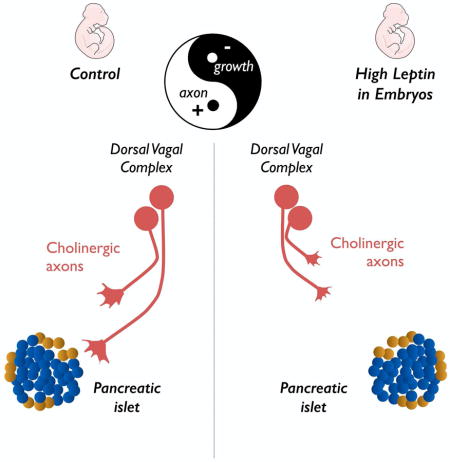
Summary
The autonomic nervous system plays a critical role in glucose metabolism through both its sympathetic and parasympathetic branches, but the mechanisms that underlie the development of the autonomic innervation of the pancreas remain poorly understood. Here, we report that cholinergic innervation of pancreatic islets develops during mid-gestation under the influence of leptin. Leptin-deficient mice display a greater cholinergic innervation of pancreatic islets beginning in embryonic life and this increase persists into adulthood. Remarkably, a single intracerebroventricular injection of leptin in embryos caused a permanent reduction in parasympathetic innervation of pancreatic β cells and long-term impairments in glucose homeostasis. These developmental effects of leptin involve a direct inhibitory effect on the outgrowth of preganglionic axons from the hindbrain. These studies reveal an unanticipated regulatory role of leptin on the parasympathetic nervous system during embryonic development and may have important implications for our understanding of the early mechanisms that contribute to diabetes.
Graphical Abstract
Introduction
The autonomic nervous system is essential for the regulation of critical physiological functions including cardiac output, body temperature and blood glucose levels (Schmidt and Thews, 1989). Classically, the autonomic nervous system is divided into two anatomically and functionally distinct branches: the sympathetic nervous system and the parasympathetic nervous system. Consistent with a role of the autonomic nervous system in the maintenance of glucose homeostasis, sympathetic and parasympathetic neurons densely innervate the endocrine pancreas and, in particular, the islets of Langerhans (Ahrén, 2000; Thorens, 2014; Woods and Porte, 1974). Notably, the sympathetic and parasympathetic systems exert distinct actions on pancreatic islets: whereas sympathetic nerve stimulation inhibits insulin secretion from pancreatic β cells and promotes glucagon secretion from pancreatic α cells, parasympathetic input stimulates the secretion of both glucagon and insulin (Bloom and Edwards, 1975). Despite the critical role of the autonomic nervous system in the regulation of islet hormone secretion, relatively little is known about the mechanisms involved in the development of the autonomic innervation of the pancreas.
The parasympathetic fibers that innervate the endocrine pancreas originate primarily from neurons in the intrapancreatic ganglia, which receive preganglionic inputs from the hindbrain via the vagus nerve (Fox and Powley, 1986). In contrast, sympathetic fibers within in the pancreas originate from preganglionic cell bodies that are located in the thoracic and upper lumbar segments of the spinal cord (Furuzawa et al., 1996). Both the parasympathetic and sympathetic systems develop before birth, and autonomic nerve fibers can be observed in various peripheral organs as early as mid-gestation (Black, 1978; Burris and Hebrok, 2007; Rinaman and Levitt, 1993). Notably, the onset of autonomic innervation coincides with stages of rapid growth, differentiation, and maturation in the embryonic pancreas (Jørgensen et al., 2007).
In addition to being controlled by the autonomic nervous system, pancreatic function is also regulated by hormonal factors, including the adipocyte-derived hormone leptin. Although leptin was initially described as a regulator of energy balance and neuroendocrine function, it has subsequently been shown to influence sympathetic tone and glucose homeostasis (Enriori et al., 2011; Marino et al., 2011; Morton and Schwartz, 2011; Simonds et al., 2014). The effects of leptin are now recognized to be mediated by a distributed neural network that includes neurons located in both the hypothalamus and hindbrain (Gautron et al., 2015; Grill and Hayes, 2012; Leinninger and Myers, 2008). In addition to the physiological effects it exerts during adult life, leptin provides trophic support to hypothalamic neural projections during development (Bouret et al., 2004). However, whether leptin influences the development of non-hypothalamic circuits remains unknown.
In this report, we investigated the development of the parasympathetic innervation of the endocrine pancreas. We demonstrated that exposure of the embryonic brain to leptin during a discrete developmental period results in permanent alterations in the cholinergic innervation of pancreatic β cells as well as long-term perturbations in glucose homeostasis. Furthermore, we showed that leptin directly inhibits cholinergic neurite outgrowth from the hindbrain, suggesting that the developmental effects of leptin on parasympathetic projections involves, at least in part, a direct effect on preganglionic neurons within the hindbrain.
