Abstract
Anopheles arabiensis Patton (Diptera: Culicidae) is considered the most efficient malaria vector in eastern Sudan. This study aims to characterize the breeding sites of An. arabiensis throughout the year in and around Kassala town, eastern Sudan. Diverse larval habitat types were visited and characterized based on the habitat type and chemical composition. Mosquito larvae were found in many diverse habitats. During the rainy season, rain pools and water bodies created by the seasonal Gash River serve as the main breeding sites. In the dry season, irrigation canals, seepage from water pipes, neglected wells, artificial containers, and man-made ditches serve as the main breeding sites. Breeding water showed a pH of 7.9 and a low concentration of the total dissolved salts. The results of this study may be considered in planning and implementing larval control programs in the area.
Keywords: Anopheles arabiensis, mosquito, malaria vector, breeding sites, Kassala town, eastern Sudan
Introduction
Anopheles arabiensis Patton (Diptera: Culicidae), a member of the Anopheles gambiae complex,1,2 is considered the dominant and most dangerous malaria vector in Sudan. It is being reported from all parts of Sudan, East Africa3 and distributed over the dry savanna belt and semiarid parts of the country. It has been reported from many localities in Kassala State, eastern Sudan.4–8
Anopheles mosquitoes generally breed in different types of water and in a wide range of larval habitats that may be natural or human made, shaded or sunny, or temporary or permanent.9 Most anopheline mosquitoes breed in static freshwater pools and each species being associated with particular environmental conditions.10 Certain environmental parameters are particularly influential in determining aquatic habitats suitability for the different anopheline vectors, including size and permanence for the water body, intensity of sunlight, water salinity, and turbidity, and presence of emergent or floating vegetation.11 Knowledge of the ecological characteristics of the mosquito breeding sites and the environmental factors affecting mosquito abundance can help in designing vector control programs.12,13 The type of habitat should be considered for effective larval control and most productive habitat type should be prioritized in the mosquito abatement program.14
The water surfaces in which mosquitoes breed vary in their chemical contents. This may be attributed to (1) the salt contents of the soil in which they are present, (2) the type and amount of fallen leaves debris or decayed organic materials, and (3) the source and previous uses of the water. The chemical nature of larval breeding site has a direct impact on the selection by gravid female mosquitoes, growth and development of immature stages, and hence mosquito distribution.15
In eastern Sudan, malaria is mesoendemic over years,16,17 and it remains the leading cause of morbidity with an incidence rate of 14 cases per 10,000 populations in Kassala State.18 The vector control remains as the best approach for protecting the community against malaria.19 Investigating and detecting the mosquito breeding sites in a particular area is important for planning and deciding the best vector control programs. Although malaria is a major public health problem and anopheline larval control is an integral part of malaria vector management in Kassala town, little is known about the larval habitats ecology of Anopheles mosquitoes in and around the town. For the first time, we conducted a preliminary investigation of An. arabiensis’s larval habitats in and around Kassala town in order to assign a baseline data of larval habitats ecology of this important malaria vector in the area. The study aims to record and describe visually the types of An. arabiensis breeding sites based on habitat origin and stability and to estimate their chemical composition. These findings will be helpful in planning prevention and control activities against this important malaria vector in eastern Sudan.
Materials and Methods
The study area
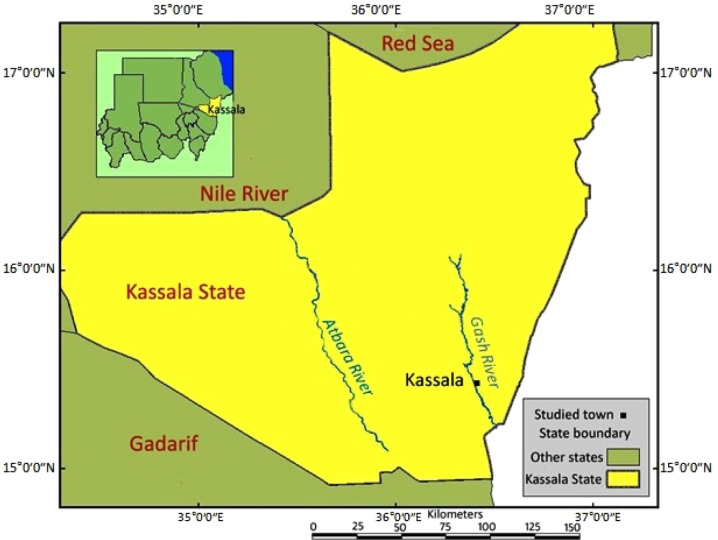
The study was carried out in and around Kassala town (15° 28′ N and 36° 24′ E), eastern Sudan (Fig. 1). The town is located under arid and semiarid climate with rainfall of varying intensity and duration and characterized by the presence of three seasons, namely, the cool dry season (November–February), the hot dry season (March–June), and the short rainy season that extends from mid-June to September with an average annual rainfall of 410 mm. January is the coolest month of the year with a mean temperature of 25°C. May is the hottest month of the year with a mean temperature of 33°C.20
Figure 1.
