Abstract
Objective
To examine the effect of intensive glycemic control on cardiovascular disease events (CVD) among the major race/ethnic groups in a post-hoc analysis of the VADT.
Materials and Methods
Participants included 1111 non-Hispanic Whites, 307 Hispanics and 306 non-Hispanic Blacks randomized to intensive or standard glucose treatment in VADT. Multivariable Cox proportional hazards models were constructed to assess the effect of intensive glucose treatment on CVD events among race/ethnic groups.
Results
Mean age was 60.4 years and median follow-up was 5.6 years. By design, modifiable risk factors were managed equally well in both treatment arms and only differed modestly between race/ethnic groups. HbA1c decreased significantly from baseline with intensive glucose treatment in each race/ethnic group, with a trend for a greater response in Hispanics (P=0.02 for overall comparison between groups). Intensive glucose treatment was associated with reduced risk of CVD events for Hispanics but not for others (hazard ratios ranged from 0.54 to 0.75 for Hispanics whereas they were consistently close to 1 for others). Sensitivity analyses with different definitions of race/ethnicity or limited to individuals free of previous known CVD yielded similar results.
Conclusions
The results of these analyses support the hypothesis that race/ethnicity is worthy of consideration when tailoring intensive treatment for individuals with long-standing type 2 diabetes. However, additional studies are needed to confirm the findings of this post-hoc analysis.
Keywords: CVD, ethnicity, intensive glycemic control, race, type 2 diabetes, VADT
Introduction
Recent large and well conducted randomized controlled trials demonstrated that intensive glycemic control does not reduce cardiovascular disease (CVD) events in people with type 2 diabetes of moderate to long duration [1–3]. However, subsequent analyses [4–8], together with results from an earlier trial conducted in new onset diabetes [9–11], have suggested that factors such as pre-existing macrovascular disease, duration of diabetes, level of glycemic control and significant comorbidities may influence whether intensive glucose control is appropriate for patients with diabetes [1, 5–7, 12–14]. Many of these factors reflect the stage of the diabetes and, in particular, the individual’s vascular health. Baseline levels of atherosclerosis may therefore be an important determinant of response to tight glucose control. In an ancillary study of the Veterans Affairs Diabetes Trial (VADT), we have previously reported intensive glycemic control significantly reduced the primary CVD endpoints in those with lower burden of atherosclerosis at baseline [4]. In addition, using different measures of subclinical atherosclerosis such as vascular calcium scores and carotid intima media thickness, we and others have shown Hispanics have lower atherosclerosis burden compared with non-Hispanic Whites in both diabetic and non-diabetic populations [15–19]. A similar phenomenon has been reported among Native American Indians [20], where CVD was reported as extremely rare, until overt diabetes and diabetic nephropathy became common in this population [21, 22]
In line with a reduced burden of atherosclerosis in Hispanics, a number of studies have reported lower rates of CVD mortality, despite a higher burden of risk factors in Hispanics compared with non-Hispanic Whites [23–28], which led to the notion of a “Hispanic Paradox”. Consistently, the most recent National Vital Statistics reported that Hispanics have the lowest age-adjusted mortality rates compared with non-Hispanic white and non-Hispanic black populations [29]. The concept of “Hispanic Paradox” is complex and has become controversial, in part because of the recognition that Hispanics are a diverse and heterogeneous population, and the idea has been contested in several prospective cohort studies [30–32]. Nevertheless, there is greater appreciation that several characteristics such as social support, optimism, and strong familial and social ties are common among Hispanics, all of which are thought to be stress buffering and may potentially be protective among Hispanics despite their higher risk profile [33]. There is also growing recognition for the role of gene polymorphisms among different ethnic populations influencing disease outcomes and drug response [34, 35]. Particularly relevant support for this concept comes from a recent UKPDS study that reported different rates of CVD events and mortality in people with new onset diabetes across various race/ethnic groups, in line with the idea that race/ethnic specific cardiovascular protective mechanisms may exist [36]. A recent comprehensive review of the literature from the science advisory from the American Heart Association [33] promotes the need to develop a culturally tailored and targeted approach for the goal of CVD risk reduction among Hispanics. However, the data on consequences of CVD risk reduction strategies in different race/ethnic groups, in particular in patient with type 2 diabetes, is lacking. Therefore, in this post-hoc analysis of the VADT we sought to determine the association between intensive glycemic control and CVD events among major race/ethnic groups.