Results
Ontogeny of the parasympathetic innervation of pancreatic islets
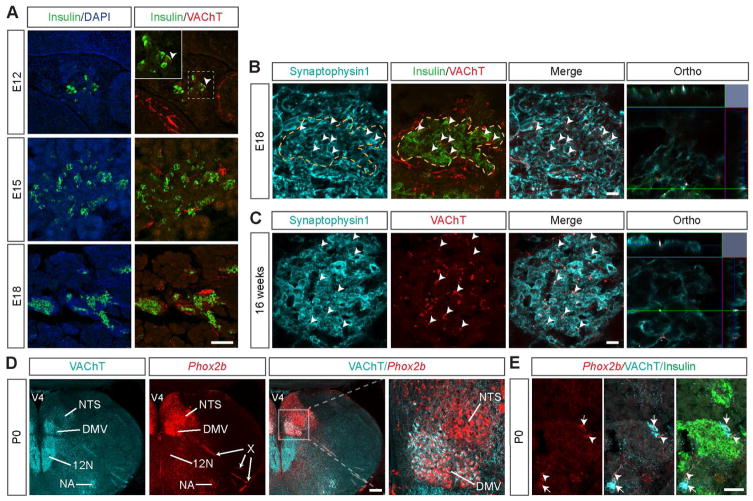
To begin to examine the development of the parasympathetic innervation of the pancreas, we first performed immunohistochemical labeling for the vesicular acetylcholine transporter (VAChT), a well-known marker of cholinergic fibers (Weihe et al., 1996), in mouse embryonic pancreatic tissues. To visualize β cells, we also immunostained for insulin. VAChT-positive fibers were found in the vicinity of pancreatic insulin-containing cells as early as embryonic day (E) 12 (Figure 1A). However, at this age, only a few cells expressed insulin, as previously described (Jørgensen et al., 2007). The relative number of insulin-containing cells was markedly increased at E15, as was the density of VAChT-positive fibers surrounding β cells (Figure 1A). By E18, the insulin-expressing cells formed adult-like islets, and the density of cholinergic axons in β-cell islets had substantially increased (Figure 1A). The VAChT-positive axons appeared to make synaptic contacts within the islets because those axons also expressed synaptophysin1, a major synaptic vesicle membrane protein (Figures 1B and 1C). As expected, adult islets were densely innervated by VAChT-immunopositive fibers (Figure 1C). Notably, projections containing tyrosine hydroxylase (TH), a marker of sympathetic neurons, developed in a pattern that mirrored that of the innervation of VAChT terminals (Figure S1A)
Figure 1. Ontogeny of the parasympathetic innervation of pancreatic islets.
(A) Confocal images of VAChT-immunopositive fibers (red fluorescence) in pancreatic islets (insulin, green fluorescence) of E12, E15 and E18 WT embryos. Arrowheads point to VAChT fibers in close proximity to insulin-positive cells. Representative images showing VAChT immunoreactivity (red fluorescence) and its colocalization with synaptophysin1 labeling (turquoise fluorescence) in pancreatic β cells (insulin, green fluorescence) of (B) E18 WT mouse embryos and (C) 16-week-old ob/ob mice. Arrowheads point to double-labeled inputs (white labeling). (D) Representative images of genetically labeled cell bodies (red fluorescence) in the hindbrain of P0 Phox2b-Cre-tdTomato mice. (E) Detail of Phox2b-Cre-tdTomato innervation of pancreatic islets (insulin, green fluorescence). Arrowheads point to td-Tomato-positive fibers that are also VAChT-positive. Arrows point to intrapancreatic ganglia containing tdTomato. DMV, dorsomedial nucleus of the vagus nerve; NA, nucleus ambiguus; NTS, nucleus of the solitary tract; X, vagus nerve; 12N, hypoglossal nucleus; V4, fourth ventricle. Scale bars, 100 um (A); 20 um (B–C); 200 um (D); and 50 um (E).
The cholinergic fibers that innervate the pancreas mainly originate from the dorsomedial nucleus of the vagus nerve (DMV). Most of these fibers run within the vagus nerve and terminate on the pancreatic ganglia, where they form synapses with interneurons (Fox and Powley, 1986). To assess the proportion of cholinergic preganglionic fibers that directly innervate the embryonic pancreatic ganglia and islets, we used a transgenic mouse in which the fluorescent protein tdTomato is selectively expressed in cell bodies and neuronal processes of a subset of nucleus of the solitary tract (NTS)/DMV neurons (Phox2b-Cre; tdTomato mice) (Figure 1D) (Scott et al., 2011). We observed that a significant proportion of the VAChT-immunoreactive fibers in the pancreatic islets also contained the red fluorescent signal of tdTomato. In addition, a number of cholinergic intrapancreatic ganglia also contain tdTomato (Figure 1E). These data suggest that the cholinergic fibers that are found in pancreatic islets might originate from hindbrain neurons as well as intrapancreatic ganglia (Figures 1D and 1E).
Lack of leptin affects the pattern of cholinergic innervation of the pancreas
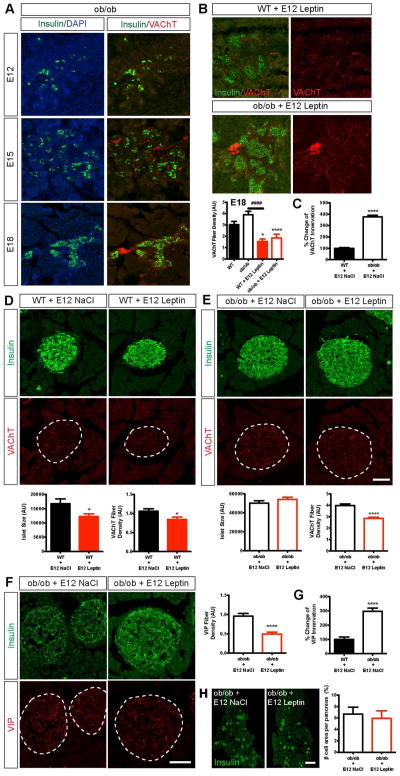
Leptin is known to regulate the activity of the autonomic nervous system during adulthood (Arteaga-Solis et al., 2013; Simonds et al., 2014) but whether leptin is involved in the development of the autonomic nervous system remains unknown. Accordingly, we next examined the density of cholinergic fibers in the islets of leptin-deficient (ob/ob) embryos. Although the overall timing of development and the distribution of VAChT fibers were relatively similar between ob/ob and WT embryos (Figures 1A and 2A), we observed clear differences in the density of fibers. There were 1.3-fold more labeled fibers in the islets of E18 ob/ob mice than in those of E18 WT mice (Figure 2B).
Figure 2. Prenatal leptin exposure impairs parasympathetic innervation of pancreatic islets.
