in the average follow-up period of 63.3 months.
Surgical clipping for AChA aneurysm has now become very safe—and the disadvantages inherent to endovascular coiling have virtually disappeared—owing to the advent of ICG-VAG and MEP monitoring. In our study, we found that the clinical outcomes of our patients in both the coil and clip groups were similarly favorable. Furthermore, we observed relatively good long-term outcomes in both groups. By carefully evaluating the aneurysmal type, the appropriate treatment method for each patient can be chosen. It appears that the best indications for coiling are type A aneurysm, a low dome-to-neck ratio, and high-grade SAH, whereas surgical clipping is safe for all types of aneurysms. In type B and C aneurysms, there is a slightly higher ratio of residual neck occurrence even after clipping. However, the long-term outcomes in our study population were good throughout the average follow-up period of 5.3 years.
CONCLUSION
In recent years, surgical clipping of AChA aneurysms has become safer because of the introduction of ICG-VAG and MEP monitoring. The selection of a treatment method—whether endovascular coiling or surgical clipping—should involve careful consideration of the relationship of the aneurysm with the neck and the AChA, as well as the presence of any AChA duplication.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Takachika Aoki, Email: takachi@med.kurume-u.ac.jp.
Masaru Hirohata, Email: hiroha@med.kurume-u.ac.jp.
Kei Noguchi, Email: noguchi_kei@med.kurume-u.ac.jp.
Satoru Komaki, Email: komaki_satoru@med.kurume-u.ac.jp.
Kimihiko Orito, Email: orito-kimihiko@kurume-u.ac.jp.
Motohiro Morioka, Email: mmorioka@med.kurume-u.ac.jp.
REFERENCES
- 1.Amagasaki K, Higa T, Takeuchi N, Kakizawa T, Shimizu T. Late recurrence of subarachnoid hemorrhage due to regrowth of aneurysm after neck clipping surgery. Neurol Med Chir. 2002;42:496–500. doi: 10.2176/nmc.42.496. [DOI] [PubMed] [Google Scholar]
- 2.Baxter BW, Rosso D, Lownie SP. Double microcatheter technique for detachable coil treatment of large, wide-necked intracranial aneurysms. AJNR Am J Neuroradiol. 1998;19:1176–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Biondi A, Janardhan V, Katz JM, Salvaggio K, Riina HA, Gobin YP. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: Strategies in stent deployment and midterm follow-up. Neurosurgery. 2007;61:460–8. doi: 10.1227/01.NEU.0000290890.62201.A9. [DOI] [PubMed] [Google Scholar]
- 4.Bohnstedt BN, Kemp WJ, 3rd, Li Y, Payner TD, Horner TG, Leipzig TJ, et al. Surgical treatment of 127 anterior choroidal artery aneurysms: A cohort study of resultant ischemic complications. Neurosurgery. 2013;73:933–9. doi: 10.1227/NEU.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 5.Cho MS, Kim MS, Chang CH, Kim SW, Kim SH, Choi BY. Analysis of clip-induced ischemic complication of anterior choroidal artery aneurysms. J Korean Neurosurg Soc. 2008;43:131–4. doi: 10.3340/jkns.2008.43.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake CG, Vanderlinden RG, Amacher AL. Carotid-choroidal aneurysms. J Neurosurg. 1968;29:32–6. doi: 10.3171/jns.1968.29.1.0032. [DOI] [PubMed] [Google Scholar]
- 7.Friedman JA, Pichelmann MA, Piepgras DG, Atkinson JL, Maher CO, Meyer FB, et al. Ischemic complications of surgery for anterior choroidal artery aneurysms. J Neurosurg. 2001;94:565–72. doi: 10.3171/jns.2001.94.4.0565. [DOI] [PubMed] [Google Scholar]
- 8.Galatius-Jensen F, Ringberg V. Anastomosis between the anterior choroidal artery and the posterior cerebral artery demonstrated by arteriography. Radiology. 1963;81:942–4. doi: 10.1148/81.6.942. [DOI] [PubMed] [Google Scholar]
