Abstract
Background
The purpose of our study was to investigate the role of microRNA (miR)-148b in cervical cancer.
Material/Methods
The expression of miR-148b was determined in HPV-16-immortalized cervical epithelial cell line CRL-2614 cells and in cervical cancer cell line HeLa cells. The miR-148b mimics or scrambled RNA were then transfected into Hela cells. Forty-eight hours after transfection, the mRNA expression of miR-148b and DNA methyltransferase 1 (DNMT1) were confirmed. Cell proliferation ability (cell viability and colony formation ability), invasion ability, and apoptosis were assessed after transfection with miR-148b mimics or scrambled RNA, as well as the protein expression of cyclin D1 and caspase-3.
Results
The expression of miR-148b was significantly downregulated in HeLa cells compared with CRL2614 cells (P<0.05), but was statistically upregulated by transfection with miR-148b mimics compared with the cells transfected with scrambled RNA (P<0.05). Also, we found that the expression of DNMT1 was significantly decreased by transfection with miR-148b mimics (P<0.05). Additionally, miR-148b mimics significantly decreased the cell proliferation ability and invasion ability, and statistically induced apoptosis. Furthermore, the expression of cyclin D1 protein was significantly decreased and the expression of caspase-3 protein was significantly increased by miR-148b mimics compared with that in the cells transfected with scrambled RNA (P<0.05).
Conclusions
Our results suggest that overexpression of miR-148b protects against cervical cancer by inducing G1/S-phase cell cycle arrest and apoptosis through caspase-3-dependent manner, and overexpression of miR-148b might develop a therapeutic intervention for cervical cancer.
MeSH Keywords: Caspase 3, Cyclin D1, Methyltransferases, MicroRNAs, Uterine Cervical Neoplasms
Background
Cervical cancer is the fourth leading cause of cancer-related death and the second most common cancer among female malignancies worldwide [1]. There are an estimated 529 800 incident cases of cancer diagnosed and 275 100 cancer deaths annually [2]. Although the mortality of cervical cancer is decreasing in developed countries, the incidence is still high in developing countries. Approximately 80% of cases occur in less developed countries, where there are no effective screening systems [3]. The molecular mechanisms of cervical cancer still remain largely unclear in spite of extensive clinical and basic research efforts. Therefore, it is necessary to understand the molecular mechanisms involved in cervical cancer and to provide new knowledge regarding the diagnosis and treatment of cervical cancer.
MicroRNAs (miRNAs) are a group of small (21–24 nucleotides) non-coding RNAs that have been identified as oncogenes or tumor suppressors by regulating their target genes [4–7]. They have the capacity to regulate gene expression at transcriptional, post-transcriptional, or post-translational levels. It has been well demonstrated that miRNAs play significant roles in cell proliferation, apoptosis, migration, and invasion [8,9]. Hence, analysis of the entire miRNAome is becoming more important in cancer studies [10]. Screening for miRNAs that are differently profiled in both normal and cancer tissues might help to detect miRNAs involved in the pathogenesis of cancer. In addition to the role of miRNAs, DNA methyltransferases (DNMTs) also have been implicated in the development and progression of multiple forms of cancer, including cervical cancer [11,12]. It has been considered as a critical regulator for epigenetic processes of chemotherapy [13], and proved to be regulated by miR-148b in pancreatic cancer cell lines [14] and in non-small cell cancer cells [15].
The functional role of miR-148b has been investigated in several types of cancers and acts as a tumor suppressor [16–18]. However, little information is available about the functional role of miR-148b in cervical cancer. In consideration of the relationship between miR-148b and DNMT1, we speculated that miR-148b might play an important role in cervical cancer. Our study might provide new insights into cervical cancer pathogenesis and strategies for cervical cancer treatment.