1. Materials and Methods
2.1. Study population
The VADT study design, exclusion/inclusion criteria, and study measures and activities have been described in detail [37]; further study information is provided in supplementary material on-line. In brief, the VADT included 1791 military veterans at 20 VA medical centers with poorly controlled type 2 diabetes who were randomized to receive either intensive or standard glucose treatment for a median duration of 5.6 years. The primary outcome was the time to the first occurrence of any one of a composite of CVD events (myocardial infarction, stroke, death from cardiovascular causes, new or worsening congestive heart failure, surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease, inoperable coronary artery disease, and amputation for ischemic gangrene) adjudicated by an end-point committee that was unaware of treatment assignment. Optimal control of blood pressure and dyslipidemia, daily aspirin use, dietary advice, and diabetes education were uniformly provided to both treatment arms. Protocol and consent forms were approved by the institutional review board at each of the 20 participating sites and all patients provided written informed consent. The current analysis included 1724 VADT participants whose responses to standard NIH-format questions concerning race and ethnicity [38] allowed categorization into one of the 3 major race/ethnic groups: non-Hispanic White, non-Hispanic Black and Hispanic. We categorized as Hispanic those reporting Hispanic ethnicity as long as such individuals did not report Black race. Similarly, individuals who reported Black race were categorized as non-Hispanic Blacks as long as Hispanic ethnicity was not reported and irrespective of other races that were reported. The non-Hispanic White group included those who reported only White race and non-Hispanic ethnicity.
2.2. Statistical Analyses
Descriptive statistics, including mean ± SD, median (25th–75th percentiles), and frequencies were calculated for all variables. Between group differences were evaluated with analyses of variance or unpaired t-tests for normally distributed variables, with Kruskal-Wallis analysis of variance and Mann-Whitney U test for variables with skewed distributions, and with chi square tests for proportions. To determine whether longitudinal responses to treatment (i.e. HemoglobinA1c (HbA1c) changes during the study) differed among race/ethnic groups, a linear mixed effects model was used (details provided in Supplementary Material). Statistical significance was recognized for two-sided P values < 0.05.
Cox proportional hazards risk analysis was used to determine associations between treatment assignment and CVD events independent of other risk factors. As our study aim was to assess treatment effects within different race/ethnic groups, and recognizing that smaller sample sizes in Hispanic and Black groups provided limited power to detect modifications of the overall treatment effect by race/ethnic groups, we performed exploratory subgroup analyses. We then assessed whether the results were consistent by performing sensitivity analyses with a series of Cox proportional hazards models within strata defined by different definitions of race/ethnic group. Sensitivity analyses included: 1) categorizing all participants into Hispanics vs. non-Hispanics regardless of race, 2) excluding individuals of mixed race from the three race/ethnic groups, 3) excluding individuals of mixed race and with prior CVD.
To identify the “best” subset of predictors for CVD events within each race/ethnic stratum, we performed forward stepwise variable selection. Treatment assignment as the primary variable of interest was forced into all models. Other variables submitted to the stepwise forward selection procedure were age, duration of diabetes, history of CVD, history of hypertension, pack-years of smoking, education level, employment status and HbA1c at baseline as well as time-dependent values for diastolic and systolic blood pressure, body mass index (BMI), and total cholesterol-to-high density lipoprotein (HDL) ratio. Selection criteria required a P-value <0.05 for a variable to enter and be retained in models. The stepwise analysis indicated the best predictors of CVD events differed somewhat among race/ethnic groups (Supplementary Table 1). In a series of Cox proportional hazards models, we included different combinations of significant predictors identified from the stepwise variable selection models. The number of CVD events within Hispanic and non-Hispanic Black categories did not allow including more than six predictors (treatment assignment and five other independent variables) in the Cox proportional hazards models, to avoid overfitting the models. We therefore combined several variables (systolic and diastolic blood pressure into mean arterial blood pressure; total cholesterol and HDL into their ratio). However, models containing more than six predictors yielded similar results.
3. Results
The 3 major race/ethnic groups consisted of 1111 non-Hispanic Whites, 307 Hispanics and 306 Non-Hispanic Blacks. Of the 307 Hispanics, 291 also identified themselves as Whites, 11 as American Indian/Alaskan Natives, 3 as Asians, and 2 as Native Hawaiian/Pacific Islanders. The Non-Hispanic Blacks included 299 individuals who identified themselves as Black only, 6 as Black and American Indian/Alaska Native, and 1 as Black and White. To avoid overlap between the major race/ethnic categories a total of 67 (3%) of VADT participants (24 Hispanic Blacks, 13 non-Hispanic Asians, 26 Non-Hispanic American Indians/or Alaska Natives, and 4 non-Hispanic Native Hawaiians or other Pacific Islanders), were excluded from the main analyses.
As shown in Table 1, within each race/ethnic group, baseline variables were generally similar for both treatment arms. Exceptions were a slightly longer mean(±SD) duration of diabetes in Hispanics in the intensive arm compared with the standard arm (13±9 vs. 11±7 years, P=0.04) and among non-Hispanic Blacks, a higher prevalence of ever smoking (76% vs. 65%, P=0.03), greater mean (±SD) pack-years of smoking (21±27 vs. 13±17, P=0.01), but a lower prevalence of insulin use (47% vs. 62%, P=0.01) for those in the intensive arm compared with the standard arm. With both treatment groups combined, the three race/ethnic groups differed in age, prevalence of diabetes, HbA1c levels, BMI, blood pressure, lipid profile, pack years of smoking, and baseline medication use. Hispanics and non-Hispanic blacks also had lower prevalence of prior CVD.
