Abstract
Soy phosphatidylinositol (PI) containing lipid nanoparticles prolong plasma survival, improve hemostatic efficacy, and decrease immunogenicity of human B-domain deleted Factor VIII (BDD FVIII) in Hemophilia A (HA) mice. We hypothesize that PI associated BDD FVIII is more potent than the free protein, and using mathematical modeling, have projected that PI associated BDD FVIII could be used for once-weekly prophylactic dosing in patients. To facilitate translation to the clinic, comparative plasma survival and ex vivo efficacy of PI associated recombinant canine FVIII (PI-rcFVIII) were evaluated in HA dogs. 2 HA dogs were administered a 50 U/kg iv dose of free or PI-rcFVIII. rcFVIII activity measurements and ex vivo efficacy analyses like whole blood clotting time (WBCT) and thromboelastography (TEG) were conducted on recovered plasma and whole blood samples. PI association decreased clearance (~25%) and increased plasma exposure (~1.4 fold) of rcFVIII. PI-rcFVIII treated animals had prolonged improvements in WBCTs and TEG parameters compared to free rcFVIII treated animals. Since rcFVIII is a BDD form of FVIII, these studies provide proof-of-principle that observations with human BDD FVIII in mice translate to higher animal species. Additionally, PI-rcFVIII has potential applications in canine HA management and as a bypass therapy in inhibitor-positive HA patients.
INTRODUCTION
Hemophilia A (HA) is an X-linked bleeding disorder caused by the genetic deficiency or dysfunction of the essential blood coagulation protein Factor VIII (FVIII). Replacement therapy with recombinant human FVIII is currently the first line of therapy in HA management. However the short half-life (~12 hours1,2) and high incidence of immune responses against the protein (nearly 30% of patients develop neutralizing antibodies3,4) have spurred the development of several next generation FVIII products 5-9.
A phosphatidylinositol (PI) containing lipidic nanoparticle for the delivery of B-domain deleted FVIII (BDD FVIII) has been developed10. These lipid nanoparticles extend the circulating half-life and hemostatic efficacy of BDD FVIII as well as mitigate immune responses against the protein, in a mouse model of HA. Lipidic BDD FVIII is more potent than the free form of the protein in HA mice10. The combined improvements in PK and PD have been predicted to substantially prolong the time above clinically established therapeutic thresholds, permitting once weekly prophylactic dosing of BDD FVIII.
The objective of this work was to evaluate whether the lipid-mediated improvements in the pharmacokinetics (PK) and pharmacodynamics (PD) of BDD FVIII observed in HA mice translate to higher species. For several decades, dogs with congenital HA have been used as a preclinical model to study the translational PK-PD of FVIII products11. These dogs replicate the genotypic and phenotypic characteristics of human HA with high fidelity, 12,13 and this animal model has proved to be an excellent predictor of clinical efficacy14. HA dogs were therefore chosen as the animal model for these studies.
The canine FVIII protein has recently been recombinantly expressed and characterized15. Recombinant canine FVIII (rcFVIII) is a BDD form of FVIII and is secreted as a ~160kDa single chain protein15. rcFVIII was used as the model protein in these studies for two reasons. First, rcFVIII has important veterinary and clinical applications. It has been proposed as an attractive option for the management of canine HA and as a potential bypass strategy in human HA patients with inhibitors15. Second, the use of rcFVIII permits a prolonged evaluation of comparative PK-PD in these dogs in a crossover study design. Such evaluations are challenging with human FVIII forms because, despite high sequence homology between human and canine FVIII16, adult HA dogs develop antibodies against human FVIII, usually shortly after a single exposure15. Limited availability of animals and ethical considerations preclude the use of large sample sizes to study comparative efficacy of control and modified FVIII formulations in a parallel study design. Because rcFVIII is a BDD form of FVIII, studies in HA dogs with rcFVIII provide proof of principle that observations with human BDD FVIII in mice may translate to higher animal species.
Comparative PK and ex vivo PD studies with free and PI associated rcFVIII (PI-rcFVIII) were conducted in HA dogs. The results of these studies indicate that PI association does, in fact, improve the PK-PD properties of rcFVIII in HA dogs.
MATERIALS AND METHODS
Materials
rcFVIII was expressed, purified, and characterized as previously described15. The specific activity of the protein was 2.25 U/μg. Dimyristoylphosphatidylcholine (DMPC) and soybean PI were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Cholesterol was purchased from Sigma-Aldrich (St.Louis, Missouri, USA). FVIII chromogenic assay detection kits were purchased from DiaPharma (Westchester, Ohio). Control plasmas and activated partial thromboplastin time (aPTT) reagents were purchased from Precision Biologics (Dartmouth, Canada) and Tcoag (Parsippany, NJ) respectively. Buffer salts were purchased from Fisher Scientific.
Liposomal encapsulation and conformational studies
PI containing lipid nanoparticles (PI/DMPC/Cholesterol molar ratio 50:50:5) were prepared as previously described17. rcFVIII was associated with the PI nanoparticles using a ‘triggered loading’ approach17. These loading conditions involve incubating the FVIII-lipid particle solution at 37°C for 30 min, which results in mild, reversible unfolding of the protein to promote interaction and loading18. The protein-to-lipid mole ratio for association was 1:20, 000 in all experiments unless stated otherwise. The activity of PI-rcFVIII was assessed by an aPTT assay prior to initiation of the study and was found to found to be comparable to that of free rcFVIII.
