Abstract
Background:
Obesity among children and adolescents in the United States has tripled since 1980, and has become a major public health concern.
Aims:
The purpose of this study was to evaluate the potential relationship between exposure to organic mercury from Thimerosal-containing hepatitis B vaccines and the children's subsequent risk of an obesity diagnosis.
Materials and Methods:
A hypothesis-testing, case-control study was undertaken to evaluate exposure to organic mercury from Thimerosal-containing hepatitis B vaccines, which were administered at specific intervals in the first 6 months of life, among cases diagnosed with childhood obesity and controls by examining automated medical records for children born from 1991 to 2000 who were continuously enrolled in the Vaccine Safety Datalink database.
Results:
This study found highly significant associations as follows. Cases diagnosed with obesity were significantly (P < 0.00001) more likely to have received greater exposure to organic mercury from Thimerosal-containing hepatitis B vaccines administered within the first month of life (odds ratio (OR) =1.511), first 2 months of life (OR = 1.486), and first 6 months of life (OR = 3.795) than the controls. Similar outcomes were observed when the overall data were separated by gender. In a dose-response manner, cases diagnosed with obesity were significantly more likely than controls to have received greater exposure to organic mercury from Thimerosal-containing hepatitis B vaccines, which were administered within the first 6 months of life (OR = 1.0375 per μg of mercury, P < 0.00001).
Conclusions:
In a dose-response manner, the present study associates an increased organic mercury exposure from Thimerosal-containing hepatitis B vaccines with an increased risk of obesity diagnosis, and suggests that Thimerosal is an obesogen. The results are biologically plausible and future studies are needed to examine this phenomenon.
Keywords: Ethylmercury, mercury, merthiolate, obesity, obesogen, Thimerosal
Introduction
The United States (US) Centers for Disease Control and Prevention (CDC) defines a child as overweight if his/her body mass index (BMI) is at or above the 85th percentile and lower than the 95th percentile compared to children of the same age and gender, and a child as obese if he/she has a BMI at or above the 95th percentile for children of the same age and gender.[1] According to the CDC, there are serious immediate and long-term risks associated with childhood obesity. With respect to the immediate health risks of childhood obesity, 70% of obese children were found to have at least one cardiovascular disease risk factor and 39% were found to have two or more such factors.[2]
Moreover, obese children have increased risks for impaired glucose tolerance, insulin resistance and type 2 diabetes;[3] breathing problems such as sleep apnea and asthma;[4,5] joint problems and musculoskeletal discomfort;[4,6] fatty liver disease, gallstones, and gastroesophageal reflux;[3,4] and greater risk of social and psychological problems such as discrimination and poor self-esteem.[7] In addition to the cited childhood risks, the later health risks from childhood obesity include an increased risk of adult obesity[8,9,10] with increased severity,[2] which is associated with a number of serious health conditions including heart disease, diabetes, and some cancers.[11]
According to the CDC, the estimated prevalence of obesity among children and adolescents in the US has tripled since 1980, and has become a major public health concern.[12] Estimates from the National Health and Nutrition Examination Survey (NHANES) show that 31.7% of children and teens in the US in 2008 were either overweight or obese.[13]
Recent advances in human genetics have revealed a number of genes influencing the susceptibility to obesity, however, their contribution to disease is likely contingent upon environmental factors because the childhood obesity epidemic has occurred over a relatively short period of time.[14] Environmental factors in obesity have recently gained attention with the hypothesis that the correlation between increasing incidence of obesity and increased chemical exposure is possibly causally related.[15] The term “obesogen” is now used to represent an environmental pollutant that adversely affects various aspects of adipose tissue functions.[15] The “obesogen hypothesis” postulates that chemical pollutants are able to promote obesity by altering homeostatic metabolic set-points and disrupting appetite controls.[16] Many chemicals have been suggested as possible obesogens, including various endocrine disrupting compounds (e.g., bisphenol A, phthalates, polybrominated diphenyl ethers, and perfluorocompounds) because they can disrupt hormonal signaling relevant to adipose tissue biology.[16]
Mercury (Hg) is also a powerful endocrine disrupter. As outlined by Tan et al.,[17] Hg has five main endocrine-disruptive outcomes: (1) Endocrine system accumulation; (2) endocrine tissue cytotoxicity; (3) hormone concentration changes; (4) sex hormones interactions; and (5) upregulation or downregulation of enzymes within the steroidogenesis pathway. Importantly, increasing Hg levels have been noted in humans over the last couple of decades.[18] One source of Hg exposure in children, particularly in the 1990s, was ethyl-Hg from Thimerosal-containing vaccines. In the US during the 1990s, infants may have been exposed to bolus doses of Hg ranging from 12.5 μg Hg to 62.5 μg Hg that collectively added up to 200 μg of Hg from Thimerosal-containing childhood vaccines during the first 6 months of life. Some estimates found that more than 50% of early childhood exposure to Hg was from routine childhood vaccination.[19]
Thimerosal-containing hepatitis B vaccines (TM-HepB) were first recommended for universal administration to US newborns in 1991, with two subsequent immunizations also administered during infancy.[20] Up until March 2000, manufactured TM-HepB contained 12.5 μg Hg per dose. Therefore, children who received the three doses were exposed to 37.5 μg Hg from TM-HepB.
