Abstract
Rat liver tryptophan (Trp), kynurenine pathway metabolites, and enzymes deduced from product/substrate ratios were assessed following acute and/or chronic administration of kynurenic acid (KA), 3-hydroxykynurenine (3-HK), 3-hydroxyanthranilic acid (3-HAA), Trp, and the kynureni-nase inhibitors benserazide (BSZ) and carbidopa (CBD). KA activated Trp 2,3-dioxygenase (TDO), possibly by increasing liver 3-HAA, but inhibited kynurenine aminotransferase (KAT) and kynureninase activities with 3-HK as substrate. 3-HK inhibited kynureninase activity from 3-HK. 3-HAA stimulated TDO, but inhibited kynureninase activity from K and 3-HK. Trp (50 mg/kg) increased kynurenine metabolite concentrations and KAT from K, and exerted a temporary stimulation of TDO. The kynureninase inhibitors BSZ and CBD also inhibited KAT, but stimulated TDO. BSZ abolished or strongly inhibited the Trp-induced increases in liver Trp and kynurenine metabolites. The potential effects of these changes in conditions of immune activation, schizophrenia, and other disease states are discussed.
Keywords: kynurenine pathway, kynurenine aminotransferase, kynureninase, tryptophan 2, 3-dioxygenase
Introduction
The essential amino acid L-tryptophan (Trp) is metabolized by four known pathways, the quantitatively most important of which is the hepatic kynurenine (K) pathway (KP)! (Fig. 1), as it accounts for at least ∼90% of overall disposal of dietary Trp under normal physiological conditions.2–4 The extrahepatic KP contributes little to Trp degradation under normal conditions, but plays a major role after immune activation.4 This latter feature has provided the stimulus for intense research interest in the KP in recent years with the demonstration of the immunosuppressive properties of some of its intermediates, notably 3-hydroxykynurenine (3-HK), 3-hydroxyanthranilic acid (3-HAA), and quinolinic acid (QA), in pregnancy and defense against infection,4–7 and the involvement of these and other kynurenine metabolites (Ks) in a variety of neurological disorders.8,9
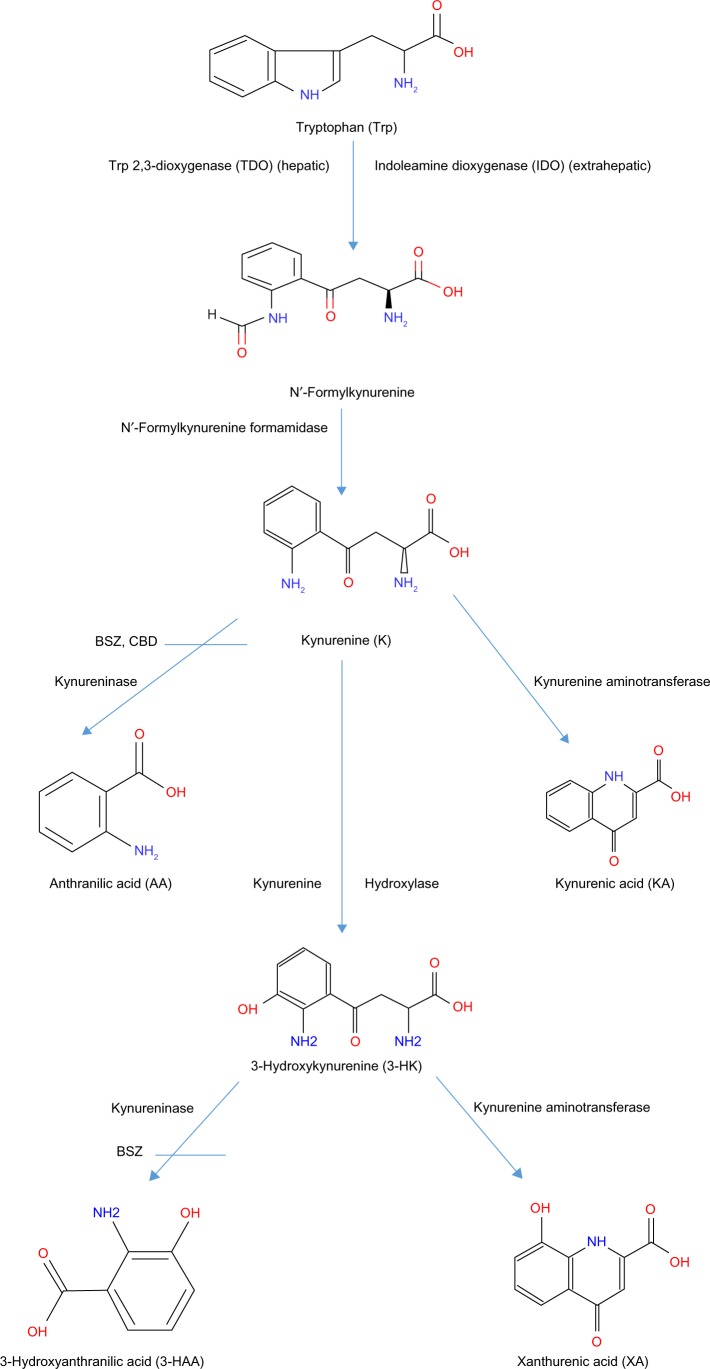
Figure 1.
The hepatic kynurenine pathway of tryptophan degradation up to the kynureninase step. Abbreviations are in parentheses. Benserazide (BSZ) and carbidopa (CBD) are shown as kynureninase inhibitors. Adapted from Ref. 1. Badawy AA-B, Bano S. Elevation of kynurenine metabolites in rat liver and serum: a potential additional mechanism of the alcohol aversive and anti-cancer effects of disulfiram? Alcohol Alcohol. 2016;51:20–5.1
Most previous studies of the hepatic KP have focused on changes in the first and rate-limiting enzyme Trp 2, 3-dioxygenase (TDO, formerly Trp pyrrolase: EC 1.13.11.11) and some subsequent enzymes in the pathway induced by Trp, various drugs and other chemicals, and nutritional deficiencies. The potential effects of intermediates of the KP on the hepatic metabolism of Trp have received little attention. Investigating such effects could throw light on feedback control mechanisms of the pathway and the potential modulation of intermediates in pathological conditions associated with elevated Ks. We have previously reported that 3-HK, 3-HAA, and kynurenic acid (KA) induce aversion to alcohol by elevating blood acetaldehyde levels secondary to inhibition of liver aldehyde dehydrogenase activity.10 Aversion to alcohol by the same mechanism and mediated by 3-HK was also demonstrated by combined administration of Trp and the kynureninase inhibitor benserazide (BSZ).11 In these latter two studies, hepatic levels of KA, 3-HK, and 3-HAA were reported after their administration,10 as was 3-HK after administration of BSZ or the other kynureninase inhibitor carbidopa (CBD) in combination with Trp.11 In the present paper, we report the effects of the above three kynurenine metabolites, and also BSZ, CBD, and Trp, on hepatic Trp metabolism by the KP.
Materials and Methods
Chemicals and materials
Trp, 3-HK, 3-HAA, KA, BSZ [(DL-serine 2(2,3,4-trihydroxybenzyl) hydrazine hydrochloride], CBD [s(S)-3-(3,4-dihydroxyphenyl)-2-hydrazino- 2-methylpropionic acid], and other kynurenine metabolites were purchased from the Sigma-Aldrich Co Ltd. and were stored as directed by the manufacturer. Water and methanol (high-performance liquid chromatography: HPLC grade) were purchased from either VWR International or Fisher Scientific. Acids and alkalis of the purest commercially available grades were purchased from VWR International and were made up in HPLC-grade water. Filtration, Eppendorf, and other tubes were purchased from Fisher or other standard suppliers.
Animals and treatments
Adult normal male Wistar rats weighing between 150 and 170 g at the start of experiments were purchased from accredited animal suppliers and were acclimatized to our standard UK Home Office-approved housing conditions (21 ± 2 °C, relative humidity 55 ± 10%, and a 12-hour/12-hour light/dark cycle) for at least one week before experiments. They were housed five per cage in conventional open-top cages with standard softwood bedding from accredited suppliers, and were allowed free access to standard laboratory RM1 diet and water. This study was performed under the auspices of Cardiff University and approved and licensed (PPL 30/2502) by the UK Home Office under the Animal (Scientific Procedures) Act 1986. All compounds were administered intra-peritoneally in 0.9% (w/v) NaCl (physiological saline). 3-HK, 3-HAA, and KA were given in single doses of 1–10 mg/kg body weight, whereas Trp was given in a 50 mg/kg dose. The kynureninase inhibitors BSZ and CBD were given in single doses of 100 and 50 mg/kg, respectively. When given repeatedly for seven days, Trp and BSZ were given in the above single doses once daily, whereas 3-HK, 3-HAA, and KA were given in a single daily 10 mg/kg dose each.
Laboratory procedures
Trp and its K metabolites were determined in liver and serum by our rapid isocratic HPLC procedure.12 Briefly, a Perkin Elmer LC200 system, consisting of a quaternary pump, a column oven, and a degasser, was used with ultraviolet and fluorimetric detection in series. The mobile phase was a methanol/sodium dihydrogen phosphate mixture (27:73 by vol.) at a final pH of 2.0 or 2.8. The system was run isocratically using a Synergi 4 μ reverse-phase Fusion-RP80 A column (250 × 4.6 mm) with a guard column (Phenomenex). Operation of the system, data processing, and handling were all performed by the associated Total Chrome software. A standard mixture of Trp and six of its kynurenine metabolites (1 μg/mL each) was used as calibrant at the start of each run. Results were corrected for full recovery. Kynureninase activity was determined in liver supernatants by measuring the conversion of kynurenine to anthranilic acid, as described previously.11 Kynurenine aminotransferase (KAT) activity was determined under the same experimental conditions by measuring the simultaneous production of KA.
