Abstract
Lower blood pressure (BP) within the normotensive range have been suggested to be deleterious in diabetic persons using anti-hypertensive drugs. We hypothesized BP<120/80mmHg and BP trajectories may predict further risk of all-cause mortality or cardiovascular events (CVEs) in normotensive diabetic individuals. We included 3,159 diabetic adults, free of hypertension, atherosclerotic cardiovascular diseases or cancer in 2006 (baseline), from a community-based cohort including 101,510 participants. A total of 831 participants with SBP <120/80 mmHg and 2,328 participants with BP of 120-139/80-89 mmHg were included. BP and other clinical covariates were repeatedly measured every 2 years. During 7 years of follow-up, we documented 247 deaths and 177 CVEs. Diabetic persons with BP<120/80mmHg had a 46% increased risk of all-cause mortality (95%CI 10-93%) compared with those with BP of 120-139/80-89mmHg at baseline. We then estimated the association between BP trajectories from 2006 to 2008 and adverse events among 2,311 diabetic persons who had both BP measures at 2006 and 2008. Relative to stable BP of 120-139/80-89mmHg, having persistently BP<120/80mmHg (HR 2.35, 95%CI 1.10-5.01) or a spontaneous decrease in BP from 120-139/80-89 to <120/80mmHg (HR 3.04, 95%CI 1.56-5.92) was significantly associated with an increased risk of all-cause mortality during 2008-2014. A rise in BP from 120-139/80-89 to ≥140/90 mmHg conferred a high risk of CVEs (HR 1.98, 95%CI 1.24-3.17). In normotensive diabetic persons, having a low BP or a decline in BP was both associated with an increased risk of all-cause mortality, while development of incident hypertension increased the risk of CVEs.
Keywords: diabetes, blood pressure, SPRINT, all-cause mortality, cardiovascular disease
Introduction
Diabetes is highly prevalent and attributed to the majority of cardiovascular mortality and morbidity worldwide, representing a huge burden on health care and social economy1. Diabetic comorbidities such as hypertension and dyslipidemia substantially increase mortality risk2-4. It has been consistently shown that blood pressure (BP) lowering therapy leads to a significant reduction in cardiovascular events (CVEs) and death in individuals with both diabetes and hypertension5. However, a “J-curve” phenomenon was observed in the Framingham Heart Study6 and several randomized controlled trials (RCTs)7-11, resulting in debate as to the meaning of this finding12. In these studies, low BP (e.g., systolic blood pressure [SBP] <130mmHg or diastolic blood pressure [DBP] <70mmHg) conferred increased risks of CVEs and/or all-cause mortality in individuals with diabetes12. It is worth noting that these RCTs for the administration of anti-hypertensive drugs restricted prescriptions mostly to diabetic persons with hypertension or at a high risk of clinical/subclinical atherosclerotic diseases. It remains unknown whether this “J-curve” phenomenon exists in untreated normotensive diabetics.
The Eighth Joint National Committee (JNC8) recommended a goal SBP <140mmHg and a goal DBP <90mmHg in adults with both diabetes and hypertension. These thresholds were the same as for the general hypertensive population aged<60 years because solid evidence for diabetic persons is scanty13. The panel also did not address the SBP range of 120-139mmHg or DBP of 80-89mmHg, which was previously called “prehypertension” in the seventh Joint National Committee (JNC7)14.
Recently, the Systolic Blood Pressure Intervention Trial (SPRINT) reported encouraging results regarding a protection from CVEs with intensive BP control (a target SBP <120mmHg) when compared with standard BP control (a target SBP<140 mmHg)15. However, individuals with diabetes or stroke were not included in the SPRINT, leaving a knowledge gap regarding whether “the lower, the better” approach to BP reduction can be applied in individuals with diabetes.
Herein, we reported our analyses from a large prospective community-based cohort study examining whether BP<120/80mmHg were associated with incident CVEs and mortality during approximate 7 years of follow-up. We also examined the potential effects of the trajectory of BP over two years (2006-2008) on subsequent risk of developing CVEs/mortality after 2008. To minimize the confounding derived from BP lowering drugs and other cardiovascular diseases (CVDs) related clinical conditions, we excluded participants with hypertension and CVDs at recruitment.