A map shows the study area, Kassala town, eastern Sudan.
Note: UNDP data, 2008 & Landsat image, 2006.
Kassala area has nearly a flat topography characterized by the presence of the backbones of mountains surrounding the eastern part. The most important water course in the area is the seasonal Gash River that originates in the Ethiopian high lands and flows across the flat plain during the rainy season. The soil consists mainly of clays or sandy clays, surrounding the basements of the hills. The availability of water in this semidesert environment supports the horticultural farms of vegetables and fruits, which are distributed along the riverbanks of Gash River.
Detection of the types of An. arabiensis breeding sites
Detection of the types of An. arabiensis breeding sites in and around Kassala town took place during the period (September 2000 to January 2002), including the dry and rainy seasons. Diverse sites were visited, including the surrounding farms, wells, and mountains, and the presence of larval An. arabiensis mosquitoes were detected by collection methods such as dipping or netting.21 Larvae of An. arabiensis were identified according to the morphological identification key.22 During the survey, a habitat was first investigated for the presence of mosquito larvae visually, when the mosquito larvae were present (at least one larva), the aquatic habitat was recorded as a type of An. arabiensis breeding habitats. Photographs of each larval habitat type were taken.
Environmental characteristics of An. arabiensis larval habitats
For each larval habitat type, two physical characteristics, including origin of habitat or habitat type (natural or human made), and habitat stability (permanent, semipermanent, or temporary), were estimated and recorded visually. Permanent habitats were considered as those that remained during the dry season, and temporary habitats were considered as those that appear only during the rainy season.
For chemical analysis of An. arabiensis breeding water, a representative sample of breeding site near a main water work tank (where water pools were created by the running water) was selected. The selected breeding site was characterized by its permanent productivity of larval stages and was not subjected to any antilarval control during the study period. Water sample was collected during the rainy season of 2001. The first water sample was filtered through a fine mesh screen (0.5–1 mm) to remove debris and aquatic organisms and then placed in a plastic bottle, which is tightly closed and transported to the Hydrology Unit, Ministry of Irrigation and Water resources, at Kassala town for chemical analysis.
The sample was analyzed for concentration of hydrogen ions (pH), total dissolved solid, total ionic conductivity, and ions such as ammonia, nitrates, nitrites, sulfate, fluoride, iron (were determined using a spectrophotometer; Drill 5), magnesium, calcium, bicarbonate, chloride (were measured via titration), and sodium. The pH and conductivity were determined using electronic conductivity (Ec)/pH meter. Chemical indicators of water quality were measured according to the procedures in the standard methods for examination of water and waste water.
Results and Discussion
Description of An. arabiensis larval habitat types
Five diverse larval habitat types were identified in the area. Two natural habitats (rain pools and seasonal Gash River pools) were temporary breeding sites and restricted to the rainy season. The other three larval habitat types were human made (irrigation canals, man-made ditches, and artificial containers) were the most permanent breeding sites throughout the year.
Temporary natural habitats during the rainy season
Rain pools: Rain pools were the main breeding sites of An. Arabiensis in and around Kassala town during the rainy season. Rain pools are standing freshwater collections that are formed after heavy rain falls. In these pools, water remains stagnant for more than one month. Rain pools include pools in plain (in ground depressions; Fig. 2A) and rock pools formed in rock depressions or in pits naturally occurring between rocks in the surrounding mountains (Fig. 2B). Previous studies in the Ethiopian Rift Valley have shown that An. arabiensis larvae preferred seasonal habitats such as sand pools, brick-making pits, and rain pools.23
Figure 2.