However, even though Hg is a known endocrine disrupter, and Hg is still used as a preservative in some vaccines today, the potential relationship between obesity and Thimerosal has not been studied. The purpose of the present study was to evaluate the potential relationship between exposure to organic Hg from TM-HepB, which were administered at specific intervals during the first 6 months of life, and the subsequent long-term risk of a child being diagnosed with obesity, by conducting a prospective, longitudinal, hypothesis-testing, case-control epidemiological study of automated medical records in the Vaccine Safety Datalink (VSD) database.
Materials and Methods
Institutional review board-approved protocol
The study protocol was approved by the US CDC, the Institutional Review Board (IRB) of Kaiser Permanente North-West (KPNW), and the IRB of Kaiser Permanente Northern California (KPNC). The data were analyzed at the secure Research Data Center of the National Center for Health Statistics in Hyattsville, Maryland. The views expressed in this study do not necessarily reflect those of the CDC or those of Kaiser Permanente.
The VSD project was created in 1991 by the National Immunization Program (NIP) of the CDC. The VSD's data collection and study methods have been previously described.[21,22,23] The project links medical event information, specific vaccine history, and selected demographic information from the computerized databases of several health maintenance organizations (HMOs).
Determining the population at risk
A cohort of over 1 million infants enrolled in the VSD project (the CDC provided data to independent investigators is updated through the end of 2000) from KPNW, Kaiser Permanente Colorado (KPC), and KPNC was examined using SAS® software (Statistical Analysis System; SAS Institute Inc., 100 SAS Campus Drive Cary, NC 27513-2414, USA). The cohort examined was restricted to the accessible records for individuals who were continuously HMO-enrolled from their date of birth and whose records specified their gender.
Determining cases
The outcome files (inpatient and outpatient diagnoses) from this population were then reviewed to find the first instance of obesity for each child, as defined by the International Classification of Disease, ninth revision (ICD-9). The obesity-associated ICD-9 diagnostic codes examined included obesity, unspecified (278.00), morbid obesity (278.01), overweight (278.02), or obesity hypoventilation syndrome (278.03). When there were multiple instances of the same diagnosis in a child, only the first instance was counted. In addition, to ensure the potential for an association between exposure and outcome for vaccinated individuals diagnosed with obesity, only those individuals diagnosed with obesity following the vaccines under study were included as cases in the present analyses.
A total of 1869 cases diagnosed with obesity (males = 909, females = 960, male/female ratio = 0.95), born during 1991–2000, were identified. These individuals diagnosed with obesity were evaluated to determine their mean age of initial diagnosis (3.94-year-old) and the standard deviation of that mean age of initial diagnosis (2.30 years).
Determining controls
Working within the restrictions of the available data, researchers sought to identify controls without a diagnosis of obesity who would have only a minimal chance of subsequently receiving such a diagnosis (i.e., to minimize the risk of misclassification of controls). The selection criterion for these controls was that they had to have been continuously enrolled from birth for at least 6.24 years (mean age of initial diagnosis of obesity plus the standard deviation of mean age of initial diagnosis of obesity). Applying this criterion, the study identified 41115 controls without an obesity diagnosis (males = 21113, females = 20002, male/female ratio = 1.05) born during 1991–1994.
Hepatitis B vaccine exposure
The vaccine file for cases and controls was then reviewed to determine the exact dates of HepB administration. Those cases and controls who received no doses of HepB vaccine were also included in the present study. Though the level of Thimerosal in a given TM-HepB vaccine dose could vary around the target value of 25 μg Thimerosal per 0.5-mL dose, in general, this specification translated into a nominal organic-derived Hg dose of 12.5 μg Hg per injection/administration. Thus, Hg exposure was 12.5 μg Hg per dose for those receiving a pediatric TM-HepB or 0 μg Hg per dose for those receiving either combined Hemophilus influenzae type b (Hib)-HepB (a TM-free hepatitis B vaccine) or neither of the aforementioned vaccines. Among the cases and controls, the maximum exposure to Hg from pediatric HepB was 37.5 μg Hg (for children who received three doses of TM-HepB) administered within the first 6 months of life.
The HepB vaccines were chosen for this study because they start being given at the earliest time in development. Unfortunately, the current study limitations in the accessible VSD preclude studying more than one type of childhood vaccine in a given study (e.g., a joint study of the combined exposures from the “hepatitis B” and “diphtheria, tetanus and pertussis” vaccines), although studies such as this, where one of the Hep B vaccines was a combination vaccine with Hib, are allowed.
Statistical methods
In all statistical analyses, a two-sided probability value (P value) of <0.05 was considered statistically significant.