- 9.Kang HS, Kwon B, Kwon OK, Jung C, Kim JE, Oh CW, et al. Endovascular coil embolization of anterior choroidal artery aneurysms. Clinical article. J Neurosurg. 2009;111:963–9. doi: 10.3171/2009.4.JNS08934. [DOI] [PubMed] [Google Scholar]
- 10.Kim BM, Kim DI, Chung EC, Kim SY, Shin YS, Park SI, et al. Endovascular coil embolization for anterior choroidal artery aneurysms. Neuroradiology. 2008;50:251–7. doi: 10.1007/s00234-007-0331-0. [DOI] [PubMed] [Google Scholar]
- 11.Kim BM, Kim DI, Shin YS, Chung EC, Kim DJ, Suh SH, et al. Clinical outcome and ischemic complication after treatment of anterior choroidal artery aneurysm: Comparison between surgical clipping and endovascular coiling. AJNR Am J Neuroradiol. 2008;29:286–90. doi: 10.3174/ajnr.A0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodama Y, Ohnishi H, Taomoto K, Kuga Y, Nakashima K, Kubota H, et al. The classification and surgery for anterior choroidal artery aneurysms based on morphological feature. Surg Cereb Stroke. 2011;39:267–71. [Google Scholar]
- 13.Lasjaunias P, Berenctein A, Ter Brugge KG. Surgical Neuroangiography 1, clinical vascular anatomy and variations. 2nd ed. Heidelberg, Berlin: Springer-Verlag; 2001. pp. 563–77. [Google Scholar]
- 14.Lee YS, Park J. Anterior choroidal artery aneurysm surgery: Ischemic complications and clinical outcomes revisited. J Korean Neurosurg Soc. 2013;54:86–92. doi: 10.3340/jkns.2013.54.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehecka M, Dashti R, Laakso A, van Popta JS, Romani R, Navratil O, et al. Microneurosurgical management of anterior choroid artery aneurysms. World Neurosurg. 2010;73:486–99. doi: 10.1016/j.wneu.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Mukherjee R, Lan Z, Liu Y, He M. Microneurosurgical management of anterior choroidal artery aneurysms: A 16-year institutional experience of 102 patients. Neurol Res. 2012;34:272–80. doi: 10.1179/1743132812Y.0000000008. [DOI] [PubMed] [Google Scholar]
- 17.Perria L, Viale GL, Rivano C. Further remarks on the surgical treatment of carotid-choroidal aneurysms. Acta Neurochir. 1971;24:253–62. doi: 10.1007/BF01405411. [DOI] [PubMed] [Google Scholar]
- 18.Piotin M, Mounayer C, Spelle L, Williams MT, Moret J. Endovascular treatment of anterior choroidal artery aneurysms. AJNR Am J Neuroradiol. 2004;25:314–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, et al. Prospective evaluation of surgical microscope–integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg. 2005;103:982–9. doi: 10.3171/jns.2005.103.6.0982. [DOI] [PubMed] [Google Scholar]
- 20.Shindou M, Acevedo JC, Turjman F. Aneurysmal remnants after microsurgical clipping: Classification and results from a prospective angiographic study (in a consecutive series of 305 operated intracranial aneurysms) Acta Neurochir. 1998;140:1153–9. doi: 10.1007/s007010050230. [DOI] [PubMed] [Google Scholar]
- 21.Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC. Balloon-assisted coil embolization of intracranial aneurysms: Incidence, complications, and angiography results. J Neurosurg. 2006;105:396–9. doi: 10.3171/jns.2006.105.3.396. [DOI] [PubMed] [Google Scholar]
- 22.Szelényi A, Langer D, Kothbauer K, De Camargo AB, Flamm ES, Deletis V. Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: Intraoperative changes and postoperative outcome. J Neurosurg. 2006;105:675–81. doi: 10.3171/jns.2006.105.5.675. [DOI] [PubMed] [Google Scholar]
- 23.Viale GL, Pau A. Carotid-choroidal aneurysms: Remarks on surgical treatment and outcome. Surg Neurol. 1979;11:141–5. [PubMed] [Google Scholar]
- 24.Yasargil MG. Microneurosurgery. II. Stuttgart: Georg Thieme; 1984. [Google Scholar]
- 25.Yasargil MG, Yonas H, Gasser JC. Anterior choroidal artery aneurysms: Their anatomy and surgical significance. Surg Neurol. 1978;9:129–38. [PubMed] [Google Scholar]