Material and Methods
Cell culture
HPV-16-immortalized cervical epithelial cell line CRL-2614 cells and cervical cancer cell line HeLa cells were obtained from the American Type Culture Collection (ATTC, Manassas, VA). CRL-2614 cells were cultured in RPMI medium 1640 (Gibco BRL Life Technologies, Gaithersburg, MD). HeLa cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Life Technologies, USA). Both of the media were supplemented with 10% fetal bovine serum (FBS, Life Technologies, US), L-glutamine (Gibco BRL), 100 IU/ml penicillin (Gibco BRL), and 100 mg/ml streptomycin (Gibco BRL) at 37°C in a 5% CO2 humidified incubator.
Transfection
The miR-148b mimics and scrambled RNA were designed and synthesized by GenePharma, Inc (Shanghai, China) according to GenePharma’s recommendations. Briefly, HeLa cells were seeded onto 24-well plates at a final concentration of 100 nM. Cells at 70% confluence were transfected with mimics and scrambled RNA using Lipofectamine® RNAiMAX reagent (Invitrogen) according to the manufacturer’s instructions. Forty-eight hours after transfection, luciferase activities were determined by means of the Dual Luciferase Reporter Assay (Promega), and the cells were harvested for further analysis.
Quantitative real-time RCR (qRT-PCR)
Total RNA was prepared from both CRL-2614 cells and Hela cells with the TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. First-strand complementary DNA (cDNA) was obtained using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). TaqMan® miRNA Assays (Applied Biosystems) was used to quantify mature miR-148b expression according to the manufacturer’s instructions using the StepOne Thermocycler (Applied Biosystems). The expression of miR-148b was normalized to U6 snRNA with the comparative 2−ΔΔCT methods.
Cell viability
The status of HeLa cell viability was monitored by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl -2-H-tetrazolium bromide (MTT) assay. Briefly, HeLa cells were adjusted to 1×105 cells/cm2 with DMEM, seeded in 96-well plates, and then incubated for 12 h. After 48 h of transfection with miR-148b mimics or scrambled RNA, 20 μl of MTT solution (5 mg/ml) was added to each well and incubated for another 4 h at 37°C. Thereafter, the media were removed and replaced with dimethylsulfoxide (DMSO) to dissolve the reduced the formazan crystals. The plate was read in an enzyme-linked immunosorbent microplate reader (Bio-Rad 2550, Bio-Rad, Hercules, CA, USA) at 540 nm. Each experiment was performed in triplicate.
Colony formation assay
HeLa cells were plated in a 6-well plate and then transfected with miR-148b or scrambled RNA and incubated for 48 h. Subsequently, the cells were washed 3 times with phosphate-buffered saline (PBS) and cultured in DMEM supplemented with 10% FBS for 10 days. Finally, the colonies were fixed with methanol and stained with 0.1% crystal violet for 10 min.
Transwell invasion assay
Cell invasion assay was determined using transwell chambers (8-μm pore size) coated with diluted Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). At 48 h post-transfection with miRNA mimics or scrambled RNA, cells were incubated with serum-free medium overnight, and then 1×105 cells in 0.5 ml serum-free medium were added to the upper chamber. DMEM containing 10% FBS was added to the lower chambers as a chemotactic attractant. Cells were then incubated at 37°C in 5% CO2 for 48 h. The media were removed, and non-migrating cells were also removed. Cells that migrated to the bottom of the membranes were fixed with 4% paraformaldehyde, following by staining with 0.5% crystal violet. Invaded cells in at least 4 randomly selected fields were counted under an inverted microscope (Nikon Ti-E). The data are expressed as the means ± standard deviation (SD).
Apoptosis assay
HeLa cells were seeded at 1×105 cells/well in 6-well plates. After 48 h of transfection, the cells were collected and cell death was detected by Annexin V-FITC staining using flow cytometry. Briefly, the cell pellets were washed and incubated with 1× binding buffer containing 10 μl Annexin V-FITC and 5 μl propidium iodide (PI) for 20 min in the dark at room temperature. Annexin V positive cells were recorded as early apoptotic cells, and cells with both Annexin V and PI positive were considered as late apoptotic cells.