In the first set of statistical tests examining different levels of organic Hg exposure from up to three doses of TM-HepB, the Fisher's exact test contained in the SAS® software was utilized for statistical analyses. The frequency of receiving maximum exposure(s) to organic Hg from TM-HepB vaccine(s) in comparison to the frequency of receiving 0 μg Hg from TM-free hepatitis B vaccine (TM-free-HepB) or no HepB vaccine within the first month of life (Test I), within the first two months of life (Test II), and within the first six months of life (Test III), among cases diagnosed with obesity and controls, was determined. The aforementioned statistical tests were repeated after separating the data so that male cases diagnosed with obesity were compared to male controls (Tests IV–VI) and female cases diagnosed with obesity were compared to female controls (Tests VII–IX). The overall null hypotheses for each of the case-control statistical tests were that there would be no difference in the frequency of exposure to organic Hg from TM-HepB between the cases (who were diagnosed with obesity) and the controls (who were not diagnosed with obesity). In the second set of statistical tests examining the potential dose-response relationship between increasing levels of organic Hg exposure from TM-HepB, the logistic regression and Fisher's exact tests contained in StatsDirect (version 2.8.0) (Stats Direct Ltd, 9 Bonville Chase, Altrincham, CHESHIRE WA14 4QA, UK) were utilized for statistical analyses. The logistic regression test was employed for each of the cases-versus-controls comparisons examined to determine the OR per μg Hg exposure from TM-HepB vaccines that were administered within the first 6 months of life. In addition, the Fisher's exact statistical test was used to determine the discrete OR for exposure to nominally 12.5 μg Hg, 25 μg Hg, or 37.5 μg Hg from TM-HepB vaccine doses in comparison to 0 μg Hg from TM-free-HepB vaccine doses and/or no HepB vaccines within the first 6 months of life among cases compared to controls. The overall null hypothesis for each of these comparisons was that there would be no difference in the frequency of exposure per μg Hg from TM-HepB vaccines between cases and the controls.
Results
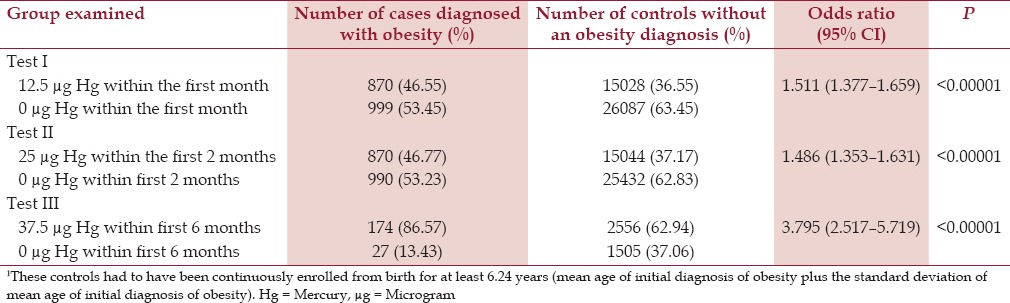
The effects observed ranged from the smallest (OR = 1.49, P < 0.00001) among cases in comparison to controls following receipt of 25 μg Hg from two doses of TM-HepB vaccine in comparison to 0 μg Hg from TM-free-HepB vaccine doses and/or no HepB vaccines, which for cases and controls were administered within the first 2 months of life, to the largest (OR = 3.79, P < 0.00001) among cases in comparison to controls following receipt of 37.5 μg Hg from the three doses of TM-HepB vaccine in comparison to 0 μg Hg from TM-free-HepB vaccine doses and/or no HepB vaccines, which for cases and controls were administered within the first 6 months of life. The relationship between cases diagnosed with obesity in comparison to controls, based on their respective doses of Hg from TM-HepB vaccines at several specific points within the first 6 months of life, as determined in Tests I–III is shown in Table 1.
Table 1.
A summary of exposure to Hg from TM-HepB vaccine administration among cases diagnosed with obesity in comparison to controls1 within the VSD database
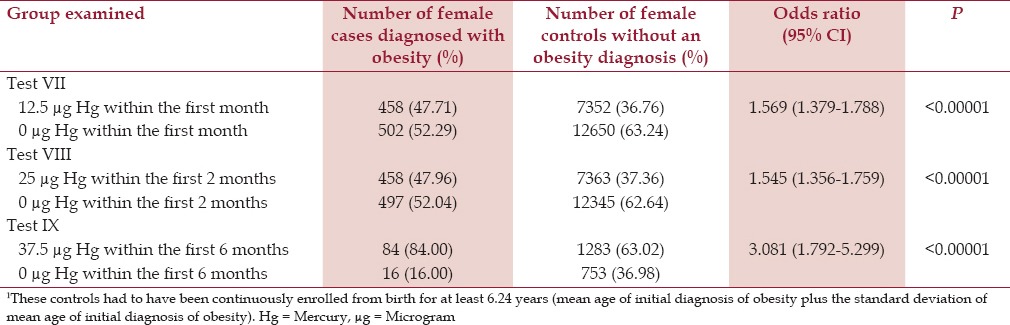
It was found that for males the effect was largest (OR = 4.833, P < 0.00001) for male cases in comparison to male controls for receipt of 37.5 μg Hg from three doses of TM-HepB vaccine in comparison to 0 μg Hg from TM-free-HepB vaccines and/or no HepB vaccines, which were administered to cases and controls within the first 6 months of life. Similarly, it was found for females the effect was largest (OR = 3.081, P < 0.00001) for female cases in comparison to female controls for receipt of 37.5 μg Hg from three doses of TM-HepB vaccine in comparison to 0 μg Hg from the TM-free-HepB vaccine and/or no HepB vaccines, which were administered within the first 6 months of life. The findings for male cases diagnosed with obesity in comparison to male controls (Tests IV–VI) and female cases diagnosed with obesity in comparison to female controls (Tests VII–IX), at the three dose levels of Hg from TM-HepB vaccines, which were administered at specific points within the first 6 months of life are shown in Tables 2 and 3, respectively.
Table 2.
