Abstract
Background:
The use of renin-angiotensin system (RAS) inhibitors in patients with chronic kidney disease, and especially in diabetic kidney disease, has been shown to provide renoprotective effects and slow progression to end-stage renal disease. However, this protective effect in kidney transplant patient populations is unclear.
Aim:
The objective of this systematic review and meta-analysis was to evaluate the effect of RAS inhibitors on kidney allograft survival.
Materials and Methods:
A literature search for randomized controlled trials (RCTs) was performed from inception through February 2016. Studies that reported relative risks or hazard ratios comparing the risks of renal graft loss in renal transplant recipients who received RAS inhibitors vs. controls were included. Pooled risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using a random-effect, generic inverse variance method.
Results:
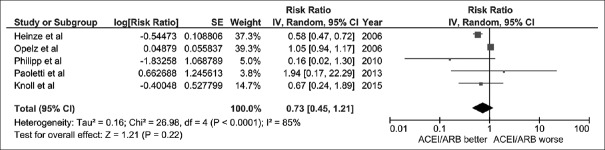
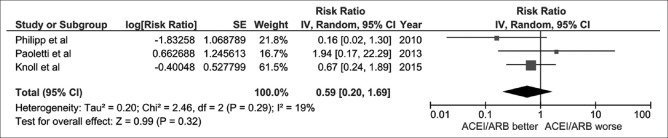
Five studies (3 RCTs and 2 cohort studies) with 20024 kidney transplant patients were included in the meta-analysis. Pooled RR of allograft failure in recipients who received RAS inhibitors was 0.73 (95% CI: 0.45–1.21). When meta-analysis was limited only to RCTs, the pooled RR of allograft failure in patients using RAS inhibitors was 0.59 (95%: CI 0.20–1.69). The risk for mortality (RR: 1.13 [95% CI: 0.62–2.07]) in patients using RAS inhibitors compared to controls was not significantly reduced.
Conclusion:
This meta-analysis demonstrated insignificant reduced risks of renal graft loss among renal transplant recipients who received RAS inhibitors. Future studies assessing the potential benefits of RAS inhibitors on allograft survival in specific kidney transplant patient populations are needed.
Keywords: Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, kidney transplantation, renin-angiotensin system inhibitors, transplantation
Introduction
The use of renin-angiotensin system (RAS) inhibitors, including angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), in patients with chronic kidney disease (CKD), and especially in diabetic kidney disease, has been shown to provide renoprotective effects and slow progression to end-stage renal disease (ESRD).[1] With these known benefits, the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines have recommended using ACEI or ARB for management of blood pressure in adult proteinuric CKD patients with and without diabetes.[2]
In kidney transplant recipients, despite significant improvements in short-term renal allograft survival,[3] long-term graft survival is still an ongoing concern.[4] Proteinuria after kidney transplantation is common and is associated with reduced allograft survival.[5,6] A thorough systematic review of RAS inhibitors in kidney transplantation demonstrated reductions in proteinuria, hematocrit, and glomerular filtration rate in renal transplant recipients with RAS inhibitors.[7] However, there was insufficient data to determine the effect on patient or graft survival. Recently, the findings from a multicentre, double-blind, randomized controlled trial (RCT) of ramipril versus placebo in 213 kidney transplant patients showed that treatment with ramipril did not lead to a significant reduction in allograft failure in proteinuric kidney transplant recipients.[8] Unfortunately, the investigators did not achieve target sample size, resulting in a potentially underpowered study.[8] Therefore, we conducted this systematic review and meta-analysis to comprehensively accumulate all allograft survival data and pool results to evaluate the effect of RAS inhibitors on kidney allograft survival.
Materials and Methods
Search strategy
Two investigators (WC and CT) independently searched published studies and conference abstracts indexed in MEDLINE, EMBASE, the Cochrane database, and ClinicalTrials.gov from inception through February 2016 using the following words: “Angiotensin-Converting Enzyme Inhibitors,” “Angiotensin Converting Enzyme Inhibitor,” “ACE inhibitor,” “ACEIs,” “Angiotensin II Type 1 Receptor Blockers,” “ARB,” “benazepril,” “captopril,” “cilazapril,” “delapril,” “enalapril,” “fosinopril,” “imidapril,” “lisinopril,” “moexipril,” “perindopril,” “quinapril,” “ramipril,” “trandolapril,” “spirapril,” “zofenopril,” “candesartan,” “eprosartan,” “irbesartan,” “losartan,” “olmesartan,” “telmisartan,” or “valsartan” AND “transplantation” AND “kidney” or “renal.” A manual search for additional relevant studies using references from retrieved articles was also performed.