Materials and Methods
Study Populations
The Kailuan Study is an ongoing longitudinal cohort study begun in 2006. Detailed study design and procedures have been previously described16. At baseline (2006-2007), a total of 101,510 Chinese individuals (81,110 men and 20,400 women) were enrolled from 11 hospitals affiliated with the Kailuan community in Tangshan (Hebei, China). Subsequently, 3 repeated follow-up examinations were conducted in 2008, 2010 and 2012. All participants were followed-up until death or until December 31, 2014, whichever came first. At the baseline and during the biennial follow-up, trained nurses and physicians employed all face to face survey on demographic characteristics, medical co-morbidities information (e.g., hypertension, diabetes and CVDs) and lifestyle behaviors (e.g., smoking status, alcohol consumption and physical activity) and performed all physical examinations according to the standardized protocol. Questionnaires contained whether the participants used medications for CVDs, hypertension or diabetes.
Diabetes were defined as a fasting blood glucose (FBG) concentration of ≥7.0mmol/L, a non-fasting blood glucose concentration of ≥11.1mmol/L, administration of glucose lowering medication or self- reported physician's diagnosis17. We identified 9,489 individuals with diabetes by questionnaires and laboratory examinations. To reduce the confounding due to the use of anti-hypertensive drugs, we further excluded 6,160 individuals with hypertension (SBP of ≥140mmHg or DBP of ≥90mmHg, physician diagnosis of hypertension, or use of anti-hypertensive drugs). Information on use of anti-hypertensive drugs and their types (i.e., diuretics, angiotensin converting enzyme [ACE] inhibitors, angiotensin receptor blockers [ARBs], β-Blockers, calcium channel blockers, α-blockers, polypill, and others) was collected via questionnaires during the surveys. Participants who reported use of any anti-hypertensive drugs, regardless of their prevalent diseases (i.e., CVDs, hypertension, nephropathy), were excluded in the current analyses.
Considering diabetic participants with CVDs might experience a more complex clinical condition and commonly used renin-angiotensin-aldosterone system blockade or β-blockers, as recommended by the guidelines18-20, we further excluded 159 diabetic participants with physician-diagnosed CVDs. None of the participants in the current analyses used anti-platelet drugs (i.e., aspirin) in our study. We also excluded 11 individuals with prevalent cancer at baseline.
Finally, we included 3,159 participants in the current analysis, including 831 participants with SBP <120mmHg and DBP <80mmHg (referred to as “BP<120/80mmHg” in this study) and 2,328 participants with SBP of 120-139mmHg or DBP of 80-89mmHg (referred to as “BP of 120-139/80-89mmHg”) were included. Hypotension (SBP<90mmHg or DBP<60mmHg) was observed in 20 participants.
In a second analysis, we excluded diabetic persons who dead (n=23) or developed CVEs (n=19) during 2006-2008, and individuals who did not have BP data in 2008 (n=806) to examine the possible association between longitudinal BP trajectory (from 2006 to 2008) and all-cause mortality/CVEs. A flow diagram depicting the stepwise selection process was listed in the online-only Data Supplement.
Similar to previous Chinese national survey21, approximate 68% of participants in the current study had undiagnosed diabetes based on laboratory examination in 2006. These participants were unaware of their condition and were untreated. We cannot obtain accurate information on diabetes duration for these participants. We thus used the diagnosis status (diagnosed vs undiagnosed diabetes) as a surrogate of diabetes duration in our analyses.
This study protocol was approved by the ethics committee of the Kailuan Hospital and the Brigham and Women's Hospital in compliance with the guidelines of the World's Association Declaration of Helsinki. All participants gave written informed consent.
Assessment of BP (exposures)
BP was measured according to the JNC7 recommendation14 every two years since the baseline. After being seated for at least 5 minutes in a chair, all participants received at least 2 measures of BP using random 0 sphygmomanometers. The average value of the multiple BP measures was used for further analysis. In the current study, we examined the baseline BP (2006) and subsequent risk of mortality/CVEs in 2006-2014. To take advantage of repeated BP assessment, we also used the BP trajectory patterns from 2006 to 2008 to predict further risks of CVEs and all-cause mortality during 2008-2014. Individuals with stable BP of 120-139/80-89mmHg from 2006 to 2008 were regarded as a reference.