Western blotting
After 48 h of transfection, HeLa cells were lysed in RIPA buffer (Thermal). The cell supernatants were collected and the protein concentrations were assessed using BCA protein assays kit (Novogen, Darmstadt, Germany) according to the manufacturer’s instructions. The protein samples were then separated to 10–12% sodium dodecyl sulfate (SDS)-PAGE gels and electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Sigma-Aldrich). Thereafter, the membranes were blocked in 5% nonfat dry milk for 2 h at room temperature and probed with the following primary antibodies overnight at 4°C: anti-cyclin D1 antibody (Sigma-Aldrich) and anti-caspase-3 antibody (Sigma-Aldrich). The membranes were then incubated with appropriate horseradish peroxidase-conjugated secondary antibodies with GAPDH as a lysate loading control. Enhanced chemiluminescence (ECL) reagent (GE Healthcare) was used to visualize the immunoreactive protein bands and the density of the bands were analyzed using the Image J software package (NIH, Bethesda, MD, USA).
Statistical analysis
All experiments were carried out at least 3 times. Statistical analysis was performed using GraphPad software (GraphPad, San Diego, CA, USA). Statistical significance was defined when P<0.05. Multiple comparisons were analyzed using the analysis of variance (ANOVA) assay.
Results
Expression of miR-148b in cervical cancer cells
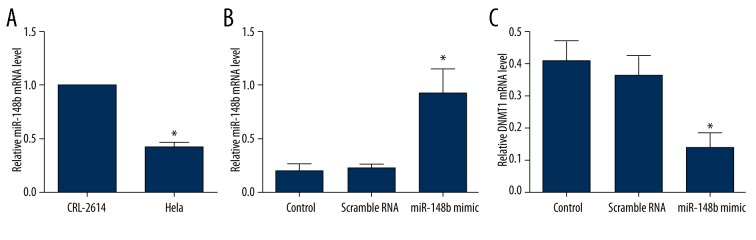
First, we determined the expression of miR-148b in human cervical cancer cell line (HeLa) and HPV-16-immortalized cervical epithelial cells (CRL2614) using qRT-PCR. U6 snRNA was used as a loading control. The results showed that the average expression of miR-148b was significantly downregulated in HeLa cells when compared with CRL2614 cells (P<0.05) (Figure 1A). To illustrate the function of miR-148b in cervical cancer, HeLa cells were transiently transfected with miR-148 mimics or scrambled RNA (negative control). We confirmed the expression of miR-148b after 48 h of transfection by using qRT-PCR. The results indicated that the expression of miR-148b was significantly upregulated by transfection with miR-148b mimics compared with the HeLa cells transfected with or without scrambled RNA (P<0.05) (Figure 1B). The mRNA expression of DNMT1was also evaluated. We found that the expression of DNMT1 was significantly decreased by transfection with miR-148b mimics compared with the HeLa cells transfected with or without scrambled RNA (P<0.05) (Figure 1C).
Figure 1.
Expression of miR-148b in cervical cancer cells. (A) TaqMan qRT-PCR detection of miR-148b expression in human cervical cancer cell line (HeLa cells) and HPV-16-immortalized cervical epithelial cell (CRL2614); (B) TaqMan qRT-PCR detection of miR-148b expression after transfection with miR-148b mimics or scrambled RNA; (C) TaqMan qRT-PCR detection of DNMT1 expression after transfection with miR-148b mimics or scrambled RNA. DNMT1, DNA (cytosine-5-)-methyltransferase 1. * P<0.05 in comparison with scrambled RNA or control group.
Effect of miR-148b mimics on cell proliferation
At 48 h after transfection, the effect of forced expression of miR-148b on cell proliferation was determined by MTT assay and colony formation assay. As shown in Figure 2, miR-148b mimics significantly decreased the cell growth activity compared with scrambled RNA or the control group (P<0.05). Additionally, the number of colony formation in HeLa cells transfected with miR-148b mimics was significantly reduced compared with scrambled RNA or the control group (P<0.05) (Figure 3). These results demonstrated that cervical cancer cell proliferation was significantly decreased by overexpression of miR-148b.