(A) Confocal images of VAChT-positive fibers (red fluorescence) in clode proximity to insulin-positive cells (green fluorescence) in E12, E15 and E18 ob/ob embryos. (B) Confocal images and quantification of the density of VAChT fibers (red fluorescence) innervating pancreatic β cells (insulin, green fluorescence) in E18 WT and ob/ob embryos that were injected with vehicle or leptin at E12 (1 ug per embryo) (n = 4 per group). (C) Percentage change in the innervation of pancreatic islets by VAChT fibers between adult WT and ob/ob mice. (D–E) Confocal images and quantification of pancreatic islet size (insulin, green fluorescence) and of the density of VAChT fibers (red fluorescence) innervating islets in 16-week-old (D) WT and (E) ob/ob mice that were injected with leptin or vehicle at E12 (n = 3–6 per group). (F) Confocal images and quantification of the density of VIP-positive fibers (red fluorescence) innervating pancreatic β cells (insulin, green fluorescence) in 16-week-old WT and ob/ob mice that were injected with vehicle or leptin at E12 (1 ug per embryo) (n = 4 per group). (G) Percentage change in the innervation of pancreatic islets by VIP fibers between adult WT and ob/ob mice. (H) Photomicrographs and quantification of the β cell area in pancreases of 16-week-old ob/ob mice that were prenatally injected with leptin or vehicle (n = 4 per group). Scale bars, 100 um (A–F); and 1 mm (G). *P < 0.05 versus WT (B), and versus WT + NaCl at E12 (D); ****P < 0.0001 versus ob/ob (B), versus WT + NaCl at E12 (C) and versus ob/ob + NaCl at E12 (E, F); ####P < 0.0001 between the indicated groups (B). Values are shown as the mean ± SEM.
To determine whether the changes in the cholinergic innervation of islets that were observed in the ob/ob embryos were permanent, we studied pancreatic islet innervation in adult mice. The density of VAChT fibers that was observed in the islets of adult ob/ob mice was 3-fold higher than that observed in those of WT mice (Figure 2C). We also examined the density of fibers that contained vasoactive intestinal polypeptide (VIP), another marker of parasympathetic innervation, and found a 3-fold increase in the density of VIP-labeled fibers in the islets of adult ob/ob mice (Figure 2G).
A single prenatal leptin injection has long-term structural effects on islet innervation
If leptin is a critical regulator of the cholinergic innervation of the pancreas, exposing embryos to leptin should change VAChT fiber density. We therefore injected leptin directly in the ventricular cavity of WT and ob/ob embryos at E12. The density of VAChT-positive fibers was reduced by 1.9-fold and 2.1-fold in the leptin-treated WT and ob/ob embryos, respectively, compared to their control littermates (Figure 2B). The effects of prenatal leptin on parasympathetic innervation appear to be permanent because the density of VAChT fibers was also reduced by 1.4-fold and 1.3-fold in adult WT and ob/ob animals, respectively, that were prenatally treated with leptin (Figures 2D and 2E). Similarly, a single prenatal leptin injection reduced the density of VIP fibers in pancreatic islets by 2-fold (Figure 2F). In contrast, prenatal leptin did not appear to influence the sympathetic innervation of the pancreas: the density of TH-labeled fibers was similar between leptin- and vehicle-treated ob/ob mice (Figure S1B). In addition, prenatal leptin treatment did not influence islet size (Figure 2E) or β cell area (Figure 2H) in ob/ob mice but did reduce islet size by 1.4-fold in WT mice (Figure 2D). Because the parasympathetic system is also involved in glucagon secretion (Patel, 1984; Thorens, 2014), we also examined the parasympathetic innervation of glucagon-producing α cells. The density of VAChT fibers innervating pancreatic α cells was similar between leptin- and vehicle-treated mice (Figures S2A–S2C). Together, these data indicate that prenatal leptin exposure has long-term effects on the parasympathetic innervation of pancreatic β cells but does not lead to a widespread disruption of the autonomic inputs to the pancreas.
Injection of leptin during embryonic life causes longterm disturbances in glucose homeostasis
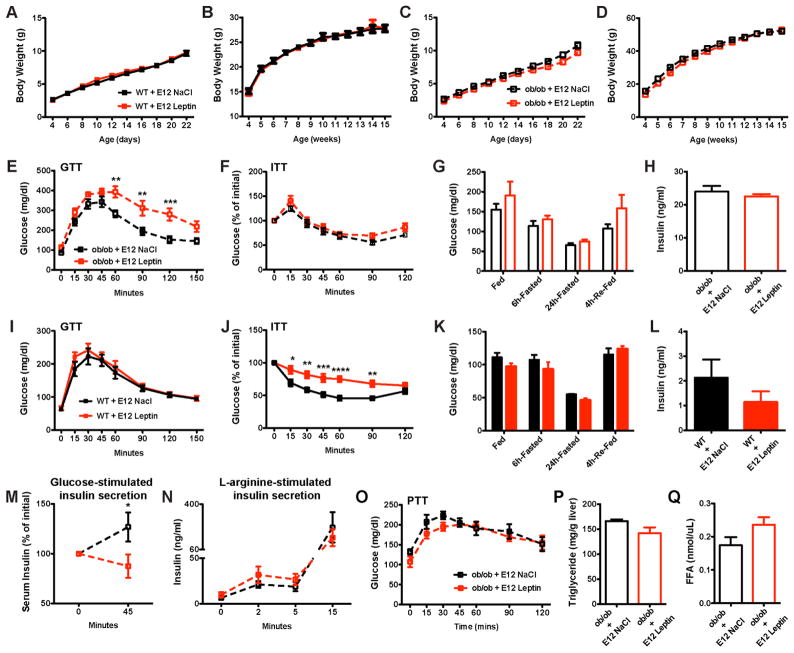