Table 1.
Baseline Characteristics of Major Race/Ethnic Groups by Treatment Arm
| Non-Hispanic Whites | Non-Hispanic Blacks | Hispanics | ||||
|---|---|---|---|---|---|---|
| Standard (n=572) |
Intensive (n=539) |
Standard (n=151) |
Intensive (n=155) |
Standard (n=145) |
Intensive (n=162) |
|
| Age (years) | 62±9 | 61±9 | 58±8 | 60±8 | 58±8 | 59±8 |
| Diabetes duration (years) | 11±7 | 11±7 | 12±7 | 11±7 | 11±7 | 13±9a |
| Prior CVD (%) | 48 | 46 | 32 | 30 | 25 | 28 |
| Hypertension (%) | 72 | 70 | 76 | 78 | 69 | 69 |
| HbA1c (%) | 9.2±1.5 | 9.2±1.3 | 9.8±1.9 | 9.8±1.7 | 9.6±1.4 | 9.8±1.5 |
| HbA1c (mmol/mol) | 77±16 | 77±14 | 84±20 | 84±17 | 81±15 | 84±16 |
| BMI (kg/m2) | 31.4±4.4 | 31.5±4.3 | 31.1±4.3 | 31.4±4.6 | 30.6±4.2 | 30.4±4.2 |
| Systolic blood pressure (mmHg) | 131±16 | 131±16 | 134±18 | 134±18 | 131±17 | 130±15 |
| Diastolic blood pressure (mmHg) | 75±10 | 75±11 | 79±10 | 79±10 | 78±11 | 77±10 |
| Total cholesterol (mmol/l) | 4.7±1.5 | 4.6±1.0 | 4.8±1.1 | 4.7±0.9 | 4.8±1.0 | 4.8±1.2 |
| Triglycerides (mmol/l) | 2.8±4.7 | 2.4±1.9 | 1.6±1.6 | 1.6±1.2 | 1.7±1.2 | 2.4±1.6 |
| LDL (mmol/l) | 2.6±0.9 | 2.7±0.8 | 3.0±0.9 | 2.9±0.8 | 2.9±0.9 | 2.8±0.8 |
| HDL (mmol/l) | 0.9±0.3 | 0.9±0.2 | 1.1±0.3 | 1.0±0.3 | 0.9±0.2 | 0.9±0.2 |
| Total cholesterol-to-HDL ratio | 5.6±2.4 | 5.4±1.8 | 4.8±1.7 | 4.8±1.8 | 5.6±2.1 | 5.4±1.7 |
| Ever smoker (%) | 74 | 74 | 65 | 76a | 72 | 65 |
| Pack-years of smoking | 30±32 | 33±37 | 13±17 | 21±27a | 17±26 | 13±21 |
| TZD use (%) | 21 | 19 | 17 | 11 | 17 | 23 |
| Insulin use (%) | 50 | 53 | 62 | 47a | 52 | 54 |
| Anti-hypertensive use (%) | 85 | 85 | 85 | 54 | 78 | 78 |
| Statin use (%) | 60 | 62 | 54 | 59 | 48 | 49 |
Data presented as mean (± SD) or frequencies (%).
P< 0.05 for the difference between treatment group within race/ethnic groups by t-test for continues variables with normal distribution, Mann-Whitney U test for variables with skewed distribution (triglycerides and pack-years of smoking), and chi square test for percentages.
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; HbA1c, hemoglobin A1c; HDL, high density lipoprotein; LDL, low density lipoprotein; TZD, thiazolidinedione
Number of missing values: diabetes duration=18, blood pressure=8, total cholesterol=3, triglycerides=3, LDL=91, HDL=5, total cholesterol-to-HDL ratio =6, and smoking=14.
Most cardiovascular risk factors improved in each race/ethnic group in both treatment arms (Supplementary Table 2). The exceptions was BMI, which increased in the intensive arm in all race/ethnic groups (P <0.05), and tended to increase most in Hispanics (P=0.08 for overall comparisons between race/ethnic groups in intensive arm). Mean HbA1c levels decreased in both treatment arms, stabilized at 6 months, and the pre-specified goal of an absolute between-treatment arm difference of 1.5% points (16.4 mmol/mol) was achieved for all race/ethnic groups (Supplementary Figure 1). Although the absolute between-treatment arm differences in HbA1c were similar in each race/ethnic group throughout the study, at the end of the study the average HbA1c decline tended to be more pronounced among Hispanics in the intensive treatment arm (−2.2±1.9% in Hispanics, 2.0±1.9 % in non-Hispanic Blacks, and −1.7±1.7% in non-Hispanic Whites, P=0.02 for overall comparisons between race/ethnic groups). Despite the improvement in HbA1c and in other modifiable CVD risk factors in all race/ethnic groups, the baseline differences persisted at the end of the study among race/ethnic groups; Non-Hispanic Whites had the lowest HbA1c levels throughout the study (P=0.01 for heterogeneity for HbA1c levels over time between race/ethnic groups), and non-Hispanic Blacks had a better lipid profile, but the highest blood pressure compared to others in both treatment arms (Supplementary Table 2).
