Abstract
Molecular imprinting within cross-linked micelles using 4-vinylphenylboronate derivatives of carbohydrates provided water-soluble nanoparticle receptors selective for the carbohydrate templates. Complete differentiation of d-aldohexoses could be achieved by these receptors if a single inversion of hydroxyl occurred at C2 or C4 of the sugar or if two or more inversions took place. Glycosides with a hydrophobic aglycan displayed stronger binding due to increased hydrophobic interactions.
Graphical abstract
Carbohydrates are one of the most important classes of biomolecules and involved in numerous biological processes including cell–cell interaction, immune response, and viral and bacterial infection.1,2 Synthetic analogues of carbohydrate-binding lectins are powerful tools for the detection of biologically important sugars and intervention of carbohydrate-mediated interactions.3 Although receptors based on both covalent4-8 and noncovalent9-12 interactions have been reported, a general method for selectively binding carbohydrates in water remains elusive.13,14
As building blocks of oligo- and polysaccharides, the eight d-aldohexoses differ only in the stereochemistry of 1–3 hydroxyls. Unfortunately, the hydroxyl group is not a good functional group handle from the supramolecular viewpoint, because its strong solvation by water makes it difficult to use hydrogen bonds to bind a sugar. Having the same number of carbon and hydroxyls, the aldohexoses have only minute differences in hydrophobicity, mainly in the axial/equatorial distribution of the hydroxyls. Although the difference has been used successfully to distinguish glucose/glucoside from other monosaccharides,11,15 it is not clear how the other d-aldohexoses can be differentiated in this regard.
Despite these tremendous challenges, literature suggests boronic acid-based covalent receptors could potentially overcome the difficulty. Boronic acid-functionalized molecularly imprinted polymers (MIPs) were reported in the 1970s by Wulff as the stationary phase to separate sugar derivatives by chromatography.16 Monoboronic acids generally bind fructose more strongly than glucose due to its higher percentage of furanose that contains the preferred synperiplanar 1,2-diol for boronate formation.17 This selectivity, interestingly, could be reversed with diboronic acids preorganized to form boronate with the particular hydroxyls of glucose, whether the furanose or pyranose form.18-21 The preorganization comes from the organic scaffold whose structure and conformation dictate how the diboronic acids interact with its guests. Strongest binding is obtained when the maximum number of boronate ester bonds are formed with minimal strain.
The above results suggest that a general method to recognize carbohydrates might result if the number, distance, and orientation of boronic acids on the receptor can be tuned precisely to match the hydroxyl groups on the guest. Such structural control, however, is difficult to imagine given the minute structural differences of the carbohydrates.
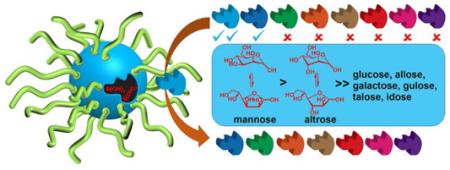
Herein, we report that this level of structural precision can be readily obtained through covalent molecular imprinting within cross-linked micelles. The molecularly imprinted nanoparticles (MINPs) obtained practically could distinguish all eight d-aldohexoses based on the configurations of the hydroxyls. This work lays the foundation for the construction of receptors to bind more complex carbohydrates, since different hydroxyls contributed quite differently to the binding.
With a tripropargylammonium headgroup, surfactant 1 could be cross-linked in the micellar form on the surface by click chemistry with diazide 2 (Scheme 1), to afford alkynyl-functionalized surface-cross-linked micelles (i.e., alkynyl-SCM).22-24 Template 3 was solubilized in water together with DVB and a photoinitiator (DMPA) by 1 in the very beginning. It was prepared from 4-vinylphenylboroxine and glucose according a literature procedure.25 Because the diboronate product contains different isomers (e.g., 3a, 3b, etc.) that undergo transesterification during repeated crystallization, the material obtained from a single crystallization was used without further purification.26 After surface-cross-linking, azide 3 was added to decorate the resulting alkynyl-SCMs with a layer of hydrophilic ligands. Free radical polymerization was subsequently initiated by UV irradiation in the core to polymerize/cross-link the methacrylate of 1, DVB, and the polymerizible styrenyl groups of 3. The doubly cross-linked micelles were recovered by precipitation from acetone. Glucose was removed by repeated washing with acetone/water, methanol/acetic acid, and acetone.27 Template 3 has a free hydroxyl. As demonstrated previously, hydrophilic groups on the template anchor the template near the surface of the micelle/MINP and is helpful to its removal/rebinding.28,29
Scheme 1.