Because prenatal leptin exerted an enduring effect on β-cell innervation, we next examined whether it was associated with disturbances in glucose homeostasis. The pre-and post-weaning growth curves (body weights) of prenatally injected WT and ob/ob mice were undistinguishable from those of vehicle-injected mice (Figures 3A–3D). Consistent with these data, daily food intake and body composition were similar between the leptin-treated and control mice (Figures S3A–S3C). However, clear differences were observed in several indices of glucose homeostasis. Compared with vehicle-treated ob/ob mice, ob/ob mice that were prenatally treated with leptin displayed elevated levels of glucose 60–120 minutes following a glucose challenge (Figure 3E). However, glucose tolerance test results of the leptin-treated WT mice were similar to those of the controls (Figure 3I). Nevertheless, WT mice that were prenatally exposed to leptin displayed impaired insulin tolerance compared to control mice (Figure 3J). These differences were not observed in leptin-treated ob/ob mice (Figure 3F). Moreover, leptin-treated WT and ob/ob mice displayed normal fed, fasting and re-fed glucose levels (Figures 3G and 3K). In addition, serum insulin (Figures 3H and 3L), glucagon (Figure S2D), and C-peptide levels (Figure S3D) were similar between leptin-treated and control ob/ob mice. However, the glucose-stimulated insulin secretion in leptin-treated ob/ob mice was decreased compared to that in control mice when measured 45 min after the glucose injection (Figure 3M). In contrast, this defect in insulin secretion in leptin-injected embryos was not observed upon L-arginine stimulation (Figure 3N). Furthermore, hepatic gluconeogenesis appeared normal because injection of pyruvate resulted in similar glucose responses in leptin-treated and control mice (Figure 3O). Other markers of liver function, such as hepatic triglyceride content and free fatty acid levels, also appeared normal in prenatally treated mice (Figures 3P and 3Q).
Figure 3. Prenatal leptin exposure causes lifelong dysregulation of glucose homeostasis.
(A, C) Pre- and (B, D) post-weaning growth curves (body weights) of (A, B) WT and (C, D) ob/ob mice that were injected with leptin or vehicle at E12 (n ≥ 10 per group). (E, I) Glucose and (F, J) insulin tolerance tests of 10- to 12-week-old (E, F) ob/ob and (I, J) WT mice that were injected with leptin or vehicle at E12 (n = 5–8 per group for the GTT and n = 7–8 per group for the ITT). Blood glucose levels in 13-week-old (G) ob/ob and (K) WT mice that were prenatally injected with leptin or vehicle (n = 3–7 per group). Plasma insulin levels in 16-week-old (H) ob/ob and (L) WT mice that were injected with leptin or vehicle at E12 (n = 5–7 per group). (M) Glucose- and (N) L-arginine-stimulated insulin secretion in adult ob/ob mice that were injected with leptin or vehicle at E12 (n = 3–5 per group). (O) Pyruvate tolerance test of 8-week-old ob/ob mice that were prenatally injected with leptin or vehicle (n = 6 per group). (P) Plasma triglyceride and (Q) free fatty acids (FFA) levels in 16-week-old ob/ob mice that were injected with leptin or vehicle at E12 (n = 4–7 per group). *P < 0.05 versus ob/ob + NaCl at E12 (M); **P < 0.01 versus ob/ob + NaCl at E12 (E); ***P < 0.001 versus ob/ob + NaCl at E12 (E), and versus WT + NaCl at E12 (J); and ****P < 0.0001 versus WT + NaCl at E12 (J). Values are shown as the mean ± SEM.
Leptin acts directly on cholinergic neurons in the hindbrain to blunt axon growth
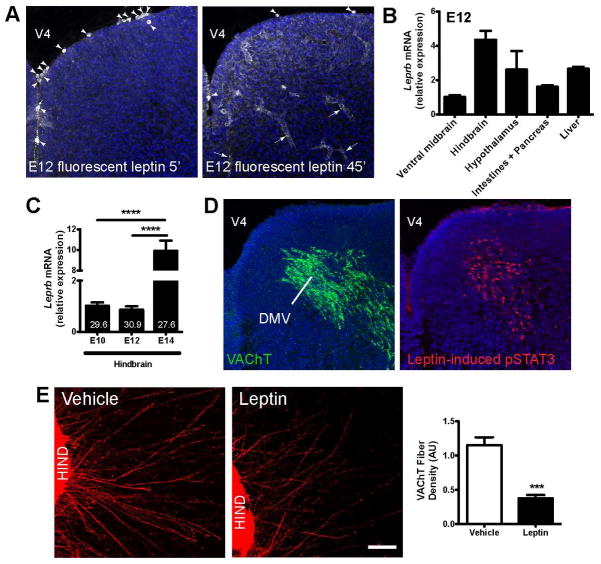
The parasympathetic nerve fibers that innervate the pancreas mainly originate from hindbrain neurons located in the DMV (Fox and Powley, 1986). Most of these fibers travel within the vagus nerve and terminate on the pancreatic ganglia (Fox and Powley, 1986). To identify leptin’s potential site of action, we first examined where leptin was taken up by intracerebroventricularly injecting fluorescently labeled bioactive leptin (16 KDa) into embryos (Balland et al., 2014; Vauthier et al., 2013). As early as 5 min after injection, fluorescent leptin was observed in cells in the wall of the fourth ventricle (Figure 4A). By 45 min after injection, a significant amount of fluorescently labeled leptin was detected in the hindbrain parenchyma and vessels (Figure 4A). Fluorescent leptin was also observed in the parenchyma and vessels of the spinal cord near the cholinergic neurons of the ventral motor column (Figure S4A). Less fluorescent leptin was observed in other brain regions, including the hypothalamus (Figure S4B). Fluorescently labeled leptin was also observed in peripheral tissue, including the liver and pancreas, 45 min after injection (Figures S4C and S4D).
