Abstract
Background
There is a paucity of data on incidence, prevalence and mortality associated with non-CF bronchiectasis.
Methods
Using Clinical Practice Research Datalink (CPRD) for participants registered between 1st January 2004 and 31st December 2013, we determined incidence, prevalence and mortality associated with bronchiectasis in the UK and investigated changes over time.
Results
The incidence and point prevalence of bronchiectasis increased yearly during the study period. Across all age groups, the incidence in women increased from 21.2/100,000 person-years in 2004 to 35.2/100,000 person-years in 2013, and in men from 18.2/100,000 person-years in 2004 to 26.9/100,000 person-years in 2013. The point prevalence in women increased from 350.5/100,000 in 2004 to 566.1/100,000 in 2013 and in men from 301.2/100,000 to 485.5/100,000 in 2013. Comparing morality rates in women and men with bronchiectasis in England and Wales (n=11,862) to mortality rates in the general population from ONS data, showed that in women the age adjusted mortality rate for the bronchiectasis population was 1437.7/100,000 and for the general population 635.9/100,000; (comparative mortality figure of 2.26). In men, the age adjusted mortality rate for the bronchiectasis population was 1914.6/100,000 and for the general population 895.2/100,000; (comparative mortality figure of 2.14).
Conclusion
Bronchiectasis is surprisingly common and is increasing in incidence and prevalence in the UK, particularly in older age groups. Bronchiectasis is associated with a markedly increased mortality.
Keywords: Bronchiectasis, Incidence, Prevalence, Mortality
Introduction
Bronchiectasis is a long term respiratory condition characterised by persistent airway infection and recurrent exacerbations in the presence of structurally abnormal bronchi. Although worldwide the commonest cause of bronchiectasis is previous acute lung infection, there is a large range of other causes and bronchiectasis could remain a relatively common condition even in high-income countries. However, there are few recent, comprehensive data on the epidemiology and associated mortality of bronchiectasis in a high-income country. No large study has been performed in the UK since the 1950s and there are only limited relevant data from non-UK sources.[1–5] Bronchiectasis can co-exist and/or be associated with other long term respiratory conditions such as chronic obstructive pulmonary disease (COPD) and asthma, as well as many non-respiratory diseases including rheumatoid arthritis and HIV infection. Data on the co-existence of bronchiectasis with these diseases is lacking but is necessary to help inform management.
Using primary care data (Clinical Practice Research Datalink (CPRD), we aimed to establish the incidence and prevalence of non-CF bronchiectasis in UK adults from 2004 to 2013. Additionally, we investigated the health care burden of bronchiectasis through mortality.
Methods
Data sources
CPRD is the world’s largest validated computerized database of anonymized longitudinal medical records for primary care.[6,7] Data comprise approximately 14 million patients with around 5.4 million of these being currently alive and registered from 660 primary care practices spread throughout the UK. Records are derived from a widely used GP software system and contain complete prescribing and coded diagnostic and clinical information as well as information on tests requested, laboratory results and referrals made at or following on from each consultation.[8] The population of patients within CPRD have been shown to be representative of the UK population with respect to age, gender and geographical distribution.[9]
Study population to determine incidence and prevalence of bronchiectasis
Incidence was determined within financial years, with individuals aged ≥18 years between 1st April 2004 and 31st March 2013 eligible for inclusion. To determine prevalence, the study population was the whole CPRD-HES linked population over the age of 18 enrolled in a CPRD practice on a specific date in each year between 2004 and 2013, stratified by age and gender.
Bronchiectasis cohort study population and follow up time
A dynamic cohort of individuals who were alive and contributing to linked CPRD at any time between 1st January 2004 and 31st December 2013 and who had a diagnosis of bronchiectasis were identified using pre-specified Read codes (on line supplement). We excluded individuals with co-existing Read codes for cystic fibrosis (CF) to reduce misclassification of CF as non-CF bronchiectasis, as well as anyone aged less than 18 years at time of diagnosis of bronchiectasis and people who only had a record of bronchiectasis posthumously. Follow-up began at the latest of the study start date, patients’ 18th birthday, the date CPRD deemed the submitting general practice was ‘up to standard’ (i.e. the practice had passed a number of quality checks and deemed suitable for research use), the date a patient joined the practice + 365 days or at bronchiectasis diagnosis (for patients who received their first bronchiectasis code after start of the practice being up to standard on or after the 1st January 2004). The additional 365 days was added to the date the patient joined the practice, in order to exclude retrospective recording of historical bronchiectasis diagnoses reported when visiting a new GP. [10] Follow-up ended at the earliest of the study end date, death, transfer out of the practice or the practices’ last data collection date. Patients who contributed at least one day of follow-up were included in the study.