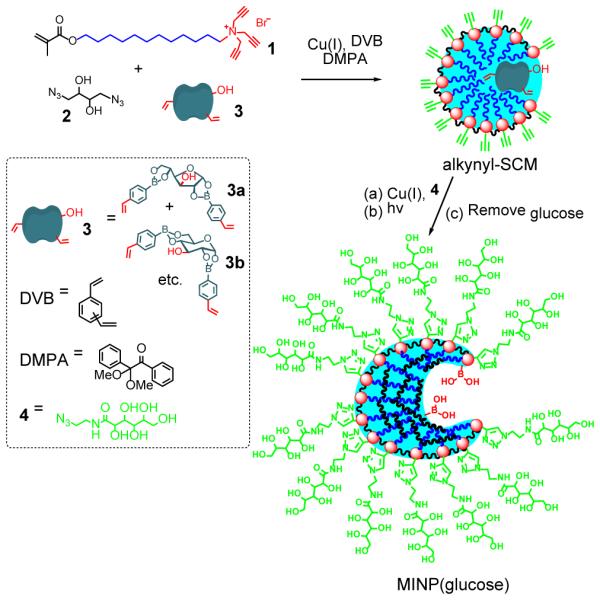
Preparation of boronic acid-functionalized MINP(glucose).
The MINP preparation was adapted from our published procedures.28-31 Cross-linking of the micelles and formation of the MINPs likewise were monitored by 1H NMR spectroscopy and dynamic light scattering (DLS), as shown by Figures S1 and S2 in the Supporting Information. DLS also allowed us to estimate the molecular weight of the nanoparticles. SCMs were characterized previously also by transmission electron microscopy (TEM) and mass spectrometry (after cleaving the surface-cross-linkages).22
To understand the potential of MINPs in carbohydrate recognition, we prepared boronic acid-functionalized receptors first for glucose, mannose, and galactose. Among the eight d-aldohexoses, these three are most biologically relevant, serving as building blocks for many naturally occurring oligo- and polysaccharides. We also prepared MINP(5), using the diboronate derivative of 4-nitrophenyl α-d-mannopyranoside 5 as the template. We are interested in the glycoside because the added hydrophobic aglycan potentially could contribute to the binding. In addition, the glycoside will be bound in the pyranoside form, different from the free sugars.
All the bindings were studied by isothermal titration calorimetry (ITC). The method previously was found to yield similar binding constants (Ka) as fluorescence titration for fluorescently labelled templates.28-31 As shown in Table 1, Ka between MINP(glucose) and glucose was 1.18 × 103 M−1 in 10 mM HEPES buffer at pH 7.4. This binding affinity compares favorably with those between lectins and their monosaccharide ligands (typically 103–104 M−1).1,2 Importantly, the MINP receptor displayed excellent selectivity, showing negligible binding toward other d-aldohexoses except allose (Ka = 0.52 × 103 M−1). Binding with selected non-aldohexoses was very weak, with Ka = 0.06 and 0.003 × 103 M−1 for fructose and xylose, respectively. Thus, covalent imprinting within the cross-linked micelles must have positioned the boronic acids quite accurately to match the hydroxyls on the templating sugar. Our MINPs were prepared with a 1:1 surfactant/DVB ratio. This amount of DVB was the maximum that could be solubilized by the micelle and corresponds to ~50 molecules of DVB per micelle. The high cross-linking density was found previously to be critical to the MINP binding selectivity.28
Table 1.