Statistics
Test results were compared with those of control groups by the unpaired t-test using Sigma Plot (Systat), version 11, with which the graphics were prepared. For multiple group comparisons using this program, the Holm–Sidak test was applied, as it is more powerful than the Tukey or Bonferroni tests and can be used for both pairwise comparisons and those versus a control group. Where the data failed the normality (Shapiro–Wilk) test, Kruskal–Wallis one-way ANOVA on ranks was performed. A two-tailed level of significance (P) was set at 0.05.
Results and Discussion
Effects of kynurenine metabolites on liver trypto-phan metabolism
The three kynurenine metabolites used in the present work (3-HK, 3-HAA, and KA) have previously been shown in our alcohol aversion study10 to be potent inhibitors of aldehyde dehydrogenase. The acute time-course data reported below were obtained over a four-hour period, as this was found10 to cover the maximum increases in their hepatic concentrations.
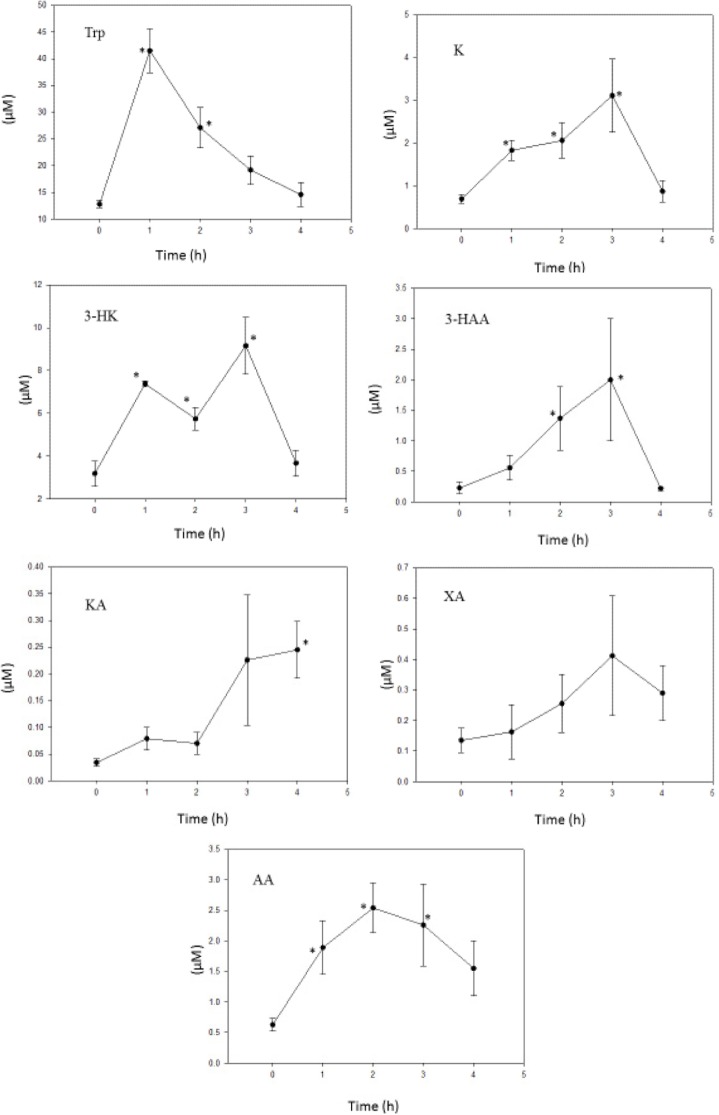
Kynurenic acid
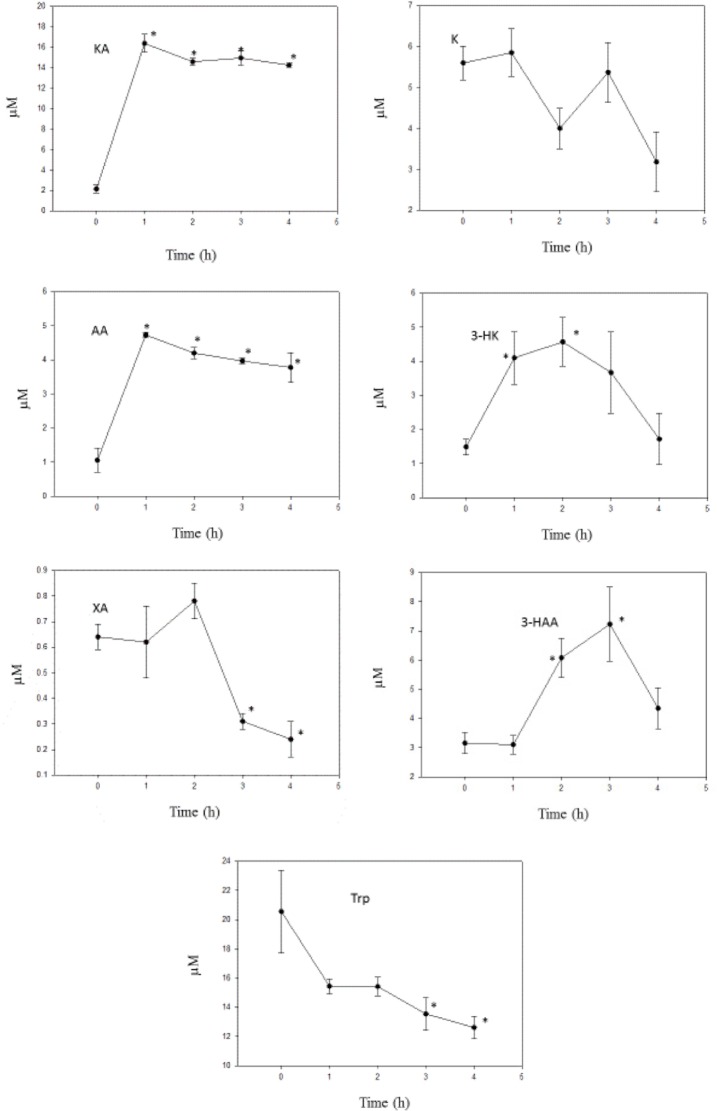
As the data in Figure 2 show, liver [KA] was rapidly and maximally increased at 1 hour following administration of a 10 mg/kg dose. The sustained high level over the subsequent 3 hours suggests that KA does not undergo extensive metabolism. In rats, mice, and some other mammalian species, KA is largely (80%–100%) excreted in urine within 24 hours, with little conversion to QA and quinaldylglycine.13 In bacteria, KA is hydroxylated to 7,8-dihydroxykynurenic acid,14 but it is not known whether this occurs in mammals.
Figure 2.
Time course of effects of administration of KA on liver tryptophan and kynurenine metabolite concentrations.
Notes: KA (10 mg/kg body wt) was administered intraperitoneally at zero time, and liver tryptophan and kynurenine metabolite concentrations were determined as described in the Materials and Methods section at the time intervals indicated. Values are mean ± SEM for five rats. The significance of the differences from the control zero-time value is indicated by an asterisk, with levels of significance (P) of 0.05–0.001.
Abbreviations: Trp, tryptophan; K, kynurenine; 3-HK, 3-hydroxykynurenine; 3-HAA, 3-hydroxyanthranilic acid; KA, kynurenic acid; XA, xanthurenic acid; AA, anthranilic acid.
The data with kynurenines in Figure 2 show that KA did not exert a significant effect on [K], and the 44% decrease at four hours did not reach a significant level (P = 0.055). This suggests that KA does not inhibit the KAT reaction from K to KA (KAT A). This is further suggested by the absence of a change in the [KA]/[K] ratio if the increase in [KA] at 1–4 hours is excluded from the calculation. The KAT B reaction (that from 3-HK to XA) was, however, inhibited by KA by 60%–81%. This is due to a combination of a [3-HK] elevation not matched by one in [XA] at 1–2 hours and a later decrease in [XA]. The mechanism of this KAT B inhibition by KA remains to be investigated. Little is known about the potential effects of KA on KAT, but one possible mechanism of KAT B inhibition is product inhibition by KA.
[AA] was elevated by KA by 2.57–3.46-fold, suggesting that the kynureninase (kynase) A reaction (that from K to AA) is enhanced, and this is reflected in the several-fold increase in the [AA]/[K] ratio over the four-hour study period. By contrast, the kynase B reaction (that from 3-HK to 3-HAA) was decreased over the first two hours after KA administration. Thus, there is a preferential vulnerability in the conversion of 3-HK to both XA and 3-HAA. The results in Figure 2 demonstrate and suggest that KA increases the hepatic concentrations of 3-HK and 3-HAA, both of which possess immune-modulatory properties (see above) and this may be related to the ability of KA to protect against lipopolysaccharide-induced toxicity in mice15 and to exhibit anti-inflammatory activity toward activated mouse splenocytes.16 The KA theory of schizophrenia17 postulates a state of glutamatergic hypoactivity induced by increased levels of cerebrospinal fluid (CSF) KA, the endogenous antagonist of the N-methyl-d-aspartate (NMDA) receptors of the excitatory amino acid glutamate.18 The neuronal excitability state is thought to involve the balance between the NMDA receptor antagonist KA and agonist QA. As the latter is not decreased in schizophrenia,19 it may be concluded that the glutamatergic hypoactivity is solely due to the raised KA. In the present work with KA, the increased [3-HAA] suggests that QA production is not impaired by a raised KA level, thus supporting the situation in schizophrenia.19 Thus the NMDA hypoactivity in schizophrenia may be accompanied by an anti- inflammatory component.
It has been suggested20 that a decrease in the ratio of [3-HAA]/[AA] is associated with many neurological diseases and may represent a protective response to limit primary and secondary damage, particularly in brain. In nonsurviving acute stroke patients, levels of KA are increased along with the decrease in the above ratio.21 In the present work, KA administration induced a decrease in this ratio in liver from a control value at zero time of 2.98 to 0.66, 1.45, 1.82, and 1.18 at 1, 2, 3, and 4 hours, respectively. Linear regression analysis showed a highly significant correlation between liver [KA] and [AA] (r = 0.931; P < 0.001), but not between liver [KA] and [3-HAA] (r = 0.303; P = 0.141). Thus, it appears that KA itself may be capable of decreasing this ratio by its stimulation of AA production. A similar situation exists in schizophrenia, wherein a strong (4.1-fold) elevation of serum [AA] has recently been observed.22 [3-HAA] was not measured in this latter study, though its level in anterior cingulate samples from patients with schizophrenia was found in another study23 to be 1.68-fold higher than in controls. Thus, in all probability, the [3-HAA]/[AA] ratio will also be decreased in schizophrenia. Whether this potential decrease is also caused by the elevated KA in schizophrenia remains to be established.