Codes used to define variables of interest
Information on demographic factors (age at study start, gender, ethnicity, and individual level socio-economic status), smoking status and other diseases associated with bronchiectasis (asthma, chronic obstructive pulmonary disease (COPD), Human Immunodeficiency Virus (HIV), rheumatoid arthritis, other connective tissue diseases, inflammatory bowel disease, bone marrow transplant, hypogammaglobulinaemia and allergic bronchopulmonary aspergillosis (ABPA)) were obtained from the CPRD data using Read codes. Bronchiectasis was deemed to be idiopathic or post-infectious if it was not associated with any of the co-morbidities listed above, and it was not possible within this database to further separate those two groups.
Primary outcomes
The outcome was incident or prevalent bronchiectasis, stratified by age, gender and year.
Statistical Analyses
Incidence and Point Prevalence
Incidence rates were calculated by year, gender and age by dividing the number of new cases of bronchiectasis by the person time at risk. Point prevalence was calculated by dividing the number of currently registered patients active in the dataset who had a bronchiectasis diagnosis on or before a randomly selected date (16th February of each year (2004 to 2013)) by the total adult study population on that date. Age was grouped as those less than 30 years and then in ten-year age bands up to 80 years, then as ≥80 years.
Cohort study
The cohort of patients with prevalent bronchiectasis in the study period were used to estimate the prevalence of co-morbid illnesses potentially associated with bronchiectasis. The presence of these co-morbidities was ascertained both before and after the first bronchiectasis record.
Mortality rates
We calculated crude all-cause mortality rates by gender and 10 year age bands in the bronchiectasis population in 2010 by calculating the number of deaths in people with bronchiectasis in the linked CPRD-HES dataset in 2010 and dividing this by the midpoint bronchiectasis population in 2010. We used CPRD-HES linked patients only in this analysis. We compared results to Office of National Statistics (ONS) published data on all-cause mortality figures for the same gender and age bands in England and Wales in 2010. Additionally, using direct standardisation, we calculated comparative mortality figures separately for men and women. [11,12]
All statistical analyses were conducted using Stata version 13 (Texas).
Ethics