A summary of exposure to organic Hg from TM-HepB vaccine administration among male cases diagnosed with obesity in comparison to male controls1 within the VSD database
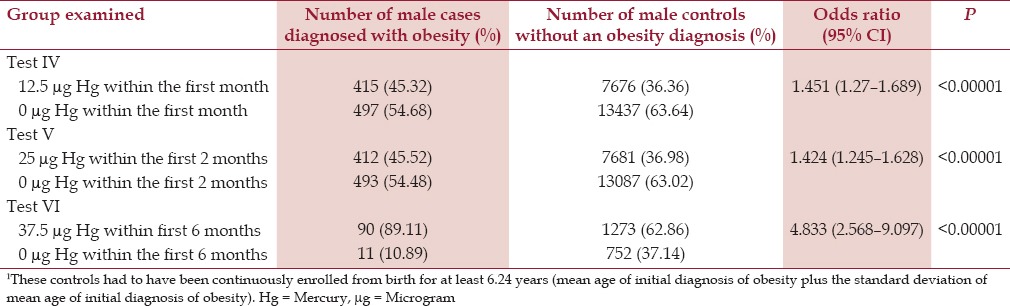
Table 3.
A summary of exposure to organic Hg from TM-HepB vaccine administration among female cases diagnosed with obesity in comparison to female controls1 within the VSD database
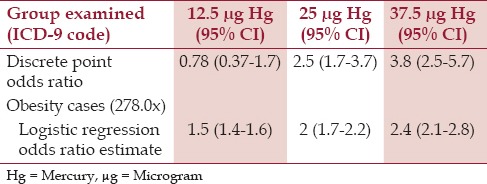
Based on logistic-regression analysis, cases were significantly more likely than controls to receive higher doses of Hg from TM-HepB vaccines, which were administered within the first 6 months of life (OR = 1.038 per μg Hg, P < 0.00001) [Table 4]. When evaluating exposure to Hg from TM-HepB vaccines, which were administered within the first 6 months of life, among cases diagnosed with obesity in comparison to controls, the discrete-point OR were similar to those predicted by the logistic-regression OR estimates [Table 5].
Table 4.
A summary of the logistic-regression results of the effect of organic Hg exposure from TM-HepB vaccine administration within the first six months of life for the cases diagnosed with obesity in comparison to controls1
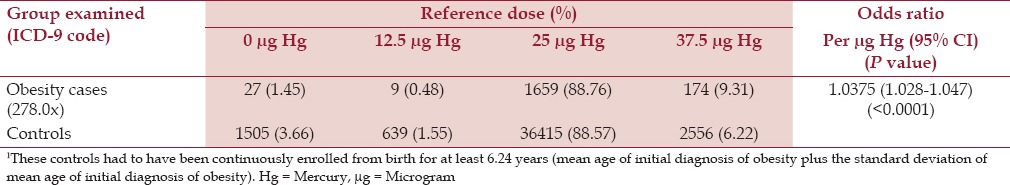
Table 5.
A summary of exposure to organic Hg from TM-HepB vaccine administration within the first six months of life for the cases diagnosed with obesity and the controls by both the discrete-point odds-ratio estimates and the logistic regression odds-ratio estimates
Discussion
The results of the present study support the hypothesis that the risk of obesity in the US during the 1990s was at least partially contingent on the environmental exposure of organic Hg exposure from TM-HepB vaccines administered within the first 6 months of life. It was observed in the present study that increasing organic Hg exposure from TM-HepB vaccines administered to both males and females within specific intervals within the first 6 months of life significantly increased the long-term risk of a child being diagnosed with obesity. Further, the risk was modified by the timing of administration and the total dose a child received.
It is also important to note that recent time-trend analyses of the prevalence of childhood obesity in the US during the 1980–2000s support the epidemiological findings of the present study. Specifically, organic Hg exposure significantly increased in the US during the 1980s and 1990s as additional Thimerosal-containing vaccines were routinely recommended for administration to infants, and, subsequently, decreased following the July 7, 1999 recommendation by the US Public Health Service (USPHS) and American Academy of Pediatrics (AAP) that Thimerosal should be removed from all vaccines as reduced Thimerosal-containing vaccines were approved by the US Food and Drug Administration (FDA) in the early 2000s.[24] It was observed in time-trend analyses of the prevalence of childhood obesity in the US following the epidemic rise in the prevalence of diagnosed childhood obesity during the 1980–1990s, the prevalence of childhood obesity remained stable between 2003–2004, 2009–2010, and 2011–2012. It was also observed that there was a significant decrease in obesity among 2 to 5-year-old children during this latter period.[25] Similarly, between 2008 and 2011, significant decreases were observed among preschool-aged children participating in federal nutrition programs in 18 states and the US Virgin Islands.[25] In addition, between 2003 and 2008, a decrease in obesity prevalence among children younger than 6-year-old was reported in a multisite pediatric practice in eastern Massachusetts.[26] These time-trend observations in the prevalence of childhood obesity in the US roughly correlate with the introduction/reduction of Thimerosal in vaccines.