Consistent with a low baseline prevalence of prior CVD, Hispanics as a whole (combining both treatment groups) continued to have the lowest rates of new CVD events (20%) during the study, with relatively similar rates in non-Hispanic Blacks (22%) and notably higher rates in non-Hispanic Whites (32%). Of note, the type of new CVD events was comparable between race/ethnic groups (Supplementary Table 3). Moreover, in a univariate Cox proportional hazards model with both treatment arms pooled, both Hispanics and non-Hispanic Blacks were at significantly lower risk for future CVD events. Compared with non-Hispanic Whites as reference group (hazard ratio=1), HR (95% confidence interval) was 0.54 (0.41–0.71) in Hispanics, and 0.65 (0.50–0.84) in non-Hispanic Blacks respectively. Of note, this pattern persisted even in the subset of participants without known baseline CVD.
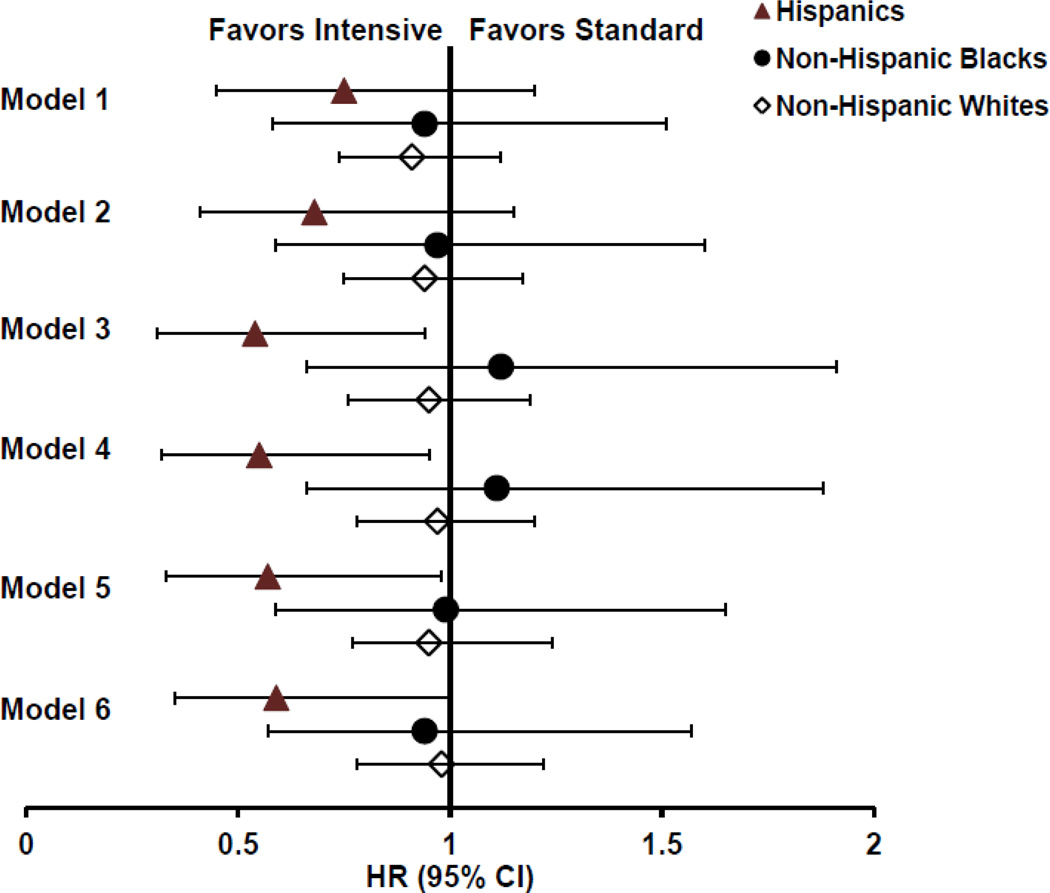
Despite an overall reduced rate of future CVD events in both non-Hispanic Blacks and Hispanics compared with non-Hispanic Whites, intensive glucose control appeared to be associated with a lower risk of CVD events only in Hispanics. As shown in Figure 1 (Model 1), the unadjusted HR (95% CI) for the effect of intensive glucose treatment in Hispanics was 0.75 (0.45–1.25), whereas it was 0.94 (0.58–1.51) in non-Hispanic Blacks, and 0.91 (0.74–1.12) in non-Hispanic Whites, respectively. Notably, the HR for intensive glucose treatment in Hispanics remained consistently and clearly below 1 after adjustment for combinations of the many relevant CVD risk factors, identified from stepwise variable selection models (shown in Supplementary Table 1), whereas it was close to 1 in other race/ethnic groups (Models 2–6). Although confidence intervals for the treatment estimate in Hispanics were relatively wide in the unadjusted (Model 1) and baseline risk factor adjusted model (Model 2), including time-varying on-trial variables such as BMI, total cholesterol-to-HDL ratio, and mean arterial blood pressure (Models 3–6) further reduced the variation in treatment estimates for Hispanics. To explore if the effect of intensive glucose control on incident CVD in Hispanics was explained by better glucose control during the study, we compared a model including baseline HbA1c (Model 6) with an otherwise similar model including on-trial HbA1c instead of the baseline HbA1c value. However, this latter model only modestly increased the HR for incident CVD with intensive glucose control from 0.59 (0.36–1.01, in Model 6) to 0.64 (0.35–1.17) in Hispanics. This relatively small change in HR is not altogether surprising as on-trial HbA1c was only weakly associated with CVD in the same model (1.06, 0.89–1.28).