Ethics approval was obtained from ISAC (the Independent Scientific Advisory Committee overseeing CPRD (protocol 12_016)) and the LSHTM Ethics Committee. Following being granted ethics approval by ISAC, the length of the study was extended for a further two years (from end 2011 to 2013). This was considered a minor amendment and did not require further ISAC approval.
Results
Incidence of bronchiectasis
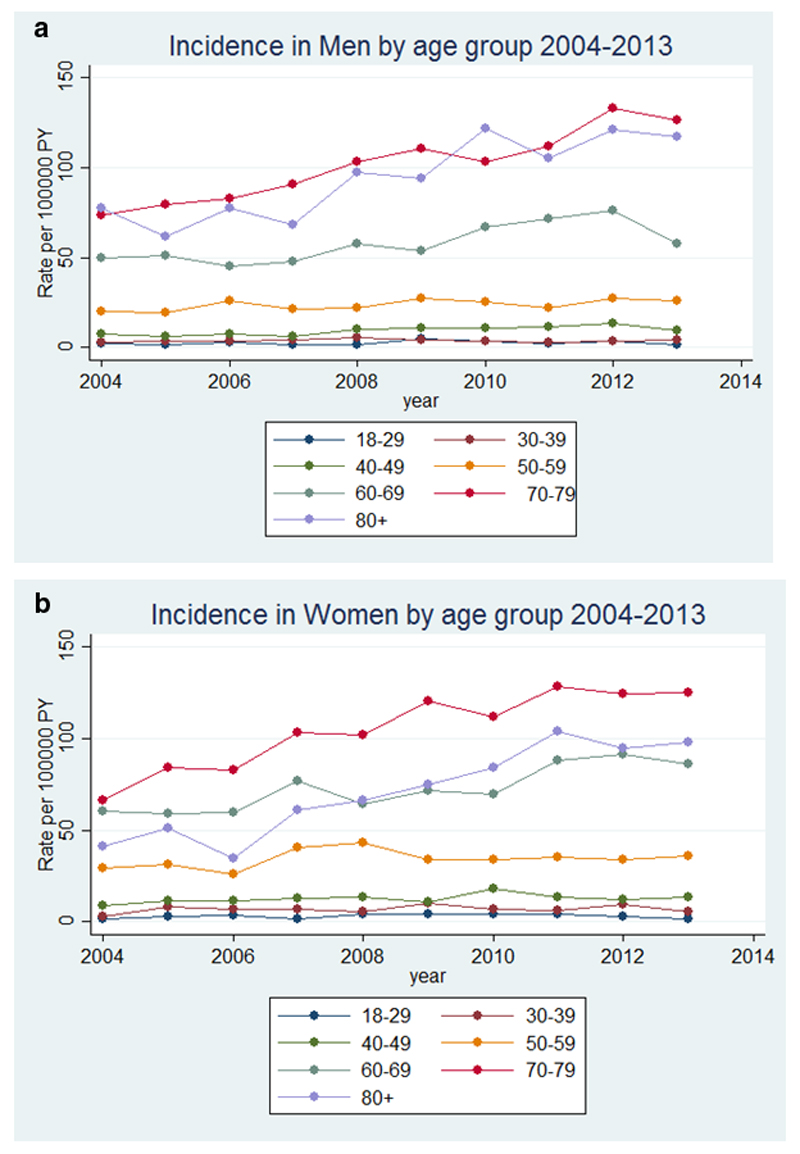
Across all age groups, the incidence of bronchiectasis in women increased overall from 21.24 per 100,000 person-years in 2004 to 35.17 per 100,000 person-years in 2013, and in men from 18.19 per 100,000 person-years in 2004 to 26.92 per 100,000 person-years in 2013. The details of age-specific rates are given in Table 1. The incidence of bronchiectasis in men and women in 2004 and in 2013 increased with increasing age, except in men in 2013 in the ≥80 age group, and in women ≥80 in 2004 and 2013 in whom the incidence decreased compared to the 70-79 year old age groups (table 1). The yearly incidence of bronchiectasis stratified by age and sex from 2004 to 2013 is shown in Figure 1.
Table 1.
Incidence of bronchiectasis in 2004 and 2013 stratified by gender and age group (confidence intervals in brackets)
| Age Groups (years) | Rate in men per 100,000 person-years | Rate in women per 100,000 person-years | Rate overall per 100,000 person-years | |||
|---|---|---|---|---|---|---|
| 2004 | 2013 | 2004 | 2013 | 2004 | 2013 | |
| 18-29 | 2.44 (0.696, 8.56) | 1.48 (0.296, 7.41) | 1.54 (0.317, 7.47) | 1.55 (0.321, 7.482) | 2.01 (0.51, 8.01) | 1.52 (0.31, 7.45) |
| 30-39 | 3.08 (1.01, 9.41) | 4.14 (1.58, 10.85) | 3.18 (1.06, 9.55) | 5.36 (2.30, 12.50) | 3.13 (1.03, 9.47) | 4.75 (1.93, 11.68) |
| 40-49 | 7.74 (3.29, 15.66) | 9.28 (4.88, 17.66) | 8.95 (4.65, 17.23) | 13.63 (8.02, 23.18) | 8.34 (4.23, 16.44) | 11.44 (6.41, 20.42) |
| 50-59 | 19.91 (12.83, 30.89) | 26.36 (18.00, 38.61) | 29.51 (20.57, 42.33) | 35.96 (25.94, 49.86) | 24.66 (16.62, 36.59) | 31.13 (21.91, 44.24) |
| 60-69 | 49.68 (37.62, 65.60) | 58.06 (44.90, 75.09 | 60.55 (47.07,77.89) | 86.07 (69.69, 106.3) | 55.17 (42.38, 71.82) | 72.31 (57.43, 91.05) |
| 70-79 | 73.82 (58.77, 92.73) | 126.66 (106.43,150.74) | 66.32 (52.14, 84.36) | 124.94 (104.86, 148.87) | 69.72 (55.14, 88.16) | 125.74 (105.59, 149.74) |
| ≥80 | 77.68 (62.20, 97.02) | 117.47 (98.05, 140.74) | 41.57 (30.68, 56.34) | 97.85 (80.27, 119.28) | 54.02 (41.38, 70.52) | 105.38 (87.08, 127.54) |
Figure 1.
