Abstract
BACKGROUND
Cytologic findings of pancreatic oncocytic-type intraductal papillary mucinous neoplasms (IPMNs)/intraductal oncocytic papillary neoplasms (IOPNs) are largely unknown.
METHODS
Five IOPNs encountered by the authors were analyzed.
RESULTS
Four IOPNs were located in the pancreatic head, and 1 was located in the pancreatic body/tail in 2 men and 3 women ages 56 to 84 years (mean age, 66 years). Radiologic diagnoses included pancreatic ductal adenocarcinoma (PDAC) in 2 patients, invasive cancer associated with IPMN in 1 patient, IPMN versus mucinous cystic neoplasm in 1 patient, and cystic mass in 1 patient. Cytologic findings included: hypercellular smears (4 of 5 cases) containing well formed clusters of oncocytic cells (5 of 5 cases) with prominent, slightly eccentric nucleoli (4 of 5 cases), predominantly arranged in sheets/papillary units (5 of 5 cases), with punched-out intercytoplasmic spaces (4 of 5 cases), and with occasional 3-dimensional groups and focal necrosis (3 of 5 cases). The intracytoplasmic mucin and thick extracellular mucin typical of other IPMNs were observed only in 2 cases and were very limited. The mean size on resection was 4.5 cm. Invasion was observed in 3 cases (0.1, 0.3, and 2.0 cm) of tubular-type IPMN. Initial cytologic evaluation was performed by the authors in 4 of 5 cases, which were diagnosed as IOPN (n = 3) and IPMN versus cystic PDAC (n = 1). One case was initially misdiagnosed as PDAC and, on resection, proved to be noninvasive IOPN.
CONCLUSIONS
Cytologic features of IOPNs are classical, similar to their histologic counterparts, and differ significantly from other IPMN subtypes. Because of their highly complex appearance, they are often radiologically misdiagnosed as PDAC; thus, failure to recognize their characteristic features on fine-needle aspiration may lead to inappropriate treatment. Patients with IOPN have an incomparably better prognosis than patients with ordinary PDAC, even when their neoplasms are invasive.
Keywords: cytology, fine-needle aspiration, intraductal oncocytic papillary neoplasm (IOPN), intraductal papillary mucinous neoplasm (IPMN), oncocytic, oncocytic intraductal papillary mucinous neoplasm, pancreas
INTRODUCTION
The vast majority of clinically relevant pancreatic masses are pancreatic ductal adenocarcinomas (PDACs), which have a dismal prognosis and a 5-year survival rate of less than 5%. PDAC is currently the fourth (and is estimated to become the third) leading cause of cancer-related death in the United States.1 More Americans die of pancreatic cancer than prostate cancer, and it is estimated that, in 2015, pancreatic cancer will surpass breast cancer as the third leading cause of cancer mortality in the United States.1 Because of its relative frequency and dire clinical consequences, the foremost task in the differential diagnosis of pancreatic lesions is the exclusion of PDAC; and, if it is radiologically complex, the case is typically assigned this diagnosis.
The vast majority of PDACs present as solid lesions; consequently, PDAC is seldom considered in the differential diagnosis of cystic pancreatic lesions, which are increasingly being encountered in clinical practice. In fact, because of advances in imaging technology, incidental pancreatic cysts are discovered in nearly 14% of the elderly.2 Fortunately, most cystic pancreatic lesions are either benign or low-grade malignant neoplasms. The most common cystic pancreatic neoplasms are intraductal papillary mucinous neoplasms (IPMNs), which are defined as cystic, mass-forming, intraductal tumors characterized by proliferating, mucinous epithelium and result in cystic dilatation of the main and/or branch ducts.3-7 Similar to PDACs, IPMNs typically arise in patients aged ≥60 years and are much more common in the pancreatic head. Although the vast majority of IPMNs present as innocuous-appearing cystic lesions, a subset (the entity originally described as intraductal oncocytic papillary neoplasms [IOPNs] and now classified as the oncocytic variant of IPMN in the 2010 World Health Organization series), frequently presents as complex tumors that are often clinically interpreted as cancerous.8,9 However, recent studies indicate that, in fact, IOPNs are quite different from IPMNs not only in their clinical presentation but also at the molecular level.9-11 In this report, we present cytologic findings from 5 examples of the oncocytic variant of IPMN (also known as IOPN) in which an accurate cytologic diagnosis on fine-needle aspiration biopsy (FNAB) was crucial in ensuring proper patient management and avoiding detrimental chemoradiation.
MATERIALS AND METHODS
Of 2600 pancreatectomy specimens from 1979 to 2014 that included 1690 adenocarcinomas (and variants) as well as 185 verified IPMNs (including 10 resected IOPNs), 5 specimens had cytologic material available for analysis. In addition, 11 IOPNs that were originally reported by 1 of the authors (V.A.) in 1996 (from the files of Memorial Sloan-Kettering Cancer Center) were also reviewed, but none of those had FNAB material available, probably because of incomplete clinical histories or because most were from an era (before 1995) when pancreatic FNABs were not as common. This study was approved by the institutional review board.
All patients underwent endoscopic ultrasound-guided FNAB with rapid on-site evaluation (ROSE) and 3 to 6 passes per patient (mean, 3.7 passes per patient), all of which were deemed adequate. Cyst fluid analysis and molecular studies were not performed on any of the specimens.
All slides of the cytology specimens were reviewed. On average, 7 slides were available per case (range, 2-19 slides per case), including both Papanicolaou-stained slides (range, 1-8 per case) and Diff-Quik–stained slides (range, 1-8 per case). Cell-block sections were available for review in 4 of 5 cases.
All 5 follow-up resection specimens were reviewed by the authors. For the consultation cases, follow-up clinical information was obtained from the submitting outside pathologists.
The clinicocytopathologic characteristics of the 5 cases were compared with the findings documented in the literature for ordinary (nononcocytic) IPMNs, including our own publications in which we documented both the clinical and pathologic features of nononcocytic IPMNs4 and also performed a separate, focused analysis of the distinct subtypes.5,12
RESULTS
Clinical Characteristics
The 5 patients included 2 men and 3 women who ranged in age from 56 to 84 years (mean age, 66 years). Four patients had tumors arising in the pancreatic head, and 1 patient had a tumor arising in the pancreatic body/tail.
Radiologic Findings
Tumors ranged in size from 2.5 to 6.6 cm (mean size, 4.5 cm) and had radiologic diagnoses of PDAC (n = 2; cases 1 and 5); mucinous cystic neoplasm versus IPMN (n = 1; case 4); main duct-IPMN with invasive component (n = 1; case 2); and cystic mass, not otherwise specified (n = 1; case 3). It is noteworthy that only case 2 was definitively diagnosed as IPMN on imaging (based on marked dilatation of the main pancreatic duct [3.3 cm]) and was also interpreted as having an invasive component on imaging, but it proved to be noninvasive on resection. Clinicopathologic and radiologic findings are summarized in Table 1.
TABLE 1.
