Abstract
Tumor lymphangiogenesis is a major prognostic indicator of gastric cancer. Tumor‐induced inflammation has been shown to attract tumor‐associated macrophages that affect lymphangiogenesis. However, detailed mechanisms of macrophage‐induced lymphangiogenesis have not been elucidated. Here, we evaluated the interaction between tumor‐associated macrophages and lymphatic endothelial cells (LECs) derived from lymph nodes (LNs) of human gastric cancer. Lymphatic endothelial cells were directly or indirectly cocultured with macrophages from healthy human blood, with or without the supernatant of the gastric cancer cell line, OCUM‐12. We analyzed the effect of cancer pretreated macrophages and of macrophages from metastatic LNs of gastric cancer on LECs. We observed morphological changes of LECs in coculture and assessed the gene expression of possible lymphangiogenic molecules of macrophages and LECs after contact coculture, and of cancer pretreated macrophages, by quantitative RT‐PCR. Specimens of metastatic LN of gastric cancer were immunofluorescently stained. We found that tubulogenesis of LECs was observed only in the contact coculture model. OCUM‐12 cells promoted macrophage‐induced tubulogenesis of LECs. Relative gene expression of MMP and adhesion molecules was significantly upregulated in both capillary‐forming LECs and cocultured macrophages. Cancer pretreated macrophages upregulated lymphangiogenic factors including inflammatory cytokines, MMPs, adhesion molecules, and vascular endothelial growth factor‐C. Blocking of intercellular adhesion molecule‐1 and macrophage activation suppressed tubulogenesis of LECs. Immunohistochemistry showed macrophages localized around lymphatic vessels. Our results suggested that interaction between LECs and macrophages may be an important initial step of tumor lymphangiogenesis developing LN metastasis. Understanding of its mechanisms could be useful for future therapeutics of gastric cancer.
Keywords: Cancer‐related inflammation, gastric cancer, lymphangiogenesis, macrophage, tumor microenvironment
Lymphatic metastasis is one of the major processes of distant metastasis in gastric cancer.1 Tumor lymphangiogenesis is an essential process for tumor metastatic spread to LNs in various human cancers.2 The remodeling of lymphatic vessels, including the generation of new lymphatic vessels and lymphatic enlargement, requires the coordination of complex cellular events such as proliferation, migration, and tube formation, which resembles angiogenesis.3 We previously reported that lymphangiogenesis in human tumor‐draining LNs is augmented before development of overt nodal metastasis of gastric cancer.4 Our data suggested that gastric cancer cells produce lymphangiogenic factors such as VEGF‐A and ‐C, and that new lymphatic vessels are formed in tumor‐draining LNs. It is well known that there is a correlation between macrophage‐mediated inflammation and cancer initiation.5 Once tumors are established, macrophages are educated to become protumoral.6 Recent studies have shown that tumor and immune cells including TAMs release lymphangiogenic factors into the tumor microenvironment.7 Macrophages are essential metastatic promoters that act both to prepare sites for, and to promote, extravasation and growth of metastatic cells.8 Consistent with previous reports, we also showed the association of lymphangiogenesis with infiltrating TAMs of regional LNs in gastric cancer and that TAMs extended along the lymphatic flow in premetastatic nodes of gastric cancer.9 Previous studies using murine angiogenetic models have shown that macrophages function as cellular chaperones or as a bridge to guide endothelial cell anastomosis, which leads to vascular anastomosis.10, 11 However, detailed cellular mechanism of tumor lymphangiogenesis induction remains unclear. We recently identified “tumor‐associated LECs” that had the capacity to induce inflammation in tumor‐draining LNs.12 Based on these results, we hypothesized that macrophages might be directly involved in induction of tumor lymphangiogenesis in LNs. Here, we examined the interaction between TAMs and LECs and we sought to clarify whether the cancer‐related inflammatory condition could affect macrophage‐induced lymphangiogenesis.
Materials and Methods
Ethics statement
Our investigation was carried out according to the Declaration of Helsinki. This study was approved by the ethics committee of Osaka City University Graduate School of Medicine (Approval No. 3138; Osaka, Japan). Written informed consent was obtained from all of the patients.