ITC binding data for boronic acid-functionalized MINPs.a
| Entry | Host | Guest |
Ka
(103M−1) |
−ΔG
(kcal/mol) |
N |
|---|---|---|---|---|---|
| 1 | MINP(glucose) | glucose | 1.18 ± 0.20 | 4.19 | 1.0 ± 0.1 |
| 2 | MINP(glucose) | mannose | 0.003b | ||
| 3 | MINP(glucose) | allose | 0.52 ± 0.02 | 3.7 | 0.9 ± 0.1 |
| 4 | MINP(glucose) | galactose | 0.002b | --b | --b |
| 5 | MINP(glucose) | altrose | 0.004b | --b | --b |
| 6 | MINP(glucose) | gulose | 0.009b | --b | --b |
| 7 | MINP(glucose) | talose | 0.011b | --b | --b |
| 8 | MINP(glucose) | idose | 0.004b | --b | --b |
| 9 | MINP(glucose) | fructose | 0.06 ± 0.01 | 2.4 | 1.0 ± 0.1 |
| 10 | MINP(glucose) | xylose | 0.003b | --b | --b |
| 11 | MINP(glucose) | glucosec | 0.54 ± 0.17 | 3.7 | 1.1 ± 0.1 |
| 12 | MINP(glucose) | glucosed | 1.20 ± 0.30 | 4.2 | 0.6 ± 0.1 |
| 13 | MINP(mannose) | glucose | 0.012b | --b | --b |
| 14 | MINP(mannose) | mannose | 0.94 ± 0.06 | 4.1 | 1.1 ± 0.1 |
| 15 | MINP(mannose) | allose | 0.003b | --b | --b |
| 16 | MINP(mannose) | galactose | 0.003b | --b | --b |
| 17 | MINP(mannose) | altrose | 0.56 ± 0.02 | 3.8 | 0.9 ± 0.1 |
| 18 | MINP(mannose) | gulose | 0.001b | --b | --b |
| 19 | MINP(mannose) | talose | 0.013b | --b | --b |
| 20 | MINP(mannose) | idose | 0.001b | --b | --b |
| 21 | MINP(5) | 5 | 26.3 ± 3.0 | 6.0 | 0.5 ± 0.1 |
| 22 | MINP(5) | 6 | 8.1 ± 0.16 | 5.3 | 1.1 ± 0.1 |
| 23 | MINP(5) | 7 | 4.1 ± 0.15 | 4.9 | 1.1 ± 0.1 |
| 24 | MINP(5) | mannose | 0.80 ± 0.07 | 4.0 | 0.6 ± 0.1 |
The titrations were performed in 10 mM HEPES buffer at pH 7.4. The ITC titration curves are reported in the Supporting Information, including the binding enthalpy and entropy.
Binding was extremely weak. Because the binding constant was estimated from ITC, -ΔG and N are not listed (see SI for details).
The binding was in 10 mM HEPES buffer at pH 6.5.
The binding was in 10 mM HEPES buffer at pH 8.5.
To our delight, the binding selectivity was reproduced when mannose and galactose were used as the templates. MINP(mannose), for example, bound its sugar template with Ka of 0.94 × 103 M−1 and no other d-aldohexoses except altrose (Ka = 0.56 × 103 M−1) (Table 1, entries 13–20; see Chart 1 for structures). MINP(galactose), likewise, only bound its template (Ka = 1.41 × 103 M−1) and one other sugar (gulose, Ka = 0.80 × 103 M−1) among the d-aldohexoses (Table 1S).
Chart 1.
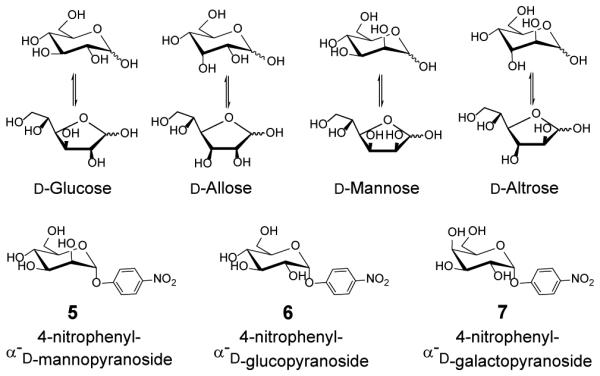
Selected template/guest molecules in this study.
Molecular imprinting has become a very powerful technique to create guest-complimentary materials.32-43 Conventional typical MIPs, however, are intractable highly cross-linked polymers. In our case, because polymerization and cross-linking largely occurred within the boundary of the micelles, the MINPs were completely soluble in water due to their nanosize and hydrophobic/hydrophilic core–shell structure.28-31 Note that most MINPs in Table 1 had a single binding site per nanoparticle on average.44 The number of binding site per nanoparticle is controlled by the surfactant/template ratio used in the MINP preparation and, as demonstrated earlier, is fully tunable if desired.28 This number was determined by the ITC titration from the guest/host ratio.45 Previously, the ITC-determined binding stoichiometry was found to agree well with those determined by the Job plots for fluorescently labeled substrates.46
It is very interesting that a consistent binding selectivity was displayed by all three MINPs. Strong binding for the template sugar was fully expected. Why did the MINP bind only one of the remaining d-aldohexoses? The answer becomes clear when the following trends are considered.