Clinicopathologic Characteristics of Patients With Oncocytic Intraductal Papillary Mucinous Neoplasms
| Case | Age, y |
Sex | Location | Imaging Modality |
Size on Imaging, cm |
Additional Radiologic Findings |
Radiologic Diagnosis |
Cytologic Diagnosis |
Histologic Diagnosis | Size of Invasion, cm |
Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | Man | Head | MRI | 6.6 | Mass communicates with pancreatic duct (7.5 mm) |
Cystic and solid mass PDAC |
PDAC with intraductal component |
IOPN, 6.5 cm; negative margins |
None identified | ANED at 38 mo |
| 2 | 56 | Woman | Head | CT | 5.6 | Cystic mass in main and branch PDs with papillary projections and dilatation of PD to 3.3 cm |
MD-IPMN with invasive component |
IPMN vs cystic PDAC |
IOPN, 8.7 cm, with minute focus of PDAC; margin positive for IPMN, LNs negative, confined to pancreas |
0.3 with focal extravasated mucin |
ANED at 15 mo |
| 3 | 68 | Woman | Head | CT | 4.3 | Cystic mass in pancreatic head with pancreatic duct dilatation to 7 mm |
Cystic mass | IOPN, with high-grade atypia |
IPMN, 3.3 cm, with high-grade dysplasia/carcinoma in situ; negative margins and LNs |
None | LTF/U |
| 4 | 84 | Man | Head | MRI | 2.5 | Dilated CBD 7 mm and pancreatic duct 6 mm; thickened septae and thick fluid observed |
Mucinous cyst: MCN vs IPMN |
IOPN | IOPN, 2.2 cm, minute focus suspicious for PDAC; negative margins, LNs |
0.1 | Perioperative death, 23 d postsurgery |
| 5 | 64 | Woman | Body | MRI | 3.7 | Solid, circumscribed mass with central cystic component; PD dilated to 6 mm |
PDAC | IOPN, with high-grade atypia, suspicious for invasion |
IOPN, 4.5 cm, multifocal PDAC; negative margins, 5 of 17 LNs positive |
<2 cm aggregate microscopic measurement |
ANED at 9 mo, receiving chemotherapy |
Abbreviations: ANED, alive with no evidence of disease; CBD, common bile duct; CT, computerized tomography scan; IOPN, intraductal oncocytic papillary neoplasm; LNs, lymph nodes; LTF/U, lost to follow-up; MCN, mucinous cystic neoplasm; MD-IPMN, main duct-intraductal papillary mucinous neoplasm; MRI, magnetic resonance imaging; PD, pancreatic duct; PDAC, pancreatic ductal adenocarcinoma.
Cytologic Findings
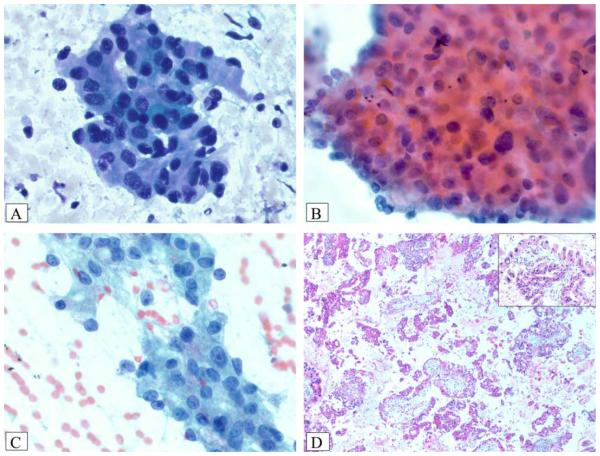
Diff-Quik smears were hypercellular in the majority of cases (4 of 5 cases; 80%), and limited amounts of thick magenta or blue extracellular mucin were present in the smear background in only 2 of 5 cases (40%). Neoplastic cells were observed predominantly in large, flat sheets (100% of cases) with only minimal crowding and occasional papillae (Fig. 1). In some areas, there were 3-dimensional groups (3 of 5 cases; 60%) with complex branching, papillary configuration (100%), and focal fibrovascular cores (80%) (Fig. 1). All 5 cases had numerous neoplastic cells that displayed oncocytic features (Fig. 2). These cells were intermediate to large with well defined borders, dense granular cytoplasm, large central nuclei with predominantly rounded nuclear borders, and prominent but relatively small, slightly eccentric nucleoli (100%) (Fig. 2). Intracytoplasmic mucin was not readily evident but was identifiable on careful inspection in 4 of 5 cases (80%) (Fig. 3). Focal oncotic cells were observed in the extracellular mucin and smear background in 30% of cases, indicative of cyst contents (Fig. 4). Necrotic debris was observed in the smear background in 3 of 5 cases (60%). Single, intact oncocytic cells were identified in 4 of 5 cases (80%), and rare psammomatous calcifications were observed in the smear background of case 5.
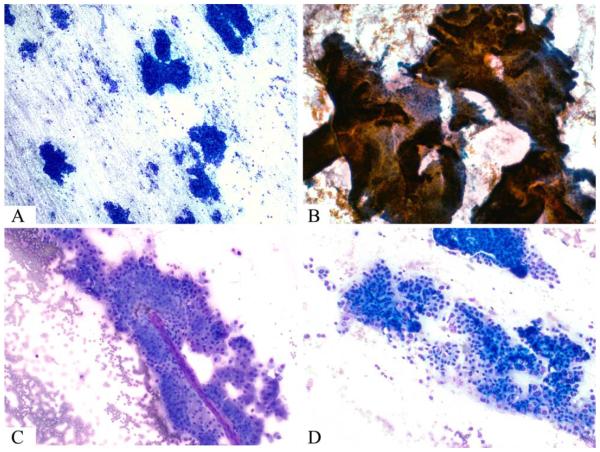
Figure 1.
(A) A hypercellular smear is composed of numerous sheets of epithelial cells (Diff-Quik stain). (B) Large, complex papillary units have an “antler-like” branching pattern similar to that observed in fibroadenoma of the breast (Papanicolaou stain). (C) Magenta-colored fibrovascular cores traversed some tumor cell clusters (Diff-Quik stain). (D) A hypercellular smear is composed of crowded sheets and singly dispersed oncocytic tumor cells (Diff-Quik stain).
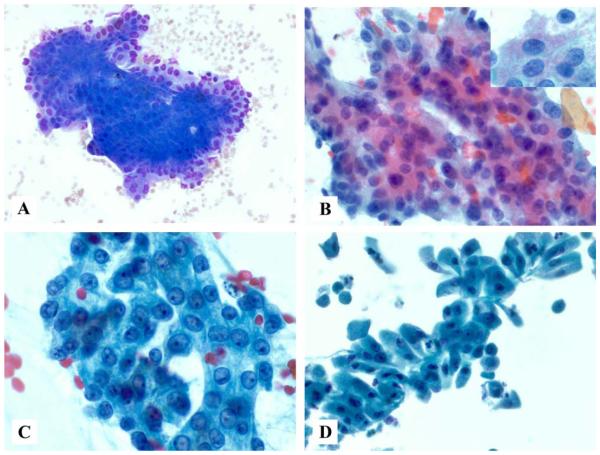
Figure 2.