Figure 1.
| Model 1 | Unadjusted model. Number of events/participants and P-value for intensive treatment: Hispanics, 60/307, P=0.27; non-Hispanic Blacks (NHB), 68/306, P=0.78; non-Hispanic white (NHW), 354/111, P=0.38 |
| Model 2 | Adjusted for age, prior cardiovascular disease (CVD), history of hypertension, duration of diabetes and pack-years of smoking. Hispanics, 59/292, P= 0.15; NHB, 67/302, P=0.89; NHW, 344/1086, P=0.60 |
| Model 3 | Adjusted for age, prior CVD, and on-trial values for body mass index (BMI) and total cholesterol-to-HDL ratio and mean arterial pressure. Hispanics, 59/296, P= 0.02; NHB, 62/285, P=0.66; NHW, 317/1035, P=0.69 |
| Model 4 | Adjusted for age, prior CVD, history of hypertension, on-trial total BMI and cholesterol-to-HDL ratio. Hispanics, 59/294, P= 0.03; NHB, 62/285, P=0.69; NHW, 316/1034, P=0.82 |
| Model 5 | Adjusted for age, prior CVD, history of hypertension, on-trial values for and total cholesterol-to-HDL ratio and mean arterial pressure. Hispanics, 59/294, P= 0.04; NHB, 62/285, P=0.99; NHW, 316/1034, P=0.67 |
| Model 6 | Adjusted for age, prior CVD, history of hypertension, baseline HbA1c and on-trial total cholesterol-to-HDL ratio. Hispanics, 59/294, P= 0.05; NHB, 62/285; P=0.83; NHW, 316/1034 P=0.85 |
Importantly, the consistency of these results was supported in several sensitivity analyses. For example, categorizing all VADT participants into Hispanics vs. non-Hispanics regardless of race, provided a HR for intensive glucose control after adjustment for age, prior CVD and on-trial variables (BMI, mean arterial pressure and cholesterol-to-HDL ratio) that was 0.58 (0.34–0.98) in Hispanics and 0.94 (0.77–1.15) in non-Hispanics, respectively. Furthermore, excluding individuals of mixed race from the race/ethnic groups or excluding both mixed races and those with prior CVD did not appreciably change the point estimates for intensive treatment (Supplementary Table 4).
Discussion
The results of these analyses support the hypothesis that Hispanics may have an “enhanced cardiovascular response” to glucose lowering therapy. Although this was a post-hoc examination of race/ethnic subgroups, there was remarkable consistency in the findings. Intensive glycemic control was associated with 25%–45% lower risk of CVD events in Hispanics across multiple models containing different predictor variables and in several sensitivity analyses that tested different racial/ethnic definitions and/or excluded individuals with known CVD.
Although there were a few differences in baseline or on trial CVD risk factors between the race/ethnic groups, the differences did not account for the reduced risk of CVD events with intensive glucose treatment in Hispanics. Non-Hispanic Whites had notably higher CVD history and pack-years of smoking.at baseline. However, after adjusting for these baseline differences or excluding participants with known baseline CVD, the subset of individuals most aggressively treated with risk modifying medications (e.g., statins, aspirin and anti-hypertensive medications), the results did not change. Of note, the use of non-diabetes medications to improve CVD risk factor profiles increased similarly in both treatment arms during the study [3]. Importantly, as these analyses focused on comparing the response between treatment groups, the only significant baseline difference between treatment groups in Hispanics was a longer duration of diabetes in the intensive arm. However, this would be expected to attenuate the association between treatment intensity and CVD events. Nevertheless, adjustment for duration of diabetes did not change the results. By design, modifiable non-glycemic risk factors, such as lipids and blood pressure, improved during the study in participants from both treatment arms. Importantly, adjustment for these on-trial risk factors in different multivariable models strengthened the association between intensive treatment and CVD outcomes, and contributed to supplementary benefit of intensive treatment in Hispanics [3].