Incidence of bronchiectasis in the UK from 2004 to 2013 stratified by age in a) men and b) women
Prevalence of bronchiectasis
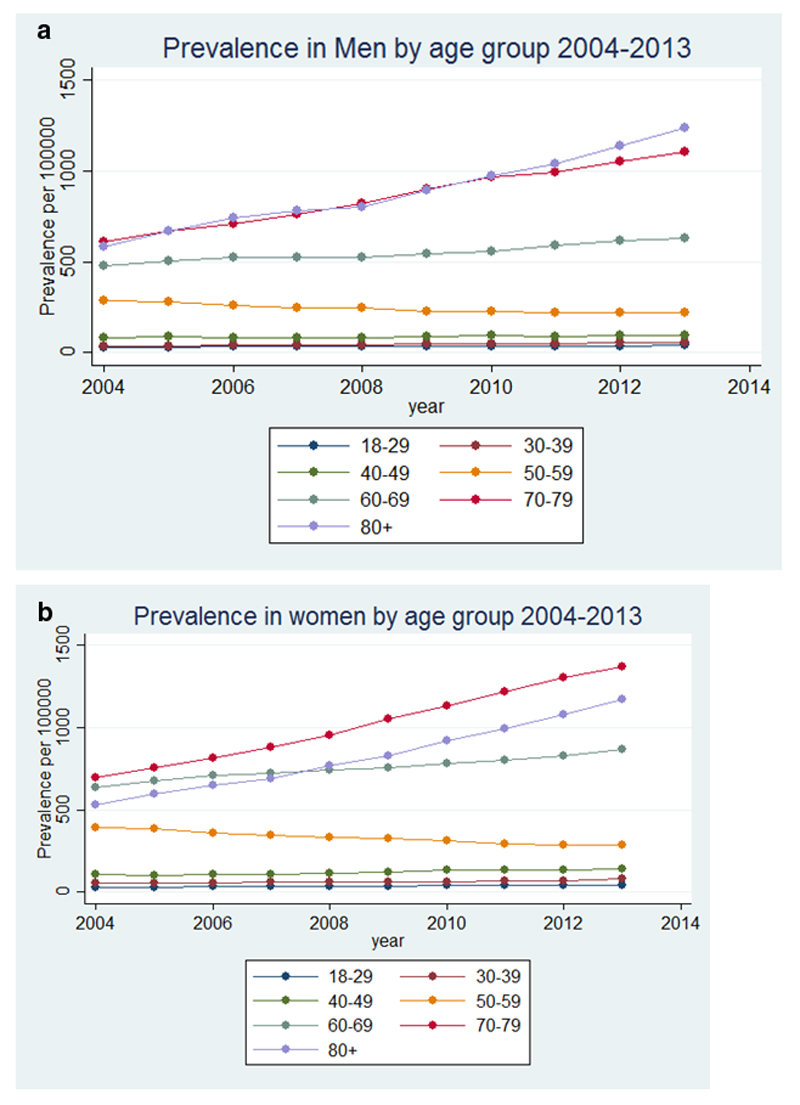
Across all age groups, the point prevalence of bronchiectasis in women increased from 350.5 per 100,000 in 2004 to 566.1 per 100,000 person-years in 2013, and in men from 301.2 per 100,000 in 2004 to 485.5 per 100,000 in 2013. The details of prevalence by age are given in Table 2. The recorded prevalence of bronchiectasis increased over time within each age group in both men and women, remaining uncommon in the under 40s but reaching a very high prevalence in the elderly of around 1% by 2013. The exception was the 50-59 year age group in whom the prevalence of bronchiectasis slowly declined for both men and women over time (Table 2 and Figure 2). The recorded prevalence of bronchiectasis was higher in men in increasing age groups in the later years of the study and in women for all age groups except in the over 80s (Figure 2).
Table 2.