Figure 2.
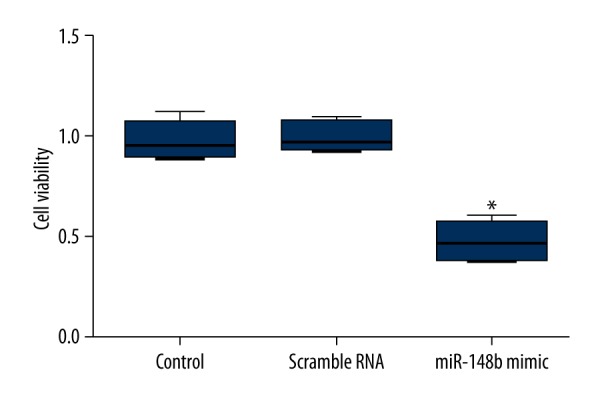
Effect of miR-148b mimics on cell viability. At 48 h of transfection, MTT analysis showed that miR-148b mimics significantly decreased the growth activity of HeLa cells. MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide. * P<0.05 in comparison with scrambled RNA or control group.
Figure 3.
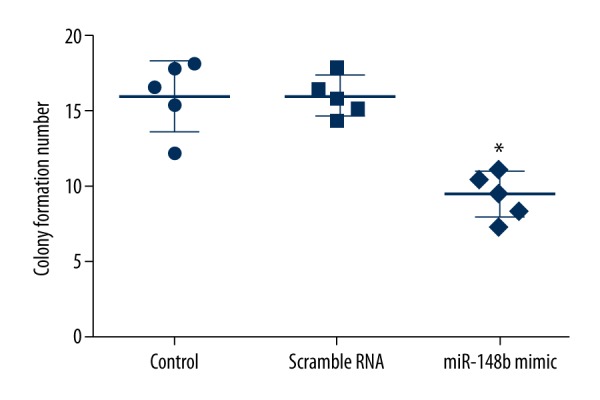
Effect of miR-148b mimics on colony formation ability. Results of the colony formation analysis demonstrated that miR-148b mimics significantly reduced the colony formation ability of HeLa cells. * P<0.05 in comparison with scrambled RNA or control group.
Effect of miR-148b mimics on invasion and apoptosis
We investigated the effect of forced miR-148b expression on invasion and apoptosis of cervical cancer cells by using the Transwell invasion assay and Annexin V-FITC staining, respectively. The Transwell invasion assay revealed that HeLa cells transfected with miR-148b mimics showed a substantially reduced invasive capacity compared with scrambled RNA or the control group (P<0.05) (Figure 4). Additionally, we observed that apoptotic rates of HeLa cells transfected with miR-148b mimics were significantly increased compared with scrambled RNA or control group (P<0.05) (Figure 5). Thus, the results indicated that miR-148b overexpression decreased the invasion capacities but increased the apoptosis of cervical cancer cells.
Figure 4.
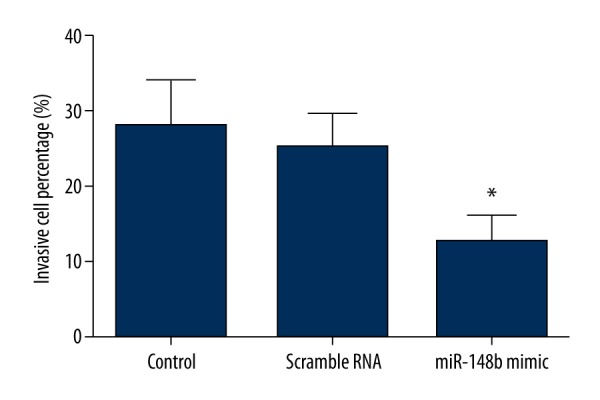
Effect of miR-148b mimics on invasion. Results of the Transwell invasion assay indicated that miR-148b mimics significantly reduced the invasion ability of HeLa cells. * P<0.05 in comparison with scrambled RNA or control group.