Encapsulation efficiency of rcFVIII with the PI particle was determined with discontinuous dextran gradient centrifugation19. Briefly, PI-rcFVIII was loaded into the bottom layer of a 0%/10%/20% dextran gradient and subjected to ultracentrifugation at 190,000 X g for 30 minutes. FVIII activity of each layer was measured after centrifugation in duplicate with the aPTT assay and compared to a standard curve of known rcFVIII activity. Encapsulation efficiency was determined by comparing FVIII activity in the upper layers to the lowest layer.
The tertiary structural changes in rcFVIII upon association with PI liposomes were assessed using fluorescence spectroscopy as previously described17. Free or PI-rcFVIII samples (5 μg/mL) were excited at 265 or 280 nm and emission scans were collected in the wavelength range of 300-400 nm. Slit widths were set at 4 nm for both the excitation and emission paths. The spectra were acquired on a PTI-Quantamaster fluorescence spectrophotometer (Photon Technology International, Lawrenceville, New Jersey, USA). The contribution of PI vesicles on the emission spectra of the protein was corrected by subtracting the spectra acquired for the vesicles alone and by using a long pass filter on the emission path.
Animals
All animal work was conducted at the Francis Owen Blood Research Lab at the University of North Carolina (UNC), Chapel Hill, NC. The HA dogs used in these studies are an outbred model of severe hemophilia A due to an intron 22 inversion defect13. All experimental procedures were performed in accordance with the protocol approved by the Institutional Animal Care and Use Committee of UNC. The animals (n=2) used in these studies were female, inhibitor-negative and weighed~20kg each. Baseline characteristics of the animals used are presented in Table 1.
Table 1.
Baseline characteristics of study dogs
| Animal ID | Sex | Weight (kg) | FVIII:C (U/mL)a | WBCT (min) | TEG | |||
|---|---|---|---|---|---|---|---|---|
| R(min) | K(min) | Angle(°) | MA(mm) | |||||
| Daisy | F | 19.7 | <0.006 | 41 | >60 | nab | na | na |
| Nora | F | 19.4 | <0.006 | 39.5 | 58.9 | 45.5 | 4 | 24.4 |
Determined using a two-stage chromogenic assay
Not available
Preparation of free and PI-rcFVIII injections
PI lipid particles were prepared at the University at Buffalo and shipped to UNC overnight at 2-8°C. rcFVIII was associated with lipid particles at UNC immediately prior to administration. It has previously been established that the duration and conditions of transit do not impact the stability or association efficiency of the PI particles. rcFVIII injections were prepared by dilution into saline for the free protein, or into Tris-NaCl buffer (25mM Tris, 150mM NaCl, pH 7.0) for the lipid-associated protein.
Study Design
Studies were conducted in a crossover design. The 2 HA dogs had not received any FVIII for at least 2 weeks and were then first administered a single i.v. injection of free rcFVIII at a dose of 50 U/kg. Serial plasma and blood samples were collected from these animals at fixed time points post-injection (5min to 15d). After a ~3 week washout period, animals were administered a 50 U/kg i.v. PI-rcFVIII dose. Samples were collected at identical time points as after the free rcFVIII dose until whole blood clotting times (WBCT) values returned to baseline.
WBCT and thromboelastography (TEG) were performed using whole blood samples to evaluate ex vivo PD. WBCTs provide a rough measure of the activity of all intrinsic clotting factors in the absence of tissue factors. TEG provides information about the kinetics of clot formation using parameters such as R (reaction time; time of latency from the start of the test to initial clot formation), K (time taken to achieve a certain level of clot strength) is a measure of the rate of clot formation, Alpha measures the speed at which fibrin build up and crosslinking takes place (hence is also a measure of the rate of clot formation), and MA (maximum amplitude) represents the ultimate strength of the fibrin-cross linked clot. WBCT and TEG assessments were performed in real time, and plasma PK analyses were performed in batch after all the infusions and sample collections were completed. Plasma samples were stored at −80°C until analysis. Prior to administration of PI-rcFVIII at the end of the washout period, rcFVIII plasma concentrations were below the limit of quantification, and WBCTs and thromboelastography (TEG) parameters were confirmed (in real time) to have returned to baseline.
rcFVIII activity assays
Plasma rcFVIII activity levels were analyzed using both a one-stage aPTT assay optimized for this purpose20 and a commercially available two-stage chromogenic assay. Briefly, the aPTT assay was performed as follows: samples and standards of rcFVIII were diluted 1:10 with imidazole buffered saline. The diluted plasma was then mixed 1:1 with FVIII deficient human plasma. A standard aPTT test was performed on 100 μl of this solution. For both assays, activities of free and PI-rcFVIII in plasma samples from the study animals were estimated from calibration curves constructed using a concentrate of corresponding form of the protein, spiked into pooled plasma from naïve HA dogs.
PK-PD modeling
Non-compartmental analysis (NCA) was performed on the resulting PK profiles in each individual dog using Phoenix WinNonlin v6.3 (Pharsight Corporation, Sunnyvale, CA) to compute basic PK parameters including: area under the curve (AUC), half-life (t1/2), clearance (CL), volume of distribution (Vss), and mean residence time (MRT). This was followed by compartmental modeling using various structural models to elucidate the disposition of free and PI-rcFVIII in HA dogs.