Cell preparation
The OCUM‐12 cell line was previously generated from a scirrhous gastric cancer specimen at our department in 2004.13 OCUM‐12 cells were cultured in DMEM (Wako, Osaka, Japan) supplemented with 10% FBS (Sigma‐Aldrich, St. Louis, MO, USA) and penicillin (100 U/mL)/streptomycin (100 μg/mL) (both Wako).
Human LECs were isolated from metastatic LNs obtained from patients who had previously undergone surgery for gastric cancer at Osaka City University Hospital. Briefly, LNs were made into a single‐cell suspension. Collected cells were resuspended in EGM‐2 (Lonza, Basle, Switzerland) supplemented with penicillin/streptomycin and seeded on collagen I‐coated dishes (Corning, New York, NY, USA) that were incubated at 37°C in 5% CO2. Adherent cells were subcultured several times until a sufficient number of cells was obtained. When the cells reached approximately 80% confluence, CD31+ cells were positively isolated using magnetic cell sorting with anti‐CD31+ microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer's instructions. After several passages, LECs were isolated using an anti‐human podoplanin antibody (ReliaTech, Wolfenbüttel, Germany) as the primary antibody and an anti‐mouse IgG antibody (Miltenyi Biotec) as the secondary antibody. Monocytes were isolated from the blood of healthy donors by low density gradient centrifugation. Blood was diluted two‐fold with Hanks’ Balanced Salt solution (Sigma‐Aldrich). The diluent was layered on top of the Lymphocyte Separation Medium (PromoCell, Heidelberg, Germany) and centrifuged at 440g for 40 min. Mononuclear cells were aspirated and CD14+ monocytes were positively isolated by magnetic cell sorting, using anti CD14‐microbeads (Miltenyi Biotec); 1 × 107 monocytes resuspended in RPMI‐1640 with l‐glutamine (Wako) supplemented with heat‐inactivated 10% FBS (Sigma‐Aldrich), penicillin (100 U/mL), streptomycin (100 μg/mL), and 25 ng/mL M‐CSF (Miltenyi Biotec) were seeded into a 10‐cm culture dish. The cells were incubated for 24 h and were then used for further experiments. Stimulation with M‐CSF was continued until the end of the experiments to induce differentiation into macrophages. Macrophages were also isolated from metastatic LNs by magnetic cell sorting as described above. Macrophages were pretreated with gastric cancer cells, OCUM‐12. In brief, 2 × 106 isolated monocytes were cultured in a 10‐cm culture dish for 5 days. Differentiated macrophages were then cocultured with 1.5 × 106 OCUM‐12 cells, which had been seeded into the upper chamber of a Transwell system with a 3‐μm‐pore polycarbonate membrane filter (Millipore, Danvers, MA, USA), for the next 72 h, and were termed cancer pretreated macrophages. Control macrophages were cultured without OCUM‐12 for an additional 72 h.
Direct coculture model
The LECs were grown to confluence in collagen I‐coated dishes and were then washed twice with PBS. A total of 2 × 106 peripheral blood macrophages, cancer pretreated macrophages, or macrophages obtained from metastatic LNs were resuspended in EBM‐2 (Lonza) supplemented with 25 ng/mL M‐CSF and then individually directly cocultured with LECs at 37°C in 5% CO2 for 72 h. As a control experiment, 2 × 106 macrophages were seeded into the upper chamber of a Transwell system and were then indirectly cocultured with LECs. OCUM‐12 cells (1.5 × 106 cells) were seeded into the upper chamber of a Transwell system and were indirectly cocultured with macrophages and LECs under the same coculture condition for 72 h. Images were collected every 24 h using a phase contrast microscope (Primo Vert; Zeiss, Baden‐Württemberg, Germany). Macrophages and LECs were dissociated using anti‐human CD14 microbeads at 72 h from initiation of the coculture, and the cells were used for qRT‐PCR analysis as described later. Direct coculture of macrophages and LECs with or without OCUM‐12 cells seeded into the upper chamber was examined five times under the same culture condition. Capillary structures were microscopically inspected at ×40 magnification 72 h later, and three areas with the maximal number of capillary structures were determined. The number of closed circles, which indicates closed intercellular components of LECs, was counted and the average number was calculated, as previously described.14 Rabbit anti‐ICAM‐1 antibody (SouthernBiotech, Birmingham, AL, USA) and SN50 (Wako), an NF‐κB inhibitor, were used for blockade experiments.