Figure 5.
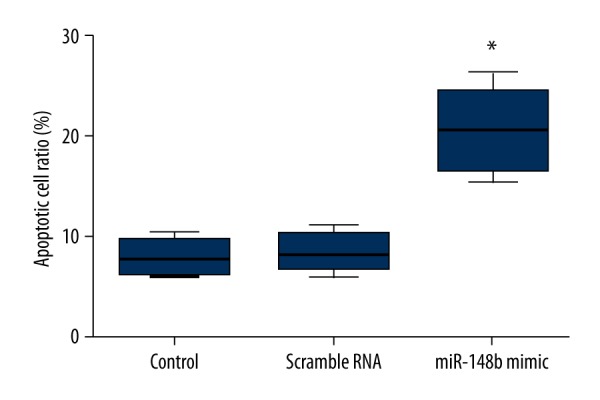
Effect of miR-148b mimics on apoptosis. Results of Annexin V-FITC staining showed that miR-148b mimics significantly elevated the apoptosis of HeLa cells. * P<0.05 in comparison with scrambled RNA or control group.
Effect of miR-148b mimics on the expression of cyclin D1 and caspase-3
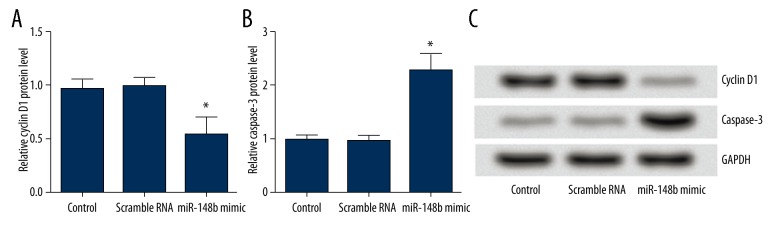
We also evaluated the underlying mechanism of cell proliferation and apoptosis. As shown in Figure 6A–6C, the results showed that the expression of cyclin D1 protein in HeLa cells transfected with miR-148b mimics was significantly decreased compared with that in the cells transfected with scrambled RNA (P<0.05). However, the expression of caspase-3 protein in HeLa cells transfected with miR-148b mimics was significantly increased (P<0.05). From these data, we concluded that overexpression of miR-148b inhibited cervical cancer cells proliferation, possibly by inducing G1/S-phase cell cycle. Overexpression of miR-148b induced apoptosis of cervical cancer cells, apparently in a caspase-3-dependent manner.
Figure 6.
(A–C) Effect of miR-148b mimics on expression of cyclin D1 and caspase-3. Results of Western blotting suggested that miR-148b mimics significantly decreased the expression of cyclin D1 protein but significantly increased the expression of caspase-3 protein. * P<0.05 in comparison with scrambled RNA or control group.
Discussion
Accumulating evidence has shown that miRNAs play significant roles in many human malignant cancers, including cervical cancer [19,20]. Several miRNAs, including miR-182 [21], miR-1246 [22], and miR-29a [23], have been reported to be implicated in cervical cancer. In the present study, we showed that miR-148b was downregulated in cervical cancer, and overexpression of miR-148b significantly decreased the expression of DNMT1. Also, overexpression of miR-148b inhibited the cell viability, growth, and invasion of cervical cancer cells, as well as the induction of cell apoptosis. These effects might occur by regulating the expression of cyclin D1 protein and caspase-3 protein.