Ascertainment of myocardial infarction, stroke and death (outcomes)
The main outcomes included the all-cause mortality and CVEs (i.e., a composite of myocardial infarction [MI] and stroke events). Death information was collected from provincial vital statistics offices. Information on physician-diagnosed CVEs was collected in the biennial interview. To further identify potential CVEs, we also investigated discharge lists from the 11 hospitals. All suspected CVEs were ascertained annually via reviewing medical records after recruitment by a committee of three experienced physician adjudicators who were blinded to the study design. Incident MI was diagnosed according to the World Health Organization's Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) criteria on basis of clinical symptoms and dynamic changes in cardiac enzymes/biomarkers concentrations and electrocardiogram22. Stroke was diagnosed according to the World Health Organization criteria23, based on signs, symptoms, neuroimages (from computed tomography or magnetic resonance imaging) and other diagnostic reports, as detailed previously24.
Assessment of potential covariates
Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Smoking status and alcohol drinking status were divided into 4 categories: “never”, “former”, “occasional”, or “daily”. Physical activity was assessed by questions on lifestyle behaviors that combined occupational and discretionary physical activities (inactive, moderately active and vigorously active25).
Whole blood samples were drawn from all participants, generally after an overnight fast and analyzed in the Central Laboratory of Kailuan General Hospital at the same day. The biochemical parameters, including blood glucose, creatinine, triglyceride, high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C), were measured using an auto-analyzer (Hitachi 747; Hitachi, Tokyo, Japan), as detailed previously26. Plasma high-sensitivity C reactive protein (hs-CRP) concentrations were measured using a high-sensitivity particle-enhanced immunonephelometry assay (Cias Latex CRP-H, Kanto Chemical Co. Inc, Japan). Estimated Glomerular Filtration Rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation27.
Statistical analysis
Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using the Cox proportional hazards regression models for the risk of CVEs and all-cause mortality, according to baseline BP status and BP trajectory patterns. The initial models were adjusted for age, sex and glucose-lowering therapy. The fully adjusted models further included variables which could be associated with both the exposure and outcomes, including smoking and drinking status, BMI, physical activity, education, occupation, eGFR, and blood concentrations of FBG, triglyceride, HDL-C, LDL-C and hs-CRP.
Given that anti-diabetic drugs (e.g., metformin) and lipid-lowering drugs (e.g., statins) may provide additional effect on CVEs, we conducted several sensitivity analyses by excluding diabetic persons receiving glucose-lowering or lipid-lowering therapy or those with hypotension, respectively. Chronic renal failure is common in diabetics and worsens cardiovascular outcomes28 and individuals with chronic kidney disease (CKD) stage 4-5 may not use ACE inhibitors or ARBs due to hypotension or hyperkalemia. We thus did a sensitivity analysis by excluding individuals with eGFR <30 mL/min/1.73m2 (CKD stage 4-5) to estimate the contribution of BP in diabetics with normal renal function or mild-to-moderate renal impairment. To understand whether the possible association between BP trajectories and mortality was attributed to CVDs or hypertension occurred after 2008 (the baseline of the trajectory analysis), we also conducted sensitivity analyses by excluding participants who developed CVDs or hypertension during 2008-2012, separately. Because it was unclear whether hypotension in our study was a physiological state or due to subclinical diseases, we further excluded those with hypotension at baseline in the sensitivity analysis. Given BP<120/80mmHg may be due to chronic illnesses (i.e., malignancies) and also be related to a decline in BMI, we sequentially remove individuals developed cancers during follow-up, individuals who had annual weight loss >5% from 2006 to 2008 and individuals with BMI<18.5 kg/m2 at baseline. Interaction between BP trajectories and sex, BMI, alcohol consumption, diabetic duration for all-cause mortality and CVEs were also explored.
All statistical tests were 2-sided, and P<0.05 was regarded as significant. Statistical analyses were performed using SAS 9.3 (SAS Institute Inc; Cary, NC, USA).
Results
Individuals with BP<120/80mmHg at baseline, were more likely to be women, had lower BMI, lower concentrations of triglyceride and HDL-C, and a higher prevalence of smoking and drinking habit and prescription of anti-diabetic drugs (Table1).