Time‐lapse imaging
The LECs were cultured with EGM‐2 and grown to confluence in a 96‐well culture plate. The LEC monolayers were washed twice with PBS, and 1 × 104 macrophages resuspended in EBM‐2 supplemented with 25 ng/mL M‐CSF were seeded onto the LEC monolayer and incubated for 30 min to allow macrophages to sufficiently contact with the LECs. Subsequently, morphological changes were monitored using IncuCyte ZOOM (Essen BioScience, Ann Arbor, MI, USA) at 37°C in 5% CO2 and images were recorded at 1‐h intervals for 89 h.
Confocal microscopic imaging of capillary morphogenesis
The CellVue Claret Far Red and PKH67 Fluorescent Cell Linker Kits were obtained from Sigma‐Aldrich. Cultrex reduced growth factor basement membrane extract was obtained from Trevigen (Gaithersburg, MD, USA). The LECs were labeled with CellVue Claret and macrophages were labeled with PKH67 according to the manufacturer's instructions. Both LECs (5 × 104 cells) and macrophages (1 × 105 cells) were resuspended in EBM‐2 supplemented with 25 ng/mL M‐CSF seeded onto basement membrane extract‐coated 35‐mm glass bottom dishes and were incubated for 24 h at 37°C. Cells were fixed with 4% paraformaldehyde for 1 h, and then covered with glass using Dapi‐Fluoromount‐G (SouthernBiotech). Cells were analyzed by using an LSM 700 microscope system equipped with the objectives ×40 (Carl Zeiss, Jena, Germany). The Zen software (Carl Zeiss) was used for analysis, processing, and projection of the confocal image stacks. To detect the tubule structure, XYZ image stacks were displayed using the “Ortho Slice” function which gives a cross‐section at any location, cover slip (start point) to the outside.
Quantitative real‐time RT‐PCR analysis
Isolated normal macrophages and LECs after direct coculture for 3 days, as well as cancer pretreated macrophages, were compared with no cocultured macrophages and LECs using qRT‐PCR analysis. Total RNA derived from each of the cells was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and was used for qRT‐PCR analysis using 96‐well Extracellular Matrix and Adhesion Molecules RT2 Profiler PCR Arrays (Qiagen) and primers (TaqMan Gene Expression Assays; Applied Biosystems, Carlsbad, CA, USA) as follows: IL1‐β (Hs01555410_m1), IL‐6 (Hs00985639_m1), IL‐10 (Hs00961622_m1), IL‐12 (Hs01073447_m1), IL‐18 (Hs01038788_m1), TGF‐β (Hs00998133_m1), VEGF‐A (Hs00900055_m1), VEGF‐C (Hs00153458_m1), VEGFR‐2 (Hs00911700_m1), VEGFR‐3 (Hs01047677_m1), CXCL1 (Hs00605382_gH), CXCL2 (Hs00601975_m1), CXCL6 (Hs00605742_g1), and CXCR2 (Hs01891184_s1). Quantitative RT‐PCR was carried out using the ABI Prism 7000 (Applied Biosystems) for the RT2 Profiler PCR arrays and using StepOnePlus (Applied Biosystems) for the other samples. Data were normalized against the GAPDH (Sigma‐Aldrich) internal control transcript and were analyzed according to the comparative threshold cycle method.