Epigenetic alterations have been reported to be associated with the development and progression of cancers. DNA methylation is the major type of epigenetic modification, which is catalyzed by maintenance DNMT1, and/or DNMT3A and DNMT3B [24]. Dysregulated expression of DNMT1 has been implicated in multiple forms of cancer [25,26], including cervical cancer [27]. However, the exact mechanisms underlying this link are not yet well defined. One of the possible mechanisms involves the alterations in the expression of regulatory miRNAs [28]. Several miRNAs, such as miR-21, miR-148a, miR-152, miR-148b, and miR-140, have been identified as important regulators of DNMTs translation by directly and indirectly targeting DNMT1 expression [14,29,30]. MiR-148b belongs to the miR-148/152 family, which is involved in various biological processes [31]. It has been well demonstrated that miR-148b is responsible for tumorigenesis, distant metastasis, or poor prognosis in cancer [14,32,33], as well as suppression of proliferation and induction of apoptosis [16,34]. However, its expression and potential functional role in cervical cancer are still poorly understood. Considering the relationships between DNMT1 and cervical cancer, and between DNMT1 and miR-148b, we focused on the expression of miR-148b and hypothesized the functional role of miR-148b in cervical cancer.
To confirm the hypothesis, we first evaluated the expression of miR-148b in cervical cancer cell line HeLa cells. The results showed a lower expression of miR-148b in HeLa cells compared to the normal cervical cells, indicating that miR-148b might be involved in cervical cancer. We further overexpressed the level of miR-148b, and then the expression of DNMT1 was evaluated. From these data, we confirmed that DNMT1 was also regulated by miR-148b in cervical cancer, which was in line with previous studies [14,15]. Thereafter, the function role of miR-148b in cervical cancer was explored. The cell proliferation (cell viability and growth), invasion, and apoptosis were determined after transfection with miR-148b mimics. We observed that overexpression of miR-148b not only inhibited cell proliferation and invasion, but also induced apoptosis, indicating that miR-148b also serves as a potential tumor suppressor in cervical cancer. We then investigated the underlying mechanism of cell proliferation and apoptosis. The protein expression levels of cyclin D1 and caspase-3 were measured. Cyclin D1 is an important cell cycle regulator that is required for cell cycle progression in G1 [35]. Increasing evidence has demonstrated that aberrant expression of cyclin D1 is implicated in tumorigenesis and tumor progression in several human cancers, including cervical cancer [36]. Therefore, enhancement of efforts to inhibit cyclin D1 expression has received great attention as targeted therapeutics. Currently, many miRNAs have been reported to be associated with cancer by targeting cyclin D1. A previous study has suggested that miR-195 inhibits proliferation of cervical cancer cells by targeting cyclin D1a [37]. Similarly, in our study we also confirmed that miR-148b protected against cervical cancer by regulating the expression of cyclin D1. Moreover, activation of caspases leads to cleavage of proteins that are critical for cell survival [38]. Caspases-3, one of the most significant executioners, has the ability to cleave many important cellular substrates and plays a critical role in the pathogenesis and treatment of a variety of malignancies [39]. In our study, we also observed that miR-148b significantly decreased the expression of caspase-3, indicating that miR-148b acts as a tumor suppressor by inducing caspase-3-dependent apoptosis. However, further research should be performed in animal studies to confirm the efficacy of miR-148b in decreasing tumor cell proliferation and inducing apoptosis in vivo.