PD modeling was performed on the WBCT and selected TEG (K) data. The final structural pharmacokinetic model and parameters (from modeling the chromogenic assay data) were fixed and used to drive the PD model. Several modifications of the Hill function were evaluated:
With E0 as the mean WBCT or K value in the absence of treatment, Emax is the maximum response, EC50 is the concentration of rcFVIII at which 50% response is observed, and the γ is the Hill coefficient. Model selection and evaluation of goodness of fit were guided by precision of the parameter estimates, objective measures like the Akaike information criteria, F-test and subjective measures like visual inspection of fitted profiles and residuals.
RESULTS
Encapsulation efficiency of rcFVIII with PI particle and conformation of PI-rcFVIII
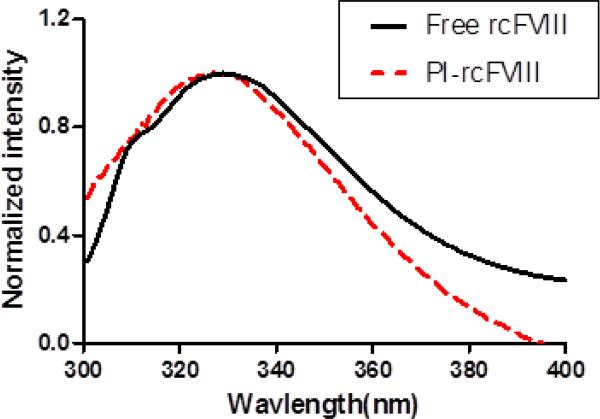
The association efficiency of rcFVIII with PI nanoparticles was determined using discontinuous dextran gradient centrifugation. Fluorescence spectroscopy was used to evaluate tertiary structural changes in rcFVIII as a result of association with PI nanoparticles. Association of rcFVIII with PI particles was found to be less efficient than human BDD FVIII10, with 39.4 ± 3.2 % of recovered protein found in association with lipid particles. A blue shift in the fluorescence emission maximum of rcFVIII was observed in the presence of PI particles (Fig. 1), indicating a tertiary structural change attributable to hydrophobic interactions and deep penetration of the protein into the particle.
Figure 1. Normalized florescence emission spectra of free and PI-rcFVIII.
Normalized fluorescence emission spectra of free rcFVIII (solid line) and PI-rcFVIII (dashed line) acquired in the range of 300–400 nm. The excitation monochromator was set at 280 nm. The protein concentration used was 5 μg/mL.
Pharmacokinetics
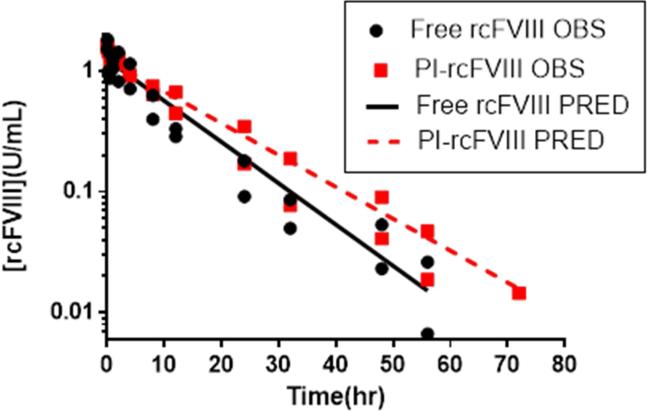
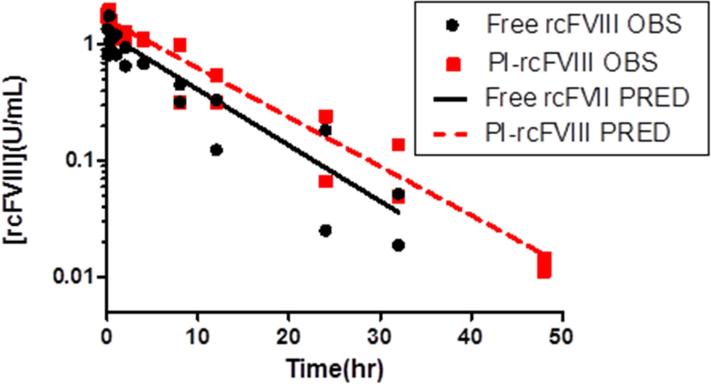
To investigate whether association with the PI particle prolongs plasma survival of rcFVIII, comparative PK studies were conducted at a dose of 50 U/kg IV. The PK profiles (Fig. 2) indicate that association of rcFVIII with the PI particle results in PK improvements. Whereas initial recoveries of plasma rcFVIII after free and PI-rcFVIII doses were comparable, the profiles diverged a few hours post-injection. FVIII activity in PI-rcFVIII treated animals was consistently greater than in animals treated with free rcFVIII. Meaningful FVIII activity levels (>0.01 U/mL) could be detected in animals treated with PI-rcFVIII 8 hr (aPTT) to 16 hr (chromogenic assay) after FVIII activity became undetectable in animals treated with free rcFVIII.