(a) Inversion of two (or more) hydroxyl groups of the templating sugar turns off the binding essentially completely. This trend was seen from glucose to any of the diaxial sugars, and from mannose to allose/galactose/idose. The anomeric hydroxyl at C1 was not considered because it exchanges between the α and β form as the hemiacetal.
(b) The effect of a single hydroxyl inversion depends on the position of the hydroxyl. Consistently for all three MINPs, inversion at either C2 or C4 turned off the binding—e.g., from glucose to mannose/galactose or from mannose to glucose/talose. Inversion at C3, however, weakened the binding by 40–60%, e.g., from glucose to allose, mannose to altrose, or galactose to gulose. As shown by xylose, missing the C6 hydroxyl also caused a complete loss of binding.
When monoboronic acids bind free monosaccharides, literature generally agrees that binding of the first boronic acid occurs through the C1,2 hydroxyls of the sugar.4,47,48 Binding of the second boronic acid, however, could differ depending on the reaction conditions (e.g., aqueous or nonaqueous solvent, solution pH, concentration) and the structure of the boronic acid. The situation is complicated further by pyranose–furanose interconversion. Furanose, having the preferred synperiplanar 1,2-diols for boronate formation,17 is generally the minor component, sometimes representing <1% of the mixture. Nonetheless, two or all three of the C3,5,6 hydroxyls are frequently involved in the second boronate formation for furanose.4 There is also evidence that, for certain sugars, additional conformations (e.g., twist boat) may play roles in the binding, at least in aqueous alkaline media.48
In our case, the hydroxyls at C2, C4, and C6 were critical to the binding and the C3 hydroxyl played a secondary role. The results suggest that, under our conditions, the boronic acids probably bound two pairs of hydroxyls, at C1,2 and C4,6, respectively. Because the boronate template was synthesized in organic solvent under azeotropic distillation, neutral trivalent boronate esters instead of negatively charged tetravalent structures are expected.4
Solution pH has a large effect on the binding of small-molecule boronic acids. A change of pH from 6.5 to 8.5 increased the binding constant between phenyl boronic acid and glucose by over an order of magnitude.49 The pH effect came from the acid–based equilibria involved in the binding and the tetrahedral vs. trigonal forms of boronic acid/boronate. In our case, the same pH change barely had any effect on the MINP binding (compare entries 1, 11, and 12 of Table 1). Presumably, the overall hydrophobicity of the diboronate template means that the boronic acids would reside in a relatively hydrophobic region of the MINP. With poor solvent exposure, neither the boronic acids nor the boronate esters formed after binding would be very sensitive to the solution pH.
4-Nitrophenyl α-d-mannopyranoside 5 should interact with the MINP receptor through hydrophobic interactions, in addition to two boronate esters through the C2,3 and C5,6 hydroxyls.25 After template removal, a binding site is expected to form in the hydrophobic core of the cross-linked micelle, complementary to the 4-nitrophenyl group in size and shape. The hydrophobic imprinting worked very well for many substrates in our previous work.28-31 Consistent with the added hydrophobic interactions, binding between 5 and MINP(5) was over twenty times stronger than those between the sugars and their MINPs (Table 1, entry 21). Encouragingly, significant selectivity was found for the binding of similar glycosides. Glucopyranoside 6 was bound by MINP(5) with <1/3 of the binding affinity and mannopyranoside 7 with ~1/6. Apparently, inversion of one or two hydroxyl groups could still be easily distinguished, even for the pyranosides. Lastly, MINP(5) was also able to bind mannose with Ka = 0.5 × 103 M−1 (entry 24). The binding constant was similar to that between mannose and its own MINP (entry 14). Thus, the binding site imprinted from the mannopyranoside was able to bind mannose, most likely in the pyranose form.
The most significant discovery of this research is the general applicability of the MINP receptors, which are completely water-soluble and similar to proteins in size. It is very important that the MINP receptors displayed a very clear and consistent trend in the binding, mainly controlled by the C2, C4, and C6 hydroxyls of the d-aldohexoses. The milli- and submillimolar binding affinities already approached those found in natural lectins for monosaccharides. The observed binding selectivity suggests that the cross-linked micelles can be used as a platform to precisely position and orient binding groups even to distinguish minute structural changes in carbohydrates. If the same holds true for oligo- and polysaccharides, a general method for selective binding of carbohydrates will become available.
Supplementary Material
ACKNOWLEDGMENT
We thank the National Institute of General Medical Sciences of the National Institutes of Health (R01GM113883) for financial support of this research.