Conclusions
In conclusion, the present study suggests that miR-148b functions as a tumor suppressor of cervical cancer by inducing G1/S-phase cell cycle arrest and apoptosis in a caspase-3-dependent manner. Overexpression of miR-148b may be a promising new therapeutic intervention for cervical cancer.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Source of support: Departmental sources
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin H-R, Bray F, et al. GLOBOCAN 2008, Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Vol. 2010. Lyon, France: International Agency for Research on Cancer; 2010. p. 29. [Google Scholar]
- 3.Sankaranarayanan R. Overview of cervical cancer in the developing world. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S205–10. doi: 10.1016/S0020-7292(06)60035-0. [DOI] [PubMed] [Google Scholar]
- 4.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–33. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg E, Hershkovitz L, Itzhaki O, et al. Regulation of cancer aggressive features in melanoma cells by microRNAs. PLoS One. 2011;6:e18936. doi: 10.1371/journal.pone.0018936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho WC. MicroRNAs: potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int J Biochem Cell Biol. 2010;42:1273–81. doi: 10.1016/j.biocel.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Bueno MJ, Perez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle. 2008;7:3143–48. doi: 10.4161/cc.7.20.6833. [DOI] [PubMed] [Google Scholar]
- 9.Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: Key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–74. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 10.Cummins JM, Velculescu VE. Implications of micro-RNA profiling for cancer diagnosis. Oncogene. 2006;25:6220–27. doi: 10.1038/sj.onc.1209914. [DOI] [PubMed] [Google Scholar]
- 11.Wang JT, Ding L, Jiang SW, et al. Folate deficiency and aberrant expression of DNA methyltransferase 1 were associated with cervical cancerization. Curr Pharm Des. 2014;20:1639–46. doi: 10.2174/13816128113199990543. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Chen FQ, Sun YH, et al. Effects of DNMT1 silencing on malignant phenotype and methylated gene expression in cervical cancer cells. J Exp Clin Cancer Res. 2011;30:98. doi: 10.1186/1756-9966-30-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang M, Xu W, Wang Q, et al. Potential of DNMT and its epigenetic regulation for lung cancer therapy. Curr Genomics. 2009;10:336–52. doi: 10.2174/138920209788920994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azizi M, Teimoori-Toolabi L, Arzanani MK, et al. MicroRNA-148b and microRNA-152 reactivate tumor suppressor genes through suppression of DNA methyltransferase-1 gene in pancreatic cancer cell lines. Cancer Biol Ther. 2014;15:419–27. doi: 10.4161/cbt.27630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sui C, Meng F, Li Y, Jiang Y. miR-148b reverses cisplatin-resistance in non-small cell cancer cells via negatively regulating DNA (cytosine-5)-methyltransferase 1(DNMT1) expression. J Transl Med. 2015;13:132. doi: 10.1186/s12967-015-0488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song YX, Yue ZY, Wang ZN, et al. MicroRNA-148b is frequently down-regulated in gastric cancer and acts as a tumor suppressor by inhibiting cell proliferation. Mol Cancer. 2011;10:1. doi: 10.1186/1476-4598-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao G, Zhang JG, Liu Y, et al. miR-148b functions as a tumor suppressor in pancreatic cancer by targeting AMPKalpha1. Mol Cancer Ther. 2013;12:83–93. doi: 10.1158/1535-7163.MCT-12-0534-T. [DOI] [PubMed] [Google Scholar]
- 18.Ge H, Li B, Hu WX, et al. MicroRNA-148b is down-regulated in non-small cell lung cancer and associated with poor survival. Int J Clin Exp Pathol. 2015;8:800–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, Schwarz JK, Lewis JS, Jr, et al. A microRNA expression signature for cervical cancer prognosis. Cancer Res. 2010;70:1441–48. doi: 10.1158/0008-5472.CAN-09-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JW, Choi CH, Choi JJ, et al. Altered MicroRNA expression in cervical carcinomas. Clin Cancer Res. 2008;14:2535–42. doi: 10.1158/1078-0432.CCR-07-1231. [DOI] [PubMed] [Google Scholar]