Temporary natural breeding sites of An. arabiensis during the short rainy season: (A) rain pool, (B) rock pool in Kassala mountains, (C) residual pool in the seasonal Gash riverbed, and (D) pool left over by the receding water of the seasonal Gash River.
Water bodies created by the seasonal Gash River: Immature stages of An. arabiensis were found in different types of pools formed by water of the Gash River and its tributaries: residual pools formed in the riverbed after the end of the flood season when water flow stops (Fig. 2C) and riverside pools created as a result of seeping of river water in nearby man-made ditches or in depressions in the ground (Fig. 2D). Usually, they are lower than the level of the river and formed during the flood season only. These pools vary in size from very small ditches to large pools in which water was retained for about one to three months after the end of the flood season and creates suitable sites for mosquito breeding.
Temporary human-made breeding sites during the rainy season
Human-made ditches: Human activities make water bodies suitable for mosquito breeding, eg, man-made ditches used by the inhabitants for building purposes (Fig. 3A), puddles left on the ground by wheel tracks of vehicles (Fig. 3B), and man-made ditches used for the collection of rainwater during the rainy season. Rainwater was collected in these ditches during the rainy season. In the later pools, water remains for about six months after the end of the rainy season, and it is used by the nomadic people for obtaining water for household purposes and for drinking for their domestic animals during the dry season.
Figure 3.
Temporary human-made breeding sites of An. arabiensis during the rainy season. (A) Human-made ditches used by the inhabitants for building purposes. (B) Puddles left on the ground by vehicles wheel tracks.
Permanent human-made habitats
Water from irrigation canals: Agricultural practices in the horticultural farms provided the species with excellent opportunities to breed throughout the year. Between two successive irrigation operations, a number of puddles were formed. These puddles formed from the stagnant water that was left in the beds of the earth lined parts of the irrigation canals (Fig. 4A). The seepage or overflow of water from irrigation canals due to incomplete or bad construction results in the formation of puddles. Puddles also formed near the irrigation canals as a result of misuse of water sources by the inhabitants or by animals. Neglected wells, which are scattered throughout the horticulture farms, were found to serve as ideal breeding sites for the mosquito species throughout the year (Fig. 4B).
Figure 4.
Permanent human-made breeding sites of An. arabiensis during the dry season. (A) Irrigation canal, (B) neglected well, (C) artificial cement container, (D) artificial metal barrel container, (E) leakages of drinking water pipe, and (F) pools close to a water tank.
Artificial containers: Immature stages of An. arabiensis were found in a cement container (Fig. 4C) and in a metal barrel used for keeping water for household purposes (Fig. 4D). Other breeding sites include leakages of drinking water pipes (Fig. 4E). Also pools close to water tanks (Fig. 4F) were found to harbor aquatic stages of An. arabiensis. These pools were formed by water collecting on the ground due to continuous flow of water as a result of misuse of water closing devices by the inhabitants.
Dry season larval habitat types (broken water pipes, leakages from irrigation canals, and water excavations for human and animal use) were previously reported from the New Halfa town, eastern Sudan.6 The inefficient irrigation system in the cultivated areas gave rise to a complicated health situation by spreading breeding sites, particularly during the dry season. Improvement of these systems would check and reduce mosquito breeding sites. Artificial larval habitats are more important for anopheline mosquito breeding because these habitats prevent water flow and create pools that offer ideal situation for the mosquito proliferation.24 Community participation is necessary to facilitate integrated control of the disease and decreases vector populations by reducing artificial and man-made breeding sites. Water bodies resulting from leakage of pipes formed important breeding sites in the area during dry season. A better maintenance of the pipes network will be an excellent measure to decrease mosquito populations.
The dry season larval habitats that are responsible for continuous production of the adult vectors throughout most of the year need to be considered in larval vector control operations. So that antilarval measures against them are very well suited during the dry season, thus reducing the overall mosquito population before the increased larval habitats during the rainy season.23
Larval habitat characterizations
Chemical analysis of breeding water of An. arabiensis showed a pH of 7.9, total ionic conductivity of 1049 mho/cm at 28°C, and a low concentration of the total dissolved salts (734.3 mg/L). Ammonia (3.29 mg/L), nitrates (83.6 mg/L), and bicarbonate ions (366 mg/L) were found in comparatively high concentrations. Fluoride (0.85 mg/L), chloride (83.78 mg/L), nitrites (0.413 mg/L), sulfate (60 mg/L), iron (0.45 mg/L), calcium (35.2 mg/L), magnesium (15.52 mg/L), and sodium ions (151.34 mg/L) were found in comparatively low concentrations.