KA administration did not alter liver [Trp] over the first two hours, but caused significant (P 0.05–0.027) decreases of 34% and 39% at three and four hours, respectively. The flux of Trp down the KP was assessed from the sum of kynurenines, excluding the high [KA] at 1–4 hours, and was found to be minimally altered by KA (sums at 0, 1, 2, 3, and 4 hours were 14.22, 16.45, 15.42, 17.60, and 11.57 μM, respectively). Although this is consistent with the absence of any significant changes (P > 0.1) in TDO activity estimated from the [K]/[Trp] ratio over the four-hour study period (data not shown), the decrease in liver [Trp] can still suggest a TDO activation, with the absence of a corresponding increase in [K] being attributed to enhanced K hydroxylation to 3-HK and hydrolysis to AA. Calculating the ratio of [K + 3-HK + AA] to [Trp] shows a >2-fold increase. As will be suggested below, TDO may be activated by 3-HAA and it is therefore possible that the above decrease in liver [Trp] after KA administration is mediated by 3-HAA.
KA dose–response experiments were also performed to assess the levels of serum and liver Trp and K metabolites at one hour. This time interval was chosen because liver [KA] was maximally increased in our previous study10 on aversion to alcohol. There were distinct differences between liver and serum levels. The data in Figure 2 show that concentrations of liver 3-HK, KA, and AA were increased at one hour after administration of the 10 mg/kg dose of KA. The dose–response changes at one hour given in Table 1 show that concentrations of 3-HK, 3-HAA, and KA were increased in liver by most of the KA doses, whereas that of AA was increased only by the 7.5 mg/kg dose. In serum, by contrast, only [KA] was increased significantly by all doses of KA, though to a lesser extent than in liver especially at the lower doses, whereas [3-HK] was increased only by the 2.5 mg/kg dose, 3-HAA was not increased, and AA was detectable in only one or two rats in any one treatment group. These results clearly demonstrate that serum levels generally do not reflect those in liver. However, a correlation between the two is more likely under steady-state conditions.
Table 1.
Effects of acute administration of various doses of KA on concentrations of some kynurenine metabolites in rat liver and serum.
| DOSE OF KA (mg/kg) | 3-HK | 3-HAA | KA | AA | |
|---|---|---|---|---|---|
| 0 | Liver | 2.86 ± 0.68 | 0.10 ± 0.01 | 0.07 ± 0.02 | 0.16 ±0.06 |
| Serum | 3.79 ± 0.32 | 0.95 ± 0.12 | 0.85 ± 0.18 | UD | |
| 1 | Liver | 3.47 ± 0.65 | 0.18 ± 0.06 | 28.1 ± 1.2* | 0.53 ±0.21 |
| Serum | 3.46 ± 0.27 | 0.75 ± 0.08 | 3.20 ± 0.59* | (0.05, 0.41) | |
| 2.5 | Liver | 18.6 ± 1.0* | 1.13 ± 0.32* | 30.6 ± 0.8* | 0.27 ± 0.14 |
| Serum | 4.87 ± 0.32* | 0.61 ± 0.09 | 3.28 ± 0.09* | UD | |
| 5 | Liver | 11.6 ± 3.9* | 1.67 ± 0.74 | 35.1 ± 5.2* | 0.89 ± 0.54 |
| Serum | 3.55 ± 0.44 | 0.81 ± 0.18 | 4.01 ± 0.45* | (0.10, 0.01) | |
| 7.5 | Liver | 16.0 ± 1.0* | 1.62 ± 0.74 | 37.4 ± 18.8* | 1.01 ± 0.35* |
| Serum | 4.59 ± 0.47 | 0.61 ± 0.13 | 11.2 ± 0.2* | (0.24) | |
| 10 | Liver | 18.8 ± 0.1* | 0.84 ± 0.25* | 54.5 ± 9.6* | 0.39 ± 0.11 |
| Serum | 4.90 ± 0.43 | 0.52 ± 0.06 | 26.4 ± 1.4* | (0.01, 0.19) |
Notes: Rats were given an intraperitoneal injection of various doses of KA or physiological saline and were killed one hour later. Values are mean ± SEM for each group of five rats, except for individual values in parentheses. The significance of the differences from the zero dose control group is indicated by an asterisk (*) (P = 0.042–0.001 by t-test).
3-Hydroxykynurenine
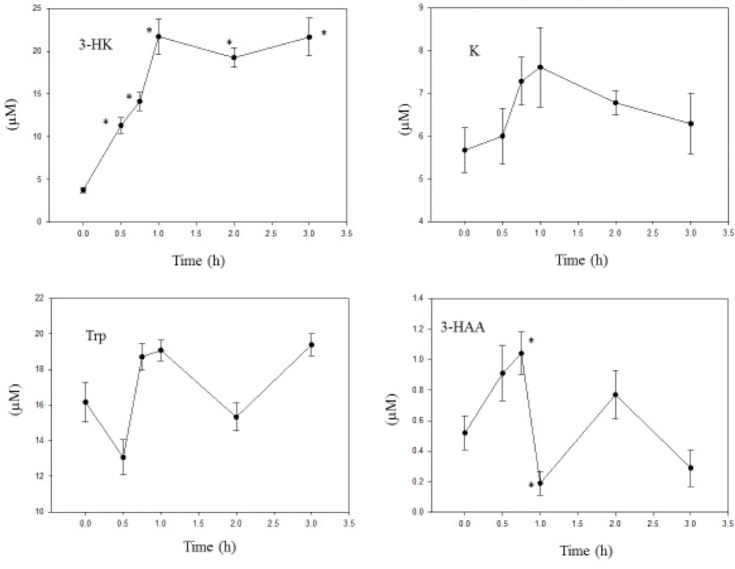
The time course of changes in liver Trp and kynurenines after acute administration of a 10 mg/kg dose of 3-HK is shown in Figure 3. As expected, liver [3-HK] was increased by 3-HK administration, maximally at one hour. The absence of a decline at two to three hours suggests that its further metabolism to either 3-HAA or XA is impaired. We were unable to establish with certainty the levels of XA or KA due to experimental factors preventing their chromatographic separation. Also, AA was undetectable in most animals. As far as we could ascertain, no previous studies of the potential effects of 3-HK administration on K metabolites have been reported. We can, however, suggest that the absence of detectable AA may involve kynase A inhibition by 3-HK. Such inhibition has been reported with the human recombinant enzyme.24 It is unlikely that 3-HK inhibited KAT A or B under our experimental conditions. The rat brain KAT A activity is inhibited by 50% only at the very high concentration of 2 mM.25
Figure 3.
Time course of effects of administration of 3-hydroxykynurenine on liver tryptophan and kynurenine metabolite concentrations.
Notes: 3-Hydroxykunurenine (10 mg/kg body weight) was administered intraperitoneally at zero time, and liver tryptophan and kynurenine metabolite concentrations were determined as described in the Materials and Methods section at the time intervals indicated. Values are mean ± SEM for five rats. The significance of the differences from the control zero-time value is indicated by an asterisk, with levels of significance (P) of 0.033–0.001.
Abbreviations: Trp, tryptophan; K, kynurenine; 3-HK, 3-hydroxykynurenine; 3-HAA, 3-hydroxyanthranilic acid.
However, [3-HAA] was elevated up to 0.75 hours after 3-HK administration before declining to lower than control values at 1 and 3 hours. It may be concluded that these latter decreases are due to kynase inhibition by 3-HK. The [3-HAA]/[3-HK] ratio %, indicative of kynase B activity, was decreased from the zero-time control value of 13.8 to 8.0, 7.4, 0.9, 4.0, and 1.3 at 0.5, 0.75, 1, 2, and 3 hours, respectively. This is consistent with the previously reported 3-HK inhibition of human recombinant kynase.24
3-HK did not influence significantly liver [K], [Trp], or the [K]/[Trp] ratio, thus suggesting that TDO activity is not inhibited at the 10 mg/kg dose level. 3-HK has previously been shown to inhibit rat liver TDO activity in vitro by 67% and 95% at concentrations 5 and 50 μM, respectively.26 However, as inhibitory levels were reached in the present work, it can only be concluded that other factors operating in vivo must play a role in overcoming a potential TDO inhibition. Perhaps, the increase in [3-HAA] could have overcome a potential inhibition, as this latter K metabolite stimulates TDO (see below).
3-Hydroxyanthranilic acid
The time course of changes in liver Trp and kynurenines after acute administration of a 10 mg/kg dose of 3-HAA is shown in Figure 4. As expected, [3-HAA] increased rapidly, by 4.7-fold at 1 hour and 5.4-fold at 4 hours, whereas there was no change in [3-HK]. 3-HAA increased [K] significantly over the first three hours. The K transamination product KA was also increased, suggesting that 3-HAA did not inhibit KAT A activity. Similarly, KAT B activity was also not decreased, though there was a trend toward an increase. The increased formation of KA reflected only the increased availability of the K substrate, rather than enhancement of KAT A activity, as there were no increases in the [KA]/[K] ratio (data calculated from the results in Fig. 4). By contrast with KAT, kynase A activity was decreased by 3-HAA. Thus, despite the increase in [K], there was a steady and significant decrease in [AA]. This suggests that 3-HAA may exert a product inhibition of kynase activity. Kynase inhibition by 3-HAA in pig liver had been demonstrated previously,27 with 0.1 and 1 mM concentrations causing inhibition in vitro of 53% and 79%, respectively.
Figure 4.
Time course of effects of administration of 3-hydroxyanthtranilic acid on liver tryptophan and kynurenine metabolite concentrations.