(A) A smear composed of oncocytic cells arranged in large, flat sheets with prominent nucleoli is shown (Diff-Quik stain). (B) Tumor cells have round to oval nuclei, prominent nucleoli, and abundant pink cytoplasmic granules, as highlighted in the inset (Papanicolaou stain). (C) Case 2 had large vesicular nuclei with slightly eccentric, prominent nucleoli and more nuclear contour irregularity than that observed in B (Papanicolaou stain). (D) Oncocytic features of the tumor cells are easily identifiable on this ThinPrep slide (Papanicolaou stain).
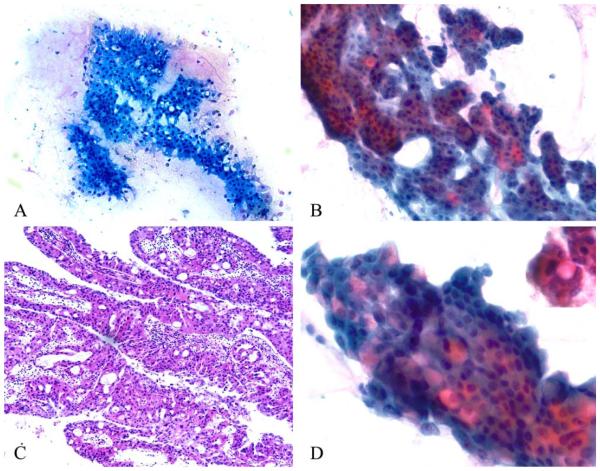
Figure 3.
Rigid, punched-out, intercellular spaces are visible on (A) Diff-Quik–stained and (B) Papanicolaou-stained smears and give a vaguely cribriform appearance to cell clusters. (C) Identical punched-out spaces are observed in this resected oncocytic intraductal papillary mucinous neoplasm composed of complex, branching, edematous papillae lined by oncocytic epithelium (H&E stain). (D) Intracytoplasmic pink mucin is observed in occasional tumor cells, as highlighted in the inset (Papanicolaou stain).
Figure 4.
Case 1 was an oncocytic intraductal papillary mucinous neoplasm that was misdiagnosed as pancreatic ductal adenocarcinoma on cytology. (A) Smears revealed crowded sheets of oncocytic cells (Diff-Quik stain). (B) Thick, colloid-like mucin was present and contained necrotic debris and clusters of atypical cells with prominent cytoplasmic vacuoles (Papanicolaou stain). (C) Occasional 3-dimensional groups with high nuclear-to-cytoplasmic ratio and irregular nuclear contours highly concerning for adenocarcinoma were present (Papanicolaou stain). (D) A corresponding cell block revealed numerous papillae with hyalinized fibrovascular cores lined by atypical oncocytic cells and no adenocarcinoma component (H&E stain).
Papanicolaou-stained smears and ThinPrep slides revealed large oncocytic cells, some with ill-defined cell borders and a vague, syncytial arrangement (Figs. 2, 3). The cells had dense, granular cytoplasm and round-to-oval central nuclei with prominent, slightly eccentric nucleoli. Cytoplasmic granules ranged in color from greenish-blue to orange-pink (Figs. 2, 5). Chromatin was relatively fine and homogenous but ranged from predominantly hyperchromatic in most cases to hypochromatic in others (Fig. 2). Nuclei were predominantly round and smooth, but nuclear irregularity was appreciable in 2 cases (cases 2 and 5) (Figs. 4, 5; Table 2). Despite their overt cellularity and striking architectural complexity with sheet-like or papillary configuration, cytologically, the tumor cells displayed some degree of uniformity. Conversely, on careful inspection, anisonucleosis could also be observed focally in 4 of 5 cases (80%; mild in cases 2 and 3 and marked in case 5). Case 5 also had hyperchromatic nuclei, a high nuclear-to-cytoplasmic ratio, and single-cell and background necrosis (Fig. 5), and it had an invasive (<2 cm) component on resection. Punched-out intercytoplasmic spaces (Fig. 3) were observed focally within sheets. Most of these were empty and did not contain mucin. However, intracytoplasmic mucin was identified in occasional tumor cells (Fig. 3).
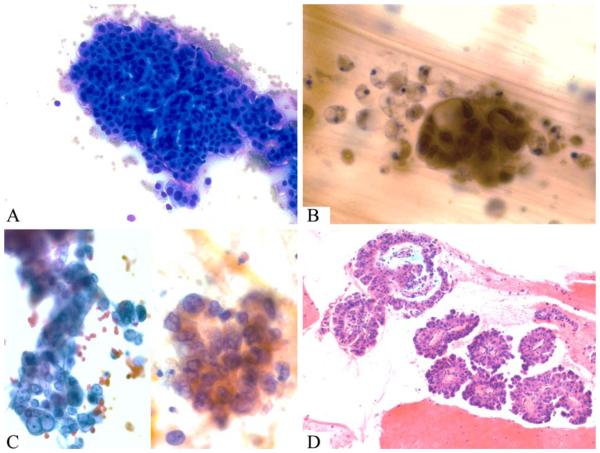
Figure 5.
Case 5 was an oncocytic intraductal papillary mucinous neoplasm that had an invasive adenocarcinoma component identified on resection. (A) Smears reveal numerous 3-dimensional clusters of oncocytic cells with background necrotic debris (Diff-Quik stain). (B) There is marked nuclear pleomorphism and single-cell necrosis (Papanicolaou stain). (C) Prominent nucleoli and nuclear irregularity were striking in some areas (Papanicolaou stain). (D) A cell block revealed multiple, broad papillae with striking basophilic edema lined by oncocytic cells with occasional intranuclear inclusions (inset) and large, hyperchromatic nuclei (H&E stain).
TABLE 2.
Cytologic Findings in Oncocytic Intraductal Papillary Mucinous Neoplasms
| Case |
||||||
|---|---|---|---|---|---|---|
| Cytologic Findings |
1 | 2 | 3 | 4 | 5 | No./Total No. (%) |
| Hypercellular | Yes | Yes | Yes | No | Yes | 4/5 (80) |
| Extracellular mucin | Yes | No | No | Yes | No | 2/5 (40) |
| Flat sheets | Yes | Yes | Yes | Yes | Yes | 5/5 (100) |
| Single cells | No | No | Yes | Yes | Yes | 2/5 (40) |
| 3D groups | Yes | Yes | No | No | Yes | 3/5 (60) |
| Papillary groups | Yes | Yes | Yes | Yes | Yes | 5/5 (100) |
| Fibrovascular cores | Yes | No | Yes | Yes | Yes | 4/5 (80) |
| Oncocytic cells | Yes | Yes | Yes | Yes | Yes | 5/5 (100) |
| Nuclear irregularity | No | Yes | No | No | Yes | 2/5 (40) |
| Anisonucleosis | Yes | Yes | Yes | No | Yes | 4/5 (80) |
| Prominent nucleoli | No | Yes | Yes | Yes | Yes | 4/5 (80) |
| Eccentric nucleoli | Yes | Yes | Yes | Yes | Yes | 5/5 (100) |
| Intranuclear inclusions | No | No | Yes | No | No | 1/5 (20) |
| Mitotically active | No | No | No | No | No | 0/5 (0) |
| Necrosis | Yes | No | Yes | No | Yes | 3/5 (60) |
| Intercellular spaces | No | Yes | Yes | Yes | Yes | 4/5 (80) |
| Cytoplasmic mucin | Yes, F | No | Yes | Y, F | Yes, F | 4/5 (80) |
Abbreviations: 3D, 3-dimensional; F, focal.