The high concentrations of ammonia and nitrates were due to the presence of animal waste. The high concentrations of nitrogen, ammonia, and sodium and calcium carbonate accounted for low populations of An. arabiensis. On the other hand, magnesium, aluminum, chloride, potassium, calcium, and sulfate showed no obvious effect on mosquito abundance in Khartoum area.25 Vrtiska and Pappas15 ascribed the high potassium levels in some breeding sites to the amount of leaves present, as potassium is the major inorganic component of plants. Such variations have encouraged many ecologists to analyze mosquito breeding water to correlate their chemical composition with mosquito abundance. Tadesse et al26 found that dissolved oxygen, pH, water transparency, and rainfall were positively associated and important in explaining the presence and abundance of Anopheles mosquitoes. Gouagna et al27 reported the occurrence of An. arabienisis larvae in man-made and natural habitats with a pH of 8.5 and 7.8, respectively.
Knowledge of larval habitat and their distribution would be an important step for planning and implementing larval control strategies effectively. A limitation of this study is not measuring the larval habitat productivity, which could play a role in the results obtained.
Conclusion
This study demonstrates the diversity of larval habitat types of An. arabiensis in Kassala town, eastern Sudan. The information on the characteristics of these larval habitats will be useful in designing malaria control programs in the area. Further detailed study of the breeding habitats of this important malaria vector is recommended, including all the environmental variables using accurate quantitative measurements.
Acknowledgments
The authors thank the staff of Malaria Center of Kassala Unit, Kassala State, for their assistance in the field work. They also thank Ms Balghies Mohamed Ahmed of the Hydrology Unit, Ministry of Irrigation and Water resources, Kassala State, for carrying out the water analysis.
Footnotes
ACADEMIC EDITOR: Paul-Andre Calatayud, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 2,970 words, excluding any confidential comments to the academic editor.
FUNDING: This study was supported by a grant from Kassala University, Sudan. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Carried out the entomological survey and drafted the manuscript: AMH. Contributed to the reading and revising the manuscript: EAER. All the authors read and approved the submitted version of the manuscript.
REFERENCES
- 1.Service MW. Anopheles gambiae: Africa’s principal malaria vector 1902–1984. Bull Entomol Soc Am. 1985;31:8–12. [Google Scholar]
- 2.Hunt RH, Coetzee M, Fettene M. The Anopheles gambiae complex: a new species from Ethiopia. Trans R Soc Trop Med Hyg. 1998;92:231–235. doi: 10.1016/s0035-9203(98)90761-1. [DOI] [PubMed] [Google Scholar]
- 3.Petrarca V, Nugud AD, Ahmed MAH, Haridi AM, Di Deco MA, Coluzzi M. Cytogenetics of the Anopheles gambiae complex in Sudan with special reference to An. arabiensis: relationships with East and West African populations. Med Vet Entomol. 2000;14:149–164. doi: 10.1046/j.1365-2915.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- 4.Haridi AM. Partial exophily of Anopheles gambiae species B in Khashm El-Girba area in eastern Sudan. Bull World Health Org. 1972;46:39–46. [PMC free article] [PubMed] [Google Scholar]
- 5.Petrarca V, Nugud AD, Ahmed MAH, Haridi AM, Abd-El-Nur OM, Coluzzi M. Dati Preliminari sul complesso Anopheles gambiae in Sudan. Parasitologia. 1986;28:304–306. [Google Scholar]
- 6.Himeidan YE, Dukeen MY, El-Rayah E-LA, Adam I. Anopheles arabiensis: abundance and insecticide resistance in an irrigated area of eastern Sudan. East Mediterr Health J. 2004;10:167–174. [PubMed] [Google Scholar]
- 7.Hamza AM, Himeidan YE, Adam I, El-Rayah EL-A. The ecology of Anopheles arabiensis and insecticide resistance/susceptibility status in Kassala area, eastern Sudan. Gezira J Health Sci. 2005;1(2):46–54. [Google Scholar]