Immunofluorescent staining of tissue sections
Lymph node specimens were obtained from patients with gastric cancer who underwent surgery at Osaka City University Hospital. Specimens were immunohistochemically assessed by a medical pathologist. Metastatic LNs were selected for immunofluorescent staining of macrophages and lymphatic vessels. All specimens were formalin‐fixed, paraffin‐embedded, and cut into 4‐μm‐thick sections. After deparaffinization in xylene and rehydration in a graded ethanol series, the slides were washed twice (5 min per wash) in PBS. The sections were heat treated at 120°C in an autoclave for 10 min for antigen retrieval. A non‐specific staining blocking agent (Nacalai Tesque, Kyoto, Japan) was applied to prevent non‐specific binding of antibodies. The slides were incubated with a mouse monoclonal anti‐human podoplanin antibody (dilution 1:100; Dako, Glostrup, Denmark), a rabbit polyclonal anti‐LYVE1 antibody (dilution 1:100; Abcam, Cambridge, UK), and a rabbit polyclonal anti‐CD163 antibody (dilution 1:100; Bioss, Woburn, MA, USA) at 4°C overnight and washed in PBS for 10 min. The slides were subsequently incubated with Hilyte Fluor 488‐labeled goat anti‐mouse IgG antibody (dilution 1:100; AnaSpec, Fremont, CA, USA) and Hilyte Fluor 647‐labeled goat anti‐rabbit IgG antibody (dilution 1:100; AnaSpec) for 1 h at room temperature. The sections were covered with glass using Dapi‐Fluoromount‐G (SouthernBiotech). Digital images were taken with an all‐in‐one fluorescent microscope (BZ‐8000; Keyence, Osaka, Japan).
Statistical analysis
All statistical analyses were carried out using statistical software JMP 10.0.2 (SAS, Cary, NC, USA). Acquired data were compared with the Wilcoxon rank sum test or with a two‐tailed unpaired Student's t‐test. P < 0.05 was considered statistically significant.
Results
Induction of capillary morphogenesis of LECs by contact coculture with macrophages
Lymphatic endothelial cells isolated from metastatic LNs of human gastric cancer were cobblestone in shape and showed uniform size in culture. By day 3 of culture, confluent LECs showed a cobblestone‐like appearance (Fig. 1a). We then assayed the effect of added normal monocyte derived macrophages to the confluent culture of LECs in a 10‐cm dish. When the macrophages were added to the upper chamber (“contact‐free coculture”), no marked change in the cellular shape of LECs was observed (Fig. 1a). In contrast, when macrophages were directly added into the same dish as the LECs (“contact coculture”), the LECs showed capillary formation after 72 h of incubation (Fig. 1b). Close observation at higher magnification indicated that the morphology of the LECs changed to a spindle‐like form that was likely to be stretched (Fig. 1c). Time‐lapse imaging over 72 h showed that the LECs started to gradually elongate and form capillary structures once 24 h had elapsed. The size of luminal structures was extended according to the time course (Video S1).
Figure 1.
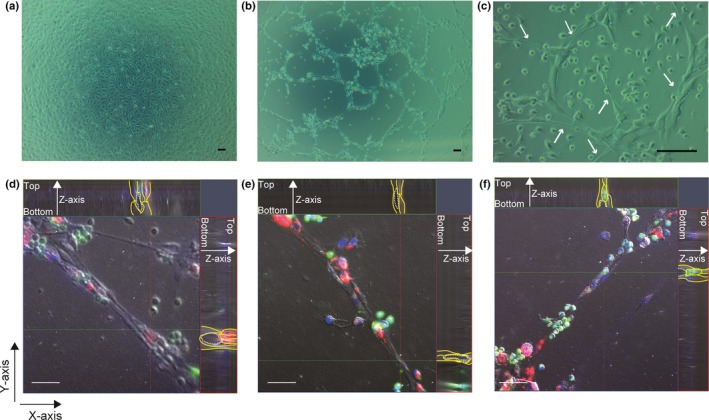
Microscopic images of coculture assays of macrophages with lymphatic endothelial cells (LECs) from metastatic lymph nodes of gastric cancer patients. Phase contrast images of the following coculture assays are shown. (a) Contact‐free coculture of normal monocyte‐derived macrophages with a confluent culture of LECs after 72 h of incubation. The LECs have grown as a cobblestone‐like monolayer. (b) Contact coculture of LECs and macrophages after 72 h of incubation. The LECs have stretched and formed macrophage‐induced capillary‐like structures. (c) Capillary structures of LECs. White arrows indicate LECs with an elongated shape. (d–f) 3‐D reconstructions from confocal Z‐stacks of immunostained LECs (red) and macrophages (green). Cultured LECs and macrophages were fluorescently labeled with CellVue Claret (red) or PKH67 (green) and were seeded onto a collagen gel‐coated glass bottom dish. LECs show the formation of tube‐like structures in vitro. Efficient formation of capillary structures incorporating macrophages was observed after 24 h of direct coculture of LECs with macrophages in vitro. Nuclear staining with DAPI (blue). Blue line, depth of Z‐axis; dashed line, inner diameter; green line, cross‐section of x‐axis; red line, cross‐section of y‐axis; yellow line, shape of cells. Scale bar = 100 μm in (a–c); 50 μm in (d–f).