Figure 4. Leptin blocks cholinergic axonal growth from the embryonic hindbrain.
(A) Representative photomicrographs of the hindbrain showing cell bodies (arrowheads) and vessels (arrows) labeled with fluorescent leptin (1ug per embryo, white labeling) 5 and 45 min after it was intracerebroventricularly injected into E12 embryos. (B) Relative expression of Leprb mRNA in various brain areas and peripheral organs of E12 WT mouse embryos (n = 3 per group). (C) Relative expression of Leprb mRNA in the hindbrain of E10, E12 and E14 WT mouse embryos (n = 3–5 per age). Numbers in bar graphs indicate Ct values. (D) Confocal images of VAChT-immunoreactive cells (green fluorescence) and leptin-induced pSTAT3-immunopositive cells (red fluorescence) in the DMV of E12 WT embryos. (E) Confocal images and quantification of the overall density of VAChT-positive fibers in organotypic cultures of isolated E12 hindbrains. The cultures were incubated with leptin (100ng/ml) or vehicle for 48h (n = 5–6 explants per group). DMV, dorsomedial nucleus of the vagus nerve; HIND, hindbrain; V4, fourth ventricle. Scale bar, 100 um. ***P < 0.001 versus vehicle (E); and ****P < 0.0001 between indicated groups (D). Values are shown as mean the ± SEM.
Consistent with a primary action of central leptin on hindbrain neurons, relatively high levels of leptin receptor (Leprb) mRNA were found in the embryonic hindbrain. At E12, Leprb mRNA levels were 2–4 times higher in the hindbrain than in other brain regions such as the ventral midbrain or hypothalamus (Figure 4B). Moreover, Leprb mRNA levels in the hindbrain in E14 embryos were 5- to 10-fold greater than those in E10-E12 embryos (Figure 4C). During embryonic life, Leprb mRNA is also expressed in several peripheral organs that are known to be innervated by the vagus nerve, including the intestines, pancreas, liver and stomach (Figure 4B).
We next examined the distribution of labeling for pSTAT3, a commonly used surrogate marker of leptin receptor activation, in embryos that received a central injection of leptin. After 45 min, robust pSTAT3 immunoreactivity was observed in the DMV, and the distribution of pSTAT3-positive cells overlapped with that of VAChT neurons (Figure 4D). In addition, central leptin increased the number of pSTAT3-labeled cells in the ventral motor column of the spinal cord, where cholinergic neurons are located (Figure S4E). In contrast, pSTAT3 immunoreactivity was not observed in other tissues that took up fluorescent leptin, such as the hypothalamus, liver and pancreas (Figures S4F–S4H). However, it remains possible that leptin might activate other canonical LepRb signaling pathways, such as MAPK and PI-3K/Akt, in regions and tissues that do not exhibit STAT3 phosphorylation after leptin administration. Nevertheless, these results suggest that central leptin might influence the parasympathetic innervation of the pancreas by primarily acting, at least in part, on hindbrain neurons.
To determine if leptin acts directly on preganglionic cholinergic neurons of the hindbrain to influence axon growth, we next performed a series of in vitro experiments in which hindbrain explants were microdissected, placed in a collagen matrix and exposed to leptin or vehicle. After 48h, the density of cholinergic axons extending from hindbrain explants in which leptin was added to the culture medium was 3.3-fold less than that in control explants (Figure 4E). Together, these data provide direct evidence that leptin acts on hindbrain neurons to inhibit cholinergic axon growth.
Discussion
Both the sympathetic and parasympathetic branches of the autonomic nervous system are critical regulators of glucose homeostasis (Ahrén, 2000; Thorens, 2014; Woods and Porte, 1974). However, the mechanisms that underlie the development of the autonomic nervous system in general, and of the parasympathetic innervation of the endocrine pancreas in particular, remain elusive. In the present study, we report that cholinergic innervation of pancreatic islets develops during mid-gestation under the influence of leptin. Our results indicate that prenatal leptin decreases parasympathetic innervation of the endocrine pancreas, which alters glucose homeostasis without affecting body weight or body composition. These data are in good agreement with a direct role of leptin in glucose regulation. Mice with a genetic leptin deficiency (ob/ob mice) or leptin receptor deficiency (db/db mice) display hyperphagia and obesity as well as insulin resistance and diabetes. While increased food intake and body adiposity clearly contribute to the impaired glucose metabolism that is observed in these mouse models, several observations suggest that leptin regulates glucose metabolism independently of its effects on energy balance. Caloric restriction has only a moderate beneficial effect on insulin sensitivity and hyperglycemia in ob/ob and db/db mice (Morton et al., 2005; Wyse and Dulin, 1970), and leptin administration to leptin-deficient mice ameliorates hyperglycemia and hyperinsulinemia (Farooqi et al., 1999; Pelleymounter et al., 1995) even when differences in food intake are controlled for pair-feeding (Hedbacker et al., 2010; Schwartz et al., 1996). The exact mechanisms underlying the insulin resistance observed in leptin-injected embryos remain to be investigated but it is possible that prenatal leptin alters glucose production in the liver and/or glucose uptake by muscle. Our data are also in good agreement with other studies that have reported an inhibitory action of leptin on the parasympathetic system. For example, central leptin administration in adult mice has been shown to reduce parasympathetic outflow, causing changes in airway diameter and lung function (Arteaga-Solis et al., 2013).