The results of this study were similar to a secondary finding of the recently published Look AHEAD study, which tested the effects of lifestyle intervention for weight loss on CVD events in overweight or obese adults with type 2 diabetes. Despite achieving sustained improvements in glycated hemoglobin levels and several other risk factors throughout the study [39] in the intensive lifestyle treated group, there was no evidence for reduced CVD events in the entire study population. However, although no interactions between intervention and pre-specified subgroups were significant in the Look AHEAD study; those data suggest that the event rates for the primary outcomes were lower in the intervention group compared with the control group in two subgroups, i.e. those participants with no history of CVD at baseline and among Hispanics.
The explanation for the apparent “enhanced cardiovascular response” of Hispanics to glucose lowering or weight loss therapy is unknown. It is possible that a lower burden of subclinical atherosclerosis in Hispanics contributed to reduced CVD outcomes in the intensive arm. Studies by several groups, including ours, have reported that Hispanics have reduced subclinical atherosclerosis when carefully measured by several different imaging modalities [15–19]. Of particular note, in an ancillary study of the VADT, intensive glycemic control significantly reduced the primary CVD endpoints in those with low CAC scores at baseline, but not in those with high scores [4]. In line with the current study, the absence of CVD at baseline was also associated with reduced CVD outcomes in the intensive therapy group in ACCORD trial [1] and with lifestyle intervention in the Look AHEAD study [39]. One may speculate that a lower atherosclerosis burden could also account for the greater effectiveness of glucose and risk factor improvement on CVD outcomes resulting from lifestyle intervention in Hispanics in the Look AHEAD study. This is consistent with the growing recognition that glucose lowering interventions may be most effective for CVD prevention when started earlier in the course of diabetes [4, 5, 9]. Although further work will be needed to clarify potential mechanism(s) accounting for the apparent enhanced cardiovascular response to glucose lowering therapy in Hispanics, behavioral or genetic predispositions may modify vascular responses that alter the development of atherosclerosis [33–35].
Although our data do not directly address whether Hispanics have reduced CVD mortality compared with other race/ethnic groups, they do appear consistent with the notion of a “Hispanic Paradox”. First, Hispanics had a lower prevalence of CVD events at baseline despite having CVD risk profiles at study entry similar to non-Hispanics. Secondly, compared with non-Hispanic Whites, Hispanics as a group had a lower overall incidence of CVD events during the study, although on-study CVD risk profiles were similarly well controlled in both groups. Finally, in response to similar intensification of glucose control in Hispanics and non-Hispanics (using identical methods and medications), intensified treatment was associated with a trend for substantially greater CVD benefit in Hispanics.
An important advantage of our study was that comprehensive health care was available and provided to all veteran participants without regard to race/ethnicity. Moreover, general diabetes care and CVD risk factor management was provided similarly to all participants according to established study algorithms. Thus, in contrast to prior studies, our finding of apparent differences in CVD health outcomes according to race/ethnicity were much less likely to be confounded by factors such as differences in access to healthcare, or the type of healthcare, or differences in socioeconomic status that mediated differences in access to care. Several other features of the study support the validity of the study findings. The VADT was a randomized controlled long-term trial with careful monitoring and adjudication of the results, thus differential reporting of CVD and mortality rates, a concern among some prior studies of health outcomes among different ethnic groups [30–32], did not occur. As all participants had type 2 diabetes, the commonly noted race/ethnic differences in prevalence of diabetes [30, 31] and their potential effects on subsequent CVD outcomes did not influence the results. Furthermore, by design, a substantial HbA1c separation was achieved between treatment arms in all race/ethnic groups using specific treatment algorithms, so that difference in insulin or other diabetes medication use [30] and cultural barriers to the successful glycemic control in Hispanics [40] were not a concern in VADT. A noteworthy finding in our study was that despite a higher burden of hyperglycemia (higher HbA1c) at baseline among Hispanics compared with non-Hispanic Whites, there was a trend for a greater response (reduction in HbA1c compared with baseline) in Hispanics in the intensive arm. These data point out that while receiving similar access to healthcare and diabetes guidance, Hispanics not only respond well to glucose lowering efforts, but may also gain additional benefit with respect to its consequences on CVD. This is both a tribute to the conduct of the VADT and a potential lesson for all diabetes care-givers.