Figure 2. Pharmacokinetics of free and PI-rcFVIII.
PK profiles of free and PI-rcFVIII in HA dogs after an i.v. bolus dose of 50 U/kg. FVIII activity in recovered plasma samples was measured using the two-stage chromogenic (2a) and one-stage aPTT assays (2b). The solid and dashed lines represent the fit of a 1 compartment model with linear elimination to free and PI-rcFVIII data respectively. PI association prolongs plasma survival of rcFVIII. PI-rcFVIII has lower CL, consistent with the hypothesis of protection against uptake by clearance receptors and proteolytic degradation. * No FVIII activity was detected in the 72hr (chromogenic assay) and 48 hr (aPTT) samples from free rcFVIII treated animals.
Parameters calculated from non-compartmental analyses of PK profiles in each dog are summarized in Table 2. Volumes of distribution of both free and PI-rcFVIII were close to the physiological plasma volume in dogs (~50 mL/kg)21. Clearance of PI-rcFVIII was reduced by ~30% (chromogenic assay) to ~40% (aPTT) compared to free rcFVIII. Plasma exposure of PI-rcFVIII was ~ 1.4-fold (chromogenic assay) to ~1.7-fold (aPTT) greater compared with free rcFVIII. The terminal half-life of PI-rcFVIII was ~1.2 fold that of free rcFVIII in these HA dogs. A 1 compartment model with linear elimination was sufficient to describe the disposition of rcFVIII in these HA dogs. Final parameter estimates from the compartmental model are shown in Table 3 and final model fitting to the data is shown in Fig. 2. As with NCA, clearance of PI-rcFVIII estimated by compartmental modeling was lower than free rcFVIII by ~25% (chromogenic assay) to ~35% (aPTT). An F-test was used to establish whether using treatment-specific clearance and volume of distribution estimates statistically improves model fitting compared to a model utilizing common estimates for both treatments (p<0.05). Additionally, confidence intervals around the clearance estimates of free and PI-rcFVIII do not overlap, suggesting statistical difference between the estimates.
Table 2.
Non-compartmental analyses parameter estimates
| Parameter | Chromogenic assay | aPTT | ||
|---|---|---|---|---|
| Free rcFVIII | PI-rcFVIII | Free rcFVIII | PI-rcFVIII | |
| Cmax (U/mL) | 1.82, 1.52 | 1.61, 1.55 | 1.76, 1.12 | 2.03, 1.69 |
| AUC0–τ (h × U/mL) | 10.8, 16.1 | 16.0, 22.2 | 7.44, 11.4 | 12.5, 19.7 |
| CL (mL/h × kg) | 4.58, 3.03 | 3.08, 2.23 | 6.59, 4.18 | 3.95, 2.52 |
| Vss (mL/kg) | 47.7, 38.7 | 36.8, 36.9 | 41.3, 47.3 | 33.6, 28.3 |
| MRT (hr) | 9.99, 11.4 | 11.1, 15.8 | 5.61, 9.56 | 7.89, 11.0 |
| T1/2 (hr) | 8.28, 9.93 | 9.26, 11.6 | 5.31, 8.18 | 7.32, 7.34 |
Cmax = maximum plasma concentration, AUC0–τ = area under the concentration-time curve, CL = clearance, Vss= volume of distribution, MRT= mean residence time, T1/2= half-life
Table 3.
Pharmacokinetic model parameter estimates
| Parameter | Chromogenic assay | aPTT | ||
|---|---|---|---|---|
| Free rcFVIII | PI-rcFVIII | Free rcFVIII | PI-rcFVIII | |
| CL (CV%) (mL/h × kg) | 3.16(5.33) | 2.44(5.39) | 4.49(8.74) | 2.97(8.37) |
| Vss (CV%) (mL/kg) | 40.2(6.05) | 40.6(11.1) | 30.6(10.6) | |
CL = clearance, Vss= volume of distribution
Pharmacodynamics