Inclusion criteria
The inclusion criteria were as follows: (1) RCTs published as original studies or conference abstracts that evaluated the effects of RAS inhibitors on kidney allograft survival, (2) studies that provided data to calculate relative risks, hazard ratios, or standardized incidence ratios with 95% confidence intervals (CIs), and (3) a reference group composed of patients who were on treatment with RAS inhibitors as control group.
Two investigators (WC and CT) independently determined study eligibility. Differing decisions were resolved by mutual consensus. The quality of each study was evaluated using the Jadad quality-assessment scale[9] for RCTs and the Newcastle–Ottawa quality assessment scale[10] for observational studies.
Data extraction
A standardized data collection form was used to extract the following information: Last name of first author, title of article, study design, year of study, country of origin, year of publication, sample size, definition of RAS inhibitors[11,12] and control groups, and outcome assessment period.
Statistical analysis
Review Manager software (Version 5.3, Copenhagen, Denmark) from the Cochrane Collaboration was used for data analysis. Point estimates and standard errors were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird.[13] Given the high likelihood of between-study variances, a random-effect model was used rather than a fixed-effect model. Statistical heterogeneity was assessed using Cochran's Q test. This statistic was complemented with the I2 statistic, which quantifies the proportion of the total variation across studies that is caused by heterogeneity rather than chance. An I2 of 0–25% represents insignificant heterogeneity, 26–50% low heterogeneity, 51–75% moderate heterogeneity, and >75% high heterogeneity.[14] The presence of publication bias was assessed by funnel plots of the logarithm of odds ratios vs. their standard errors.[15]
Results
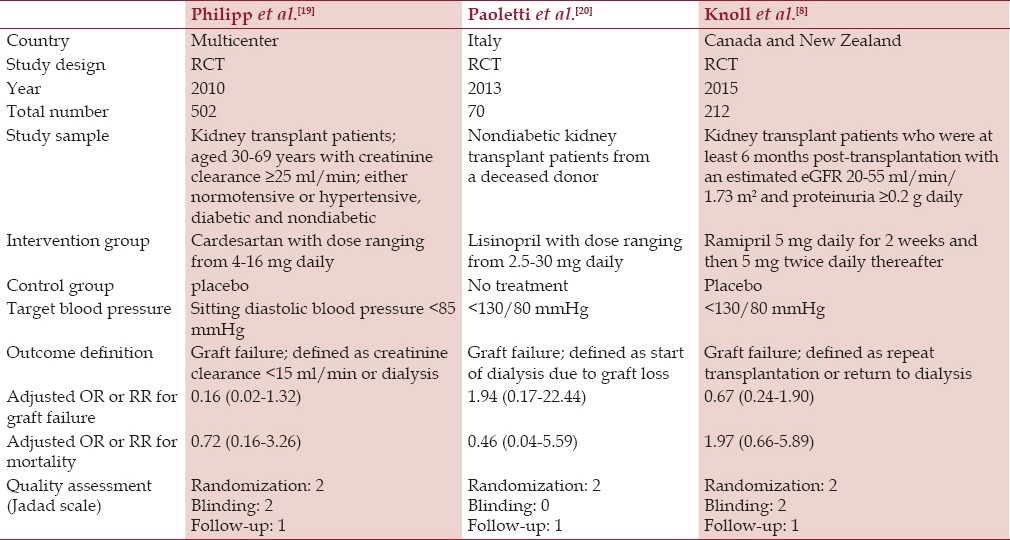
The search strategy yielded 5204 potentially relevant articles; 4951 were excluded based on the title and abstract which clearly showed that they did not fulfill inclusion criteria in terms of article type, study design, population, or outcome of interest (Item S2). The remaining 253 articles underwent full-length review, with 248 studies excluded because they were not observational studies or RCTs (n = 45) or did not report outcomes of interest (n = 203). Five studies (3 RCTs and 2 cohort studies) with 20024 kidney transplant patients were included in the meta-analysis. Tables 1 and 2 contain detailed characteristics and quality assessment of all included studies.
Table 1.