To observe 3‐D visualization of the lymphatic vasculature, we combined cell labeling and confocal microscopy. Lymphatic endothelial cells labeled with CellVue Claret showed potential for de novo tube formation and formed intercellular luminal spaces. Macrophages labeled with PKH67 were incorporated into the tubes formed by LECs (Fig. 1d–f).
Impact of gastric cancer cells on macrophage‐induced capillary formation of LECs
Next we examined the effect of gastric cancer cells on the macrophage‐induced capillary formation of LECs. OCUM‐12 cells were added to the upper chamber of a Transwell culture chamber in the above‐described LEC–macrophage contact coculture system. Morphological change and capillary formation of LECs was observed using phase contrast microscopy after 72 h of incubation but not after 24 h of incubation, regardless of the presence of OCUM‐12 (Fig. 2). However, capillary morphogenesis of LECs was more apparent in the presence (Fig. 2c,d) than in the absence of OCUM‐12 cells (Fig. 2a,b). To quantitatively compare the difference between the presence and absence of OCUM‐12 cells in this assay system, we counted the number of closed circles, which indicates closed intercellular components of LECs and reflects capillary formation, in five repeated experiments under the same conditions. The number of closed circles was significantly increased in the group exposed to OCUM‐12, compared to the control group (Fig. 2e).
Figure 2.
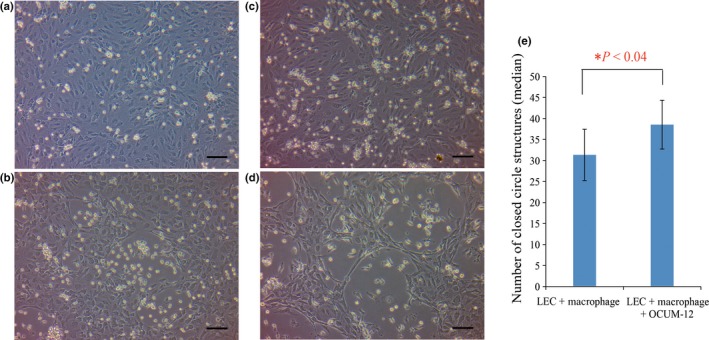
Impact of contact‐free coculture with cells of the gastric cancer cell line OCUM‐12 on macrophage‐induced capillary structures of lymphatic endothelial cells (LECs). Phase contrast images are shown of contact coculture of LECs and macrophages in the absence (a,b) or presence (c,d) of contact‐free coculture with OCUM‐12 cells for 24 h (a,c) or 72 h (b,d). There was no morphological difference in LECs after 24 h of coculture; however, after 72 h, capillary morphogenesis of LECs was more apparent in the presence (d) than in the absence (b) of OCUM‐12 cells. Scale bar = 100 μm, all panels. (e) Median number of closed ring structures in the coculture was calculated and compared after 72 h with or without OCUM‐12 cells. There was a significant increase in the number of closed rings in the coculture with OCUM‐12 cells compared to the coculture without OCUM‐12 cells (P < 0.04). Statistical comparison was by the Wilcoxon rank sum test.
To further investigate the effect of cancer cells on macrophage induction of capillary formation of LECs, we undertook a contact coculture assay using normal macrophages pretreated with cancer cells. These pretreated macrophages were then added directly into the LEC culture, which was subsequently evaluated using phase contrast microscopy for morphological changes. After a period of 72 h, we observed more capillary structures by coculture with cancer‐pretreated macrophages compared to coculture with the control, non‐pretreated macrophages (Fig. 3a,b).
Figure 3.