Cell blocks were available for review in 4 of 5 cases and were very helpful in supporting the diagnosis, because they often revealed arborizing papillae (some with striking basophilic edema) lined by large oncocytic cells (Figs. 4, 5). Mitotic figures and ovarian-type stroma were not observed on smears or cell blocks. Single-cell necrosis and areas of confluent necrosis were present in only 1 case (case 5), in which invasive carcinoma was identified on resection.
At the time of ROSE, 2 cases (cases 1 and 5) were called suspicious for adenocarcinoma. One (case 1) went on to be called PDAC (with a possible intraductal component) on final cytologic diagnosis (Fig. 4), but it proved to have no invasive component on resection. Final cytology diagnoses were made by the authors in the other 4 cases (80%) and included neoplastic cells present: oncocytic-type IPMN with high-grade atypia in 3 of 4 cases (75%; cases 3-5; all 3 were radiologically cystic) and neoplastic cells present: IPMN versus cystic pancreatic ductal adenocarcinoma in a single case (25%; case 2). For case 5, the diagnosis of suspicious for invasion was rendered in the original report based on the marked anisonucleosis, 3-dimensional clusters, and extensive single-cell and background necrosis/diathesis, features typically observed in invasive carcinomas. However, cases with such findings may be better classified as having features suspicious for the development of frank adenocarcinoma, because invasion cannot be definitively identified on cytologic specimens. This case proved to be multifocal invasive carcinoma with lymph node metastasis on resection. Cytologic findings are summarized in Table 2.
Histologic Findings
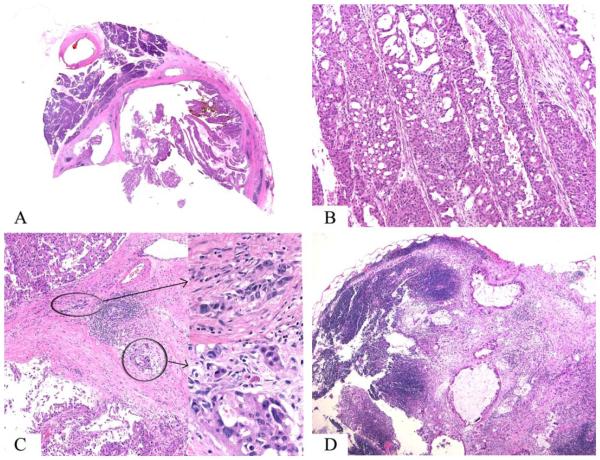
Resections included pancreatoduodenectomy (n = 4) and distal pancreatectomy (n = 1). On gross examination, tumors ranged in size from 2.2 to 6.5 cm (mean size, 4.5 cm) and were mostly composed of demarcated nodules of relatively soft, tan, friable, granular material that corresponded histologically to abundant intraductal papillary nodules with intervening atrophic/fibrotic pancreatic tissue (Figs. 3, 6).
Figure 6.
Case 5 was an oncocytic intraductal papillary mucinous neoplasm that had an invasive adenocarcinoma component identified on resection. (A) This wholemount slide reveals the intraductal location of a complex papillary neoplasm. (B) Papillae are lined by large oncocytic cells with (C) multiple, minute foci of microinvasive adenocarcinoma (see inset) and (D) lymph node metastasis (H&E stain).
Morphologically, all cases exhibited classic features of oncocytic IPMN (IOPN), with exuberant, complex, arborizing papillae and delicate fibrovascular cores lined by 2 to 5 cell layers of cells with distinctive oncocytic cytology (abundant acidophilic, granular cytoplasm; round nuclei; and single, prominent, eccentric nucleoli). Characteristic punched-out intercellular lumina formation was noted in all 5 cases (Fig. 3). Four cases were pure oncocytic IPMNs, whereas 1 (case 2) had an additional gastric-foveolar–type epithelial component (n = 1). All 4 resected IOPNs were graded (by default) as high-grade dysplasia, because of their cytoarchitectural complexity, nuclear enlargement and/or membrane irregularity, and nucleolar prominence. In addition, 3 of 5 cases (60%) had an invasive carcinoma component with a mean size of 0.8 cm (range, 0.1-2.0 cm). The invasive carcinoma component was of the tubular type (ordinary PDAC) in 2 cases but had focal extravasated stromal mucin in 1 case (Table 1). All margins were negative for invasive carcinoma, but 1 (case 2) had margin involvement by IPMN, and another (case 5) had lymph node metastasis (with 5 positive lymph nodes of 17 lymph nodes examined). Of the remaining 4 (cases 1-4), 18, 19, 14, and 23 negative lymph nodes were examined, respectively.
Follow-Up Information
Follow-up information was available for 4 patients (cases 1, 2, 4, and 5) and ranged from 23 days to 38 months. Patient 4 died of perioperative complications (pneumonia and intra-abdominal bleeding) 23 days after resection. At the last follow-up, all other patients (cases 1, 2, and 5) were alive with no evidence of disease, and patient 5 had completed 6 courses of chemotherapy. Patient 3 was lost to follow-up after surgery.
DISCUSSION
The oncocytic variant of IPMN, also known as IOPN, was first described in 1996 as a fairly distinct type of pancreatic tumor.13 Its entity-defining histomorphology is characterized by complex, arborizing, intraductal papillae lined by oncocytic epithelial cells containing mitochondria-rich eosinophilic cytoplasm and large nuclei with prominent, eccentric nucleoli and intercellular spaces, which sometimes impart an almost cribriform appearance to the epithelium.9,13,14
Because the papillary nodules that characterize these tumors are believed to represent proliferating intraductal processes, they have been designated IOPNs. Because they are intraductal and papillae-forming, they share several features with IPMNs and, thus, were included as a subset of IPMN in the 2010 World Health Organization classification, although the merit of this approach is now rightfully being questioned,8 because these oncocytic examples actually have various features that distinguish them from other IPMN epithelial subtypes (see Table 3).6,7,9,10,13 Mucin production is typically minimal and unimpressive; and, unlike intestinal-type (and other main-duct) IPMNs, virtually none of the oncocytic examples present with mucin extrusion from the ampulla of Vater. In addition, in contrast to gastric (and other branch-duct) IPMNs, these cases almost never present as innocuous cystic lesions. Instead, they typically form complex masses with solid and cystic components, and significant numbers are diagnosed radiologically as cystadenocarcinoma or PDAC with cystic change, as were 2 of our cases. An additional case had a highly infiltrative cancerous process as a part of its radiologic assessment but proved to be minimally invasive (with lymph node metastasis at resection). Another unique and interesting feature of IOPNs is their low/normal carcinoembryonic antigen levels, unlike other IPMN subtypes.15
TABLE 3.