Main characteristics of the observational studies included in this meta-analysis
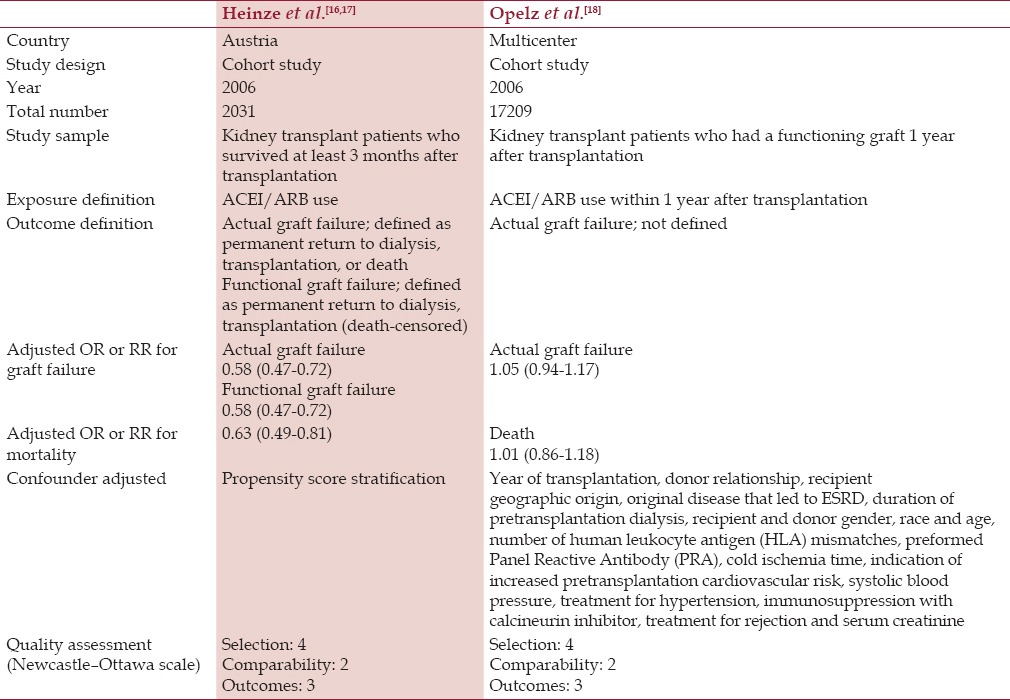
Table 2.
Main characteristics of the randomized controlled studies included in this meta-analysis
Effect of renin-angiotensin system inhibitors on kidney allograft survival
The pooled risk ratio (RR) of allograft failure in recipients who received RAS inhibitors was 0.73 (95% CI: 0.45–1.21, I2 = 85%). Figure 1 shows the forest plot of the included studies. We also performed a sensitivity analysis limited only to RCTs. The pooled RR of allograft failure in recipients using RAS inhibitors was 0.59 (95% CI: 0.20–1.69, I2 = 19%), as shown in Figure 2.
Figure 1.
Forest plot of all included studies comparing the risk of renal allograft failure in kidney transplant recipients with renin-angiotensin system inhibitors vs. control; square data markers, risk ratios (RR); horizontal lines, 95% confidence intervals (CIs), with marker size reflecting the statistical weight of the study using random-effects meta-analysis. Diamond data markers, overall RRs, and 95% CIs for outcomes of interest. IV, inverse variance; SE, standard error
Figure 2.
Forest plot of randomized controlled trails comparing the risk of renal allograft failure in kidney transplant recipients with renin-angiotensin system inhibitors vs. control; square data markers, risk ratios (RRs); horizontal lines, 95% confidence intervals (CIs), with marker size reflecting the statistical weight of the study using random-effects meta-analysis. Diamond data markers, overall RRs, and 95% CIs for outcomes of interest. IV, inverse variance; SE, standard error
Post-hoc meta-analysis assessing mortality risk was also performed. The risk for mortality was not significantly reduced in patients using RAS inhibitors compared to controls with RR of 1.13 [95% CI: 0.62–2.07].
Evaluation for publication bias
Funnel plots were constructed to evaluate publication bias regarding the risk of allograft failure in recipients using RAS inhibitors. Overall, the publication bias was insignificant.
Discussion
In this current meta-analysis of a total of 20024 kidney transplant patients, we demonstrated no significant reduction in allograft failure risk with the use of RAS inhibitors after kidney transplantation. In addition, within the selected studies, RAS inhibitors did not improve survival in kidney transplant recipients.