Leptin’s regulation of the parasympathetic innervation of the pancreas likely involves direct effects on hindbrain neurons rather than direct effects on the embryonic pancreas. Central leptin injection induced STAT3 phosphorylation in DMV neurons but not in the pancreas. In addition, the hindbrain contains the highest density of leptin receptors of any brain region, and central injection of fluorescently labeled leptin resulted in rapid accumulation of fluorescence in the hindbrain. Furthermore, direct exposure of hindbrain explants to leptin inhibited cholinergic axon growth. Notably, the developmental effects of prenatal leptin appear to be restricted to the innervation of pancreatic β cells because the cholinergic innervation of pancreatic α cells was unaffected by prenatal leptin. The parasympathetic nerve fibers that innervate the pancreas originate mainly from the DMV, and the majority of these fibers terminate on the pancreatic ganglia, which, in turn, innervate pancreatic β cells (Fox and Powley, 1986). Therefore, it is likely that the reduction in the cholinergic innervation of pancreatic β cells results from decreased innervation of the pancreatic ganglia by preganglionic autonomic fibers that originate in the hindbrain and that distinct pancreatic ganglia are responsible for the innervation of α and β cells. More generally, our data suggest that normal preganglionic innervation of pancreatic ganglia is important for normal cholinergic innervation of islets by postganglionic neurons. In addition, we cannot rule out the possibility that some of the perturbations in glucose homeostasis that were observed after prenatal leptin administration resulted from changes in the autonomic innervation of the liver, although liver function appeared normal in leptin-injected embryos.
Leptin is one of the first major metabolic hormones to appear during development. White adipose tissue (the main source of leptin production in adult animals) is minimal at early ages, yet mouse fetuses do contain significant leptin levels in their blood as early as E12 (Udagawa et al., 2006) (Ishii and Bouret, unpublished data). Various tissues produce leptin during embryonic development. On embryonic day 13, high levels of leptin gene expression are found in the fetal liver and cartilage–bone structures, followed by cardiac expression between E16 and E18 (Hoggard et al., 1997; Hoggard et al., 2000). In addition to being produced by the embryo itself, dams also contain high levels of leptin during pregnancy, but whether maternal leptin crosses the placenta and reaches the embryo during early–mid gestation (i.e., when brain development is initiated) remains unclear. Circulating leptin levels increase markedly during the postnatal period and exhibit a “surge” (Ahima et al., 1998). The inhibitory effect of leptin on parasympathetic development during embryonic life differs from its effect on hypothalamic neural projections during postnatal life. During postnatal life, leptin acts as a powerful neurotrophic agent that promotes the formation of neural circuits that originate in the arcuate nucleus (Bouret et al., 2012; Bouret et al., 2004). The mechanisms underlying the opposing effects of leptin on hypothalamic and hindbrain axon growth remain to be investigated. However, recent data have suggested that the transition from stimulatory to inhibitory effects of leptin on neuronal activity occurs in parallel with the acquisition of functional ATP-sensitive potassium channels (Baquero et al., 2014), and these data support the hypothesis that leptin activates distinct membrane-linked and intracellular pathways in hindbrain and hypothalamic neurons during development. Prenatal leptin exposure could also result in changes in insulin levels that could then, in turn, indirectly affect the autonomic innervation of the pancreas. Supporting this idea, central leptin administration in adult rats decreases glucose-stimulated insulin secretion (Muzumdar et al., 2003). In addition, recent studies have demonstrated that the effects of a perinatal high-fat diet on pancreatic parasympathetic innervation and glucose-dependent insulin secretion depended on hypothalamic insulin signaling (Vogt et al., 2014). However, our in vitro experiments, which showed that exposing isolated hindbrain explants to leptin blunted axon growth, support a direct role of leptin in cholinergic axon growth.
In conclusion, our data show the importance of an optimal hormone balance during prenatal life for lifelong glucose regulation and correct organization of parasympathetic inputs to pancreatic islets. These findings might be important for physiopathological conditions that are associated with abnormally elevated leptin levels during critical periods of growth and development. For example, maternal obesity is associated with elevated levels of leptin in both the maternal and fetal circulation (Luo et al., 2013), and epidemiological studies have demonstrated a strong association between maternal obesity and the offspring’s predisposition to diabetes (Boney et al., 2005). Better understanding of the relationship between prenatal leptin and the programming of metabolic diseases will be crucial as we seek to develop interventional studies to ameliorate and hopefully reverse such metabolic malprogramming.
Experimental Procedures
Animals
ob/ob mice and their WT littermates were produced in our breeding colony by paring heterozygous male and female mice (Jax mice stock # 000632). The day of conception (sperm-positive vaginal smear) was designated as embryonic day (E) zero (E0). For all experiments, on day one after birth, litter size was adjusted to 6–7 pups to ensure adequate and standardized nutrition until weaning. Only male mice were studied. All animal procedures were conducted in compliance with and approved by the IACUC of the Saban Research Institute of the Children’s Hospital of Los Angeles. For detailed information regarding the analytical time points, the determination of body weight and composition, the serum analyses, and the assessment of glucose homeostasis, see the Supplemental Experimental Procedures.
VAChT, synaptophysin, tyrosine hydroxylase, HNF4α, insulin, and glucagon immunohistochemistry and image analyses
For immunostaining of embryonic tissue, pregnant females were anesthetized and the embryos collected at E12, E15 and E18. The embryos were then fixed in a solution of 4% paraformaldehyde. For immunostaining of adult tissue, mice were transcardially perfused with 4% paraformaldehyde. Tissue sections were processed for immunofluorescence using standard procedures. The primary antibodies used for immunohistochemistry were as follows: rabbit anti-vesicular acetylcholine transporter (VAChT, 1:500, Synaptic Systems), mouse anti-synaptophysin1 (1:500, Synaptic Systems), rabbit anti-tyrosine hydroxylase (TH, 1:2000, Immunostar), goat anti-HNF4α (1:500, Santa Cruz), and guinea-pig anti-insulin (1:500, Abcam). For detailed information regarding image acquisition and quantification, see the Supplemental Experimental Procedures.