- 21.Tang T, Wong HK, Gu W, et al. MicroRNA-182 plays an onco-miRNA role in cervical cancer. Gynecol Oncol. 2013;129:199–208. doi: 10.1016/j.ygyno.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Yao D, Zhao S, et al. MiR-1246 promotes SiHa cervical cancer cell proliferation, invasion, and migration through suppression of its target gene thrombospondin 2. Arch Gynecol Obstet. 2014;290:725–32. doi: 10.1007/s00404-014-3260-2. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto N, Kinoshita T, Nohata N, et al. Tumor-suppressive microRNA-29a inhibits cancer cell migration and invasion via targeting HSP47 in cervical squamous cell carcinoma. Int J Oncol. 2013;43:1855–63. doi: 10.3892/ijo.2013.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li KK, Li F, Li QS, et al. DNA methylation as a target of epigenetic therapeutics in cancer. Anticancer Agents Med Chem. 2013;13:242–47. doi: 10.2174/1871520611313020009. [DOI] [PubMed] [Google Scholar]
- 25.Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: Targeting chromatin modifications. Mol Cancer Ther. 2009;8:1409–20. doi: 10.1158/1535-7163.MCT-08-0860. [DOI] [PubMed] [Google Scholar]
- 26.Singh V, Sharma P, Capalash N. DNA methyltransferase-1 inhibitors as epigenetic therapy for cancer. Curr Cancer Drug Targets. 2013;13:379–99. doi: 10.2174/15680096113139990077. [DOI] [PubMed] [Google Scholar]
- 27.Sawada M, Kanai Y, Arai E, et al. Increased expression of DNA methyltransferase 1 (DNMT1) protein in uterine cervix squamous cell carcinoma and its precursor lesion. Cancer Lett. 2007;251:211–19. doi: 10.1016/j.canlet.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki H, Maruyama R, Yamamoto E, Kai M. DNA methylation and microRNA dysregulation in cancer. Mol Oncol. 2012;6:567–78. doi: 10.1016/j.molonc.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan W, Zhu S, Yuan M, et al. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol. 2010;184:6773–81. doi: 10.4049/jimmunol.0904060. [DOI] [PubMed] [Google Scholar]
- 30.Takata A, Otsuka M, Yoshikawa T, et al. MicroRNA-140 acts as a liver tumor suppressor by controlling NF-kappaB activity by directly targeting DNA methyltransferase 1 (Dnmt1) expression. Hepatology. 2013;57:162–70. doi: 10.1002/hep.26011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Song Y, Wang Z, et al. Altered expression of MiR-148a and MiR-152 in gastrointestinal cancers and its clinical significance. J Gastrointest Surg. 2010;14:1170–79. doi: 10.1007/s11605-010-1202-2. [DOI] [PubMed] [Google Scholar]
- 32.Wang G, Cao X, Lai S, et al. Altered p53 regulation of miR-148b and p55PIK contributes to tumor progression in colorectal cancer. Oncogene. 2015;34:912–21. doi: 10.1038/onc.2014.30. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Zheng W, Hai J. MicroRNA-148b expression is decreased in hepatocellular carcinoma and associated with prognosis. Med Oncol. 2014;31:984. doi: 10.1007/s12032-014-0984-6. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Liu GL, Liu SH, et al. MicroRNA-148b enhances the radiosensitivity of non-Hodgkin’s Lymphoma cells by promoting radiation-induced apoptosis. J Radiat Res. 2012;53:516–25. doi: 10.1093/jrr/rrs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldin V, Lukas J, Marcote MJ, et al. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–21. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 36.Bae DS, Cho SB, Kim YJ, et al. Aberrant expression of cyclin D1 is associated with poor prognosis in early stage cervical cancer of the uterus. Gynecol Oncol. 2001;81:341–47. doi: 10.1006/gyno.2001.6196. [DOI] [PubMed] [Google Scholar]
- 37.Wang N, Wei H, Yin D, et al. MicroRNA-195 inhibits proliferation of cervical cancer cells by targeting cyclin D1a. Tumour Biol. 2015 doi: 10.1007/s13277-015-4292-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Burz C, Berindan-Neagoe I, Balacescu O, Irimie A. Apoptosis in cancer: Key molecular signaling pathways and therapy targets. Acta Oncol. 2009;48:811–21. doi: 10.1080/02841860902974175. [DOI] [PubMed] [Google Scholar]
- 39.Philchenkov AA. Caspases as regulators of apoptosis and other cell functions. Biochemistry. 2003;68:365–76. doi: 10.1023/a:1023635510363. [DOI] [PubMed] [Google Scholar]