Although previous systematic reviews and meta-analyses successfully showed the effectiveness of RAS inhibitors in reduction of proteinuria in patients with kidney transplantation,[7,21,22] data showing a significant benefit of RAS inhibitors on renal allograft survival were lacking.[23,24] Despite growing evidence supporting the use of RAS inhibitors to slow progression to ESRD in nontransplant patients with CKD and proteinuria,[1,2] our meta-analysis found no significant benefit of RAS inhibitors use in renal transplant recipients. Recently, Knoll et al.[8] conducted an RCT of ramipril versus placebo in 213 kidney transplant recipients with proteinuria. The investigators demonstrated a decline in proteinuria in the ramipril group. However, at 4-year follow-up, ramipril did not reduce the risk of ESRD or death in this population. A limitation of their RCT was that it was unable to achieve target sample size and was thus underpowered. The investigators had extrapolated observed event rates to a sample size of 528 kidney transplant recipients and the finding of their study still showed a nonsignificant difference in allograft survival endpoint.[8] With more statistical power, our meta-analysis confirmed no significant risk reduction in renal allograft failure with RAS inhibitor treatment. This suggests that, if any RAS inhibitor effect is present, its magnitude is likely very small.
Studies have shown a potential survival benefit with RAS inhibitors use in nonkidney transplant CKD patients,[25] especially with myocardial infarction.[26] Unfortunately, this benefit from RAS inhibitors has not translated into the kidney transplant population. Recently, Opelz et al.[27] examined cardiovascular mortality in kidney transplant recipients by using Collaborative Transplant Study retrospective data. The investigators reported no difference in the cumulative incidence of cardiovascular death 2–10 years after kidney transplantation in patients receiving RAS inhibitors versus other antihypertensive medications. Thus, with the updated evidence and findings from our meta-analysis, the current recommendations by KDIGO clinical practice guideline[28] to use RAS inhibitors for hypertensive renal transplant recipients with proteinuria do not adequately address the long-term outcomes of allograft and patient survival.
There are several limitations of the present meta-analysis. First, the objective of our meta-analysis was to assess allograft survival outcome. Thus, we did not evaluate the safety and adverse outcomes of RAS inhibitors in kidney transplant recipients. However, some of the safety outcomes have already been studied in previous meta-analyses.[7,21,22] Second, the majority of the included studies did not have available kidney allograft biopsy information, and consequently the cause of allograft dysfunction and/or failure was not known. Hence, even though RAS inhibitors did not provide a protective allograft benefit in the general transplant patient population, it is still possible that they may have a role in specific transplant subgroups such as post-transplant diabetes or recurrent/de novo glomerular diseases after transplantation.[8] Further study is needed. Finally, although the findings from our meta-analysis do not support a potentially important treatment effect, there is no data to suggest that RAS inhibitors should be avoided in kidney transplant patient populations.
In summary, this meta-analysis shows no significant reduced risk of allograft loss or mortality among renal transplant recipients treated with RAS inhibitors. Future studies that incorporate kidney allograft histology are required to evaluate if RAS inhibitors can provide potential benefits on long-term allograft survival in certain kidney transplant patient populations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin ii receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev. 2006:CD006257. doi: 10.1002/14651858.CD006257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheeler DC, Becker GJ. Summary of KDIGO guideline. What do we really know about management of blood pressure in patients with chronic kidney disease? Kidney Int. 2013;83:377–83. doi: 10.1038/ki.2012.425. [DOI] [PubMed] [Google Scholar]
- 3.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the united states, 1988 to 1996. N Engl J Med. 2000;342:605–12. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 4.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4:1289–95. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 5.Seeman T. Management of proteinuria in the transplanted patient. Pediatr Nephrol. 2015;30:889–903. doi: 10.1007/s00467-014-2876-6. [DOI] [PubMed] [Google Scholar]