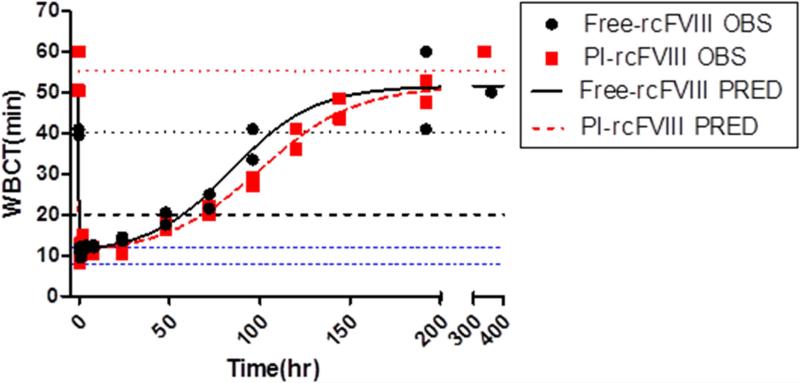
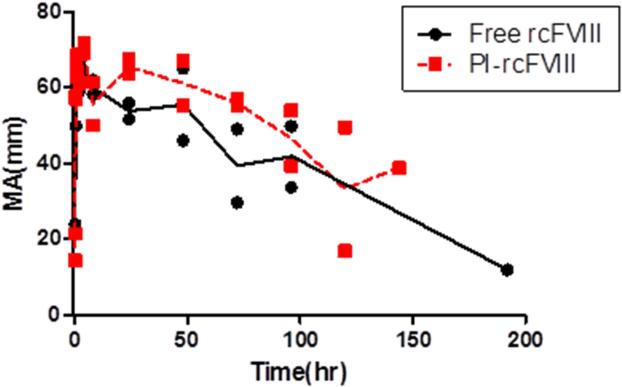
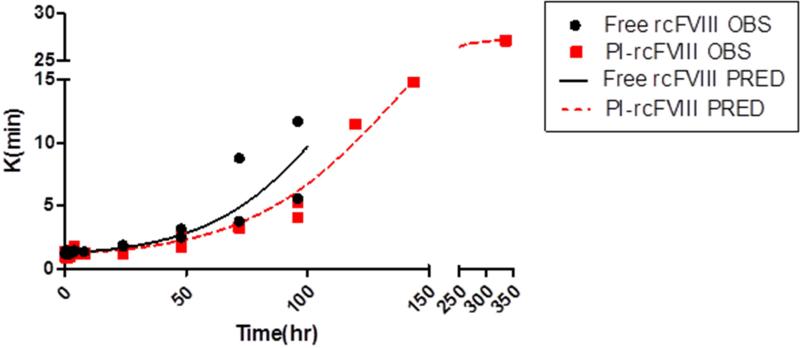
Comparative ex vivo pharmacodynamics of free and PI-rcFVIII were evaluated using WBCT and TEG. The PD profiles obtained (Fig. 3-5) suggest that PI association prolongs the hemostatic efficacy of rcFVIII in HA dogs. Immediately post-injection, both free and PI-rcFVIII corrected WBCTs to normal values (8-12 min). The return to a WBCT value of 20 min (target level below which spontaneous bleeding is usually prevented in these HA dogs) was slower in one of the two dogs when treated with PI-rcFVIII (64 hr) as compared to free rcFVIII (48 hr). In addition, return of WBCT values to pre-treatment baseline levels was slower after PI-rcFVIII administration than after free rcFVIII administration. In both dogs, TEG parameters K, Alpha (data not shown) and MA showed clear improvements after treatment with PI-rcFVIII compared to free rcFVIII. It is interesting to note that MA values, which are a measure of the strength of the clot formed, were improved after PI-rcFVIII treatment compared to the free protein.
Figure 3. WBCT data and model fit.
WBCT vs time profiles after i.v. bolus doses of 50 U/kg free and PI-rcFVIII in HA dogs. Circles represent individual observed values and the lines represent the model fit. The dashed blue line represents a WBCT of 20 min-the threshold below which spontaneous bleeding is prevented in these dogs. Dotted blue lines represent the WBCT range in dogs with a normal clotting phenotype (8-12 min). Dotted black and red lines represent mean baseline WBCT values prior to free and PI-rcFVIII administration respectively. rcFVIII treatment corrected clotting phenotype to normal levels immediately post dose followed by return to baseline over time which was more gradual after PI-rcFVIII treatment. Return to WBCT values of 20min was prolonged after PI-rcFVIII treatment in one dog.
Figure 5. Comparative MA of free and PI-rcFVIII.
TEG (MA) values over time after i.v. bolus doses of 50 U/kg free and PI-rcFVIII in HA dogs. The parameter MA provides an estimate of the strength of the clot formed. Circles represent individual observed values and the lines represent mean MA over time. The strength of the clot formed in whole blood samples was higher after treatment with PI-rcFVIII compared with the free protein.
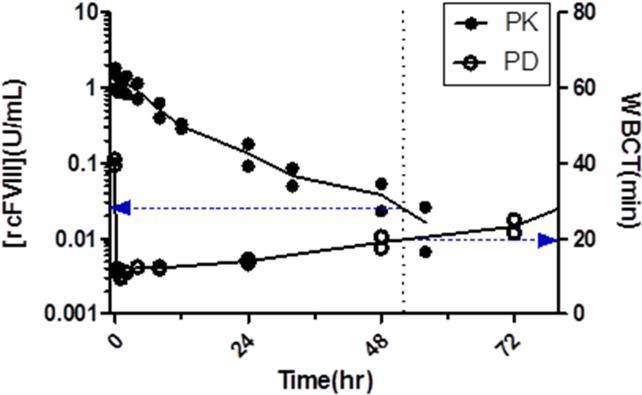
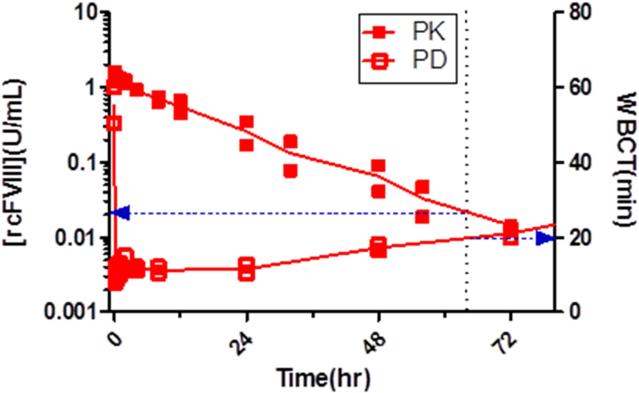
PK-PD modeling was conducted with the WBCT and TEG (K) data. Given the absence of a temporal lag between peak plasma concentrations and maximal effect (Fig 6), pharmacodynamic data were fit to a simple direct effect model. The use of this model to describe similar data in these HA dogs has previously been reported22. The final models that best described the efficacy data utilized a common EC50 value for free and PI-rcFVIII. The final model fit and estimated parameters are shown in Fig. 3 and 4 and Table 4. The available assays for FVIII plasma concentrations are not sensitive enough to follow the return of PD measures back to baseline values. The estimated EC50 values are therefore below the assay limits of quantification.