In considering the biological plausibility of the results observed in the present study, investigators previously observed that adipogenesis, the process of adipocyte development from stem-cell precursors (and mediated by glucocorticoids), occurs primarily during late fetal and early postnatal life,[27,28] which thus represents a window of vulnerability to stressors such as TM in infant vaccinations. As mentioned in the Introduction, Hg is a powerful endocrine disrupter that accumulates in and is toxic to the endocrine system and is known to disrupt hormone concentration and enzymes within the steroidogenesis pathway.[17,29] In addition, investigators have observed that blood Hg levels were significantly interrelated with overweight and obesity,[30] BMI,[31] and waist circumference values.[31]
Other investigators observed that obese and overweight persons are characterized by higher hair Hg levels.[32] Furthermore, investigators observed a significant correlation of hair Hg content, age, and body weight.[33] Other investigators observed that, among individuals coexposed to Hg and dioxins, a significant association was observed between blood Hg levels and waist circumference.[34]
As mentioned in the Introduction, it has been estimated that children receive approximately half of their early childhood Hg exposure from Thimerosal in vaccines and that it is in the form of ethyl-Hg. Ethyl-Hg is Hg with two carbon units attached to it and it is a “designer-compound” in the sense that it is man-made and not found in nature. Methyl-Hg, a related form of organic Hg, has one carbon unit attached and is found in nature. The two forms of Hg differ somewhat in their effects and levels of toxicity; however, once they enter the brain, their level of toxicity has been shown to be similar. Their cyotoxic actions, their depletion of glutathione concentrations, and their promotion of oxidative stress are similar and comparable.[35] Often questions arise as to the source and form of Hg (e.g., ethyl- or methyl-Hg or inorganic Hg) in attempts to discern the relative source of contribution of damage from exposure. However, all forms of Hg are toxic and they can also convert to another form. For example, in a study conducted by Rodrigues et al., the researchers examined the distribution of Hg (as methyl-, ethyl-, and inorganic Hg) in rat tissues (brain, heart, kidney, and liver) and blood following administration of TM or methyl-Hg. Of the total Hg found in the brain after TM exposure, 63% was in the form of inorganic Hg, 13.5% as ethyl-Hg, and 23.7% as methyl-Hg.[36] In other words, the ethyl Hg did not remain purely as ethyl-Hg. Most of what these investigators found in the brain long-term following exposure with Thimerosal was inorganic Hg, and the same phenomena has been observed for methyl-Hg exposure. It was specifically shown in a number of previous studies that exposure to methyl-Hg resulted in the long-term accumulation of inorganic Hg in the brain.[37,38] Once organic Hg compounds have been converted to inorganic Hg in the brain, it can remain in the brain for decades.[38]
Strengths/limitations
The strength of the VSD is revealed in the present study because the observations made were based on a retrospective assessment of prospectively collected medical records. Any potential independent variables that might have been associated with either enrollment or healthcare-seeking behaviors were moot because all cases were required to be enrolled from birth until obesity was diagnosed, and all controls had to be enrolled from birth for a time period sufficient to reduce the chance that an obesity diagnosis would emerge during follow-up. In addition, vaccinated cases diagnosed with obesity were specifically evaluated to ensure that only those cases diagnosed with obesity following their HepB vaccines doses were considered in the present analyses.
That the VSD data were collected independently of the present study is another strength. The VSD data records were collected as part of the routine healthcare that patients received through participation with their respective HMOs, and as such, the healthcare providers were not contemplating any possible associations between vaccine exposures and potential health outcomes.
An additional strength of the present study was that the specific methods employed to evaluate the hypothesis were also able to exploit recommendations for the timing of vaccine administration that varied widely. Specifically, differences in the cumulative doses of organic Hg received at specific intervals during the infant period were evaluated based upon the wide-ranging recommendations for routine HepB vaccination. In 1991, the Advisory Committee on Immunization Practices (ACIP) recommended that infants should receive their HepB doses as follows: first dose between birth and 2 months of age, second dose between 1 and 4 months of age, and third dose between 6 and 18 months of age.[20] Importantly, the differences in organic Hg exposures observed in all statistical tests within the present study were not the result of a small group of children receiving anomalous exposures to vaccines. Instead, the statistical tests assessed varying levels of organic Hg exposure which resulted from the varying windows recommended for administration of HepB vaccines during the first 18 months of life.
A further strength of the present study was that adequate follow-up was employed to ensure that most subjects in the control group were true controls, i.e., unlikely to be subsequently diagnosed with childhood obesity. This was achieved by setting an a priori requirement that, to be a valid control, a child had to be continuously enrolled in the VSD from birth until the child attained the mean age of initial obesity diagnosis plus the standard deviation for that mean age, which was 6.24 years of age based on the mean and standard deviation values computed for the cases. Statistically, setting the follow-up age for the control group in this manner assured a less than 16% chance that some of the controls might subsequently be diagnosed with obesity. Ideally, a longer follow-up period would have further reduced this misclassification risk. However, the limitations on the VSD patient data records that were available for review precluded a longer follow-up period because the VSD data available for examination ended in 2000. It is important to ensure that most controls will have little risk of subsequently being diagnosed, because, for outcomes like obesity that have a wide onset window, the statistical noise in the exposure signal will be minimized when a sufficient follow-up is used.
This phenomenon is illustrated by the following example from this dataset. It was observed that cases diagnosed with obesity were significantly more likely (OR = 1.511, P < 0.00001) than controls (continuously enrolled in the VSD from birth until the child attained the mean age of initial obesity diagnosis plus the standard deviation for that mean age, which was 6.24 years of age based on the distribution statistics for the cases) to have received 12.5 μg Hg in comparison to 0 μg Hg within the first month of life. By reducing the length of continuous enrollment of controls in the VSD from birth until the child attained the mean age of initial obesity diagnosis (i.e., cases and controls were followed for the same length of time from birth), which was 3.94 years of age, an 18% decrease was found in the OR of cases diagnosed with obesity being more likely (OR = 1.231, P < 0.0001) than controls to have received 12.5 μg Hg in comparison to 0 μg Hg within the first month of life. This occurred by increasing the likelihood that, with additional follow-up, the controls would be diagnosed with obesity, and thus, were misclassified with respect to their eventual disease status. Finally, by reducing the length of continuous enrollment of the controls in the VSD from birth until the child attained the mean age of initial obesity diagnosis minus the standard deviation of initial obesity diagnosis, which was 1.64 years of age, there was an even greater likelihood that, with additional follow-up, the controls would be diagnosed with obesity. This reduction in follow-up resulted in the OR of cases being diagnosed with obesity being no more likely (OR = 0.950, P > 0.25) than controls to have received 12.5 μg Hg in comparison to 0 μg Hg within the first month of life.