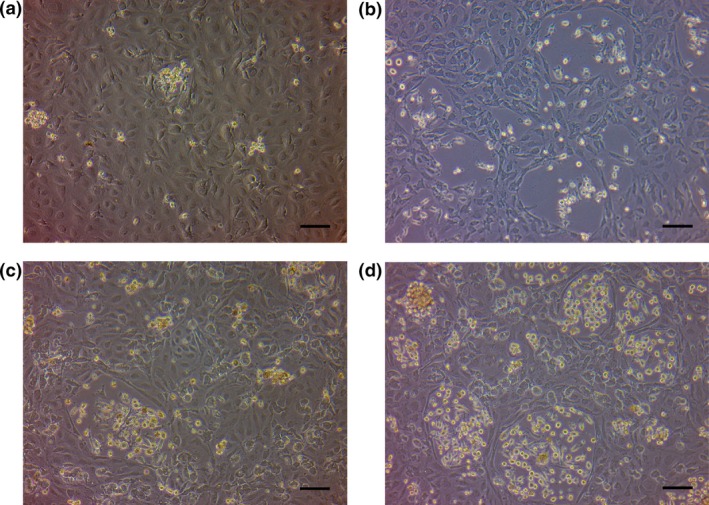
Contact coculture assays of cancer cell‐pretreated macrophages or of macrophages isolated from metastatic lymph nodes of gastric cancer, with lymphatic endothelial cells (LECs). The phase contrast images show contact coculture of LECs with control, non‐treated macrophages (a) or with OCUM‐12 cancer cell‐pretreated macrophages (b) after 72 h. Obvious capillary structures were more frequent in (b) than in (a). The phase contrast images of direct coculture of LECs with macrophages isolated from metastatic lymph nodes of gastric cancer after 72 h (c) and 96 h (d). Capillary morphogenesis similar to that observed with the pretreated macrophages in (b) was detected after 96 h. Scale bar = 100 μm, all panels.
As the macrophages in metastatic LNs should be affected by cancer cells within the nodes, we also tested the effect of isolated intranodal macrophages in the above‐described contact coculture assay with LECs. Capillary morphogenesis of LECs, similar to that induced by the OCUM‐12 cancer cell pretreated macrophages was apparent after 96 h of incubation (Fig. 3d).
Gene expression analysis of macrophages and LECs in contact coculture
To identify molecules involved in capillary formation by LECs, we compared the mRNA expression of LECs in contact and contact‐free coculture with normal macrophages using quantitative RT‐PCR. The expression of several MMPs and adhesion molecules was significantly upregulated in LECs with capillary formation compared to those without capillary formation. As shown in detail in Table 1, the mRNA expression of several MMPs, CD44, NCAM‐1, ICAM‐1, VCAM‐1, E‐selectin, and osteopontin was elevated in LECs that had formed capillaries.
Table 1.
Fold change of the gene expression of lymphatic endothelial cells directly cocultured with macrophages compared to normal lymphatic endothelial cells
| Gene groups | Fold change |
|---|---|
| Extracellular matrix molecules | |
| MMP7 | 5.77 |
| MMP9 | 138.12 |
| MMP10 | 2.30 |
| MMP12 | 8.88 |
| Cell adhesion molecules | |
| CD44 | 2.39 |
| NCAM‐1 | 2.25 |
| ICAM‐1 | 2.35 |
| VCAM‐1 | 2.27 |
| E‐selectin | 3.43 |
| Osteopontin | 121.92 |
ICAM, intercellular adhesion molecule; NCAM, neural cell adhesion molecule; VCAM, vascular cell adhesion molecule.
We also analyzed the gene expression of the cocultured macrophages themselves to examine the effect of macrophages on LEC capillary formation. The expression of the following genes was significantly upregulated in the cocultured macrophages relative to that of normal, non‐cocultured macrophages. The fold increase in expression of pro‐inflammatory cytokines was: IL‐1β, 1.3‐fold increase; IL‐6, 1.5‐fold increase; IL‐8, 3.2‐fold increase; IL‐12, 4.7‐fold increase; and IL‐18, 2.0‐fold increase. There was also a simultaneous upregulation of the anti‐inflammatory cytokine IL‐10 (2.3‐fold increase) (Fig. 4). With regard to chemokines and their receptors, CXCL2 expression was significantly upregulated (4.4‐fold increase), whereas CXCL6 (0.2‐fold decrease) and CXCR2 (0.2‐fold decrease) expression was significantly downregulated in macrophages in contact with LECs (Fig. 4).