Figure 6. PK-PD overlays.
Overlay of PK and PD profiles after free (6a) and PI-rcFVIII (6b) treatment. WBCT of 20 min requires ~0.02U/mL of both free and PI-rcFVIII. However, time to decline to mean WBCT of 20 min is prolonged with PI-rcFVIII.
Figure 4. TEG (K) data and model fit.
TEG (K) values over time after i.v. bolus doses of 50 U/kg free and PI-rcFVIII in HA dogs. The parameter K provides an estimate of the rate of clot formation. Circles represent individual observed values and the lines represent the model fit. rcFVIII treatment corrected clotting phenotype to normal levels immediately post dose followed by return to baseline over time which was more gradual after PI-rcFVIII treatment.
Table 4.
Pharmacodynamic parameter estimates
| Parameter | WBCT | K |
|---|---|---|
| E0(CV%) (min) | 51.5 (2.48) | 27.2 (3.94) |
| Emax(CV%) | 0.8 (3.23) | 0.97 (1.18) |
| EC50(CV%) (U/mL) | 0.002 (27.4) | 0.0002 (27.0) |
| γ(CV%) | 0.6 (17.5) | 0.5 (12.4) |
E0= mean WBCT or K value in the absence of treatment, Emax = maximum response, EC50 = concentration of rcFVIII at which 50% response is observed, γ= Hill coefficient
DISCUSSION
The development of a long acting, less immunogenic form of FVIII would fulfill a pressing clinical need. PI containing lipid nanoparticles can decrease the immunogenicity and prolong the plasma survival and hemostatic efficacy of human BDD FVIII in HA mice. Allometric predictions of comparative human PK suggest lipidic FVIII could be dosed once weekly for prophylactic purposes compared to thrice weekly with free FVIII, which would be a significant improvement in HA clinical management10. The objective of this work was to evaluate whether the lipid-mediated improvements in BDD FVIII PK and PD observed in HA mice translate to higher preclinical species. PK and PD studies with free and PI associated rcFVIII were conducted in HA dogs, which have long been recognized as an excellent preclinical model of the disease. Because the recently expressed rcFVIII protein is a BDD form of FVIII with important veterinary and clinical applications, this protein was used to establish proof of principle.
A smaller percentage of rcFVIII compared to human BDD FVIII associated with PI nanoparticles even at double the protein-to-lipid mole ratio. Although the cause for this has not been investigated in detail, a possible reason is that the lipid binding domain of rcFVIII might be inaccessible for interactions with the lipid particles under the ‘triggered loading’ conditions we use for association17. Alternatively, the single chain structure of rcFVIII, as opposed to the double chain structure of human BDD FVIII, could result in altered dynamics of the protein-lipid particle interaction. However, a blue shift in the fluorescence emission maximum of rcFVIII upon PI association suggested that the fraction of the protein that is associated with the particle is likely deeply embedded within the lipid bilayer. This could result in meaningful protection against catabolism of this fraction in vivo.
Plasma PK profiles following i.v. bolus doses of both free and PI-rcFVIII were characterized by a mono-exponential decrease in plasma FVIII activity. Non-compartmental and compartmental analyses indicated that PI association decreases the clearance and prolongs plasma survival of BDD FVIII. The mechanisms underlying this decrease in clearance have not yet been investigated. However, we postulate that they are similar to those reported for human BDD FVIII (i.e. PI association sterically shields rcFVIII from receptor mediated clearance and proteolytic degradation10). The fact that the prolongation in terminal half-life is not as pronounced as that observed with human BDD FVIII in HA mice10 is likely due to the lower association efficiency of rcFVIII with the PI-particle. Experimental modifications to increase this association efficiency could lead to further improvement in the plasma survival of rcFVIII in these dogs. As is, PI association prolongs the time before plasma rcFVIII activity decreases to the threshold of 0.02 U/mL by ~12 hours (Fig. 2a). This threshold represents the free rcFVIII plasma concentration required to maintain WBCT of 20 min (Fig. 6a).