Table 1. Baseline characteristics according to blood pressure in normotensive diabetic patients.
| Characteristics | SBP<120mmHg and DBP<80mmHg | SBP 120-139mmHg or DBP 80-89mmHg | P value |
|---|---|---|---|
| Number | 831 | 2,328 | |
| Age, year* | 53.6±10.7 | 54.3±10.4 | 0.10 |
| Men, % | 77.3 | 82.9 | 0.0003 |
| SBP, mmHg | 108.3±7.94 | 124.5±7.54 | <0.0001 |
| DBP, mmHg | 70.0±5.91 | 79.7±4.63 | <0.0001 |
| Fasting glucose, mmol/L | 9.65±3.58 | 9.56±3.09 | 0.50 |
| Glucose-lowering therapy, % | 29.5 | 21.5 | <0.0001 |
| Body mass index, kg/m2 | 24.6±3.30 | 25.5±3.24 | <0.0001 |
| Triglyceride, mmol/L | 1.87±1.54 | 2.18±1.98 | <0.0001 |
| HDL-C, mmol/L | 1.51±0.42 | 1.55±0.43 | 0.04 |
| LDL-C, mmol/L | 2.38±0.86 | 2.42±0.91 | 0.29 |
| eGFR, mL/min/1.73m2 | 85.1±23.0 | 83.8±25.5 | 0.20 |
| Log(hs-CRP), mg/L | 0.004±1.50 | 0.02±1.48 | 0.76 |
| Smoking status, % | |||
| Never | 56.4 | 61.5 | 0.005 |
| Former | 6.14 | 6.01 | |
| Occasional | 3.49 | 3.44 | |
| Daily | 33.6 | 29.1 | |
| Alcohol consumption, % | |||
| Never | 59.0 | 61.6 | 0.01 |
| Former | 5.17 | 5.15 | |
| Occasional | 20.5 | 17.4 | |
| Daily | 15.0 | 15.9 | |
| Physical activity, % | |||
| Inactive | 7.82 | 7.56 | 0.73 |
| Moderately active | 73.5 | 75.3 | |
| Vigorously active | 18.5 | 17.0 | |
| Education level, % | |||
| Primary | 12.2 | 12.3 | 0.07 |
| Middle/high school | 81.5 | 83.6 | |
| College/university | 6.14 | 4.04 | |
| Occupation, % | |||
| White collar | 7.82 | 6.01 | 0.26 |
| Coalminer | 29.6 | 28.7 | |
| Blue collars | 62.2 | 65.0 |
Abbreviations: SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; HDL-C: HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; eGFR: estimated glomerular filtration rate; hs-CRP: high-sensitive C-reactive protein.
Continuous variables were reported as mean ± standard deviation.
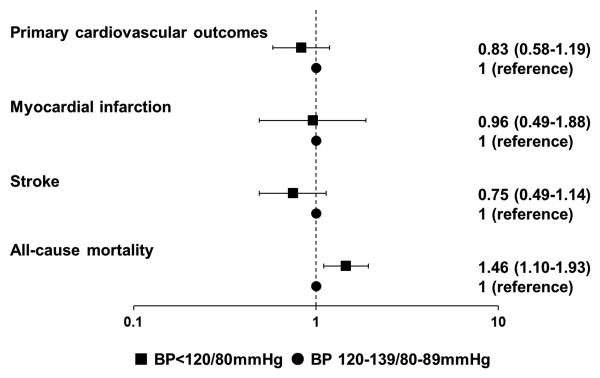
After a mean of 6.9 years follow-up, a total of 247 deaths and 177 composite CVEs were documented. One death occurred in hypotensive participants. Baseline BP <120/80mmHg was significantly associated with future risk of all-cause mortality (HR 1.46, 95%CI 1.10-1.93) but not with composite CVEs (HR 0.83, 95%CI 0.58-1.19), as compared with BP of 120-139/80-89mmHg (Figure 1). Similar results were observed in the sensitivity analyses (Table S1 in the online-only Data Supplement).
Figure 1. Adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs) of cardiovascular events and all-cause mortality during 2006-2014 according to baseline (2006) blood pressure status.
Primary cardiovascular outcomes included fatal or non-fatal myocardial infarction events and fatal or non-fatal stroke events. All models were adjusted for age (years), sex, smoking status (never, former, occasional or daily), glucose-lowering therapy, body mass index (kg/m2), high density lipoprotein cholesterol (mmol/L), low density lipoprotein cholesterol (mmol/L), triglyceride (mmol/L), alcohol consumption (never, former, occasional or daily), educational level (primary, middle/high school or college/university), occupation (white collar, coalminer or blue collar), physical activity (inactive, moderately active or vigorously active), high sensitivity C-reactive protein (mg/L), fasting blood glucose (mmol/L) and estimated Glomerular Filtration Rate (eGFR)(mL/min/1.73m2).