- 8.Hamza AM, El-Rayah EL-A, Abukashawa SM. Molecular characterization of mosquitoes of Anopheles gambiae species complex (Diptera: Culicidae) from Sudan and Republic of Southern Sudan. J Mosquito Res. 2014;4(13):1–10. [Google Scholar]
- 9.Machault V, Gadiaga L, Vignolles C, et al. Highly focused anopheline breeding sites and malaria transmission in Dakar. Malar J. 2009;8:138. doi: 10.1186/1475-2875-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White GB. Malaria vector ecology and genetics. Br Med Bull. 1982;38:207–212. doi: 10.1093/oxfordjournals.bmb.a071760. [DOI] [PubMed] [Google Scholar]
- 11.Service MW. The importance of ecological studies on malaria vectors. Bull Soc Vector Ecol. 1989;14:26–38. [Google Scholar]
- 12.Overgaard HJ, Tsuda Y, Suwonkerd W, Takagi M. Characteristics of Anopheles minimus (Diptera: Culicidae) larval habitat in northern Thailand. Env Entomol. 2001;10(1):134–141. [Google Scholar]
- 13.Surendran SN, Ramasamy R. Some characteristics of the larval breeding sites of Anopheles culicifacies species B and E in Sri Lanka. J Vector Borne Dis. 2005;42(2):39–44. [PubMed] [Google Scholar]
- 14.Ijumba JN, Lindsay SW. Impact of irrigation on malaria in Africa: paddies paradox. Med Vet Entomol. 2001;15:1–20. doi: 10.1046/j.1365-2915.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 15.Vrtiska LA, Pappas LG. Chemical analysis of mosquito larval habitats in southern Nebraska. Mosquito News. 1984;44(4):506–509. [Google Scholar]
- 16.Vande Waa JA, Jensen JB, Akood MA, Bayoumi R. Longitudinal study on the in vitro immune response to Plasmodium falciparum in Sudan. Infect Immunol. 1984;45(2):505–510. doi: 10.1128/iai.45.2.505-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Hassan IM, Hviid L, Jakobson PH, et al. High proportion of subclinical Plasmodium falciparum infections in an area of seasonal and unstable malaria in Sudan. Am J Trop Med Hygiene. 1995;53:78–83. [PubMed] [Google Scholar]
- 18.WHO . Emergency Preparedness and Humanitarian Action (EHA) Sudan: Sudan Health Highlights; 2011. [Google Scholar]
- 19.WHO A global strategy for malaria control. Bull World Health Org. 1993;71:281–284. [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Energy and Mining . N. A. F. W. Water resources assessment. 1989. (Kassala Report, Gash Basin, Final Technical Report). [Google Scholar]
- 21.WHO . Manual on Practical Entomology in Malaria: Part II, Methods and Techniques. Vol. 13. Geneva, Switzerland: WHO Offset Publications; 1975. [Google Scholar]
- 22.Gillies MT, De-Mellion B. The Anophelinae of Africa South of the Sahara. Johannesburg, South Africa: South African Institute for Medical Research; 1968. [Google Scholar]
- 23.Kenea O, Balkew M, Gebre-Michael T. Environmental factors associated with larval habitats of anopheline mosquitoes (Diptera: Culicidae) in irrigation and major drainage areas in the middle course of the Rift Valley, central Ethiopia. J Vector Borne Dis. 2011;48:85–92. [PubMed] [Google Scholar]
- 24.Soleimani-Ahmadi M, Vatandoost H, Hanafi-Bojd A, et al. Environmental characteristics of anopheline mosquito larval habitats in a malaria endemic area in Iran. Asian Pac J Trop Med. 2013;6(7):510–515. doi: 10.1016/S1995-7645(13)60087-5. [DOI] [PubMed] [Google Scholar]
- 25.Abu-Groon NAM. Studies on the Biology and Population Dynamics of Mosquitoes of Khartoum [Ph D thesis] Sudan: Department of Zoology, Faulty of Science, University of Khartoum; 1988. [Google Scholar]
- 26.Tadesse D, Mekonnen Y, Tsehaye A. Characterization of mosquito breeding sites in and in the vicinity of Tigray microdams. Ethiop J Health Sci. 2011;21(1):57–66. doi: 10.4314/ejhs.v21i1.69045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gouagna LC, Rakotondranary M, Boyer SM, Lempérière G, Dehecq J, Fontenille D. Abiotic and biotic factors associated with the presence of Anopheles arabiensis immatures and their abundance in naturally occurring and man-made aquatic habitats. Parasit Vectors. 2012;5:96. doi: 10.1186/1756-3305-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]