Ex vivo PD data indicate that lipid association prolongs hemostatic efficacy of rcFVIII in HA dogs. Both WBCTs and TEG parameters showed prolonged improvements post PI-rcFVIII administration. This prolongation is attributable to the fact that PI association prolongs plasma survival of rcFVIII (Fig. 6). The time before plasma concentrations decrease to a minimum therapeutic threshold is therefore longer for PI-rcFVIII than for the free protein. Unlike with human FVIII, there was no improvement in potency of rcFVIII upon PI association. In the case of both free and PI-rcFVIII, average WBCT values of 20 min require mean plasma rcFVIII activity levels of ~0.02 U/mL. If PI-rcFVIII was more potent than the free form of the protein, a lower concentration of the lipidic protein would have sufficed to maintain WBCTs of 20min. This is corroborated by the fact that modeling the WBCT and K data using treatment-specific EC50 parameters results in similar estimates of EC50 values for both treatments. We hypothesize that the improved activity of PI associated FVIII could be due to stabilization of the activated FVIII hetero-trimer upon association with the lipid particle10. However, it has been shown that the activated form of rcFVIII is much more stable than activated human FVIII15. It is therefore possible that the stabilizing effects of PI association on the activated hetero-trimer are not as evident with rcFVIII as they are with human FVIII. In addition, the efficacy studies reported here were conducted ex vivo. The difference in potency might become apparent in an in vivo setting, in the presence of physiologically relevant, global hemostasis machinery. Such evaluations will be the subject of future work. An important caveat to these interpretations remains that nearly 60% of the protein administered in the PI-rcFVIII treatment was unassociated with the lipid particle.
Regardless of the underlying mechanisms, these studies provide proof of principle that the lipid-mediated prolongation in BDD FVIII plasma survival we have observed in HA mice translates to HA dogs and results in prolonged hemostatic efficacy which is the clinically meaningful endpoint. The translation of the enhanced potency we have observed with human BDD FVIII in HA mice to HA dogs, would further improve this prolongation in efficacy. Such evaluations with human BDD FVIII in HA dogs will be the subject of future work.
CONCLUSIONS
In summary, the association of rcFVIII with PI-containing lipidic nanoparticles prolongs plasma survival and hemostatic efficacy of this protein in HA dogs. These findings serve as proof-of-principle that the lipid-mediated improvements in the PK properties of human BDD FVIII observed in HA mice translate to higher species. Lipid-associated rcFVIII also has direct applications in the veterinary management of canine HA. PI association protects human BDD FVIII from inactivation by inhibitors in HA mice (unpublished results). Similar protection of rcFVIII would enhance the efficacy of this protein as a bypass therapy in inhibitor-positive subjects and future studies could explore this possibility. Lipidic FVIII therefore is a promising approach to improve quality of care in both human and canine Hemophilia A.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health to Dr. Balu-Iyer (R01 HL-70227) and Dr. Nichols (HL-63098). The authors thank Pharmaceutical Sciences Instrumentation facility, University at Buffalo, State University of New York, for use of the shared microplate reader, ultracentrifuge and fluorescence spectrometer.
ABBREVIATIONS USED
- FVIII
Factor VIII
- BDD FVIII
B-domain deleted Factor VIII
- rcFVIII
recombinant canine Factor FVIII
- HA
Hemophilia A
- PK
pharmacokinetic
- PD
pharmacodynamic
- PI
phosphatidylinositol
- DMPC
dimyristoylphosphatidylcholine
- aPTT
activated partial thromboplastin time
- WBCT
whole blood clotting time
- TEG
thromboelastography
- NCA
non-compartmental analysis
- AUC
area under the curve
- t1/2
half-life
- CL
clearance
- Vss
volume of distribution
- MRT
mean residence time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Berntorp E, Bjorkman S. The pharmacokinetics of clotting factor therapy. Haemophilia. 2003;9(4):353–359. doi: 10.1046/j.1365-2516.2003.00762.x. [DOI] [PubMed] [Google Scholar]
- 2.Morfini M. Pharmacokinetics of factor VIII and factor IX. Haemophilia 9 Suppl. 2003;1:94–99. doi: 10.1046/j.1365-2516.9.s1.8.x. discussion 100. [DOI] [PubMed] [Google Scholar]
- 3.Lacroix-Desmazes S, Navarrete AM, Andre S, Bayry J, Kaveri SV, Dasgupta S. Dynamics of factor VIII interactions determine its immunologic fate in hemophilia A. Blood. 2008;112(2):240–249. doi: 10.1182/blood-2008-02-124941. [DOI] [PubMed] [Google Scholar]
- 4.Jacquemin MG, Saint-Remy JM. Factor VIII immunogenicity. Haemophilia. 1998;4(4):552–557. doi: 10.1046/j.1365-2516.1998.440552.x. [DOI] [PubMed] [Google Scholar]
- 5.Tiede A, Brand B, Fischer R, Kavakli K, Lentz SR, Matsushita T, Rea C, Knobe K, Viuff D. Enhancing the pharmacokinetic properties of recombinant factor VIII: first-in-human trial of glycoPEGylated recombinant factor VIII in patients with hemophilia A. J Thromb Haemost. 2013;11(4):670–678. doi: 10.1111/jth.12161. [DOI] [PubMed] [Google Scholar]