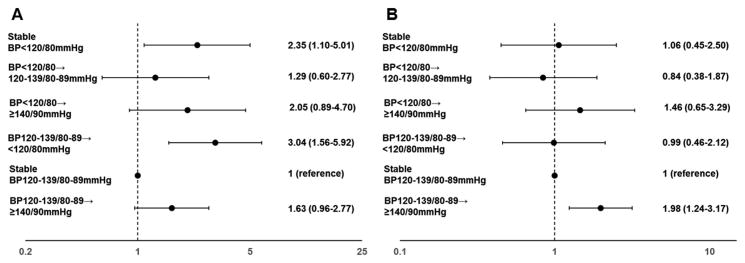
Interestingly, the likelihood of developing primary cardiovascular outcomes and all-cause mortality remarkably differed comparing the BP trajectory patterns. Relative to individuals with stable BP of 120-139/80-89 mmHg from 2006 to 2008, the adjusted HR of all-cause mortality was 2.35 (95%CI 1.10-5.01) for the persistently BP<120/80mmHg pattern and 3.04 (95%CI 1.56-5.92) for a fall in BP from 120-139/80-89 to <120/80mmHg in the fully adjusted model. There was also a non-significant trend between increase in BP and higher further risk of mortality (Figure2). Excluding diabetic persons who developed cancers during follow-up, or those who had annual weight loss >5% from 2006 to 2008, or individual with BMI<18.5kg/m2 at baseline generated similar results (Figure S2 in the online-only Data Supplement). The association between a fall in BP and all-cause mortality did not materially change even when we sequentially excluded individuals who developed CVDs (HR 3.89, 95%CI1.93-7.85) or hypertension (HR 2.26, 95%CI 1.09-4.71) during 2008-2012 or those with BP<90/60mmHg (HR 2.89, 95%CI1.48-5.64). The results did not change after exclusion of hypotension (HR 2.25, 95%CI 1.05-4.86) (Table S2 in the online-only Data Supplement). There were non-significant interactions between BP trajectories and sex, BMI, alcohol consumption, diabetic duration for all-cause mortality (Pinteraction >0.05).
Figure 2. Adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs) of all-cause mortality (Figure 2A) and cardiovascular events (Figure 2B) during 2008-2014 according to blood pressure trajectories from 2006 to 2008.
Primary cardiovascular outcomes included fatal or non-fatal myocardial infarction events and fatal or non-fatal stroke events. All models were adjusted for age (years), sex, smoking status (never, former, occasional or daily), glucose-lowering therapy, body mass index (kg/m2), high density lipoprotein cholesterol (mmol/L), low density lipoprotein cholesterol (mmol/L), triglyceride (mmol/L), alcohol consumption (never, former, occasional or daily), educational level (primary, middle/high school or college/university), occupation (white collar, coalminer or blue collar), physical activity (inactive, moderately active or vigorously active), high sensitivity C-reactive protein (mg/L), fasting blood glucose (mmol/L) and estimated Glomerular Filtration Rate (eGFR)(mL/min/1.73m2).
Increase in BP from 120-139/80-89 to ≥140/90mmHg was associated with the increased risk of CVEs (HR 1.98, 95%CI 1.24-3.17), relative to stable BP of 120-139/80-89 mmHg (Figure 2). The association became stronger when we further excluded individuals prescribed anti-diabetic drugs. Individuals with BP elevation from 120-139/80-89 to ≥140/90mmHg had an increased risk of MI (HR 3.34 95%CI 1.27-9.28). There was also a non-significant, but highly plausible tendency that BP elevation was related to further risk of stroke (HR 1.65 95%CI 0.97-2.78) (Table S3-5 in the online-only Data Supplement).
We found a significant interaction between BP trajectories and diabetic duration, but not other potential effect-modifiers, for risk of CVEs (Pinteraction=0.001). The cardiovascular hazard of BP elevation above 140/90mmHg was more pronounced in undiagnosed diabetic persons, no matter their initial BP<120/80mmHg (HR 3.49, 95%CI 1.44-8.47) or BP of 120-139/80-89mmHg (HR 2.51, 95%CI 1.37-4.61), than in those who were diagnosed previously with longer disease duration (Figure S3 in the online-only Data Supplement).