Clinicopathologic Comparison of the Oncocytic Type of Intraductal Papillary Mucinous Neoplasm (IPMN) With Other IPMN Subtypes and Pancreatic Ductal Adenocarcinomasa
| IPMN Subtype |
||||||
|---|---|---|---|---|---|---|
| Variable | PDAC | IPMN, Gastric | IPMN, Intestinal | IPMN, Pancreatobiliary | IPMN, Oncocytic | Current Patients |
| Relative frequency on resection |
65 | 7 | 2 | 0.2 | 0.4 | 0.2 |
| Age, y | 66 | 66 | 66 | Inadequate data | 63 | 66 |
| Sex: Men:women | 1.1 | 0.76 | 1.1 | Inadequate data | 1.9 | 1.5 |
| Size, cm | 3.6 | 2.7 | 4.5 | 3.3 | 6 | 4.5 |
| Imaging findings | Solid, hypodense, nonho- mogenous mass with infiltrative borders; a double duct sign may be observed |
Dilated branch duct(s) (80%); mural nodule in 30% |
Dilated main duct (63%) ± branch ducts; mural nodule in 56% |
Dilated main duct (49%) ± involvement of branch ducts; mural nodule in 57% |
Large, solid or cystic, complex mass with circumscribed borders, ± branch (62%)/main duct (38%) involve- ment; PET-avid because of high meta- bolic activity of onco- cytic cells; mural nodule in 100% |
Cystic or cystic and solid mass with main duct and/or CBD dilatation |
| Histology | Infiltrative glands in abun- dant, desmoplastic stroma; glands with luminal necrosis adja- cent to muscular arteries; variable N/C; prominent cytoplasmic vacuoles; PNI; LVI |
Short, finger-like papillae or flattened mucinous epithelium identical to gastric foveolar epithe- lium; often accompa- nied by pyloric-like, basally oriented glands; more complex papillae when high grade |
“Villous-intestinal” pattern revealing villi lined by pseudostratified, baso- philic columnar epithe- lium with apical goblet- like mucin |
Complex, arborizing papil- lae with inconspicuous fibrovascular cores, lined by low cuboidal epithelium, with high N/ C, nuclear irregularity, and scant mucin production |
Complex, arborizing papil- lae lined by 2-5 cell layers of oncocytic cells; prominent inter- cellular spaces; scant cytoplasmic mucin; clean appearance with limited necrosis |
Complex, arborizing papil- lae lined by 2-5 cell layers of oncocytic cells; prominent inter- cellular spaces; scant cytoplasmic mucin; clean appearance with limited necrosis |
| Cytology | 3D cell clusters with high N/C, 4-fold anisonu- cleosis, irregular nuclei, prominent nucleoli, cytoplasmic vacuoles, single-cell necrosis, and background necrosis |
Abundant background colloid-like mucin ± oncotic cells; mucinous epithelium in sheets ± papillae, low to inter- mediate N/C, U-shaped cytoplasmic mucin cup in superficial ⅓ of cells; mild anisonucleosis |
Abundant background colloid-like mucin ± oncotic cells; mucinous epithelium in sheets and papillae, intermedi- ate to high N/C, colum- nar cells with pencillate, pseudostra- tified nuclei, goblet-like cytoplasmic mucin; moderate to marked anisonucleosis |
Variable amount of back- ground mucin; cells with high N/C, scant cytoplasmic mucin, 3D clusters, nuclear irregu- larity, hypochromasia/ hyperchromasia, mod- erate to marked anisonucleosis |
Scant background and intracytoplasmic mucin; variable amount of oncotic cells; large oncocytic cells in sheets ± papillae, with granular cytoplasm, large central nuclei, prominent slightly eccentric nucleoli, punched-out intercellu- lar spaces; mild to moderate anisonucleosis |
Scant background and intracytoplasmic mucin; variable amount of oncotic cells; large oncocytic cells in sheets ± papillae, with granular cytoplasm, large central nuclei, prominent slightly eccentric nucleoli, punched-out intercellu- llar spaces; mild to moderate anisonucleosis |
| Frequency of high-grade dysplasia |
NA | Low | Low-moderate | High (present in all cases, by definition) |
High (present in all cases, by definition) |
High (present in all cases) |
| Frequency of invasion, % | NA (100% invasive, by definition) |
10 | 40 | 68 | 45 (but mostly microscopic) |
60 |
| Type of invasive carcinoma | Pancreatobiliary/tubular type |
Tubular type | Colloid carcinoma (most); tubular type (rare) |
Tubular type | Tubular type, oncocytic type |
3 Tubular type (0.1, 0.3, and 2.0 cm) |
| Outcome | Dismal (5-y survival, 4%) | Excellent if noninvasive (most cases), but dis- mal if invasive, because invasion is usually tubular type (in ~10%) |
Excellent when noninva- sive; for invasive dis- ease, 5-y survival >55% |
Inadequate data; 5-y sur- vival reportedly 55% |
>90% 5-y survival (even when invasive) |
Limited (1 periop death; 1 LTFU; 3 ANED at 9, 15, and 38 mo |
Abbreviations: ±, with or without; 3D, 3-dimensional; ANED, alive with no evidence of disease; CBD, common bile duct; IOPN, IPMN-oncocytic type; LTFU, lost to follow-up; LVI, lymph-vascular invasion; N/C, nuclear-to-cytoplasmic ratio; NA, not applicable; PDAC, pancreatic ductal adenocarcinoma; periop, perioperative; PET, positron emission tomography; PNI, perineural invasion.
In addition to being highly complex and nodular and, thus, closely mimicking PDAC even when they are noninvasive, IOPNs may also harbor invasive carcinoma. In our series, a convincing invasive carcinoma component was observed in 3 cases, which is in accordance with a recent publication by Marchegiani et al, who reported that 61% of their surgical cohort of 19 patients had invasive carcinoma. However, despite their overall complexity and relatively large size (the mean size was 4.5 cm in our cohort), which causes major preoperative concern for malignancy, in fact, invasive carcinoma is typically small (mean size, 0.8 cm) in these tumors. Along with the tumor cells’ distinct biology,10,11 the small size of invasion may play a role in their surprisingly good prognosis, which is now amply illustrated in the literature and suggests that deaths from these tumors are only very rarely reported, with a 5-year survival rate >90%.9,13 For this reason, it is important to diagnose IOPNs on cytology so that patients can be appropriately treated in a thoughtfully planned fashion.