However, the results of the present study potentially have a number of limitations. Theoretically, the observed results may have occurred from unknown biases or cofounders present in the datasets examined. However, this seems unlikely because other control outcomes that have no biologically plausible link to postnatal organic Hg exposure were examined, such as a diagnosis of congenital anomalies (ICD-9 code: 759.9), using the same VSD database and methodology employed for obesity, and no similar patterns of significant associations were observed for these outcomes. For example, congenital anomaly cases and controls were found to be similarly exposed to 12.5 μg Hg from a TM-HepB vaccine dose administered in the first month of life or 0 μg Hg either from TM-free-HepB vaccine or no HepB vaccine administration in the first month of life (OR = 1.03, P > 0.50).
A further theoretical limitation of the present study is that obesity is influenced by many environmental factors mainly calorie intake and physical inactivity. It is possible that children receiving additional doses of organic Hg from TM-HepB vaccination may have a different socioeconomic environment from those receiving no organic Hg from TM-HepB vaccinations or no HepB vaccines, which are closely related to calorie intake and/or physical inactivity. In considering this theoretical limitation, it is important to realize that all of the cases and controls examined were HMO-enrolled from birth for significant periods of time. As such, it is to be expected that the individuals examined in the present study had enough economic means to purchase healthcare insurance for many years, and thus, simple economic factors such as overt poverty that may be associated with reduced caloric intake and reduced vaccination rate should not have influenced the findings. Furthermore, it may be hypothesized that individuals going to their healthcare providers in order to receive additional immunizations should also receive medical guidance/interventions to help identify and reduce risk factors associated with an obesity diagnosis, and hence, they should be less likely to be eventually diagnosed with obesity than individuals who go less frequently to their healthcare providers because of receiving fewer immunizations.
Another theoretical limitation of the present study is that the results observed for obesity may be due to statistical chance. However, such a possibility would be unlikely given the limited number of statistical tests performed, the highly significant results observed, and the consistency in the direction and magnitude of the results observed.
Still other theoretical limitations of the present study include the possibilities that healthcare providers may have misdiagnosed some individuals, or some vaccine exposures may not have been appropriately recorded or classified. These possibilities should not have affected the results significantly because both cases and controls should have been affected similarly. Moreover, misclassification would tend to bias the results toward the null hypothesis because such effects would result in individuals being placed in the wrong exposure and/or outcome categories, and this would result in decreased statistical power to determine true potential exposure-outcome relationships.
Another potential theoretical limitation of the present study is that exposures to other sources of Hg were not evaluated. The children examined in the present study very likely incurred other exposures to Hg from other TM-containing vaccines, breastfeeding, formula feeding, and, possibly, dental amalgams, fish, or other environmental sources. While these other sources of Hg may play a significant role in the pathogenesis of obesity, such exposures, which were not accounted in this study, would actually tend to bias the results toward the null hypothesis by confounding the Hg exposure classifications examined. For example, individuals classified as having lower organic Hg exposure from TM-containing vaccines may have actually received high doses of Hg from other sources, and individuals having higher organic Hg exposure from TM-containing vaccines may have actually received low doses of Hg from other sources, with the net result tending to minimize the magnitude of the associations observed.
An additional potential theoretical limitation is that other types of TM-containing vaccines were not studied. Unfortunately, current study limitations in accessing the VSD preclude us from studying more than one type of childhood vaccine in a given study. As a consequence, HepB was chosen for this study because it is recommended for administration starting at the earliest time in postnatal development (i.e., the day of birth) and may potentially have the largest impact on subsequent childhood development.
The current study also suffers from the potential theoretical limitation that the analyses were not conducted to explore the precise timing and cumulative doses of organic Hg from all TM-containing childhood vaccines associated with maximum potential adverse consequences. In future studies, it would be worthwhile to explore these precise timing and cumulative-dose phenomena. In addition, evaluating other metabolic outcomes, as well as other covariates such as race, birth weight, etc., that may affect the magnitude of the adverse effects found, would be valuable.
Finally, another potential theoretical limitation is that the population within the VSD is not representative of the general US population, and as a consequence, the observed phenomena may not be generalizable to the general US population. A recent study by the US CDC specifically examined this potential theoretical limitation regarding the VSD.[39] They determined that the VSD data is representative of the general population on several key demographic and socioeconomic variables, and concluded that the VSD data is large enough to ensure significant representation of the general US population.
Conclusion
In summary, applying a hypothesis-testing, epidemiological methodology to the VSD database, organic Hg exposure from TM-containing childhood vaccines was determined to be a highly significant risk factor for the subsequent diagnosis of childhood obesity. Furthermore, early-life exposure to organic Hg is biologically plausibly linked to an increased risk of an obesity diagnosis. It is apparent that organic Hg exposure from TM-containing childhood vaccines may be an important environmental factor in the recent rapid increase in childhood obesity in the US, and this may have important consequences for the long-term risk of such children developing metabolic syndrome in the future. The results of this study support the theory that TM is an obesogen, however, additional studies should further explore the phenomena observed in the present study.