Discussion
In the current study, we observed that baseline BP<120/80mmHg was significantly associated with future risk of all-cause mortality but not with CVEs, relative to BP of 120-139/80-89 mmHg. The trajectory analysis showed a consistent result. Diabetic persons having sustained BP<120/80mmHg or a fall in BP from 120-139/80-89 to <120/80mmHg during the first 2 years of the follow-up also had an increased risk of all-cause mortality. An increased risk of CVEs was observed in diabetic persons with a rise in BP from 120-139/80-89 to ≥140/90mmHg. Our observation suggested that longitudinal BP trajectories may provide additional information in predicting adverse events besides baseline value in normotensive diabetic patients. Further, our findings indicated that the BP goal based on non-diabetic population or in general population might not be extended to diabetic population.
The observed association between persistently low BP and a drop in BP and increased all-cause mortality was independent of the anti-hypertensive drug effects and prior diseases that would be prescribed anti-hypertensive drugs such as ACE inhibitors, ARBs and β-blockers. Although we did not deliberately exclude individuals using anti-platelet drugs, none of the participants in the current study reported use of anti-platelet drugs (i.e., aspirin). This could be due to the exclusion criteria—we excluded diabetic persons with CVDs or hypertension at baseline. These participants usually used aspirin, as recommended by American Diabetes Association29. The prevalence of anti-platelet drug use could also be underestimated because we collected the data via questionnaires. Nevertheless, the impact of anti-platelet drug use on the observed association between BP and CVEs/mortality could be small. In our sensitivity analysis, exclusion of lipid-lowering drugs, glucose-lowering drugs or CKD stage 4-5 generated similar results. This would minimize the potential effects of these commonly used medications on CVDs/mortality. Hence, our results may reflect the real relationship between BP and adverse events.
BP<120/80mmHg appeared to be common in diabetic persons with lower BMI, lower concentrations of triglyceride, HDL-C and a higher prevalence of smoking and drinking habit and administrate of glucose-lowering drugs. This suggested a poor health status or an underlying illness which could be associated with increased mortality risk. Further excluding participants who developed CVDs, hypertension or cancers during follow-up generated similar significant results, suggesting that the increased hazards might be due to a poor health conditions or deterioration from subclinical diseases in these participants, not necessarily a change in atherosclerotic burden over time. Diabetic persons may suffer other diseases (e.g., infection and amyloidosis) that result in impaired cardiac output and abnormal vasodilatation, exhibiting a decrease in BP30-32. Therefore, a relative low BP may reflect the progression towards complex diabetic comorbidities and mortality.
Previous studies generally considered the initial or achieved BP (a single point in time) as a risk predictor. The potential effect of change in BP over time was not well characterized in these studies. This may pose a research problem whether the BP of 120-139/80-89 mmHg (as called “prehypertension” previously) itself is harmful or just because it developed subsequent hypertension over time. We found that the cardiovascular hazard was pronounced in diabetic persons who developed hypertension at 2008. Even in people with initial low BP, there remains a similar tendency albeit without significance. This suggests that the BP elevation over time could be relevant to further CVEs, which could be due to shear stress abnormalities and endothelium disorder, followed by vessel impairment in a high BP33-35.
Interestingly, BP elevation above 140/90 mmHg appeared more deleterious in individuals with undiagnosed diabetes than in individuals diagnosed previously, no matter of their initial BP. It was probably due to chance. Another interpretation is that previously diagnosed diabetic participants commonly used glucose-lowering drugs but undiagnosed individuals not. Some anti-diabetic drugs (e.g., metformin) provide additional cardioprotective effect and somewhat offset the adverse impact of elevated BP36. This notion was supported by a sensitivity analysis in which the cardiovascular hazard due to BP elevation became stronger after exclusion of anti-diabetic drugs. Our findings were in consistent with a recent study, in which individuals with increases in BP (modest-increasing and elevated-increasing) over a 25-years period had the highest risk of subclinical atherosclerosis compared with other trajectory groups37. The majority of these participants had an initial BP within a range of “prehypertension”. Together with this study, we suggest that not only is the achieved BP important, but longitudinal change in BP predicts high risk that are compatible or beyond the baseline value. Therefore, it is important to monitor longitudinal patterns of change in BP in clinical practice.