Isolated hindbrain explant cultures
Brains were collected from E12 embryos. The hindbrain (consisting of the DMV and NTS) was then carefully dissected from the rest of the brain under a stereomicroscope. Explants (n = 5–6 cultures per group) were cultured onto a rat tail collagen matrix (BD Biosciences) using standard procedures (Bouret et al., 2004). After a 48-h incubation with leptin (100 ng/ml; PeproTech), or with vehicle, the explants were fixed in paraformaldehyde, and cholinergic neurites extending from the explants were stained with rabbit anti-VAChT (1:1000; Synaptic Systems). See the Supplemental Experimental Procedures for additional details regarding image analysis.
Intra-embryonic leptin injections
Timed-pregnant mice carrying E12 ob/ob and WT embryos were anesthetized, and the uterine horns were gently placed outside the abdominal cavity. Using a Nanofil syringe with a 35-ga. needle attachment (World Precision Instruments), 1 ug of murine leptin (1 mg/ml, Peprotech) or vehicle (NaCl 0.9%) was then delivered into each embryo intracerebroventricularly. Each experiment included offspring from at least 11–14 litters.
Fluorescent leptin assays
Using a Nanofil syringe with a 35-ga. needle attachment (World Precision Instruments), 1 ug of fluorescently labeled bioactive leptin (Cisbio Bioassays) (Balland et al., 2014; Vauthier et al., 2013) was intracerebroventricularly injected into E12 embryos. Embryos were sacrificed 5 and 45 min later to assess tissue leptin uptake by confocal microscopy.
pSTAT3 immunohistochemistry
Leptin (1ug, Peprotech) was intracerebroventricularly injected into E12 embryos as described above. Controls received equivolume injections of vehicle (0.9% NaCl). The embryos were collected 45 min later and fixed with a solution of 2% paraformadehyde. Frozen coronal (head) or sagittal (body) sections were cut at 30 um and processed for pSTAT3 immunostaining as previously described (Bouret et al., 2012).
Statistical analysis
All values were represented as the mean ± SEM. Statistical analyses were conducted using GraphPad Prism (version 5.0a). For each experiment, slides were numerically coded to obscure the treatment group. Statistical significance was determined using unpaired 2-tailed Student’s t test and 2-way ANOVA followed by the Bonferroni post hoc test when appropriate. P ≤ 0.05 was considered statistically significant.
Supplementary Material
Highlights.
Autonomic innervation of pancreatic islets develops during mid-gestation in mice
Leptin is required for normal parasympathetic innervation of pancreatic β cells
Acute leptin injections in embryos cause lifelong disturbances in glucose homeostasis
Leptin acts directly on the hindbrain to modulate cholinergic axon growth
Acknowledgments
We thank Eric Trinquet (Cisbio Bioassays) for providing fluorescent leptin. We also would like to thank Li Liu for the expert technical assistance. This work was supported by the National Institutes of Health (Grants DK84142, DK102780, and P01ES022845 to SGB), the United States Environment Protection Agency (Grant RD83544101), and the EU FP7 integrated project (grant agreement n° 266408, “Ful l4Health”, to SGB).
Footnotes
Author contributions
S.C. and S.G.B. designed the study. S.C. and S.G.B. interpreted the results. S.C. performed experiments and analyzed the data. S.C. contributed to all figures. V.P. contributed to Figure 4 and Figure S4. S.C. and S.G.B. wrote the paper with input from the other author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahima R, Prabakaran D, Flier J. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- Arteaga-Solis E, Zee T, Emala CW, Vinson C, Wess J, Karsenty G. Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell Metab. 2013;17:35–48. doi: 10.1016/j.cmet.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A, Anouar Y, Dehouck B, Trinquet E, Jockers R, Bouret SG, Prevot V. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014;19:293–301. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquero AF, de Solis AJ, Lindsley SR, Kirigiti MA, Smith MS, Cowley MA, Zeltser LM, Grove KL. Developmental Switch of Leptin Signaling in Arcuate Nucleus Neurons. The Journal of Neuroscience. 2014;34:9982–9994. doi: 10.1523/JNEUROSCI.0933-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black IB. Regulation of autonomic development. Ann Rev Neurosci. 1978;1:183–214. doi: 10.1146/annurev.ne.01.030178.001151. [DOI] [PubMed] [Google Scholar]
- Bloom SR, Edwards AV. The release of pancreatic glucagon and inhibition of insulin in response to stimulation of the sympathetic innervation. J Physiol. 1975;253:157–173. doi: 10.1113/jphysiol.1975.sp011185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic Syndrome in Childhood: Association With Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Bates SH, Chen S, Myers MG, Simerly RB. Distinct Roles for Specific Leptin Receptor Signals in the Development of Hypothalamic Feeding Circuits. The Journal of Neuroscience. 2012;32:1244–1252. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic Action of Leptin on Hypothalamic Neurons That Regulate Feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Burris RE, Hebrok M. Pancreatic innervation in mouse development and β-cell regeneration. Neuroscience. 2007;150:592–602. doi: 10.1016/j.neuroscience.2007.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin Action in the Dorsomedial Hypothalamus Increases Sympathetic Tone to Brown Adipose Tissue in Spite of Systemic Leptin Resistance. The Journal of Neuroscience. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of Recombinant Leptin Therapy in a Child with Congenital Leptin Deficiency. New England Journal of Medicine. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- Fox EA, Powley TL. Tracer diffusion has exaggerated CNS maps of direct preganglionic innervation of pancreas. J Auton Nerv Syst. 1986;1986:1. doi: 10.1016/0165-1838(86)90079-2. [DOI] [PubMed] [Google Scholar]