Notes: 3-Hydroxykunurenine (10 mg/kg body weight) was administered intraperitoneally at zero time, and liver tryptophan and kynurenine metabolite concentrations were determined as described in the Materials and methods section at the time intervals indicated. Values are mean ± SEM for five rats. The significance of the differences from the control zero-time value is indicated by an asterisk, with levels of significance (P) of 0.035–0.001.
Abbreviations: Trp, tryptophan; K, kynurenine; 3-HK, 3-hydroxykynurenine; 3-HAA, 3-hydroxyanthranilic acid; KA, kynurenic acid; XA, xanthurenic acid; AA, anthranilic acid.
3-HAA caused a steady decrease in liver [Trp]. Coupled with the increase in [K], this suggests that 3-HAA enhances liver TDO activity. The large increase in the [K]/[Trp] ratio is consistent with this conclusion. As was the case with 3-HK,26 3-HAA had also been reported to inhibit TDO activity in vitro (by 32% at 5 μM and 85% at 50 μM).26 The hepatic concentration of 3-HAA did not reach 4 μM in this time-course experiment (Fig. 3) or 9 μM in the dose–response experiment (see below). The [K]/[Trp] ratio% was increased dose-dependently by 3-HAA in the dose range of 2.5–10 mg/kg (from 8.41 in controls to 17.18–33.02). This likely TDO enhancement must be attributed to factorsc operating in vivo, the nature of which requires investigation. In dose–response experiments, the smallest 3-HAA dose causing significant changes in the parameters affected in Figure 4 was 2.5 mg/kg, except for [3-HAA] itself, which was already elevated from the control value by 1.9-fold by the smallest (1 mg/kg) dose (data not shown).
Effects of chronic administration of kynurenine metabolites on concentrations of liver and serum trypto-phan and kynurenines
These effects are given in Table 2, from which it can be seen that elevation of liver levels of KA, 3-HK, and 3-HAA also occurs after their individual administration for eight days. None of the other kynurenines altered by acute administration of the three metabolites is altered after their chronic administration. The main chronic effects in liver are the decreases in kynase A and B by 3-HK, the decrease in kynase B, and the consequent increase in KAT B by 3-HAA (as deduced from ratios of the relevant parameters in Table 2). A glance at the parameters in serum (Table 1) shows that, apart from the raised levels of the administered kynurenines, the liver changes are not reflected in serum, thus further emphasizing the need for caution in extrapolating all changes in serum levels to those in liver.
Table 2.
Effects of chronic administration of kynurenine metabolites on concentrations of tryptophan and kynurenines in rat liver.
| PARAMETER | LIVER | SERUM | ||||||
|---|---|---|---|---|---|---|---|---|
| CONTROL | KA | 3-HK | 3-HAA | CONTROL | KA | 3-HK | 3-HAA | |
| Trp | 21.2 ± 1.8 | 20.7 ± 0.9 | 19.2 ± 1.3 | 17.0 ± 0.9 | 75 ± 4 | 68 ± 4 | 66 ± 4 | 63 ± 2* |
| K | 5.4 ± 0.3 | 6.3 ± 0.1* | 9.5 ± 0.8* | 3.6 ± 0.8* | 5.8 ± 0.5 | 5.5 ± 0.6 | 6.2 ± 0.6 | 10.9 ± 0.9* |
| KA | 4.3 ± 0.2 | 10.6 ± 0.9* | 4.4 ± 0.5 | 2.7 ± 0.4* | 0.8 ± 0.1 | 3.8 ± 0.8* | 0.23 ± 0.03* | 0.10 ± 0.04* |
| AA | 3.0 ± 0.1 | 3.4 ± 0.3 | 0.8 ± 0.1* | 5.0 ± 0.5 | 3.7 ± 0.8 | 5.1 ± 0.2 | 4.3 ± 0.1 | 3.7 ± 0.1 |
| 3-HK | 4.8 ± 0.3 | 5.6 ± 0.3 | 18.6 ± 1.7* | 14.1 ± 2.2* | 7.2 ± 0.7 | 7.5 ± 0.8 | 17.3 ± 2.2* | 8.7 ± 1.5 |
| XA | 0.02 ± 0.01 | 0.04 ± 0.02 | 1.1 ± 0.2* | 1.4 ± 0.2* | 2.1 ± 0.4 | 2.6 ± 0.2 | 4.8 ± 1.1* | 2.5 ± 0.3 |
| 3-HAA | 4.9 ± 0.4 | 5.2 ± 0.5 | 7.5 ± 1.3 | 10.3 ± 0.3* | 0.05 ± 0.01 | 0.06 ± 0.02 | 4.9 ± 1.1* | 11.0 ± 1.7* |
Notes: Rats were treated intraperitoneally once daily for 8 days with saline or a 10 mg/kg dose of kynurenine metabolites and were killed at 2 hours after the injection on the final day. Values in μM are mean ± SEM for six rats per group. The asterisk (*) denotes a significant difference from controls at P levels of 0.044–0.001.
Effects of tryptophan loading on liver tryptophan metabolism
The dose of Trp used here (50 mg/kg) does not enhance TDO activity when assayed in rat liver homogenates.28 As shown in Figure 5, this dose increased the hepatic concentrations of Trp and all six kynurenines analyzed. When TDO activity was assessed indirectly from the [K]/[Trp] ratio%, there were no significant changes at 1, 2, or 4 hours after Trp administration. Only at 3 hours was TDO activity increased significantly from a baseline value of 4.41 ± 0.71 to 10.98 ± 2.31 (mean ± SEM for five rats; P = 0.027). As the increases in concentrations of K, 3-HK, 3-HAA, and AA occurred earlier, this can be attributed to increased flux of Trp down the KP. This flux, estimated from the sums of the six kynurenines analyzed, was significantly increased during the first three hours, but not at four hours. Values (mean ± SEM for five rats) were as follows: 4.88 ± 0.55 μM, 11.90 ± 0.83 μM, 11.64 ± 0.91 μM, 14.59 ± 2.77 μM, and 6.84 ± 0.46 μM at 0, 1, 2, 3, and 4 hours, respectively.
Figure 5.
Time course of effects of administration of tryptophan on liver tryptophan and kynurenine metabolite concentrations.
Notes: Tryptophan (50 mg/kg body weight) was administered intraperitoneally at zero time, and liver tryptophan and kynurenine metabolite concentrations were determined as described in the Materials and Methods section at the time intervals indicated. Values are mean ± SEM for five rats. The significance of the differences from the control zero-time value is indicated by an asterisk, with levels of significance (P) of 0.046–0.001.
Abbreviations: Trp, tryptophan; K, kynurenine; 3-HK, 3-hydroxykynurenine; 3-HAA, 3-hydroxyanthranilic acid; KA, kynurenic acid; XA, xanthurenic acid; AA, anthranilic acid.
Also of interest is that the increases in the transamination products KA and XA occurred later and only the increase in [KA] was significant at four hours. This supports the well-known notion that K metabolism occurs mainly by oxidation (hydroxylation to 3-HK followed by hydrolysis to 3-HAA and direct hydrolysis of K to AA). Expression of enzyme activities from product to substrate ratio percentages showed that this dose of Trp did not alter the activities of K hydroxylase, kynase A or B, or KAT B. Only KAT A activity was increased significantly at 4 hours (from a control zero-time value of 5.06 ± 0.72 to 19.87 ± 3.38, mean ± SEM for five rats; P = 0.016). When KAT A activity was assayed directly, Trp caused a significant increase only at 0.5 hour (from a control zero-time value of 1.15 ± 0.03 to 1.52 ± 0.28 nmol of KA formed/mg of protein/h; P = 0.008), but not at subsequent time intervals up to 4 hours (data not shown). A larger Trp dose (300 mg/kg) had previously been reported not to alter KAT activity at 1–3 hours,29 though activity was not assessed at earlier time intervals. In our previous study, we observed a transient increase in kynase A activity at 0.5 hours after Trp administration when the enzyme activity was assayed in vitro in liver supernatants of Trp-treated rats.10 In view of the above finding with TDO, in all probability, a 50 mg/kg dose of Trp could conceivably activate TDO in humans. Accordingly, we have suggested in our accompanying paper30 that, for acute Trp loading in humans to assess the Trp flux and functional capacity of the KP in the absence of TDO activation, a 30 mg/kg body weight dose should be used, rather than the blanket 2 g dose unadjusted for body weight.
Effects of kynureninase inhibitors on liver tryptophan metabolism
The main therapeutic application of the two kynureninase inhibitors BSZ and CBD is their use as peripheral aromatic l-amino acid decarboxylase inhibitors in the treatment of Parkinson’s disease when administered jointly with l-Dopa (3,4-dihydroxyphenylalanine) to enhance cerebral dopamine synthesis by preventing peripheral Dopa decarboxylation. The effects of both drugs on Trp metabolism have been studied previously in rats and mice. Both agents inhibit liver kynase activity both in vitro and after administration.10,29,31 Additionally, BSZ inhibits KAT activity in vitro in mice.31 The results in Table 3 show that KAT A activity in rats is also inhibited in vitro by both BSZ and CBD, with the latter showing a stronger inhibition. The BSZ inhibition of KAT and the other pyridoxal 5′-phosphate-dependent enzyme kynureninase had previously been suggested to involve the inactivation of the cofactor by the formation of the hydrazone.31 By contrast, liver TDO is not inhibited by BSZ in mice in vitro by concentrations up to 200 μM or after administration of a 50 mg/kg body weight dose,32 though others have reported TDO inhibition by BSZ in rats both in vitro (at 100 μM and above) and at 0.5–1.5 hours after administration of a larger dose (400 mg/kg).33
Table 3.
Inhibition of liver KAT activity in vitro by the kynureninase inhibitors benserazide and carbidopa.
| INHIBITOR CONCENTRATION (μM) | KAT ACTIVITY | |
|---|---|---|
| BENSERAZIDE | CARBIDOPA | |
| 0 | 1.78 ± 0.26 | 1.78 ± 0.26 |
| 10 | 1.50 ± 0.18 | 1.00 ± 0.11* |
| 25 | 1.20 ± 0.16 | 0.59 ± 0.10* |
| 100 | 0.81 ± 0.17* | 0.17 ± 0.08* |
| 250 | 0.63 ± 0.20* | 0.11 ± 0.06* |
| 500 | 0.54 ± 0.18* | 0.06 ± 0.05* |
Notes: Values for KAT activity (expressed in nmol of KA formed per mg of protein/h) are mean ± SEM of liver homogenates from 4–5 different rats per group.