The strength of the current study included that using longitudinal data from a large, community-based cohort study with approximate 7 years follow-up, which allowed us to more realistically understand the association between natural process of BP and diabetic prognosis. Several limitations of our study deserve mention. First, although the current data derived from a large ongoing cohort including 101,510 adults, the sample size was relative small, especially for women. We thus did not have enough statistical power to detect small-to-modest sex difference. Second, the participants in our study were relatively young or with low BMI. This could be explained by the fact that the majority of our diabetic participants were newly detected and had not developed obvious complications. Our results may not be able to generalize to elderly diabetic persons or those with a complex clinical condition (i.e., obesity, CVDs and nephropathy). All participants were residents of the Kailuan community in Tangshan (Hebei, China), and were primarily of Northern Han Chinese ethnicity. Further studies are needed to be conducted in diabetic population of other ethnic origins. Third, we did not measure hemoglobin A1c at baseline because of its high-cost as a screening test in general population. Fourth, the follow-up period was relative short for studying BP trajectory patterns. We did not include BP measures at 2010 and 2012 for the trajectory analysis due to lack of statistical power-- few incident CVEs or deaths occurred during 2012-2014. However the sensitivity analysis by excluding those who developed hypertension during 2008-2012 generated similar results. Fifth, although we minimized the drug effects on CVDs, residual confounding from non-pharmacological factors cannot be completely excluded. Individuals with diabetes were encouraged to reduce weight, quit smoking and restrict intake of sugar and salt. These behaviors may favorably modify BP. Finally, we did not collect information on specific causes of death.
Perspectives
The spontaneous trajectory patterns of BP in participants with diabetes were associated with alerted risks of development CVEs or all-cause mortality. Dynamic BP observation may provide additional predictive information compared with the measures at one single time. Our findings need to be replicated in further studies with longer follow-up, and larger sample size, including a large proportion of women and participants with different ethnicity. If confirmed, the clinical judgment with respect to the safe BP goal should consider the within-persons change for each individual with diabetes.
Supplementary Material
Novelty and Significance.
What Is New?
Longitudinal blood pressure trajectories were significantly associated with adverse events in normotensive diabetic persons after minimizing the drug effects on cardiovascular diseases. Persistently lower blood pressure and a spontaneous drop in blood pressure were significantly associated with future risk of all-cause mortality, while development of incident hypertension increased the risk of cardiovascular events.
What Is Relevant?
Although limited sample size due to strict inclusion criteria and non-randomized allocation made our study underpowered to determine an optimal BP level against adverse health outcomes in the diabetic population, we still suggested that the BP goal in non-diabetic population or in general population might not be extended to diabetic population.
Summary.
Our findings suggested that the “J-curve” may exist in normotensive diabetic persons. Long-term blood pressure supervision are necessary in diabetic persons even they have not developed hypertension or cardiovascular diseases.
Acknowledgments
Sources of Funding: This work was supported by the youth science and technology talents “Sail” program of Shanghai Municipal Science and Technology Commission (Zhijun Wu, 14YF1402700), by the new hundred talents program of the Shanghai Municipal Health Bureau (Wei Jin, XBR2013100), by the National Natural Science Foundation of China (Wei Jin, 81370397), by the National Institutes of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award (Anand Vaidya, R01 DK107407), by Grant 2015085 from the Doris Duke Charitable Foundation (Anand Vaidya), and by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award (Anand Vaidya, K23HL111771).
Footnotes
Conflict of Interest Disclosures: No authors reported any disclosures.
References
- 1.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 3.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein M, Sowers JR. Diabetes mellitus and hypertension. Hypertension. 1992;19:403–418. doi: 10.1161/01.hyp.19.5.403. [DOI] [PubMed] [Google Scholar]
- 6.D'Agostino RB, Belanger AJ, Kannel WB, Cruickshank JM. Relation of low diastolic blood pressure to coronary heart disease death in presence of myocardial infarction: The framingham study. BMJ. 1991;303:385–389. doi: 10.1136/bmj.303.6799.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The value randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, Schumacher H, Redon J, Verdecchia P, Schmieder R, Jennings G, Yusoff K, Ryden L, Liu GL, Teo K, Sleight P, Yusuf S. Blood pressure targets recommended by guidelines and incidence of cardiovascular and renal events in the ongoing telmisartan alone and in combination with ramipril global endpoint trial (ontarget) Circulation. 2011;124:1727–1736. doi: 10.1161/CIRCULATIONAHA.110.008870. [DOI] [PubMed] [Google Scholar]