Figure 4.
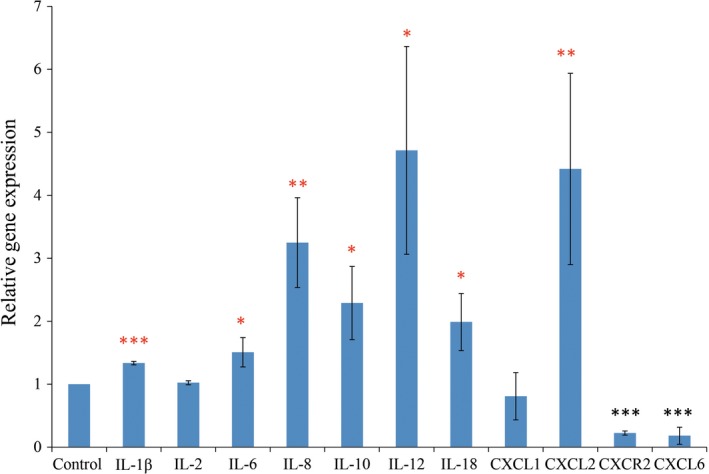
Quantitative real‐time RT‐PCR analysis of the gene expression of normal macrophages after induction of capillary structures of lymphatic endothelial cells compared to that of control macrophages. Enhanced expression of interleukin (IL)‐1β, IL‐6, IL‐8, IL‐10, IL‐12, IL‐18, and chemokine (C‐X‐C motif) ligand (CXCL)2 and downregulation of chemokine (C‐X‐C motif) receptor (CXCR)2 and CXCL6 was detected in the macrophages from the lymphatic endothelial cell contact coculture compared to the control macrophages. Statistical comparison was by a two‐tailed unpaired Student's t‐test. *P < 0.05; **P < 0.01; ***P < 0.001.
ICAM‐1 is an active participant in mediating leukocyte adhesion to endothelial cells.15 We tested the inhibitory effect of ICAM‐1 blockade on capillary formation of LECs. The ICAM‐1 blocking antibody was added into the macrophage–LEC coculture system for 72 h. As a result, morphological changes, like capillary‐forming LECs, were reduced compared with the control (Fig. 5a,b).
Figure 5.
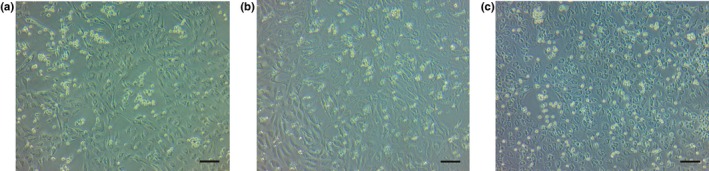
Blockade of capillary formation of lymphatic endothelial cells (LECs). The phase contrast images show contact coculture of LECs with control (a), in the presence of anti‐intercellular adhesion molecule 1 antibody (25 μg/mL) (b), and with SN50‐pretreated macrophages (c). Morphological change of LECs to capillary formation was less frequent in (b) and (c) than in (a). Scale bar = 100 μm, all panels.
Upregulation of inflammation‐related and adhesion‐related genes in macrophages by OCUM‐12 gastric cancer cells
In the next step, we used qRT‐PCR to examine the mRNA expression of macrophages that had been cocultured with OCUM‐12 cells, to evaluate whether cancer cell treatment of macrophages is involved in the upregulation of molecules that are associated with capillary formation (Table 2). Similar to the macrophages that were dissociated from the contact culture with LECs, several MMPs and inflammatory cytokines, in particular IL‐1β, IL‐6, and IL8 but not IL‐12, IL‐18, or TGF‐β, were markedly elevated in the cancer cell‐treated compared to the non‐treated macrophages. The chemokines CXCL1, CXCL2, and CXCL6, and adhesion molecules such as CD44, hyaluronan synthase 1, integrin α1 and α7, and thrombospondin 1 were also upregulated in the cancer cell‐treated compared to the non‐treated macrophages. Gene expression of VEGF‐A and VEGF‐C was additionally elevated in the cancer cell‐treated compared to the non‐treated macrophages.