Denotes significant differences from the zero control by t-test at P = 0.036–0.001.
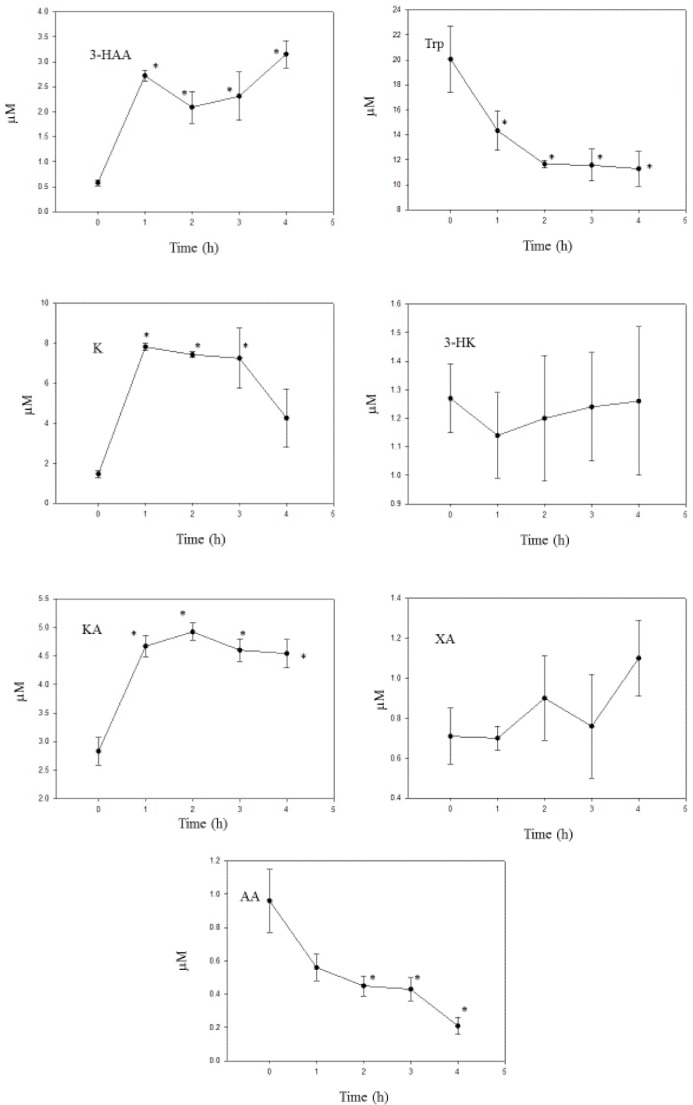
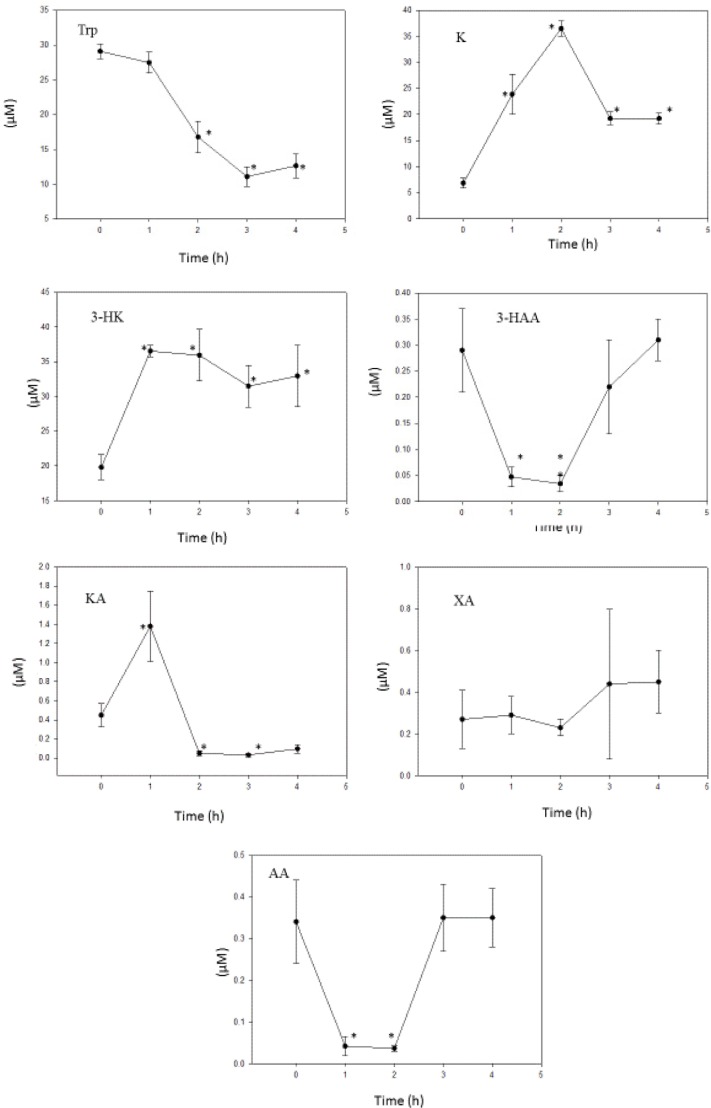
Benserazide
As shown in Figure 6, the 50 mg/kg dose of BSZ actually decreased liver [Trp] and increased [K], suggesting that the drug enhances TDO activity. However, although the [K]/[Trp] ratio% was dramatically increased, this does not reflect solely TDO enhancement, because the rise in [K] could also be due to the inhibition of kynurenine hydroxylase (see below) and to a lesser extent KAT A and kynase A. Calculating the [K]/[Trp] ratio% on the basis of unaltered [K] from the zero-time value resulted in increases of 78%, 170%, and 135% at 2, 3, and 4 hours, respectively. Thus, on the basis of this assumption and as liver [Trp] was decreased, rather than increased or remained unaltered, it is valid to conclude that TDO activity was enhanced by this dose of BSZ. A TDO enhancement by BSZ is also further suggested by an observed increase in both liver total kynurenines and total Trp oxidation (TTOX). Thus [total kynurenines] was increased by 121%, 147%, 86%, and 92% at 1, 2, 3, and 4 hours, respectively (P = 0.004–0.001) and TTOX was increased by 1.3-, 4.4-, 5.1-, and 4.5-fold, respectively (P = 0.008–0.001; data not shown).
Figure 6.
Time course of effects of administration of benserazide on liver tryptophan and kynurenine metabolite concentrations.
Notes: Benserazide (100 mg/kg body weight) was administered intraperitoneally at zero time, and liver tryptophan and kynurenine metabolite concentrations were determined as described in the Materials and methods section at the time intervals indicated. Values are mean ± SEM for five rats. The significance of the differences from the control zero-time value is indicated by an asterisk, with levels of significance (P) of 0.037–0.001.
Abbreviations: Trp, tryptophan; K, kynurenine; 3-HK, 3-hydroxykynurenine; 3-HAA, 3-hydroxyanthranilic acid; KA, kynurenic acid; XA, xanthurenic acid; AA, anthranilic acid.
Kynurenine hydroxylase, estimated from the [3-HK]/[K] ratio%, was decreased by BSZ from a control value at zero time of 324 to values of 153, 112, 164, and 171 at 1–4 hours, respectively (P = 0.027–0.005). Kynurenine hydroxylase inhibition by BSZ had previously been demonstrated in vitro.34 Elevation of liver and other tissue [K] after kynurenine hydroxylase inhibition had previously been demonstrated in rats using the inhibitor m-nitrobenzoylalanine.35 The increase in liver [K] at three hours in this latter study35 (3.00-fold) is close to that observed here with BSZ (2.82-fold) at the same time interval. As kynurenine hydroxylase stands at the start of the major route of kynurenine metabolism, it could be reasonably concluded that the BSZ inhibition of this enzyme, rather than of KAT A or kynase A, is the major contributor to the K elevation. Kynase inhibition by o-methoxybenzoylalanine increases liver [K] by only 1.31-fold.35 [KA] was significantly increased at 1 hour, and then decreased at 2–4 hours after BSZ. [AA] was also significantly decreased, at 1–2 hours, before returning to baseline levels thereafter. These results suggest that, as well as in vitro, BSZ also inhibits KAT A and kynase A activities after administration. Calculating these two enzyme activities from the [KA]/[K] and [AA]/[K] ratio percentages also confirmed these conclusions. The former was decreased by BSZ from the control zero-time value of 6.6 ± 1.2 to values of 0.15 ± 0.07, 0.16 ± 0.24, and 0.52 ± 0.20 (P = 0.009–0.001) at 2, 3, and 4 hours, whereas the latter ratio% was decreased from the control value of 3.08 ± 0.75 to values of 0.17 ± 0.06, 0.40 ± 0.01, 1.82 ± 0.51, and 1.72 ± 0.32 at 1, 2, 3, and 4 hours, respectively (P = 0.021–0.005; all mean percentages ± SEM for five rats). The strongest decrease in kynase A activity (at one hour) coincides with the inhibition of the liver enzyme activity assayed in liver supernatants from BSZ-treated rats previously reported by us.11
BSZ increased liver [3-HK] and decreased [3-HAA] but did not significantly alter [XA]. These changes suggest that KAT B activity, calculated from the [XA]/[3-HK] ratio%, was not significantly altered by BSZ, whereas kynase B activity ([3-HAA]/[3-HK] ratio%) was significantly (P = 0.008–0.005) inhibited by BSZ (from a zero-time value of 1.31 ± 0.36 to values of 0.27 ± 0.03, 0.09 ± 0.04, and 0.70 ± 0.13 at 1, 2, and 3 hours, respectively).