Footnotes
Supporting Information
Experimental details, ITC titration curves, and additional data. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interests.
REFERENCES
- (1).Kamerling JP, Boons G-J. Comprehensive glycoscience : from chemistry to systems biology. 1st Elsevier; Amsterdam ; Boston: 2007. [Google Scholar]
- (2).Wang B, Boons G-J. Carbohydrate recognition: biological problems, methods, and applications. Wiley; Hoboken, N.J.: 2011. [Google Scholar]
- (3).Jin S, Cheng Y, Reid S, Li M, Wang B. Med. Res. Rev. 2010;30:171. doi: 10.1002/med.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).James TD, Phillips MD, Shinkai S. Boronic acids in saccharide recognition. RSC Publishing; Cambridge: 2006. [Google Scholar]
- (5).Kim KT, Cornelissen JJLM, Nolte RJM, van Hest JCM. J. Am. Chem. Soc. 2009;131:13908. doi: 10.1021/ja905652w. [DOI] [PubMed] [Google Scholar]
- (6).Pal A, Bérubé M, Hall DG. Angew. Chem. Int. Ed. 2010;49:1492. doi: 10.1002/anie.200906620. [DOI] [PubMed] [Google Scholar]
- (7).Wu X, Li Z, Chen X-X, Fossey JS, James TD, Jiang Y-B. Chem. Soc. Rev. 2013;42:8032. doi: 10.1039/c3cs60148j. [DOI] [PubMed] [Google Scholar]
- (8).Bull SD, Davidson MG, Van den Elsen JMH, Fossey JS, Jenkins ATA, Jiang YB, Kubo Y, Marken F, Sakurai K, Zhao JZ, James TD. Acc. Chem. Res. 2013;46:312. doi: 10.1021/ar300130w. [DOI] [PubMed] [Google Scholar]
- (9).Ferrand Y, Crump MP, Davis AP. Science. 2007;318:619. doi: 10.1126/science.1148735. [DOI] [PubMed] [Google Scholar]
- (10).Rauschenberg M, Bomke S, Karst U, Ravoo BJ. Angew. Chem. Int. Ed. 2010;49:7340. doi: 10.1002/anie.201002847. [DOI] [PubMed] [Google Scholar]
- (11).Ke C, Destecroix H, Crump MP, Davis AP. Nat. Chem. 2012;4:718. doi: 10.1038/nchem.1409. [DOI] [PubMed] [Google Scholar]
- (12).Kobayashi K, Asakawa Y, Kato Y, Aoyama Y. J. Am. Chem. Soc. 1992;114:10307. [Google Scholar]
- (13).James TD, Sandanayake KRAS, Shinkai S. Angew. Chem. Int. Ed. 1996;35:1910. [Google Scholar]
- (14).Davis AP, James TD. Carbohydrate Receptors. Wiley-VCH Verlag GmbH & Co. KGaA; 2005. [Google Scholar]
- (15).Barwell NP, Crump MP, Davis AP. Angew. Chem. Int. Ed. 2009;48:7673. doi: 10.1002/anie.200903104. [DOI] [PubMed] [Google Scholar]
- (16).Wulff G, Vesper W. J. Chromatogr. 1978;167:171. [Google Scholar]
- (17).Lorand JP, Edwards JO. J. Org. Chem. 1959;24:769. [Google Scholar]
- (18).James TD, Sandanayake KRAS, Shinkai S. Angew. Chem. Int. Ed. Engl. 1994;33:2207. [Google Scholar]
- (19).Eggert H, Frederiksen J, Morin C, Norrild JC. J. Org. Chem. 1999;64:3846. [Google Scholar]
- (20).Yang W, He H, Drueckhammer DG. Angew. Chem. Int. Ed. 2001;40:1714. [PubMed] [Google Scholar]
- (21).Karnati VV, Gao X, Gao S, Yang W, Ni W, Sankar S, Wang B. Bioorg. Med. Chem. Lett. 2002;12:3373. doi: 10.1016/s0960-894x(02)00767-9. [DOI] [PubMed] [Google Scholar]
- (22).Zhang S, Zhao Y. Macromolecules. 2010;43:4020. [Google Scholar]
- (23).Zhang S, Zhao Y. J. Am. Chem. Soc. 2010;132:10642. doi: 10.1021/ja103391k. [DOI] [PubMed] [Google Scholar]
- (24).Zhao Y. Langmuir. 2016;32:5703. doi: 10.1021/acs.langmuir.6b01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Wulff G, Schauhoff S. J. Org. Chem. 1991;56:395. [Google Scholar]
- (26). Having a mixture of different isomers for the diboronate template is not expected to affect the molecular imprinting and binding of the MINP receptors. A binding pocket site will contibute positively to the binding as long as the boronic acids in the site can bind any isomer (furanose, pyranose, or other forms) of the sugar.