However, some limitations of this study deserve attention. Participants in the VADT were older, predominantly male veterans with longstanding type 2 diabetes, thus the findings in this study may not apply to younger female diabetes patients. Race/ethnicity was only assessed by self-report and some misclassification of race/ethnic groups may have occurred. Importantly, the VADT did not pre-specify an assessment of modification of the treatment effect by race/ethnicity and these results are a post hoc subgroup analysis. The sample size for non-Hispanic Blacks and Hispanics was substantially smaller than non-Hispanic Whites, and this presumably hampers identifying a significant interaction between treatment effect and the three race/ethnic categories, however, we cannot rule out the possibility of chance or residual confounding in this relationship. For these reasons, the findings of this study should be viewed in the context of hypothesis-generation, and hopefully will stimulate further examination of this issue. However, this post-hoc analysis provided an excellent opportunity to examine the association between intensive glucose control and CVD events among major race/ethnic groups independent of differences in access to care, ascertainment of CVD events, presence or absence of diabetes and insulin use and patient compliance.
In summary, intensive glucose control appears to be associated with reduced CVD events in Hispanics, but not in non-Hispanics. The apparent benefit was remarkably consistent in all analyses, using different classifications of race/ethnic groups, different predictor variables and excluding individuals with prior CVD. Importantly, the apparent favorable association between intensive treatment and CVD event rate in Hispanics was only partly explained by on-trial HbA1c. These findings underscore the importance of future investigation of responses to intensive glycemic control in various race/ethnic groups. This will require additional randomized trials (and re-examinations of prior trials) with sufficient power to detect modification of effects of intensive glycemic control by race/ethnicity.
Supplementary Material
Acknowledgments
This work was supported by the Office of Research and Development, Medical Research Service and Cooperative Studies Program, U.S. Department of Veterans Affairs. We gratefully acknowledge the VADT study staff and the investigators, particularly former chairmen Drs. Abraira and Duckworth, as well as other key contributors to initiation and conduct of the VADT (Domenic Reda, Madaleine McCarren, and Thomas Moritz).
Funding
Grant support: R01-067690 (P.D.R) and R01-HL-094775 (P.D.R).
Abbreviations
- RCT
randomized controlled trial
- CVD
cardiovascular disease
- VADT
Veterans Affairs Diabetes Trial
- UKPDS
the United Kingdom Prospective Diabetes Study
- HbA1c
HemoglobinA1c
- BMI
body mass index
- HDL
high density lipoprotein
- SD
standard deviation
- HR
Hazard Ratio
- CI
confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
A.S. participated in study design, statistical analysis, data interpretation and wrote the initial manuscript. D.C.S. participated in statistical analysis and interpretation and reviewed and edited the manuscript. G.B, L.G participated in data gathering and statistical analysis and reviewed and edited the manuscript. N.E participated in data collection, and reviewed and edited the manuscript and P.D.R participated in study design, data gathering, and data analysis and reviewed and edited the manuscript.
Conflict of interest
Nothing to declare.
The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 3.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 4.Reaven PD, Moritz TE, Schwenke DC, Anderson RJ, Criqui M, Detrano R, et al. Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes. 2009;58:2642–2648. doi: 10.2337/db09-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duckworth WC, Abraira C, Moritz TE, Davis SN, Emanuele N, Goldman S, et al. The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA Diabetes Trial. J Diabetes Complications. 2011;25:355–361. doi: 10.1016/j.jdiacomp.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–2298. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 8.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 9.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 12.Chokrungvaranon N, Deer J, Reaven PD. Intensive glycemic control and cardiovascular disease: are there patients who may benefit? Postgrad Med. 2011;123:114–123. doi: 10.3810/pgm.2011.11.2501. [DOI] [PubMed] [Google Scholar]
- 13.Del Prato S, LaSalle J, Matthaei S, Bailey CJ. Tailoring treatment to the individual in type 2 diabetes practical guidance from the Global Partnership for Effective Diabetes Management. Int J Clin Pract. 2010;64:295–304. doi: 10.1111/j.1742-1241.2009.02227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terry T, Raravikar K, Chokrungvaranon N, Reaven PD. Does aggressive glycemic control benefit macrovascular and microvascular disease in type 2 diabetes? Insights from ACCORD, ADVANCE, and VADT. Curr Cardiol Rep. 2011;14:79–88. doi: 10.1007/s11886-011-0238-6. [DOI] [PubMed] [Google Scholar]