Prevalence of bronchiectasis in 2004 and 2013 stratified by gender and age group (confidence intervals in brackets)
| Age Groups (years) | Prevalence in men per 100,000 | Prevalence in women per 100,000 | Prevalence Overall per 100,000 | |||
|---|---|---|---|---|---|---|
| 2004 | 2013 | 2004 | 2013 | 2004 | 2013 | |
| 18-30 | 28.8 (20.2, 33.3) | 43.3 (32.2, 58.3) | 29.7 (20.8, 42.5) | 43.6 (32.4, 58.6) | 29.3 (20.4,41.9) | 43.4 (32.3,58.4) |
| 30-39 | 38.7 (28.3, 53.0) | 58.2 (45.0, 75.2) | 53.4 (40.8, 69.8) | 79.3 (63.7, 98.8) | 46.0 (34.5,61.4) | 68.8 (54.3,87.0) |
| 40-49 | 85.2 (68.9, 105.3) | 97.4 (79.9, 118.7) | 110.7 (91.9, 133.3) | 142.1 (120.6, 167.4) | 98.0 (80.4,119.4) | 119.8 (100.2,143.2) |
| 50-59 | 286.9 (255.6, 322.0) | 224.0 (196.5, 255.3) | 391.1 (354.3, 431.7) | 285.9 (254.6, 321.0) | 339.0 (304.8,377.0) | 254.9 (225.5,288.2) |
| 60-69 | 477.4 (436.5, 522.1) | 629.3 (582.1, 680.2) | 638.4 (590.9, 689.7) | 866.0 (810.4, 925.3) | 557.9 (513.6,606.0) | 747.7 (696.1,803.0) |
| 70-79 | 608.0 (561.7, 658.1) | 1109.0 (1045.9, 1175.8) | 698.1 (648.4, 751.6) | 1370.3 (1300.1, 1444.2) | 653.0 (605.0,704.9) | 1239.7 (1172.9,1310.1) |
| ≥80 | 583.0 (537.7, 632.1) | 1236.9 (1170.2, 1307.3) | 532.3 (489.1, 579.3) | 1175.6 (1110.6,1244.3) | 557.7 (513.4,605.8) | 1206.3 (1140.5,1275.8) |
Figure 2.
Prevalence of bronchiectasis in the UK from 2004 to 2013 stratified by age in a) men and b) women
Bronchiectasis cohort study population
The bronchiectasis diagnosed study population within CPRD consisted of 18,793 individuals. Over half the individuals (58.5%) were women, and the median age at diagnosis of adult bronchiectasis was 61.8 years (Interquartile range [IQR]: 47.5 to 72.2). Among patients for whom socioeconomic status information was available (64%), as measured by Index of Multiple Deprivation, more patients belonged to the higher IMD quintile and numbers decreased with decreasing IMD. Bronchiectasis was more common in patients with higher socioeconomic status as measured by Index of Multiple Deprivation (Table 3).
Table 3.
Baseline characteristics of the bronchiectasis study population
| Characteristic | Category | Entire study population (n=18,793) (%) |
|---|---|---|
| Median age at diagnosis in years (IQR) | 61.8 (47.5-72.2) | |
| Median length of time in the study in years (IQR) | 7.2 (3.6-7.9) | |
| Gender | Male | 7798 (41.5) |
| Female | 10,995 (58.5) | |
| Smoking habit (ever, closest to diagnosis date) | Never-smoker/not recorded | 6003 (31.9) |
| Ex-smoker | 7118 (37.9) | |
| Current smoker | 5672 (30.2) | |
| Index of multiple deprivation (IMD) | 1 (Least deprived) | 3082 (16.4) |
| 2 | 3013 (16.0) | |
| 3 | 2243 (11.9) | |
| 4 | 1935 (10.3) | |
| 5 (Most deprived) | 1728 (9.2) | |
| Unavailable | 6792 (36.1) |
Co-existing co-morbidities
11,914 (63.4%) people had at least one co-existing co-morbid illness associated with bronchiectasis. Asthma was the most prevalent co-existing co-morbid illness, followed by COPD, then HIV infection, rheumatoid arthritis, and connective tissue disorders (Table 4). ABPA was only present in 1.8% of patients. Compared to those with co-morbid illnesses, patients with idiopathic or post-infectious bronchiectasis were slightly older at the time of diagnosis (62.7 years vs. 60.0; p<0.001).
Table 4.