It is important to note that these findings continue to have important relevance in preventive medicine today because despite a call for the removal of TM from all vaccines in the US on July 7, 1999 by the AAP and USPHS, this dosing pattern continues unabated in many developing nations to the present day and many children in the US continue to receive significant doses of Hg from the routinely recommended administration of TM-containing influenza vaccines (where more than 50% of all doses of influenza vaccine continue to contain 0.01% TM).[40,41,42,43,44] The findings of this study suggest that the removal of Hg from vaccines could influence the rates of childhood obesity and all the medical complications that are subsequent to childhood obesity.
Routine childhood vaccination is an important public health tool to reduce the morbidity and mortality associated with infectious diseases.[45] However, the addition of TM to vaccines, as a preservative, needs to be reconsidered in light of these and other findings.
Financial support and sponsorship
Nil.
Conflicts of interest
All but one of the authors (KGH) have been involved in vaccine/biologic litigation, but none involving Thimerosal exposure and obesity.
Acknowledgements
This study was financially supported by the Dwoskin Family Foundation, the Selz Foundation, the Institute of Chronic Illnesses, Inc., and CoMeD, Inc.
References
- 1.Barlow SE Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120:S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 2.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The Bogalusa Heart Study. J Pediatr. 2007;150:12–7. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 3.Whitlock EP, Williams SB, Gold R, Smith PR, Shipman SA. Screening and interventions for childhood overweight: A summary of evidence for the US Preventive Services Task Force. Pediatrics. 2005;116:e125–44. doi: 10.1542/peds.2005-0242. [DOI] [PubMed] [Google Scholar]
- 4.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–48. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland ER. Obesity and asthma. Immunol Allergy Clin North Am. 2008;28:589–602. doi: 10.1016/j.iac.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor ED, Theim KR, Mirch MC, Ghorbani S, Tanofsky-Kraff M, Adler-Wailes DC, et al. Orthopedic complications of overweight in children and adolescents. Pediatrics. 2006;117:2167–74. doi: 10.1542/peds.2005-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swartz MB, Puhl R. Childhood obesity: A societal problem to solve. Obesity Rev. 2003;4:57–71. doi: 10.1046/j.1467-789x.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 8.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91:1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;37:869–73. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 10.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med. 1993;22:167–77. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 11.National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services; 1998. [Google Scholar]
- 12.Pan L, Blanck HM, Sherry B, Dalenius K, Grummer-Strawn LM. Trends in the Prevalence of Extreme Obesity Among US Preschool-Aged Children Living in Low-Income Families, 1998–2010. JAMA. 2012;308:2563–5. doi: 10.1001/jama.2012.108099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among U.S. children and adolescents, 1999–2010. JAMA. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demerath EW. The genetics of obesity in transition. Coll Antropol. 2012;36:1161–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Decherf S, Demeneix BA. The obesogen hypothesis: A shift of focus from the periphery to the hypothalamus. J Toxicol Environ Health B Crit Rev. 2011;14:423–48. doi: 10.1080/10937404.2011.578561. [DOI] [PubMed] [Google Scholar]
- 16.Grün F. Obesogens. Curr Opin Endocrinol Diabetes Obes. 2010;17:453–9. doi: 10.1097/MED.0b013e32833ddea0. [DOI] [PubMed] [Google Scholar]
- 17.Tan SW, Meiller JC, Mahaffey KR. The endocrine effects of mercury in humans and wildlife. Crit Rev Toxicol. 2009;39:228–69. doi: 10.1080/10408440802233259. [DOI] [PubMed] [Google Scholar]
- 18.Laks DR. Assessment of chronic mercury exposure within the U.S. population, National Health and Nutrition Examination Survey, 1999-2006. Biometals. 2009;22:1103. doi: 10.1007/s10534-009-9261-0. [DOI] [PubMed] [Google Scholar]
- 19.Bingham M, Copes R. Thiomersal in vaccines: Balancing the risk of adverse effects with the risk of vaccine-preventable disease. Drug Saf. 2005;28:89–101. doi: 10.2165/00002018-200528020-00001. [DOI] [PubMed] [Google Scholar]
- 20.Hepatitis B virus: A comprehensive strategy for eliminating transmission in the United States of through universal childhood vaccination: Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep. 1991;40(No. RR-13):1–25. [PubMed] [Google Scholar]
- 21.Chen RT, DeStefano F, Davis RL, Jackson LA, Thompson RS, Mullooly JP, Black SB, et al. The Vaccine Safety Datalink: Immunization research in health maintenance organizations in the USA. Bull World Health Organ. 2000;78:186–94. [PMC free article] [PubMed] [Google Scholar]
- 22.Chen RT, Glasser JW, Rhodes PH, Davis RL, Barlow WE, Thompson RS, et al. Vaccine Safety Datalink project: A new tool for improving vaccine safety monitoring in the United States. The Vaccine Safety Datalink Team. Pediatrics. 1997;99:765–73. doi: 10.1542/peds.99.6.765. [DOI] [PubMed] [Google Scholar]