Although the cytologic features of IPMNs are well established,15,18-21 the cytologic features of IOPNs (oncocytic-type IPMNs) are described only rarely and mainly in isolated reports.19,21-23 This is probably related in part to the rarity of the entity, but it is also somewhat attributable to a lack of recognition, which we hope the current study will alleviate to some degree. It is noteworthy that, among the 21 IOPNs we encountered, only 5 had documented FNABs, but this may have been because many of these patients were from an era when pancreatic FNAB was not as common. We suspect that these lesions will be encountered increasingly in the future.
Because the preresection diagnosis of IPMN typically relies on imaging studies and a demonstration of thick mucin, and because IOPNs often lack both of these (see Table 3), it is crucial for cytopathologists to be aware of and rely on the cytomorphologic findings in these tumors, namely, their oncocytic cytology, their distinctive cellular complexity, a papillary architecture that differs from that of other IPMNs, and the presence of punched-out intercellular spaces. If such findings are encountered in a given case, then a more careful inspection with close cytoradiologic correlation would become crucial in making an accurate diagnosis and 1) ruling out other IPMN subtypes (because they are biologically and clinically different) but also 2) excluding ordinary PDACs, with which these tumors may be confused.
In addition to the sheets or papillary groups of characteristically large oncocytic cells with abundant granular cytoplasm, the unique cytologic features of these tumors also include their well defined cell borders, large central nuclei, prominent eccentric nucleoli, and focal intercellular punched-out spaces. Oncocytic papillae may be observed in ThinPrep solutions, smears, and cell blocks, but they are most easily appreciated on cell-block sections, which preserve papillary architecture and can better aid in diagnosis.19 In a study of cell-block material from 20 IPMNs, Monzen et al demonstrated that cell-block examination had 100% specificity in predicting IPMN subtype (including 2 oncocytic examples).19 Similar results were obtained by Hibi and colleagues, who subtyped 19 IPMNs (3 of them oncocytic) on pancreatic juice cytology.21
For reporting of IPMNs, Pitman et al proposed the term high-grade epithelial atypia to describe the spectrum of changes observed in neoplastic mucinous cysts with high-grade dysplasia and invasive carcinoma,24,25 which are of utmost importance in the diagnosis of these tumors. All oncocytic-type IPMNs, by default, qualify as exhibiting high-grade atypia by virtue of their architectural and cellular complexity, large nuclei, and prominent nucleoli. However, these tumors are also strikingly monotonous, with relatively mild anisokaryosis except for more degenerative/symplastic atypia akin to that observed in oncocytic neoplasms at other sites (eg, Hürthle cell tumors). Thus, it is not clear whether the high-grade atypia observed in these tumors is truly reflective of a more aggressive behavior or of a different cellular biology that does not necessarily translate into a malignant outcome. Recent studies have confirmed the unexpectedly good prognosis of these neoplasms, with very few patients dying of their tumors,9,13 illustrating that the very distinct nature of these neoplasms diverges from that of both conventional IPMNs and PDACs.
Differential Diagnosis
Compared with mucinous cystic neoplasms and other IPMN subtypes, we and others have observed that IOPNs (oncocytic IPMNs) produce far less intracellular mucin and have less extracellular colloid-like mucin on both cytologic and histologic examination, features typically reported in other IPMN subtypes.4-6,8,9,21 However, their distinctive oncocytic features separate them from other epithelial IPMN subtypes. A paucity of mucin combined with the cellularity and complex architecture of IOPNs, unfortunately, can lead to their misdiagnosis as PDAC, acinar tumors, pancreatic neuroendocrine tumors with oncocytic features, or metastases.
It should be kept in mind that, unlike classically cystic IPMN, oncocytic examples often have a more solid appearance on imaging, which can lead to misdiagnosis as adenocarcinoma by radiologists and gastroenterologists.13 Of the cases in our cohort, only 2 were correctly diagnosed as fundamentally intraductal neoplasms (neoplastic mucinous cysts) on imaging, whereas 2 were suspected of being PDACs. However, it should be noted here that even the cases that were radiologically interpreted as solid (suspect PDAC) were diagnosable as IOPNs because of their distinctive cytomorphologic features. It is also worth noting that an interesting radiologic feature of oncocytic IPMNs is their high uptake on positron emission tomography, likely because of the oncocytic cells’ high metabolic activity.26,27 The latter is potentially exploitable in their radiologic distinction from PDACs and other IPMN subtypes.28
Because of their complexity, large size, and often solid appearance, IOPNs are prone to be misdiagnosed clinically as cystic PDACs, as in 2 of our cases. Attention to the cytopathologic characteristics elucidated in this study should allow the distinction of these tumors from PDAC. PDAC often exhibits cytologic features of frank adenocarcinoma, including 3-dimensional clusters and singly dispersed malignant cells with high nuclear-to-cytoplasmic ratio, 4-fold anisonucleosis, marked nuclear irregularity, cytoplasmic mucin vacuoles, and background necrosis. In contrast, IOPNs have relatively monotonous cells and the distinctive cytology of abundant, acidophilic, granular cytoplasm and nuclei with single, prominent eccentric nucleoli in a backdrop of relatively uniform, pale chromatin. IOPNs also typically form larger sheets and papillary units, whereas the sheets in (well differentiated and large-duct variants of) PDACs are typically associated with significantly more disorganization (drunken honeycombs), nuclear contour irregularity, and chromatin clumping or hypochromasia.29
Tumors with acinar differentiation (acinar cell carcinoma and mixed acinar cell-neuroendocrine carcinoma) may also resemble IOPN on FNAB, because they also have relatively monotonous nuclei with single, prominent nucleoli and abundant, granular cytoplasm in some cases.30,31 In some of our cases, this differential was given serious consideration. Acinar cell carcinomas also are often large, perhaps even larger than oncocytic IPMNs (average, 10 cm), and often exhibit cystic degeneration.32 More problematically, a subset of acinar carcinomas present with prominent intraductal growth, closely mimicking IPMN.30,31,33-36 However, acinar neoplasms are more mitotically active and necrotic than IOPNs. Intracellular and extracellular mucin, papillae, and cytoplasmic vacuoles/spaces also are not features of acinar cell carcinoma,37 which stain positively for pancreatic enzymes (trypsin, chymotrypsin, and lipase) and BCL10, markers not typically expressed by IOPNs.31,38 Mixed acinar cell-neuroendocrine carcinoma may exhibit cytology and immunohistochemistry similar to those of acinar cell carcinoma (in addition to expressing neuroendocrine markers), which helps with its distinction from IOPN.31
Another close mimic is intraductal tubulopapillary neoplasm. This entity is defined by its tubular configuration, solid growth,6,39 and cytologic features (abundant cytoplasm and prominent nucleoli), which may be indistinguishable from those of IOPN. The close resemblance of intraductal tubulopapillary neoplasms to pancreatobiliary-type IPMNs and oncocytic intraductal neoplasms is not only an issue at the cytologic level but is also problematic in histologic sections. In fact, we have observed cases in which we were unable to clearly make this distinction (unpublished data).