- 9.Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, LaRosa JC, Treating to New Targets Steering C Investigators J-curve revisited: An analysis of blood pressure and cardiovascular events in the treating to new targets (tnt) trial. Eur Heart J. 2010;31:2897–2908. doi: 10.1093/eurheartj/ehq328. [DOI] [PubMed] [Google Scholar]
- 10.Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, Pepine CJ. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–68. doi: 10.1001/jama.2010.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berl T, Hunsicker LG, Lewis JB, et al. Impact of achieved blood pressure on cardiovascular outcomes in the irbesartan diabetic nephropathy trial. J Am Soc Nephrol. 2005;16:2170–2179. doi: 10.1681/ASN.2004090763. [DOI] [PubMed] [Google Scholar]
- 12.Banach M, Aronow WS. Blood pressure j-curve: Current concepts. Curr Hypertens Rep. 2012;14:556–566. doi: 10.1007/s11906-012-0314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.Group SR, Wright JT, Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Cui L, Wang Y, Vaidya A, Chen S, Zhang C, Zhu Y, Li D, Hu FB, Wu S, Gao X. Resting heart rate and the risk of developing impaired fasting glucose and diabetes: The kailuan prospective study. Int J Epidemiol. 2015;44:689–699. doi: 10.1093/ije/dyv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical practice recommendations 2005. Diabetes Care. 2005;28(Suppl 1):S1–79. doi: 10.2337/diacare.28.suppl_1.s1. [DOI] [PubMed] [Google Scholar]
- 18.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. Aha/accf secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: A guideline from the american heart association and american college of cardiology foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 19.Jneid H, Anderson JL, Wright RS, et al. 2012 accf/aha focused update of the guideline for the management of patients with unstable angina/non-st-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2012;126:875–910. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]
- 20.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 accf/aha guideline for the management of st-elevation myocardial infarction: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 22.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the world health organization monica project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 23.Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the who task force on stroke and other cerebrovascular disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, Li N, Bian L, Wu J, Jia Q, Wu S, Zhao X. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44:2451–2456. doi: 10.1161/STROKEAHA.113.678839. [DOI] [PubMed] [Google Scholar]
- 25.Nordstrom CK, Dwyer KM, Merz CN, Shircore A, Dwyer JH. Leisure time physical activity and early atherosclerosis: The los angeles atherosclerosis study. Am J Med. 2003;115:19–25. doi: 10.1016/s0002-9343(03)00242-0. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Li N, Chang S, Bassig BA, Guo L, Ren J, Su K, Li F, Chen S, Wu S, Zou Y, Dai M, Zheng T, He J. A prospective follow-up study of the relationship between c-reactive protein and human cancer risk in the chinese kailuan female cohort. Cancer Epidemiol Biomarkers Prev. 2015;24:459–465. doi: 10.1158/1055-9965.EPI-14-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rognant N, Lemoine S, Laville M, Hadj-Aissa A, Dubourg L. Performance of the chronic kidney disease epidemiology collaboration equation to estimate glomerular filtration rate in diabetic patients. Diabetes Care. 2011;34:1320–1322. doi: 10.2337/dc11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley RN. Clinical epidemiology of cardiovascular disease in chronic kidney disease. J Ren Care. 2010;36(Suppl 1):4–8. doi: 10.1111/j.1755-6686.2010.00171.x. [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association. Executive summary: Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S5–13. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 30.Sharma N, Howlett J. Current state of cardiac amyloidosis. Curr Opin Cardiol. 2013;28:242–248. doi: 10.1097/HCO.0b013e32835dd165. [DOI] [PubMed] [Google Scholar]
- 31.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 32.Seymour CW, Rosengart MR. Septic shock: Advances in diagnosis and treatment. JAMA. 2015;314:708–717. doi: 10.1001/jama.2015.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khder Y, Briancon S, Petermann R, Quilliot D, Stoltz JF, Drouin P, Zannad F. Shear stress abnormalities contribute to endothelial dysfunction in hypertension but not in type ii diabetes. J Hypertens. 1998;16:1619–1625. doi: 10.1097/00004872-199816110-00008. [DOI] [PubMed] [Google Scholar]
- 34.Sowers JR, Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. An update. Hypertension. 1995;26:869–879. doi: 10.1161/01.hyp.26.6.869. [DOI] [PubMed] [Google Scholar]
- 35.Mayet J, Hughes A. Cardiac and vascular pathophysiology in hypertension. Heart. 2003;89:1104–1109. doi: 10.1136/heart.89.9.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong J, Zhang Y, Lai S, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36:1304–1311. doi: 10.2337/dc12-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, Jacobs DR, Jr, Liu K, Lloyd-Jones D. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311:490–497. doi: 10.1001/jama.2013.285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.