- 15.Reaven PD, Thurmond D, Domb A, Gerkin R, Budoff MJ, Goldman S. Comparison of frequency of coronary artery calcium in healthy Hispanic versus non-Hispanic white men by electron beam computed tomography. Am J Cardiol. 2003;92:1198–1200. doi: 10.1016/j.amjcard.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Reaven PD, Sacks J. Reduced coronary artery and abdominal aortic calcification in Hispanics with type 2 diabetes. Diabetes Care. 2004;27:1115–1120. doi: 10.2337/diacare.27.5.1115. [DOI] [PubMed] [Google Scholar]
- 17.Saremi A, Moritz TE, Anderson RJ, Abraira C, Duckworth WC, Reaven PD. Rates and determinants of coronary and abdominal aortic artery calcium progression in the Veterans Affairs Diabetes Trial (VADT) Diabetes Care. 2010;33:2642–2647. doi: 10.2337/dc10-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 19.Breton CV, Wang X, Mack WJ, Berhane K, Lopez M, Islam TS, et al. Carotid artery intima-media thickness in college students: race/ethnicity matters. Atherosclerosis. 2011;217:441–446. doi: 10.1016/j.atherosclerosis.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard BV, Lee ET, Cowan LD, Fabsitz RR, Howard WJ, Oopik AJ, et al. Coronary heart disease prevalence and its relation to risk factors in American Indians. The Strong Heart Study. Am J Epidemiol. 1995;142:254–268. doi: 10.1093/oxfordjournals.aje.a117632. [DOI] [PubMed] [Google Scholar]
- 21.Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, et al. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99:2389–2395. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- 22.Pavkov ME, Sievers ML, Knowler WC, Bennett PH, Nelson RG. An explanation for the increase in heart disease mortality rates in diabetic Pima Indians: effect of renal replacement therapy. Diabetes Care. 2004;27:1132–1136. doi: 10.2337/diacare.27.5.1132. [DOI] [PubMed] [Google Scholar]
- 23.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 24.Sorlie PD, Backlund E, Johnson NJ, Rogot E. Mortality by Hispanic status in the United States. JAMA. 1993;270:2464–2468. [PubMed] [Google Scholar]
- 25.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2010;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willey JZ, Rodriguez CJ, Moon YP, Paik MC, Di Tullio MR, Homma S, et al. Coronary death and myocardial infarction among Hispanics in the Northern Manhattan Study: exploring the Hispanic paradox. Ann Epidemiol. 2012;22:303–309. doi: 10.1016/j.annepidem.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez MV, Yaw TS, Myers J, Froelicher VF. Prognostic value of the computerized ECG in Hispanics. Clin Cardiol. 2007;30:189–194. doi: 10.1002/clc.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz JM, Steffen P, Smith TB. Hispanic mortality paradox: a systematic review and meta-analysis of the longitudinal literature. Am J Public Health. 2013;103:e52–e60. doi: 10.2105/AJPH.2012.301103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS data brief. 2014:1–8. [PubMed] [Google Scholar]
- 30.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP, Hazuda HP. All-cause and cardiovascular mortality among Mexican-American and non-Hispanic White older participants in the San Antonio Heart Study- evidence against the "Hispanic paradox". Am J Epidemiol. 2003;158:1048–1057. doi: 10.1093/aje/kwg249. [DOI] [PubMed] [Google Scholar]
- 31.Hunt KJ, Williams K, Resendez RG, Hazuda HP, Haffner SM, Stern MP. All-cause and cardiovascular mortality among diabetic participants in the San Antonio Heart Study: evidence against the "Hispanic Paradox". Diabetes Care. 2002;25:1557–1563. doi: 10.2337/diacare.25.9.1557. [DOI] [PubMed] [Google Scholar]
- 32.Lerman-Garber I, Villa AR, Caballero E. Diabetes and cardiovascular disease. Is there a true Hispanic paradox? Rev Invest Clin. 2004;56:282–296. [PubMed] [Google Scholar]
- 33.Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, et al. Status of cardiovascular disease and stroke in hispanics/latinos in the United States: a science advisory from the american heart association. Circulation. 2014;130:593–625. doi: 10.1161/CIR.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wassel CL, Pankow JS, Rasmussen-Torvik LJ, Li N, Taylor KD, Guo X, et al. Associations of SNPs in ADIPOQ and subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis (MESA) Obesity. 2011;19:840–847. doi: 10.1038/oby.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson JA. Ethnic differences in cardiovascular drug response: potential contribution of pharmacogenetics. Circulation. 2008;118:1383–1393. doi: 10.1161/CIRCULATIONAHA.107.704023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis TM, Coleman RL, Holman RR. Ethnicity and long-term vascular outcomes in Type 2 diabetes: a prospective observational study (UKPDS 83) Diabet Med. 2014;31:200–207. doi: 10.1111/dme.12353. [DOI] [PubMed] [Google Scholar]
- 37.Abraira C, Colwell JA, Nuttall FQ, Sawin CT, Nagel NJ, Comstock JP, et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995;18:1113–1123. doi: 10.2337/diacare.18.8.1113. [DOI] [PubMed] [Google Scholar]
- 38.NIH Policy on reporting race and ethnicity data: National Institute of Health Subjects in clinical research. 2001 2001 ed. http://grants.nih.gov/grants/guide/notice-files/NOT-OD-01-053.html.
- 39.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campos C. Addressing cultural barriers to the successful use of insulin in Hispanics with type 2 diabetes. South Med J. 2007;100:812–820. doi: 10.1097/SMJ.0b013e3180f609c4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.