Pancreatic neuroendocrine tumors may also exhibit striking oncocytic cytology,40-42 leading to misdiagnosis and potential confusion with IOPN.43 Tumor cells are typically plasmacytoid with granular cytoplasm and eccentric, round nuclei, which may contain prominent nucleoli. Their salt-and-pepper chromatin is unlike oncocytic IPMNs, and nucleoli (if present) are more central than in IOPNs. Oncocytic pancreatic neuroendocrine tumors also express neuroendocrine markers (synaptophysin, chromogranin, and CD56), whereas IOPNs do not.13 Numerous tumors may metastasize to the pancreas,44,45 including hepatocellular46,47 and Hürthle cell carcinoma. These may be confused with IOPN and, despite being very uncommon in the pancreas, should be kept in mind.
Conclusion
The diagnosis of IOPN (oncocytic IPMN) is possible on cytologic samples because of its distinctive cytomorphologic features. These include sheets and papilla of large oncocytic cells with dense, granular cytoplasm; large central nuclei; a high nuclear-to-cytoplasmic ratio; and large, typically peripherally located nucleoli that may or may not touch the nuclear membranes. It is important to recognize these characteristics, because these tumors differ biologically, prognostically, and molecularly from other IPMNs as well as PDACs, for which they are clinically mistaken. Despite their cytologic atypia, once resected, these tumors have a predominantly indolent clinical course. Accurate recognition on FNAB is paramount to the appropriate triage of patients and can prevent unnecessary chemoradiation, especially in those with noninvasive disease.
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
We thank Drs. Mauricio Zapata, Clinton McElroy, and Scott Whitworth for their assistance in providing clinical information on these patients.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2015. American Cancer Society; Atlanta, GA: 2015. Available at: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 18, 2015. [Google Scholar]
- 2.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2084. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 3.Kloppel G, Basturk O, Schlitter AM, Konukiewitz B, Esposito I. Intraductal neoplasms of the pancreas. Semin Diagn Pathol. 2014;31:452–466. doi: 10.1053/j.semdp.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Adsay NV, Conlon KC, Zee SY, Brennan MF, Klimstra DS. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- 5.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–848. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Schlitter AM, Jang KT, Kloppel G, et al. Intraductal tubulopapillary neoplasms of the bile ducts: clinicopathologic, immunohistochemical, and molecular analysis of 20 cases. Mod Pathol. 2015;28:1249–1264. doi: 10.1038/modpathol.2015.61. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Adsay NV, Fukushima N, Furukawa T, et al. Intraductal neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. IARC Press; Lyon, France: 2010. pp. 304–313. [Google Scholar]
- 9.Marchegiani G, Mino-Kenudson M, Ferrone CR, Warshaw AL, Lillemoe KD, Fernndez-del Castillo C. Oncocytic-type intraductal papillary mucinous neoplasms: a unique malignant pancreatic tumor with good long-term prognosis. J Am Coll Surg. 2015;220:839–844. doi: 10.1016/j.jamcollsurg.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 10.Basturk O, Bhanot U, Berger M, et al. Intraductal oncocytic papillary neoplasm of the pancreas have distinct molecular characteristics than intraductal papillary mucinous neoplasm [abstract] Mod Pathol. 2015;28(2S):439A. [Google Scholar]
- 11.Xiao HD, Yamaguchi H, Dias-Santagata D, et al. Molecular characteristics and biological behaviours of the oncocytic and pancreatobiliary subtypes of intraductal papillary mucinous neoplasms. J Pathol. 2011;224:508–516. doi: 10.1002/path.2875. [DOI] [PubMed] [Google Scholar]
- 12.Basturk O, Khayyata S, Klimstra DS, et al. Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol. 2010;34:364–370. doi: 10.1097/PAS.0b013e3181cf8bb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adsay NV, Adair CF, Heffess CS, Klimstra DS. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980–994. doi: 10.1097/00000478-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Liszka L, Pajak J, Zielinska-Pajak E, et al. Intraductal oncocytic papillary neoplasms of the pancreas and bile ducts: a description of five new cases and review based on a systematic survey of the literature. J Hepatobiliary Pancreat Sci. 2010;17:246–261. doi: 10.1007/s00534-010-0268-2. [DOI] [PubMed] [Google Scholar]
- 15.Yoon WJ, Daglilar ES, Mino-Kenudson M, Morales-Oyarvide V, Pitman MB, Brugge WR. Characterization of epithelial subtypes of intraductal papillary mucinous neoplasm of the pancreas with endoscopic ultrasound and cyst fluid analysis. Endoscopy. 2014;46:1071–1077. doi: 10.1055/s-0034-1377629. [DOI] [PubMed] [Google Scholar]
- 16.Koh YX, Zheng HL, Chok AY, et al. Systematic review and meta-analysis of the spectrum and outcomes of different histologic subtypes of noninvasive and invasive intraductal papillary mucinous neoplasms. Surgery. 2015;157:496–509. doi: 10.1016/j.surg.2014.08.098. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M, Fukushima N, Noda N, Shibahara J, Kokudo N, Fukayama M. Intraductal oncocytic papillary neoplasm of the bile duct: clinicopathologic and immunohistochemical characteristics of 6 cases. Hum Pathol. 2009;40:1543–1552. doi: 10.1016/j.humpath.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Michaels PJ, Brachtel EF, Bounds BC, Brugge WR, Pitman MB. Intraductal papillary mucinous neoplasm of the pancreas: cytologic features predict histologic grade. Cancer. 2006;108:163–173. doi: 10.1002/cncr.21838. [DOI] [PubMed] [Google Scholar]
- 19.Monzen M, Shimizu K, Hatori T, Furukawa T, Shiratori K. Usefulness of cell block cytology for preoperative grading and typing of intraductal papillary mucinous neoplasms. Pancreatology. 2013;13:369–378. doi: 10.1016/j.pan.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Ono J, Yaeger KA, Genevay M, Mino-Kenudson M, Brugge WR, Pitman MB. Cytological analysis of small branch-duct intraductal papillary mucinous neoplasms provides a more accurate risk assessment of malignancy than symptoms [serial online] Cytojournal. 2011;8:21. doi: 10.4103/1742-6413.90084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hibi Y, Fukushima N, Tsuchida A, et al. Pancreatic juice cytology and subclassification of intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2007;34:197–204. doi: 10.1097/MPA.0b013e31802dea0. [DOI] [PubMed] [Google Scholar]