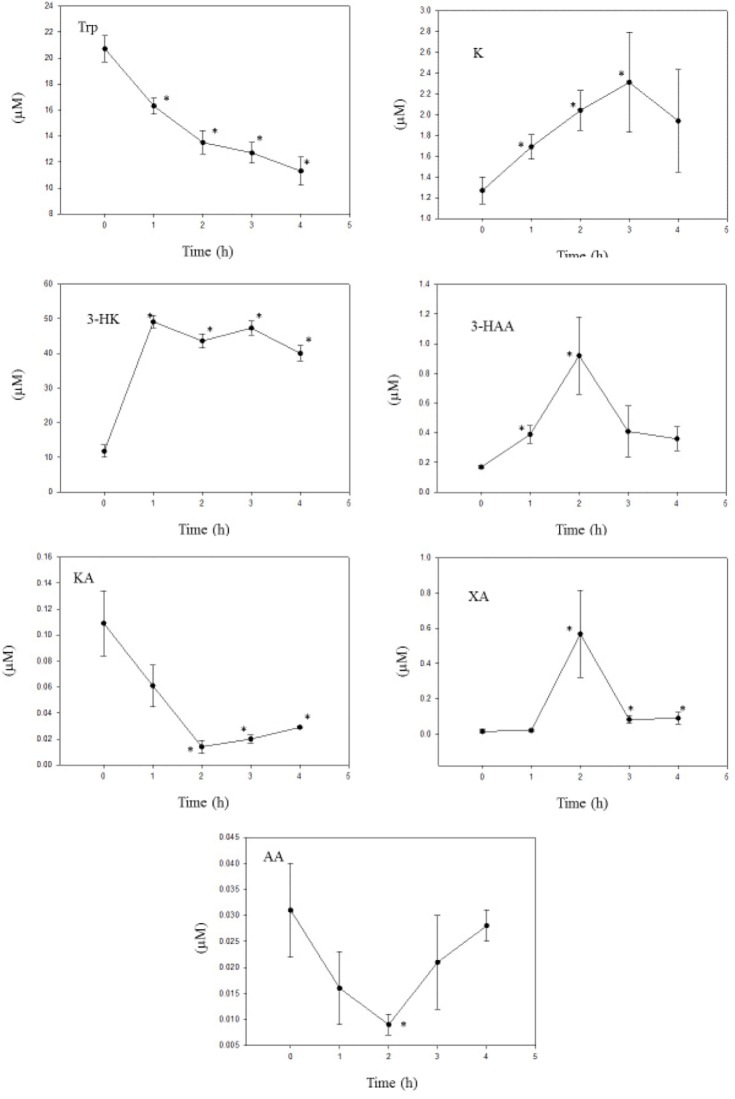
Carbidopa
As was the case with BSZ, the other kynureninase inhibitor CBD also decreased liver [Trp] and increased liver [K] (Fig. 7), thus suggesting a TDO enhancement. Expressed as the [Trp]/[K] ratio%, TDO was increased from a zero-time value of 6.14 by 69%, 146%, 196%, and 180%, respectively, at 1, 2, 3, and 4 hours. In line with this TDO enhancement, liver [total kynurenines] and total Trp oxidation were both enhanced by CBD by 217%–282% and 383%–508%, respectively (data derived from Fig. 7).
Figure 7.
Time course of effects of administration of carbidopa on liver tryptophan and kynurenine metabolite concentrations.
Notes: Carbidopa (50 mg/kg body weight) was administered intraperitoneally at zero time, and liver tryptophan and kynurenine metabolite concentrations were determined as described in the Materials and methods section at the time intervals indicated. Values are mean ± SEM for five rats. The significance of the differences from the control zero-time value is indicated by an asterisk, with levels of significance (P) of 0.05–0.001.
Abbreviations: Trp, tryptophan; K, kynurenine; 3-HK, 3-hydroxykynurenine; 3-HAA, 3-hydroxyanthranilic acid; KA, kynurenic acid; XA, xanthurenic acid; AA, anthranilic acid.
From these results, it appears that administration of BSZ and CBD enhances liver TDO activity. Administration of another hydrazine compound, BSZ, had previously been shown36 to activate TDO by promoting the conjugation of the apoenzyme with heme. Although these three chemicals share structural similarities, which may confer this TDO- activating property, not all hydrazine compounds activate TDO.36 Unlike BSZ, CBD did not inhibit kynurenine hydroxylase activity, as the [3-HK]/[K] ratio% was in fact increased by 2.20–3.13-fold (P = 0.001) at 1–4 hours. This apparent stimulation of kynurenine hydroxylase may explain the less strong elevation of liver [K] (1.33–1.83-fold), and the relatively large increase in [3-HK] by CBD, compared with those by BSZ. Perhaps because of this greater elevation of [3-HK], [3-HAA] was increased by CBD, but kynase B activity, estimated from the [3-HAA]/[3-HK] ratio%, was only little affected by CBD. Kynase A activity was, however, decreased by CBD by 61%, 82%, 63%, and 41%, respectively, at 1, 2, 3, and 4 hours. CBD decreased KAT A activity by 58%, 92%, 90%, and 83% at 1–4 hours, but inhibited KAT B activity only at 1 hour, by 69%, with a stimulation at 2–4 hours (data calculated from Fig. 7).
Chronic tryptophan–benserazide administration
In our previous study of the effects of joint acute administration of Trp and the above two kynureninase inhibitors,11 we reported the unexpected observation that aversion to alcohol occurs only when Trp is given with BSZ, but not with CBD. Aversion was then shown to involve a greater increase in liver [3-HK] by the Trp–BSZ but not by the Trp–CBD combination, because Trp prevented the kynureninase inhibition by CBD. Accordingly, we proceeded to examine the aversive effect of the Trp–BSZ combination after repeated daily administration for eight days. The changes in liver and serum Trp and K metabolites under these conditions are given in Table 4.
Table 4.
Effects of chronic administration of tryptophan and benserazide separately and in combination on concentrations of tryptophan and kynurenine metabolites in rat liver and serum.
| PARAMETER | LIVER | SERUM | ||||||
|---|---|---|---|---|---|---|---|---|
| CONTROL | BSZ | TRP | BSZ + TRP | CONTROL | BSZ | TRP | BSZ + TRP | |
| Trp | 12.3 ± 0.42 | 14.0 ± 0.7 | *26.7 ± 1.9 | *20.0 ± 1.7*¶ | 70 ± 3 | *58 ± 2 | *155 ± 9 | *96 ± 2*¶ |
| K | 7.3 ± 0.8 | *11.8 ± 1.0 | *11.8 ± 1.5 | *12.4 ± 1.4 | 3.3 ± 0.4 | 3.7 ± 0.4 | *6.8 ± 0.5 | 4.8 ± 0.6* |
| KA | 0.46 ± 0.14 | 0.58 ± 0.03 | *1.32 ± 0.28 | 0.42 ± 0.10* | 0.44 ± 0.16 | 0.24 ± 0.07 | *1.61 ± 0.32 | 0.36 ± 0.18* |
| AA | 1.99 ± 0.62 | 1.94 ± 0.50 | *4.39 ± 0.11 | 1.40 ± 0.33* | 2.59 ± 0.64 | 3.87 ± 0.37 | *7.83 ± 1.10 | 1.71 ± 0.22*¶ |
| 3-HK | 5.5 ± 1.5 | *52.6 ± 8.8 | *61.7 ± 9.2 | *112.5 ± 5.4*¶ | 8.8 ± 0.5 | *23.4 ± 1.0 | *28.5 ± 3.8 | *100.7 ± 5.7*¶ |
| XA | 0.17 ± 0.08 | 0.16 ± 0.02 | *1.50 ± 0.22 | *0.47 ± 0.04*¶ | 0.56 ± 0.07 | *0.09 ± 0.02 | 0.57 ± 0.07 | 0.58 ± 0.06¶ |
| 3-HAA | 2.43 ± 0.54 | 2.51 ± 0.70 | *7.42 ± 1.36 | 3.58 ± 0.66* | 0.01 ± 0.005 | 0.07 ± 0.04 | *6.79 ± 1.30 | *2.38 ± 1.03*¶ |
| KOHase | 88 ± 31 | *483 ± 111 | *564 ± 100 | *987 ± 114*¶ | 264 ± 50 | *626 ± 69 | 421 ± 82 | *2120 ± 199*¶ |
| KAT A | 4.5 ± 0.5 | 5.2 ± 0.6 | *9.3 ± 1.5 | 3.2 ± 0.5* | 13.2 ± 3.4 | 6.4 ± 2.6 | *26.0 ± 7.6 | 7.6 ± 4.6 |
| KAT B | 6.7 ± 1.4 | *0.3 ± 0.07 | *2.6 ± 0.5 | *0.4 ± 0.04* | 6.4 ± 0.9 | *0.4 ± 0.2 | *2.0 ± 0.3 | *0.6 ± 0.08* |
| Kynase A | 34 ± 8 | *18 ± 5 | 39 ± 3 | *12 ± 3* | 78 ± 34 | *17 ± 2 | 116 ± 19 | *36 ± 5* |
| Kynase B | 51.6 ± 6.6 | *5.6 ± 1.7 | *12.0 ± 1.3 | *3.0 ± 0.6* | 0.13 ± 0.07 | 0.30 ± 0.17 | *23.4 ±6.2 | 2.36 ± 1.05* |
| TDO | 59 ± 7 | *86 ± 10 | *44 ± 4 | 61 ± 3* | 4.8 ± 0.7 | 6.4 ± 0.9 | 4.4 ± 0.3 | 4.9 ± 0.5 |
| Ks | 3.0 ± 0.3 | *11.6 ± 1.4* | 16.0 ± 2.9 | *24.1 ± 1.4* | 2.6 ± 0.1 | *5.2 ± 0.2 | *8.7 ± 0.7 | *18.5 ± 0.9*¶ |
| TTOX | 24.4 ± 2.9 | *83.1 ± 8.5 | *59.3 ± 8.2 | *123.3 ± 10.4*¶ | 3.7 ± 0.3 | *9.0 ± 0.5 | 5.6 ± 0.7 | *19.2 ± 0.9*¶ |
Notes: Rats were treated intraperitoneally once daily for 8 days with saline, BSZ (100 mg/kg), Trp (50 mg/kg), or a combination of the latter two agents and were killed at 2 hours after the Trp injection on the final day. Saline and BSZ were given 1 hour or 15 minutes before Trp. Values in μM or ratio percentages are mean ± SEM for six rats per group. Asterisks on the left denote significant differences from controls, whereas those on the right denote significant differences between the Trp + BSZ group and the Trp group.