Co-existing diagnoses associated with bronchiectasis.
| Co-existent illness | Entire study population (n=18,793) (%*) |
|---|---|
| Asthma | 7988 (42.5) |
| Chronic Obstructive Pulmonary Disease | 6774 (36.1) |
| Human Immunodeficiency Virus | 1300 (6.9) |
| Rheumatoid Arthritis | 1163 (6.2) |
| Other Connective tissue diseases | 969 (5.2) |
| Inflammatory Bowel Disease | 527 (2.8) |
| Bone Marrow Transplant | 20 (0.11) |
| Hypogammaglobulinaemia | 172 (0.9) |
| ABPA | 339 (1.8) |
| No co-morbidities above | 6422 (34.2) |
% add up to more than 100 as some patients had multiple co-morbidities
Mortality rates
Comparing age specific morality rates in men and women with bronchiectasis to age specific mortality rates calculated in the general population in England and Wales from ONS data, we found that in all age bands mortality rates were substantially higher in people with a diagnosis of bronchiectasis compared to the general population. The crude mortality rates by age group in 2010 per 1,000 population are given in Table 5. Using direct standardisation, in women the age adjusted mortality rate for the bronchiectasis population was 1437.7 per 100,000 and for the general population 635.9 per 100,000; (comparative mortality figure of 2.26). In men, the age adjusted mortality rate for the bronchiectasis population was 1914.6 per 100,000 and for the general population 895.2 per 100,000; (comparative mortality figure of 2.14). This indicates that mortality for both men and women with bronchiectasis is more than twice the mortality in the general population, independent of age differences between the two populations.
Table 5. Crude mortality rate by age group in 2010 per 1,000 population (95% CI).
Rates have been calculated using mid-year population estimate for 2010.
| Age group | Men | Women | ||
|---|---|---|---|---|
| General population | Bronchiectasis cohort | General population | Bronchiectasis cohort | |
| 18-49 | 1.3 (1.3-1.4) | 13.1 (3.4-22.8) | 0.8 (0.7-0.8) | 6.4 (0.8-12.0) |
| 50-59 | 5.1 (5.0-5.2) | 10.0 (2.6-17.3) | 3.4 (3.4-3.5) | 7.8 (2.4-13.2) |
| 60-69 | 12.5 (12.3-12.6) | 29.5 (20.6-38.4) | 7.9 (7.8-8.0) | 16.0 (10.6-21.5) |
| 70-79 | 33.77 (33.4-33.9) | 58.6 (46.4-70.7) | 22.8 (22.6-23.0) | 43.9 (34.9-52.8) |
| 80+ | 111.8 (111.1-112.5) | 144.6 (115.4-173.9) | 98.9 (98.4-99.4) | 160.1 (136.1-184.1) |
Rates have been calculated using mid-year population estimate for 2010.
Discussion
This study provides detailed estimates of the recorded incidence and prevalence of bronchiectasis in the UK over time, the prevalence of associated diseases, and an assessment of the burden on healthcare resources in terms of mortality. Data from the USA, Germany and previously from the UK have all suggested that the clinical importance of bronchiectasis is increasing, with increases in prevalence [3,13], hospital admissions [4], and mortality.[5] Our data provide additional insight beyond these papers, with the large number of patients included in the cohort and the long study period enabling us to give accurate estimates of bronchiectasis incidence and prevalence stratified by age groups and gender. Although general practices are self-selecting with regards to contributing to CPRD, they are broadly representative of the UK population,[9] and previous studies have established the validity of medical diagnoses [14,15] and prescribing records in CPRD. [16] Hence the cohort of patients included in our study is likely to be representative of patients with a diagnosis of bronchiectasis in their medical records across the UK. Overall this study provides important and previously unavailable information regarding bronchiectasis in the UK. The data are also likely to be representative for trends in other industrialised countries and are in keeping with data form a previous British Thoracic Society Bronchiectasis audit. [23]
The advent of antibiotics and improved standards of living in industrialised countries has led to large reductions in severe childhood respiratory tract infections, perhaps leading to an assumption that bronchiectasis was a disease of the past in these countries. However, we found that the recorded incidence and prevalence of bronchiectasis in the UK has increased year on year during the study period, with an increase in almost all age groups but with the most rapid increase seen in women over the age of 70 years. In the older population, bronchiectasis was a common disease affecting over 1% of both men and women aged over 70. Bronchiectasis was also surprisingly common in younger adults as well, with a prevalence in 2013 of over 40 per 100000 even in the under 30 year old population. The exception for the increasing prevalence of bronchiectasis in the UK over time is the 50-59 year old group, for which prevalence slowly declined between 2004 and 2013. The reasons for this discrepancy are not clear – one potential explanation is that a proportion of bronchiectasis in the 50-59 age group is due to childhood disease that has become less common with time. In contrast, the increase in prevalence of bronchiectasis in older age groups is driven by adult-onset causes of bronchiectasis that are increasingly affecting older age groups. The reasons for the overall increased incidence and prevalence of bronchiectasis are not clear. It may be due to changes in the prevalence of causes of bronchiectasis, but also due to improved diagnosis rates with increased use of CT scans to assess patients with lung disease. It is also possible that changes in diagnostic labelling with respect to other associated respiratory diseases has contributed to the increased incidence. Comparing these data with published data available on other respiratory diseases, the prevalence of COPD has increased by 50% from 2000 to 2009 but incidence has remained static. [17] Overall, with bronchiectasis, the increased incidence and prevalence over time is likely to be at least partly driven by increased case ascertainment and increased investigation of the elderly population. Nonetheless, age specific mortality rates were higher in people with bronchiectasis than the general population (including in younger patients), indicating that a diagnosis of bronchiectasis is associated with important health effects. Whether the increased mortality in the bronchiectasis cohort is due to complications from co-existing illnesses or directly attributable to bronchiectasis is not known. We were only able to investigate all cause mortality. It is not clear from these data whether the increased mortality in people with bronchiectasis was due to non-respiratory causes, or indeed whether mortality was higher in those with co-morbidities. More studies are needed to characterise the health burden associated with bronchiectasis by investigating GP visits for exacerbations and hospital admissions, and to investigate the mechanisms of the increased mortality of patients with bronchiectasis.