- 6.Mahlangu J, Powell JS, Ragni MV, Chowdary P, Josephson NC, Pabinger I, Hanabusa H, Gupta N, Kulkarni R, Fogarty P, Perry D, Shapiro A, Pasi KJ, Apte S, Nestorov I, Jiang H, Li S, Neelakantan S, Cristiano LM, Goyal J, Sommer JM, Dumont JA, Dodd N, Nugent K, Vigliani G, Luk A, Brennan A, Pierce GF, Investigators AL. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317–325. doi: 10.1182/blood-2013-10-529974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turecek PL, Bossard MJ, Graninger M, Gritsch H, Hollriegl W, Kaliwoda M, Matthiessen P, Mitterer A, Muchitsch EM, Purtscher M, Rottensteiner H, Schiviz A, Schrenk G, Siekmann J, Varadi K, Riley T, Ehrlich HJ, Schwarz HP, Scheiflinger F. BAX 855, a PEGylated rFVIII product with prolonged half-life. Development, functional and structural characterisation. Hamostaseologie 32 Suppl. 2012;1:S29–38. [PubMed] [Google Scholar]
- 8.Powell JS, Nugent DJ, Harrison JA, Soni A, Luk A, Stass H, Gorina E. Safety and pharmacokinetics of a recombinant factor VIII with pegylated liposomes in severe hemophilia A. J Thromb Haemost. 2008;6(2):277–283. doi: 10.1111/j.1538-7836.2008.02856.x. [DOI] [PubMed] [Google Scholar]
- 9.Muto A, Yoshihashi K, Takeda M, Kitazawa T, Soeda T, Igawa T, Sakamoto Y, Haraya K, Kawabe Y, Shima M, Yoshioka A, Hattori K. Anti-factor IXa/X bispecific antibody (ACE910): hemostatic potency against ongoing bleeds in a hemophilia A model and the possibility of routine supplementation. J Thromb Haemost. 2014;12(2):206–213. [PubMed] [Google Scholar]
- 10.Shetty KA, Kosloski MP, Mager DE, Balu-Iyer SV. Soy Phosphatidylinositol Containing Nanoparticle Prolongs Hemostatic Activity of B-Domain Deleted Factor VIII in Hemophilia A Mice. J Pharm Sci. 2015;104(2):388–395. doi: 10.1002/jps.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols TC, Dillow AM, Franck HW, Merricks EP, Raymer RA, Bellinger DA, Arruda VR, High KA. Protein replacement therapy and gene transfer in canine models of hemophilia A, hemophilia B, von willebrand disease, and factor VII deficiency. ILAR J. 2009;50(2):144–167. doi: 10.1093/ilar.50.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham JB, Buckwalter JA, et al. Canine hemophilia; observations on the course, the clotting anomaly, and the effect of blood transfusions. J Exp Med. 1949;90(2):97–111. doi: 10.1084/jem.90.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lozier JN, Dutra A, Pak E, Zhou N, Zheng Z, Nichols TC, Bellinger DA, Read M, Morgan RA. The Chapel Hill hemophilia A dog colony exhibits a factor VIII gene inversion. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):12991–12996. doi: 10.1073/pnas.192219599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monahan PE. The expanding menagerie: animal models of hemophilia A. J Thromb Haemost. 2010;8(11):2469–2471. doi: 10.1111/j.1538-7836.2010.04053.x. [DOI] [PubMed] [Google Scholar]
- 15.Sabatino DE, Freguia CF, Toso R, Santos A, Merricks EP, Kazazian HH, Jr., Nichols TC, Camire RM, Arruda VR. Recombinant canine B-domain-deleted FVIII exhibits high specific activity and is safe in the canine hemophilia A model. Blood. 2009;114(20):4562–4565. doi: 10.1182/blood-2009-05-220327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron C, Notley C, Hoyle S, McGlynn L, Hough C, Kamisue S, Giles A, Lillicrap D. The canine factor VIII cDNA and 5' flanking sequence. Thromb Haemost. 1998;79(2):317–322. [PubMed] [Google Scholar]
- 17.Peng A, Straubinger RM, Balu-Iyer SV. Phosphatidylinositol containing lipidic particles reduces immunogenicity and catabolism of factor VIII in hemophilia a mice. AAPS J. 2010;12(3):473–481. doi: 10.1208/s12248-010-9207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramani K, Miclea RD, Purohit VS, Mager DE, Straubinger RM, Balu-Iyer SV. Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A. J Pharm Sci. 2008;97(4):1386–1398. doi: 10.1002/jps.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heath TD, Macher BA, Papahadjopoulos D. Covalent attachment of immunoglobulins to liposomes via glycosphingolipids. Biochimica et biophysica acta. 1981;640(1):66–81. doi: 10.1016/0005-2736(81)90532-0. [DOI] [PubMed] [Google Scholar]
- 20.Mischke R. Activated partial thromboplastin time as a screening test of minor or moderate coagulation factor deficiencies for canine plasma: sensitivity of different commercial reagents. J Vet Diagn Invest. 2000;12(5):433–437. doi: 10.1177/104063870001200507. [DOI] [PubMed] [Google Scholar]
- 21.Moore FD, Muldowney FP, Haxhe JJ, Marczynska AW, Ball MR, Boyden CM. Body composition in the dog. I. Findings in the normal animal. J Surg Res. 1962;2:245–253. doi: 10.1016/s0022-4804(62)80017-1. [DOI] [PubMed] [Google Scholar]
- 22.Agerso H, Stennicke HR, Pelzer H, Olsen EN, Merricks EP, Defriess NA, Nichols TC, Ezban M. Pharmacokinetics and pharmacodynamics of turoctocog alfa and N8-GP in haemophilia A dogs. Haemophilia. 2012;18(6):941–947. doi: 10.1111/j.1365-2516.2012.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]