Denotes the differences between the combined group and the BSZ group. Symbols denote significant differences at P levels of 0.044–0.001.
In liver, BSZ alone increased only [K] and [3-HK]. By contrast, Trp increased all analytes. However, when BSZ was administered with Trp, the Trp-induced increases were altered in three different ways: (1) The increase in [Trp] was minimized by BSZ, whereas that in [K] was not. This is consistent with the TDO enhancement by BSZ and the consequent increase in the Trp flux down the KP (assessed from total kynurenines) and in Trp oxidation (TTOX). (2) The Trp-induced increases in concentrations of KA, AA, XA, and 3-HAA were either abolished or strongly prevented by BSZ, thus providing further support to the drug-induced inhibition of KAT A and B and kynase A and B activities. (3) The Trp-induced increase in [3-HK] was potentiated by BSZ because of its inhibition of kynase B, as reported previously after acute administration.11
In serum, BSZ alone decreased the concentrations of Trp, KA, and XA and increased that of 3-HK but did not alter those of K, AA, or 3-HAA, thus confirming the inhibition of KAT A and B and kynase B and activation of TDO. Total kynurenines (Ks) and TTOX were also increased by BSZ. Trp alone increased the levels of all serum parameters, except XA. These increases were either prevented or strongly decreased when BSZ was administered jointly with Trp, except 3-HK, whose serum levels were potentiated. From the results in Table 4, it is clear that changes in liver Trp and kynurenine metabolite profiles are generally reflected in serum as are the effects of BSZ on these changes under these steady-state conditions.
The results in Table 5 show that both KAT A and kynase A activities were inhibited significantly by chronic BSZ administration but not by Trp. The BSZ inhibition of both enzyme activities was even stronger when Trp was co-administered. KAT A inhibition by BSZ was stronger than that of kynase A.
Table 5.
Effects of chronic administration of tryptophan and benserazide separately or in combination on liver kynureninase and KAT activities.
| TREATMENT GROUP | KYNURENINASE | KYNURENINE AMINOTRANSFERASE |
|---|---|---|
| Control | 7.46 ± 0.28 | 1.12 ± 0.03 |
| Benserazide | *6.12 ± 0.42 | *0.44 ± 0.02 |
| Tryptophan | 7.40 ± 0.39 | 0.97 ± 0.09 |
| Benserazide + tryptophan | *5.56 ± 0.73* | *0.32 ± 0.01*¶ |
Notes: Treatment details are as in Table 3. Values are mean ± SEM for five rats per group. Enzyme activities are expressed in nmol of product formed/min per mg of protein. The asterisk on the left denotes a significant difference from controls. Symbols on the right denote significant differences between the BSZ + Trp group and the Trp (*) or BSZ (¶) group. Symbols denote significance levels (P) of 0.037–0.001.
General Conclusions and Comments
The results of the present study, some of which were unexpected, raise a number of issues of importance in relation to control of the hepatic kynurenine pathway and its role in health and disease. Feedback control of the KP is generally thought to involve allosteric inhibition of TDO activity by the end products NAD(P)H.37 Additionally, it has been suggested2,26 that TDO could be inhibited by 3-HK and 3-HAA at concentrations (5 μM) close to physiological or pathological levels. However, the present results suggest that administration of 3-HK does not influence TDO activity, whereas that of 3-HAA actually enhances it. KA also enhances TDO possibly via 3-HAA. Rat liver TDO activity had previously been shown to be enhanced by the administration of 3-HAA and also K, XA, picolinic acid, and quinolinic acid,38 though at doses of 10 (K) or 25 mg/kg (all others). In situations in which KA (schizophrenia), 3-HK, 3-HAA, or QA (immune activation) are increased, we could expect a further increase in the flux of Trp through TDO, resulting in the production of more immunosuppressive kynurenines. If Trp is additionally administered, the flux could be even greater to an extent that pathological immunosuppression could result with negative health consequences.7 We therefore suggest that Trp should not be used in immune conditions. In schizophrenia, clinical trials have demonstrated39,40 that Trp does not improve core symptoms of schizophrenia even though it ameliorates aggressive symptoms and improves memory function. By contrast, a low-Trp diet improves scores on certain tests of brain function and also psychotic symptoms.41 As [KA] is elevated in schizophrenia, further stimulation of KAT A by exogenous Trp can only perpetuate the glutamatergic hypoactivity state of this illness. However, the elevation of 3-HK and 3-HAA by KA could have positive consequences in conditions requiring immunosuppression.
The kynureninase inhibitors BSZ and CBD exert unexpected effects on the KP, notably stimulation of TDO activity. In alcoholism, we proposed the use of a combination of BSZ and Trp as an alcohol aversion therapy, but with potential effects on NMDA receptor function to combat the hyperexcitability state of acute alcohol withdrawal.11 Inhibition of KAT A in addition to that of kynureninase offers potential opportunities for BSZ and CBD, especially the former. KAT inhibition is a desirable goal in the treatment of schizophrenia.42 and the potential benefit from BSZ has not been adequately explored. Two clinical trials of BSZ alone43 or jointly with Trp44 gave negative results, but the decrease in [KA] by BSZ reported in Table 3 warrants exploration in humans. Data presented here support the suggestion in the preceding paper30 that a suitable Trp dose for Trp loading to assess the KP in humans in the absence of TDO activation should be <50 mg/kg, closer to the 2 g traditional dose, but based on body weight, namely, 30 mg/kg.
Although most of the data in the present study were obtained in liver and therefore apply to the hepatic KP including among others the first and most rate-limiting enzyme TDO, data in serum must reflect total body KP activity, including any likely contribution of indoleamine 2,3-dioxygenase (IDO) and subsequent enzymes distributed in different organs and tissues. Whereas most studies have investigated the effects of IDO induction on K metabolites, as far as we could ascertain, very little has been done to assess the potential effects of these metabolites on IDO activity. It is possible that K metabolites exert immunomodulatory effects, resulting in changes in IDO activity. For example, KA administration to mice exerts anti-inflammatory effects and lowers the levels of proinflammatory cytokines in splenocytes,45 which is likely to decrease IDO activity. However, such a potential decrease is unlikely to influence plasma or tissue Trp levels because such levels are not influenced by pharmacological inhibition of activity of IDO46 or its gene deletion.47 Whether administration of other immunomodulatory K metabolites, such as 3-HK, 3-HAA, or QA, can influence IDO remains to be assessed in future studies.
Acknowledgments
AA-BB holds an honorary professorial position at Cardiff Metropolitan University and thanks Alex Steptoe for skillful technical assistance and Cardiff University for provision of facilities.
Abbreviations
- AA
anthranilic acid
- BSZ
benserazide
- CBD
carbidopa
- HPLC
high-performance liquid chromatography
- 3-HAA
3-hydroxyanthranilic acid
- 3-HK
3-hydroykynurenine
- IDO
indoleamine 2,3-dioxygenase
- KA
kynurenic acid
- Kynase
kynureninase
- Kynase A
kynureninase from K → AA
- Kynase B
kynureninase from 3-HK → 3-HAA
- K
kynurenine
- Ks
total kynurenines
- KAT
kynurenine aminotransferase
- KAT A
kynurenine aminotransferase from K → KA
- KAT B
kynurenine aminotransferase from 3-HK → XA
- KP
kynurenine pathway
- QA
quinolinic acid Trp: tryptophan
- TDO
tryptophan 2,3-dioxygenase
- XA
xanthurenic acid
Footnotes
ACADEMIC EDITOR: Gilles Guillemin, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 492 words, excluding any confidential comments to the academic editor.
FUNDING: This work was funded by a project grant to AA-BB from the Wellcome Trust ((069301). SB acknowledges financial support from the British Commonwealth Authority. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflict of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Designed the experiments and performed them: AA-BB, SB. Analyzed the data and wrote the first draft of the manuscript: AA-BB. Both authors revised and agreed with manuscript results and conclusions, and approved the final version of the manuscript.