- Furuzawa Y, Ohmori Y, Watanabe T. Anatomical localization of sympathetic postganglionic and sensory neurons innervating the pancreas of the cat. J Vet Med Sci. 1996;58:243–248. doi: 10.1292/jvms.58.243. [DOI] [PubMed] [Google Scholar]
- Gautron L, Elmquist Joel K, Williams Kevin W. Neural Control of Energy Balance: Translating Circuits to Therapies. Cell. 2015;161:133–145. doi: 10.1016/j.cell.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev. 2001;22:565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- Grill Harvey J, Hayes Matthew R. Hindbrain Neurons as an Essential Hub in the Neuroanatomically Distributed Control of Energy Balance. Cell Metabolism. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM. Antidiabetic Effects of IGFBP2, a Leptin-Regulated Gene. Cell Metabolism. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, Mercer JG. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. PNAS. 1997;94:11073–11078. doi: 10.1073/pnas.94.20.11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard N, Hunter L, Lea R, Trayhurn P, Mercer J. Ontogeny of the expression of leptin and its receptor in the murine fetus and placenta. Br J Nutr. 2000;83:317–326. doi: 10.1017/s0007114500000398. [DOI] [PubMed] [Google Scholar]
- Jørgensen MC, Ahnfelt-Rønne J, Hald J, Madsen OD, Serup P, Hecksher-Sørensen J. An Illustrated Review of Early Pancreas Development in the Mouse. Endocrine Reviews. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Myers MG. LRb signals act within a distributed network of leptin-responsive neurones to mediate leptin action. Acta Physiologica. 2008;192:49–59. doi: 10.1111/j.1748-1716.2007.01784.x. [DOI] [PubMed] [Google Scholar]
- Luo ZC, Nuyt AM, Delvin E, Fraser WD, Julien P, Audibert F, Girard I, Shatenstein B, Deal C, Grenier E, Garofalo C, Levy E. Maternal and fetal leptin, adiponectin levels and associations with fetal insulin sensitivity. Obesity. 2013;21:210–216. doi: 10.1002/oby.20250. [DOI] [PubMed] [Google Scholar]
- Marino JS, Xu Y, Hill JW. Central insulin and leptin-mediated autonomic control of glucose homeostasis. Trends Endocrinol Metab. 2011;22:275–285. doi: 10.1016/j.tem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metabolism. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Schwartz MW. Leptin and the Central Nervous System Control of Glucose Metabolism. 2011 doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar R, Ma X, Yang X, Atzmon G, Berstein J, Karkania G, Barzilai N. Physiologic effect of leptin on insulin secretion is mediated mainly through central mechanisms. The FASEB Journal. 2003;17:1130–1132. doi: 10.1096/fj.02-0991fje. [DOI] [PubMed] [Google Scholar]
- Patel D. Role of parasympathetic nervous system in glucagon response to insulin-induced hypoglycemia in normal and diabetic rats. Metabolism. 1984;33:1123–1127. doi: 10.1016/0026-0495(84)90098-2. [DOI] [PubMed] [Google Scholar]
- Pelleymounter M, Cullen M, Baker M, Hecht R, Winters D, Boone T, FC Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Levitt P. Establishment of vagal sensorimotor circuits during fetal development in rats. J Neurobiol. 1993;24:641–659. doi: 10.1002/neu.480240509. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Leinninger GM, Myers MG., Jr Molecular and neural mediators of leptin action. Physiology & Behavior. 2008;94:637–642. doi: 10.1016/j.physbeh.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Thews G. Autonomic Nervous System. In: Janig W, editor. Human Physiology. New York: Springer-Verlag; 1989. pp. 333–370. [Google Scholar]
- Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Woods S, Seeley RJ, Weigle DS. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. The Journal of Clinical Investigation. 2011;121:2413–2421. doi: 10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds Stephanie E, Pryor Jack T, Ravussin E, Greenway Frank L, Dileone R, Allen Andrew M, Bassi J, Elmquist Joel K, Keogh Julia M, Henning E, Myers Martin G, Jr, Licinio J, Brown Russell D, Enriori Pablo J, O’Rahilly S, Sternson Scott M, Grove Kevin L, Spanswick David C, Farooqi IS, Cowley Michael A. Leptin Mediates the Increase in Blood Pressure Associated with Obesity. Cell. 2014;159:1404–1416. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B. Neural regulation of pancreatic islet cell mass and function. Diabetes Obes Metab. 2014;16(Suppl 1):87–95. doi: 10.1111/dom.12346. [DOI] [PubMed] [Google Scholar]
- Udagawa J, Hashimoto R, Suzuki H, Hatta T, Sotomaru Y, Hioki K, Kagohashi Y, Nomura T, Minami Y, Otani H. The Role of Leptin in the Development of the Cerebral Cortex in Mouse Embryos. Endocrinology. 2006;147:647–658. doi: 10.1210/en.2005-0791. [DOI] [PubMed] [Google Scholar]
- Vauthier V, Derviaux C, Douayry N, Roux T, Trinquet E, Jockers R, Dam J. Design and validation of a homogeneous time-resolved fluorescence-based leptin receptor binding assay. Anal Biochem. 2013;436:1–9. doi: 10.1016/j.ab.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Vogt MC, Paeger L, Hess S, Steculorum SM, Awazawa M, Hampel B, Neupert S, Nicholls HT, Mauer J, Hausen AC, Predel R, Kloppenburg P, Horvath TL, Bruning JC. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell. 2014;156:495–509. doi: 10.1016/j.cell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe E, Tao-Cheng JH, Schäfer MK, Erickson JD, Eiden LE. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3547–3552. doi: 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Porte D., Jr Neural control of the endocrine pancreas. Physiol Rev. 1974;54:596–619. doi: 10.1152/physrev.1974.54.3.596. [DOI] [PubMed] [Google Scholar]
- Wyse BM, Dulin WE. The influence of age and dietary conditions on diabetes in the db mouse. Diabetologia. 1970;6:268–273. doi: 10.1007/BF01212237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.