- 22.Thompson K, Castelli MJ, Gattuso P. Metastatic papillary oncocytic carcinoma of the pancreas to the liver diagnosed by fine-needle aspiration. Diagn Cytopathol. 1998;18:291–296. doi: 10.1002/(sici)1097-0339(199804)18:4<291::aid-dc9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Jurczyk MF, Zhu B, Villa C, DeFrias D, Lin X. Cytomorphology of intraductal oncocytic papillary neoplasm of the liver. Diagn Cytopathol. 2014;42:895–898. doi: 10.1002/dc.23073. [DOI] [PubMed] [Google Scholar]
- 24.Pitman MB, Genevay M, Yaeger K, et al. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than “positive” cytology. Cancer Cytopathol. 2010;118:434–440. doi: 10.1002/cncy.20118. [DOI] [PubMed] [Google Scholar]
- 25.Pitman MB, Centeno BA, Daglilar ES, Brugge WR, Mino-Kenudson M. Cytological criteria of high-grade epithelial atypia in the cyst fluid of pancreatic intraductal papillary mucinous neoplasms. Cancer Cytopathol. 2014;122:40–47. doi: 10.1002/cncy.21344. [DOI] [PubMed] [Google Scholar]
- 26.Kato Y, Nakagouri T, Konishi M, et al. Intraductal oncocytic papillary neoplasm of the pancreas with strong accumulation on FDG-PET. Hepatogastroenterology. 2008;55:900–902. [PubMed] [Google Scholar]
- 27.Noji T, Kondo S, Hirano S, et al. Intraductal oncocytic papillary neoplasm of the pancreas shows strong positivity on FDG-PET. Int J Gastrointest Cancer. 2002;32:43–46. doi: 10.1385/IJGC:32:1:43. [DOI] [PubMed] [Google Scholar]
- 28.Fischer MA, Donati O, Heinrich S, et al. Intraductal oncocytic papillary neoplasm of the pancreas: a radio-pathological case study. JOP. 2010;11:49–54. [PubMed] [Google Scholar]
- 29.Bagci P, Andea AA, Basturk O, Jang KT, Erbarut I, Adsay V. Large duct type invasive adenocarcinoma of the pancreas with microcystic and papillary patterns: a potential microscopic mimic of non-invasive ductal neoplasia. Mod Pathol. 2012;25:439–448. doi: 10.1038/modpathol.2011.181. [DOI] [PubMed] [Google Scholar]
- 30.Toll AD, Hruban RH, Ali SZ. Acinar cell carcinoma of the pancreas: clinical and cytomorphologic characteristics. Korean J Pathol. 2013;47:93–99. doi: 10.4132/KoreanJPathol.2013.47.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigel CS, Klimstra DS. Cytomorphologic and immunophenotypical features of acinar cell neoplasms of the pancreas. Cancer Cytopathol. 2013;121:459–470. doi: 10.1002/cncy.21279. [DOI] [PubMed] [Google Scholar]
- 32.Adsay NV, Klimstra DS. Cystic forms of typically solid pancreatic tumors. Semin Diagn Pathol. 2000;17:81–88. [PubMed] [Google Scholar]
- 33.Basturk O, Zamboni G, Klimstra DS, et al. Intraductal and papillary variants of acinar cell carcinomas: a new addition to the challenging differential diagnosis of intraductal neoplasms. Am J Surg Pathol. 2007;31:363–370. doi: 10.1097/01.pas.0000213376.09795.9f. [DOI] [PubMed] [Google Scholar]
- 34.Ban D, Shimada K, Sekine S, et al. Pancreatic ducts as an important route of tumor extension for acinar cell carcinoma of the pancreas. Am J Surg Pathol. 2010;34:1025–1036. doi: 10.1097/PAS.0b013e3181e2bc11. [DOI] [PubMed] [Google Scholar]
- 35.Toll AD, Mitchell D, Yeo CJ, Hruban RH, Witkiewicz AK. Acinar cell carcinoma with prominent intraductal growth pattern: case report and review of the literature. Int J Surg Pathol. 2011;19:795–799. doi: 10.1177/1066896909339912. [DOI] [PubMed] [Google Scholar]
- 36.Stelow EB, Bardales RH, Shami VM, et al. Cytology of pancreatic acinar cell carcinoma. Diagn Cytopathol. 2006;34:367–372. doi: 10.1002/dc.20450. [DOI] [PubMed] [Google Scholar]
- 37.Stelow EB, Shaco-Levy R, Bao F, Garcia J, Klimstra DS. Pancreatic acinar cell carcinomas with prominent ductal differentiation: mixed acinar ductal carcinoma and mixed acinar endocrine ductal carcinoma. Am J Surg Pathol. 2010;34:510–518. doi: 10.1097/PAS.0b013e3181cfcac7. [DOI] [PubMed] [Google Scholar]
- 38.Hosoda W, Sasaki E, Murakami Y, Yamao K, Shimizu Y, Yatabe Y. BCL10 as a useful marker for pancreatic acinar cell carcinoma, especially using endoscopic ultrasound cytology specimens. Pathol Int. 2013;63:176–182. doi: 10.1111/pin.12045. [DOI] [PubMed] [Google Scholar]
- 39.Klimstra DS, Adsay NV, Dhall D, et al. Intraductal tubular carcinoma of the pancreas: clinicopathologic and immunohistochemical analysis of 18 cases [abstract] Mod Pathol. 2007;20:285A. [Google Scholar]
- 40.Volante M, La Rosa S, Castellano I, Finzi G, Capella C, Bussolati G. Clinico-pathological features of a series of 11 oncocytic endocrine tumours of the pancreas. Virchows Arch. 2006;448:545–551. doi: 10.1007/s00428-006-0154-0. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen-Ho P, Nguyen GK, Jewell LD. Oncocytic neuroendocrine carcinoma of the pancreas. Report of a case with needle aspiration cytology, immunocytochemistry and electron microscopy. Acta Cytol. 1994;38:611–613. [PubMed] [Google Scholar]
- 42.Pacchioni D, Papotti M, Macri L, Forte G, Bussolati G. Pancreatic oncocytic endocrine tumors. Cytologic features of two cases. Acta Cytol. 1996;40:742–746. doi: 10.1159/000333950. [DOI] [PubMed] [Google Scholar]
- 43.Chen S, Wang X, Lin J. Fine needle aspiration of oncocytic variants of pancreatic neuroendocrine tumor: a report of three misdiagnosed cases. Acta Cytol. 2014;58:131–137. doi: 10.1159/000357035. [DOI] [PubMed] [Google Scholar]
- 44.Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004;444:527–535. doi: 10.1007/s00428-004-0987-3. [DOI] [PubMed] [Google Scholar]
- 45.Olson MT, Wakely PE, Jr, Ali SZ. Metastases to the pancreas diagnosed by fine-needle aspiration. Acta Cytol. 2013;57:473–480. doi: 10.1159/000352006. [DOI] [PubMed] [Google Scholar]
- 46.Texler ML, Pierides J, Maddern GJ. Case report: a hepatocellular carcinoma metastasis in the distal pancreas. J Gastroenterol Hepatol. 1998;13:467–470. doi: 10.1111/j.1440-1746.1998.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 47.Woo SM, Park JW, Han SS, et al. Isolated pancreatic metastasis of hepatocellular carcinoma after curative resection. World J Gastrointest Oncol. 2010;2:209–212. doi: 10.4251/wjgo.v2.i4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]