REFERENCES
- 1.Badawy AA-B, Bano S. Elevation of kynurenine metabolites in rat liver and serum: a potential additional mechanism of the alcohol aversive and anti-cancer effects of disulfiram? Alcohol Alcohol. 2016;51:20–5. doi: 10.1093/alcalc/agv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender DA. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6:1–97. doi: 10.1016/0098-2997(83)90005-5. [DOI] [PubMed] [Google Scholar]
- 3.Badawy AA-B. Tryptophan metabolism in alcoholism. Nutr Res Rev. 2002;1:123–52. doi: 10.1079/NRR200133. [DOI] [PubMed] [Google Scholar]
- 4.Badawy AA-B. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep. 2015;35:e00261. doi: 10.1042/BSR20150197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247–65. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 6.Yeung AWS, Terentis AC, King NCJ, Thomas SR. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci. 2015;129:601–72. doi: 10.1042/CS20140392. [DOI] [PubMed] [Google Scholar]
- 7.Badawy AA-B, Namboodiri AMA, Moffett JR. Hypothesis: the end of the road for the tryptophan depletion concept in pregnancy and infection. Clin Sci (Lond) 2016;130(15):1327–33. doi: 10.1042/CS20160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:1–13. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. 2009;2:1–19. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badawy AA-B, Bano S, Steptoe A. Tryptophan in alcoholism treatment I: kynurenine metabolites inhibit the rat liver mitochondrial low Km aldehyde dehydrogenase activity, elevate blood acetaldehyde concentration and induce aversion to alcohol. Alcohol Alcohol. 2011;46:651–60. doi: 10.1093/alcalc/agr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badawy AA-B, Bano S, Steptoe A. Tryptophan in alcoholism treatment II: inhibition of the rat liver mitochondrial low Km aldehyde dehydrogenase activity, elevation of blood acetaldehyde concentration and induction of aversion to alcohol by combined administration of tryptophan and benserazide. Alcohol Alcohol. 2011;46:661–71. doi: 10.1093/alcalc/agr135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badawy AA-B, Morgan CJ. Rapid isocratic liquid chromatographic separation and quantification of tryptophan and six kynurenine metabolites in biological samples with ultraviolet and fluorimetric detection. Int J Tryptophan Res. 2010;3:175–86. doi: 10.4137/IJTR.S6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murachi T, Tsukada K, Hayaishi O. Metabolic fate of kynurenic acid C14 intraperitoneally administered to animals. Biochemistry. 1963;2:304–8. doi: 10.1021/bi00902a021. [DOI] [PubMed] [Google Scholar]
- 14.Taniuchi H, Hayaishi O. Studies on the metabolism of kynurenic acid III. Enzymatic formation of 7,8-dihydroxykynurenic acid from kynurenic acid. J Biol Chem. 1963;238:283–93. [PubMed] [Google Scholar]
- 15.Moroni F, Cozzi A, Sili M, Mannaioni G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm. 2012;119:133–9. doi: 10.1007/s00702-011-0763-x. [DOI] [PubMed] [Google Scholar]
- 16.Małaczewska J, Siwicki AK, Wójcik RM, Turski WA, Kaczorek E. The effect of kynurenic acid on the synthesis of selected cytokines by murine splenocytes – in vitro and ex vivo studies. Exp Immunol. 2016;41(1):39–46. doi: 10.5114/ceji.2016.58815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linderholm KR, Skogh E, Olsson SK, et al. Increased levels of kynurenine and kynurenic acid in the CSF acid in the CSF of patients with schizophrenia. Schizophrenia Bull. 2012;3:426–32. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone W. The neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–85. [PubMed] [Google Scholar]
- 19.Schwarcz R, Tamminga CA, Kurlan R, Shoulson I. Cerebrospinal fluid levels of quinolinic acid in Huntington’s disease and schizophrenia. Ann Neurol. 1988;24:580–2. doi: 10.1002/ana.410240417. [DOI] [PubMed] [Google Scholar]
- 20.Darlington LG, Forrest CM, Mackay GM, et al. On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. Int J Tryptophan Res. 2010;3:51–9. doi: 10.4137/ijtr.s4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darlington LG, Mackay GM, Forrest CM, Stoy N, George C, Stone TW. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur J Neurosci. 2007;26:2211–21. doi: 10.1111/j.1460-9568.2007.05838.x. [DOI] [PubMed] [Google Scholar]
- 22.Oxenkrug G, van der Hart M, Roeser J, Summergrad P. Anthranilic acid: a potential biomarker and treatment target for schizophrenia. Ann Psychiatry Ment Health. 2016;4:1059. [PMC free article] [PubMed] [Google Scholar]
- 23.Miller CL, Llenos IC, Cwik M, Walkup J, Weis S. Alterations in kynurenine precursor and product levels in schizophrenia and bipolar disorder. Neurochem Int. 2008;52:1297–303. doi: 10.1016/j.neuint.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Walsh HA, Botting NP. Purification and biochemical characterization of some of the of some of the properties of recombinant human kynureninase. Eur J Biochem. 2002;269:2069–74. doi: 10.1046/j.1432-1033.2002.02854.x. [DOI] [PubMed] [Google Scholar]
- 25.Okuno E, Schmidt W, Parks DA, Nakamura M, Schwarcz R. Measurement of rat brain kynurenine aminotransferase at physiological kynurenine concentrations. J Neurochem. 1991;57:533–40. doi: 10.1111/j.1471-4159.1991.tb03783.x. [DOI] [PubMed] [Google Scholar]
- 26.Wagner C. Regulation of the tryptophan-nicotinic acid DPN pathway in the rat. Biochem Biophys Res Commun. 1964;17:668–73. [Google Scholar]
- 27.Tanizawa K, Soda K. Inducible and constitutive kynureninases: control of the Inducible enzyme activity by transamination and inhibition of the constitutive enzyme by 3-hydroxyanthranilate. J Biochem. 1979;86:499–508. doi: 10.1093/oxfordjournals.jbchem.a132550. [DOI] [PubMed] [Google Scholar]
- 28.Badawy AA-B, Evans M. The regulation of rat liver tryptophan pyrrolase by its cofactor haem – experiments with haematin and 5-aminolaevulinate and comparison with the substrate and hormonal mechanisms. Biochem J. 1975;150:511–20. doi: 10.1042/bj1500511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi F, Shibata Y. Kynurenine metabolism in vitamin-B-6-deficient rat liver after tryptophan injection. Biochem J. 1984;220:693–9. doi: 10.1042/bj2200693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badawy AA-B, Dougherty DM. Assessment of the human kynurenine pathway: comparisons and clinical implications of ethnic and gender differences in plasma tryptophan, kynurenine metabolites and enzyme expressions at baseline and after acute tryptophan loading and depletion. Int J Tryptophan Res. 2016:9. doi: 10.4137/IJTR.S38189. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bender DA, Smith WRD, Humm RP. Effects of benserazide on tryptophan metabolism in the mouse. Biochem Pharmacol. 1977;26:1619–23. doi: 10.1016/0006-2952(77)90078-8. [DOI] [PubMed] [Google Scholar]
- 32.Bender DA. Inhibition in vitro of the enzymes of the oxidative pathway of tryptophan metabolism and of nicotinamide nucleotide synthesis by benserazide, carbidopa and isoniazid. Biochem Pharmacol. 1980;29:707–12. doi: 10.1016/0006-2952(80)90544-4. [DOI] [PubMed] [Google Scholar]
- 33.Young SN, St-Arnaud-McKenzie D, Sourkes TL. Importance of tryptophan pyrrolase and aromatic amino acid decarboxylase in the catabolism of tryptophan. Biochem Pharmacol. 1977;27:763–7. doi: 10.1016/0006-2952(78)90517-8. [DOI] [PubMed] [Google Scholar]
- 34.Bender DA, Smith WRD. Inhibition of kynurenine hydroxylase by benserazide, carbidopa and other aromatic hydrazine derivatives: evidence for subclinical iatrogenic niacin deficiency. Biochem Soc Trans. 1978;6:120–2. doi: 10.1042/bst0060120. [DOI] [PubMed] [Google Scholar]
- 35.Chiarugi A, Carpenedo R, Molina MT, Mattoli L, Pellicciari R, Moroni F. Comparison of the neurochemical and behavioral effects resulting from the inhibition of kynurenine hydroxylase and kynureninase. J Neurochem. 1995;65:1176–83. doi: 10.1046/j.1471-4159.1995.65031176.x. [DOI] [PubMed] [Google Scholar]
- 36.Satoh T. Activation of tryptophan pyrrolase by benzylhydrazine. Acta Vitaminol Enzymol. 1975;29:283–6. [PubMed] [Google Scholar]
- 37.Cho-Chung YS, Pitot HC. Feedback control of rat liver tryptophan pyrrolase I. End product inhibition of tryptophan pyrrolase activity. J Biol Chem. 1967;212:1192–8. [PubMed] [Google Scholar]
- 38.Hardeland R. Complexity of in vivo induction of tryptophan oxygenase and tyrosine aminotransferase: effects of tryptophan metabolites. Comp Biochem Physiol. 1972;41B:39–44. doi: 10.1016/0305-0491(72)90005-3. [DOI] [PubMed] [Google Scholar]
- 39.Levkovitz Y, Ophir-Shaham O, Bloch Y, Treves I, Fennig S, Grauer E. Effect of L-tryptophan on memory in patients with schizophrenia. J Nerv Ment Dis. 2003;191:568–73. doi: 10.1097/01.nmd.0000087182.29781.e0. [DOI] [PubMed] [Google Scholar]
- 40.Gillin JC, Kaplan JA, Wyatt RJ. Clinical effects of tryptophan in chronic schizophrenic patients. Biol Psychiat. 1976;11:635–9. [PubMed] [Google Scholar]
- 41.Rosse RB, Schwartz BL, Zlotolow S, et al. Effect of a low-tryptophan diet as an adjuvant to conventional neuroleptic therapy in schizophrenia. Clin Neuropharmacol. 1992;15:129–41. doi: 10.1097/00002826-199204000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Wu H-Q, Okuyama M, Kajii Y, Pocivavsek A, Bruno JP, Schwarcz R. Targeting kynurenine aminotransferase II in psychiatric diseases: promising effect of an orally active enzyme inhibitor. Schizophrenia Bull. 2014;40(suppl 2):S2152–8. doi: 10.1093/schbul/sbt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chouinard G, Annable L, Serrano M, Charette R. A controlled study of a dopa decarboxylase inhibitor (benserazide) in the treatment of schizophrenic patients. Int Pharmacopsychiatry. 1977;12:1–8. doi: 10.1159/000468279. [DOI] [PubMed] [Google Scholar]
- 44.Chouinard G, Annable L, Young SN, Sourkes TL. A controlled study of tryptophan- benserazide in schizophrenia. Commun Psychopharmacol. 1978;2:21–31. [PubMed] [Google Scholar]
- 45.Małaczewska J, Siwicki AK, Wójcik RM, Turski WA, Kaczorek E. The effect of kynurenic acid on the synthesis of selected cytokines by murine splenocytes – in vitro and ex vivo studies. Cent Eur J Immunol. 2016;41:39–46. doi: 10.5114/ceji.2016.58815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wirthgen E, Kanitz E, Tuchscherer M, et al. Pharmacokinetics of 1-methyl-L-tryptophan after single and repeated subcutaneous application in a porcine model. Exp Anim. 2016;65:147–55. doi: 10.1538/expanim.15-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Too LK, Li KM, Suarna C, et al. Deletion of TDO2, IDO-1 and IDO-2 differentially affects mouse behavior and cognitive function. Behav Brain Res. 2016;312:102–17. doi: 10.1016/j.bbr.2016.06.018. [DOI] [PubMed] [Google Scholar]