Bronchiectasis has a significant sex bias, being more common in women than in men. Bronchiectasis has a wide range of causes and associated co-morbidities. These include both asthma and irreversible airways obstruction, which given the spirometric criteria used to diagnose COPD in the UK could be misdiagnosed as COPD. Conversely, bronchiectasis is increasingly recognised as a complication of asthma and COPD. [18–20] In our cohort of patients with bronchiectasis, both asthma and COPD were very common, associated with 43% and 36% of subjects respectively. It is unclear in what proportion of these patients the diagnosis of bronchiectasis was secondary to the airways disease rather than the primary disease. One interesting finding was the fact that asthma associated bronchiectasis was more common than COPD associated bronchiectasis. While some of this may be due to misclassification of COPD as asthma in the database, it warrants further investigation. The low prevalence of ABPA associated bronchiectasis in this cohort is likely due to under recording of ABPA in primary care. One new and unexpected finding was the relatively high proportion of patients with bronchiectasis who were HIV positive (6.9%), had rheumatoid arthritis or another connective tissue disease (11.4%), or IBD (2.8%). Although these conditions are known causes of bronchiectasis, they were not prominent contributors in previous case series of bronchiectasis associations from secondary or tertiary referral centres.[21] According to national figures, the estimated prevalence of HIV is around 1.5 per 1000 of the UK population. [22] The increase in the association of HIV infection, rheumatoid arthritis / connective tissue disease, and inflammatory bowel disease with bronchiectasis in our cohort may reflect the strength of population studies for establishing more accurate information on disease associations. We believe that the CPRD population is representative and not richer in HIV than expected, it is likely that more CTs are done in the HIV population and so the rate of bronchiectasis is higher in this group. This may mean that the rate of bronchiectasis in this group is actually more accurate than in the non-HIV group.
Limitations
In order to improve sensitivity and specificity of the diagnosis only a small number of specific Read codes were used to identify subjects with bronchiectasis, and individuals had to be over 18 years of age when they were first diagnosed. As a consequence, some patients with bronchiectasis would have been excluded. Bronchiectasis was deemed to be idiopathic if it was not associated with any of the co-morbidities previously listed. While some of the bronchiectasis codes could be post infectious, we included individuals with such codes in the idiopathic bronchiectasis group as these codes were only used in 2% of our study population and over half of those patients also had a Read code for “Bronchiectasis” (see appendix). We are unable to confirm that the diagnosis of bronchiectasis in the GP records is accurate. We did not have access to CT scan data and therefore cannot be sure that that in each recorded case of bronchiectasis the diagnosis was attained according to current guidelines. However, as the diagnosis is usually made in a secondary care setting and requires a CT scan,[23,24] it is unlikely that a diagnosis of bronchiectasis will be recorded by the GP without confirmation from secondary care.
Conclusions and clinical implications
There are major deficits in our understanding of the pathophysiology and management of bronchiectasis,[25] yet our data show that bronchiectasis is increasingly common and is associated with a substantially raised mortality. Bronchiectasis remains an important cause of respiratory disease in the UK and this should be reflected in the provision of clinical care for these patients.