- (27). As seen from the ITC binding studies, boronate esters equilibrate rapidly in protic solvents. The fact that most MINPs prepared in this study had an average of one binding site per nanoparticle suggests that the washing conditions were sufficient for the template removal.
- (28).Awino JK, Zhao Y. J. Am. Chem. Soc. 2013;135:12552. doi: 10.1021/ja406089c. [DOI] [PubMed] [Google Scholar]
- (29).Awino JK, Zhao Y. Chem.-Eur. J. 2015;21:655. doi: 10.1002/chem.201404919. [DOI] [PubMed] [Google Scholar]
- (30).Awino JK, Zhao Y. Chem. Commun. 2014;50:5752. doi: 10.1039/c4cc01516a. [DOI] [PubMed] [Google Scholar]
- (31).Awino JK, Zhao Y. ACS Biomater. Sci. Eng. 2015;1:425. doi: 10.1021/acsbiomaterials.5b00042. [DOI] [PubMed] [Google Scholar]
- (32).Wulff G. Angew. Chem. Int. Ed. Engl. 1995;34:1812. [Google Scholar]
- (33).Wulff G. Chem. Rev. 2001;102:1. doi: 10.1021/cr980039a. [DOI] [PubMed] [Google Scholar]
- (34).Haupt K, Mosbach K. Chem. Rev. 2000;100:2495. doi: 10.1021/cr990099w. [DOI] [PubMed] [Google Scholar]
- (35).Ye L, Mosbach K. Chem. Mater. 2008;20:859. [Google Scholar]
- (36).Shea KJ. Trends Polym. Sci. 1994;2:166. [Google Scholar]
- (37).Sellergren B. Molecularly imprinted polymers: man-made mimics of antibodies and their applications in analytical chemistry. Elsevier; Amsterdam: 2001. [Google Scholar]
- (38).Komiyama M. Molecular imprinting: from fundamentals to applications. Wiley-VCH; Weinheim: 2003. [Google Scholar]
- (39).Yan M, Ramström O. Molecularly imprinted materials: science and technology. Marcel Dekker; New York: 2005. [Google Scholar]
- (40).Alexander C, Andersson HS, Andersson LI, Ansell RJ, Kirsch N, Nicholls IA, O'Mahony J, Whitcombe MJ. J. Mol. Recognit. 2006;19:106. doi: 10.1002/jmr.760. [DOI] [PubMed] [Google Scholar]
- (41).Sellergren B, Hall AJ. Supramol Chem. John Wiley & Sons, Ltd; 2012. [Google Scholar]
- (42).Haupt K, Ayela C. Molecular Imprinting. Springer; Heidelberg ; New York: 2012. [Google Scholar]
- (43).Zimmerman SC, Lemcoff NG. Chem. Commun. 2004:5. doi: 10.1039/b304720b. [DOI] [PubMed] [Google Scholar]
- (44).(a) Zimmerman SC, Wendland MS, Rakow NA, Zharov I, Suslick KS. Nature. 2002;418:399. doi: 10.1038/nature00877. Single-sited receptors have been obtained by molecular imprinting within dendrimers, see: [DOI] [PubMed] [Google Scholar]; (b) Zimmerman SC, Zharov I, Wendland MS, Rakow NA, Suslick KS. J. Am. Chem. Soc. 2003;125:13504. doi: 10.1021/ja0357240. [DOI] [PubMed] [Google Scholar]
- (45).Schmidtchen FP. Supramol Chem. John Wiley & Sons, Ltd; 2012. [Google Scholar]
- (46).Awino JK, Hu L, Zhao Y. Org. Lett. 2016;18:1650. doi: 10.1021/acs.orglett.6b00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Norrild JC, Eggert H. J. Am. Chem. Soc. 1995;117:1479. [Google Scholar]
- (48).Nicholls MP, Paul PKC. Org. Biomol. Chem. 2004;2:1434. doi: 10.1039/b312760e. [DOI] [PubMed] [Google Scholar]
- (49).Yan J, Springsteen G, Deeter S, Wang B. Tetrahedron. 2004;60:11205. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