- 6.Amer H, Cosio FG. Significance and management of proteinuria in kidney transplant recipients. J Am Soc Nephrol. 2009;20:2490–2. doi: 10.1681/ASN.2008091005. [DOI] [PubMed] [Google Scholar]
- 7.Hiremath S, Fergusson D, Doucette S, Mulay AV, Knoll GA. Renin angiotensin system blockade in kidney transplantation: A systematic review of the evidence. Am J Transplant. 2007;7:2350–60. doi: 10.1111/j.1600-6143.2007.01928.x. [DOI] [PubMed] [Google Scholar]
- 8.Knoll GA, Fergusson D, Chasse M, Hebert P, Wells G, Tibbles LA, et al. Ramipril versus placebo in kidney transplant patients with proteinuria: A multicentre, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:318–26. doi: 10.1016/S2213-8587(15)00368-X. [DOI] [PubMed] [Google Scholar]
- 9.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 11.Cheungpasitporn W, Thongprayoon C, Chiasakul T, Korpaisarn S, Erickson SB. Renin-angiotensin system inhibitors linked to anemia: A systematic review and meta-analysis. QJM. 2015;108:879–84. doi: 10.1093/qjmed/hcv049. [DOI] [PubMed] [Google Scholar]
- 12.Cheungpasitporn W, Thongprayoon C, Srivali N, O’Corragain OA, Edmonds PJ, Ungprasert P, et al. Preoperative renin-angiotensin system inhibitors use linked to reduced acute kidney injury: A systematic review and meta-analysis. Nephrol Dial Transplant. 2015;30:978–88. doi: 10.1093/ndt/gfv023. [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 16.Heinze G, Mitterbauer C, Regele H, Kramar R, Winkelmayer WC, Curhan GC, et al. Angiotensin-converting enzyme inhibitor or angiotensin ii type 1 receptor antagonist therapy is associated with prolonged patient and graft survival after renal transplantation. J Am Soc Nephrol. 2006;17:889–99. doi: 10.1681/ASN.2005090955. [DOI] [PubMed] [Google Scholar]
- 17.Heinze G, Collins S, Benedict MA, Nguyen LL, Kramar R, Winkelmayer WC, et al. The association between angiotensin converting enzyme inhibitor or angiotensin receptor blocker use during postischemic acute transplant failure and renal allograft survival. Transplantation. 2006;82:1441–8. doi: 10.1097/01.tp.0000244587.74768.f7. [DOI] [PubMed] [Google Scholar]
- 18.Opelz G, Zeier M, Laux G, Morath C, Dohler B. No improvement of patient or graft survival in transplant recipients treated with angiotensin-converting enzyme inhibitors or angiotensin ii type 1 receptor blockers: A collaborative transplant study report. J Am Soc Nephrol. 2006;17:3257–62. doi: 10.1681/ASN.2006050543. [DOI] [PubMed] [Google Scholar]
- 19.Philipp T, Martinez F, Geiger H, Moulin B, Mourad G, Schmieder R, et al. Candesartan improves blood pressure control and reduces proteinuria in renal transplant recipients: Results from secret. Nephrol Dial Transplant. 2010;25:967–76. doi: 10.1093/ndt/gfp581. [DOI] [PubMed] [Google Scholar]
- 20.Paoletti E, Bellino D, Marsano L, Cassottana P, Rolla D, Ratto E. Effects of ace inhibitors on long-term outcome of renal transplant recipients: A randomized controlled trial. Transplantation. 2013;95:889–95. doi: 10.1097/TP.0b013e3182827a43. [DOI] [PubMed] [Google Scholar]
- 21.Cross NB, Webster AC, Masson P, O’Connell P J, Craig JC. Antihypertensives for kidney transplant recipients: Systematic review and meta-analysis of randomized controlled trials. Transplantation. 2009;88:7–18. doi: 10.1097/TP.0b013e3181a9e960. [DOI] [PubMed] [Google Scholar]
- 22.Jennings DL, Taber DJ. Use of renin-angiotensin-aldosterone system inhibitors within the first eight to twelve weeks after renal transplantation. Ann Pharmacother. 2008;42:116–20. doi: 10.1345/aph.1K471. [DOI] [PubMed] [Google Scholar]
- 23.Cross NB, Webster AC. Angiotensin-converting enzyme inhibitors-beneficial effects seen in many patient groups may not extend to kidney transplant recipients. Transplantation. 2016;100:472–3. doi: 10.1097/TP.0000000000001140. [DOI] [PubMed] [Google Scholar]
- 24.Toto RD. Transplantation: The role of raas blockade in kidney transplantation. Nat Rev Nephrol. 2016;12:129–31. doi: 10.1038/nrneph.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molnar MZ, Kalantar-Zadeh K, Lott EH, Lu JL, Malakauskas SM, Ma JZ, et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63:650–8. doi: 10.1016/j.jacc.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans M, Carrero JJ, Szummer K, Akerblom A, Edfors R, Spaak J, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in myocardial infarction patients with renal dysfunction. J Am Coll Cardiol. 2016;67:1687–97. doi: 10.1016/j.jacc.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 27.Opelz G, Dohler B. Cardiovascular death in kidney recipients treated with renin-angiotensin system blockers. Transplantation. 2014;97:310–5. doi: 10.1097/01.TP.0000437672.78716.28. [DOI] [PubMed] [Google Scholar]
- 28.Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int. 2010;77:299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]