Acknowledgments
Funding: JKQ was funded on a MRC Population Health Scientist Fellowship (G0902135), SLT was funded by a National Institute for Health Research Career Development Fellowship [CDF 2010-03-32]. UCLH/UCL received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centre’s funding scheme. VN is funded by a National Institute for Health Research Academic Clinical Fellowship.
Footnotes
Competing Interests: None declared
References
- 1.Seitz AE, Olivier KN, Steiner CA, et al. Trends and Burden of Bronchiectasis-Associated Hospitalizations in the United States, 1993-2006. CHEST. 2010;138(4):944–949. doi: 10.1378/chest.10-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loebinger MR, Wells AU, Hansell DM, et al. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J. 2009;34:843–849. doi: 10.1183/09031936.00003709. [DOI] [PubMed] [Google Scholar]
- 3.Seitz AE, et al. Trends in bronchiectasis among medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142(2):432–9. doi: 10.1378/chest.11-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ringshausen FC, et al. Bronchiectasis-associated hospitalizations in Germany, 2005-2011: a population-based study of disease burden and trends. PloS one. 2013;8(8):e71109. doi: 10.1371/journal.pone.0071109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respiratory Medicine. 2010;104:981–e985. doi: 10.1016/j.rmed.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 6. http://www.cprd.com accessed February 28,2015.
- 7.Williams T, VanStaa T, Padmanabhan S, et al. Recent advances in utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf. 2012;3:88–99. doi: 10.1177/2042098611435911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tate AR, Beloff N, Al-Radwan B, et al. Exploiting the potential of large databases of electronic health records for research using rapid search algorithms and an intuitive query interface. J Am Med Inform Assoc. 2014;21(2):292–8. doi: 10.1136/amiajnl-2013-001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrett E, Gallagher AM, Bhaskaran K, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD) International Journal of Epidemiology. 2015:1–10. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JD, et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiology and Drug Safety. 2005;14(7):443–451. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 11. http://www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-227638 Accessed March 2015.
- 12. http://www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-315018 accessed march 2015.
- 13.Weycker D, E J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005;4:205–209. [Google Scholar]
- 14.Hansell A, et al. Use of the General Practice Research Database (GPRD) for respiratory epidemiology: a comparison with the 4th Morbidity Survey in General Practice (MSGP4) Thorax. 1999;54(5):413–9. doi: 10.1136/thx.54.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrett E, et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollowell J. The General Practice Research Database: quality of morbidity data. Popul Trends. 1997;87:36–40. [PubMed] [Google Scholar]
- 17.James GD, Petersen I, Donaldson GC, Wedzicha JA. Longitudinal changes in the rate and mean age of incidence and prevalence of COPD in the UK, 2000-2009. Thorax. 2011 Dec;66(Suppl 4):212. [Google Scholar]
- 18.Martínez-García MA, Soler-Cataluña JJ, Sanz YD, et al. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;1405:1130–1137. doi: 10.1378/chest.10-1758. [DOI] [PubMed] [Google Scholar]
- 19.Wedzicha JA, Hurst JR. Structural and functional co-conspirators in chronic obstructive pulmonary disease exacerbations. Proc Am Thorac Soc. 2007;48:602–605. doi: 10.1513/pats.200707-106TH. [DOI] [PubMed] [Google Scholar]
- 20.Ip MS, So SY, Lam WK, Yam L, Liong E. High prevalence of asthma in patients with bronchiectasis in Hong Kong. The European Respiratory Journal. 1992;5(4):418–423. [PubMed] [Google Scholar]
- 21.Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162:1277–e84. doi: 10.1164/ajrccm.162.4.9906120. [DOI] [PubMed] [Google Scholar]
- 22.Public Health England. HIV in the United Kingdom: 2013 report. 2013 https://www.gov.uk/government/collections/hiv-surveillance-data-and-management
- 23.Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65(Suppl 1):i1–58. doi: 10.1136/thx.2010.136119. [DOI] [PubMed] [Google Scholar]
- 24.Hill AT, Routh C, Welham S. National BTS bronchiectasis audit 2012: is the quality standard being adhered to in adult secondary care? Thorax. 2013 doi: 10.1136/thoraxjnl-2013-203739. [DOI] [PubMed] [Google Scholar]
- 25.De Soyza A, Brown JS, Loebinger MR, et al. Research priorities in bronchiectasis. Thorax. 2013;68:695–696. doi: 10.1136/thoraxjnl-2012-202893. [DOI] [PubMed] [Google Scholar]