- 23.Wassilak SG, Glasser JW, Chen RT, Hadler SC. Utility of large-linked databases in vaccine safety, particularly in distinguishing independent and synergistic effects. The Vaccine Safety Datalink Investigators. Ann N Y Acad Sci. 1995;754:377–82. doi: 10.1111/j.1749-6632.1995.tb44473.x. [DOI] [PubMed] [Google Scholar]
- 24.Geier DA, King PG, Hooker BS, Dorea JG, Kern JK, Sykes LK, et al. Thimerosal: Clinical, epidemiologic and biochemical studies. Clin Chim Acta. 2015;444:212–20. doi: 10.1016/j.cca.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC). Vital signs: Obesity among low-income, preschool-aged children-United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2013;62:629–34. [PMC free article] [PubMed] [Google Scholar]
- 26.Wen X, Gillman MW, Rifas-Shiman SL, Sherry B, Kleinman K, Taveras EM. Decreased prevalence of obesity among young children in Massachusetts from 2004 to 2008. Pediatrics. 2012;12:823–31. doi: 10.1542/peds.2011-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Entringer S, Wadhwa PD. Developmental programming of obesity and metabolic dysfunction: Role of prenatal stress and stress biology. Nestle Nutr Inst Workshop Ser. 2013;74:107–20. doi: 10.1159/000348454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens JM. The fat controller: Adipocyte development. PLoS Biol. 2012;10:e1001436. doi: 10.1371/journal.pbio.1001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geier MR, Geier DA. The potential importance of steroids in the treatment of autistic spectrum disorders and other disorders involving mercury toxicity. Med Hypotheses. 2005;64:946–54. doi: 10.1016/j.mehy.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Cho S, Jacobs DR, Jr, Park K. Population correlates of circulating mercury levels in Korean adults: The Korea National Health and Nutrition Examination Survey IV. BMC Public Health. 2014;14:527. doi: 10.1186/1471-2458-14-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang D, Lee K. The relationships between blood mercury concentration and body composition measures using 2010 Korean National Health and Nutrition Examination Survey. Korean J Obes. 2013;22:237–42. [Google Scholar]
- 32.Skalnaya MG, Tinkov AA, Demidov VA, Serebryansky EP, Nikonorov AA, Skalny AV. Hair toxic element content of adult men and women in relation to body mass index. Biol Trace Elem Res. 2014;161:13–9. doi: 10.1007/s12011-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 33.Tinkob AA, Ajsuvakova OP, Skalnaya MG, Popova EV, Sinitskii AI, Nemereshina ON, et al. Mercury and metabolic syndrome: A review of experimental and clinical observations. Biometals. 2015;28:231–54. doi: 10.1007/s10534-015-9823-2. [DOI] [PubMed] [Google Scholar]
- 34.Chang JW, Chen HL, Su HJ, Liao PC, Guo HR, Lee CC. Simultaneous exposure of non-diabetics to high levels of dioxins and mercury increases their risk of insulin resistance. J Hazard Mater. 2011;185:749–55. doi: 10.1016/j.jhazmat.2010.09.084. [DOI] [PubMed] [Google Scholar]
- 35.Ueha-Ishibashi T, Oyama Y, Nakao H, Umebayashi C, Nishizaki Y, Tatsuishi T, et al. Effect of thimerosal, a preservative in vaccines, on intracellular Ca2+concentration of rat cerebellar neurons. Toxicology. 2004;195:77–84. doi: 10.1016/j.tox.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues JL, Serpeloni JM, Batista BL, Souza SS, Barbosa F., Jr Identification and distribution of mercury species in rat tissues following administration of thimerosal or methylmercury. Arch Toxicol. 2010;84:891–6. doi: 10.1007/s00204-010-0538-4. [DOI] [PubMed] [Google Scholar]
- 37.Charleston JS, Body RL, Bolender RP, Mottet NK, Vahter ME, Burbacher TM. Changes in the number of astrocytes and microglia in the thalamus of the monkey Macaca fascicularis following long-term subclinical methylmercury exposure. Neurotoxicology. 1996;17:127–38. [PubMed] [Google Scholar]
- 38.Eto K, Takizawa Y, Akagi H, Haraguchi K, Asano S, Takahata N, Tokunaga H. Differential diagnosis between organic and inorganic mercury poisoning in human cases-The pathologic point of view. Toxicol Pathol. 1999;27:664–71. doi: 10.1177/019262339902700608. [DOI] [PubMed] [Google Scholar]
- 39.Sukumaran L, McCarthy NL, Li R, Weintraub ES, Jacobsen SJ, Jambdige SJ, et al. Demographic characteristics of members of the Vaccine Safety Datalink (VSD): A comparison with the United States population. Vaccine. 2015;33:4446–50. doi: 10.1016/j.vaccine.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bridges CB, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2002;51(RR-03):1–31. [PubMed] [Google Scholar]
- 41.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2004;53(RR-06):1–40. [PubMed] [Google Scholar]
- 42.Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, et al. Advisory Committee on Immunization Practices. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR10):1–42. [PubMed] [Google Scholar]
- 43.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and Control of Seasonal Influenza with Vaccines Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR08):1–52. [PubMed] [Google Scholar]
- 44.Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46(RR-9):1–25. [PubMed] [Google Scholar]
- 45.Geier MR, Geier DA. The state of polio vaccination in the world: The case for continuing routine vaccination. Toxicol Mech Methods. 2002;12:221–8. doi: 10.1080/15376520208951158. [DOI] [PubMed] [Google Scholar]