Table 2.
Fold change of the gene expression of macrophages cocultured with OCUM‐12 gastric cancer cells compared to normal macrophages
| Gene groups | Fold change |
|---|---|
| Extracellular matrix molecules | |
| MMP1 | 6.34 |
| MMP7 | 6.99 |
| MMP8 | 20.46 |
| MMP10 | 2.59 |
| MMP11 | 2.96 |
| MMP12 | 44.46 |
| MMP14 | 4.97 |
| TIMP metallopeptidase inhibitor 1 | 8.60 |
| Laminin, α3 | 6.25 |
| Laminin, β1 | 2.94 |
| Versican | 143.45 |
| Cell adhesion molecules | |
| CD44 | 3.69 |
| Hyaluronan synthase 1 | 53.24 |
| Integrin, α1 | 11.83 |
| Integrin, α7 | 8.31 |
| Thrombospondin 1 | 6.94 |
| Cytokines | |
| IL‐1β | 114.31 |
| IL‐6 | 2026.25 |
| IL‐8 | 117.68 |
| IL‐10 | 1.43 |
| IL‐12 | 0.54 |
| IL‐18 | 0.15 |
| TGF‐β | 0.43 |
| Growth factors and receptors | |
| VEGF‐A | 4.98 |
| VEGF‐C | 6.45 |
| VEGFR‐2 | 0.06 |
| VEGFR‐3 | 0.22 |
| Chemokines and receptor | |
| CXCL1 | 143.84 |
| CXCL2 | 6.01 |
| CXCL6 | 1611.56 |
| CXCR2 | 0.07 |
CXCL, chemokine (C‐X‐C motif) ligand; CXCR, chemokine (C‐X‐C motif) receptor; IL, interleukin; TGF‐β IL, transforming growth factor‐β interleukin; TGF‐ receptorg; TIMP, tissue inhibitor of metalloproteases; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Expression of ICAM‐1 is mediated by the transcription factor NF‐κB.16 SN50 is a cell‐permeable peptide that blocks translocation of NF‐κB active complex into the nucleus. The results showed that capillary formation of LECs with macrophages was suppressed by SN50 treatment at the dose of 100 μg/mL (Fig. 5c).
Location of macrophages in relation to lymphatic vessels in metastatic LNs of human gastric cancer
To confirm that macrophages and LECs colocalize in tumor‐draining LNs, we used immunofluorescent staining of human metastatic LNs of gastric cancer (Figs. 6,S1). As we previously reported,4 lymphatic vessels are easily detectable in human LNs. Immunofluorescence staining using anti‐podoplanin antibody and anti‐LYVE1 antibody showed that the macrophages present in the LNs surrounded the lymphatic vessels (Fig. 6).
Figure 6.
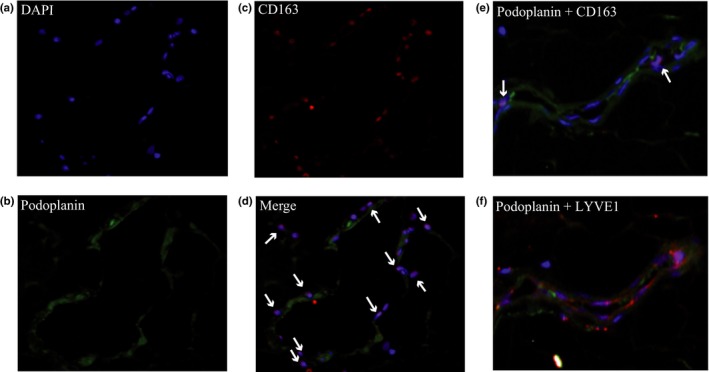
Immunofluorescent staining for intranodal macrophages adhered to lymphatic vessels of human metastatic lymph nodes. Nuclei of cells in metastatic lymph nodes were stained with DAPI (a). Images of lymphatic vessels stained with anti‐podoplanin antibody (green) (b), macrophages stained with anti‐CD163 antibody (red) (c), and merged images (d) are shown. White arrows indicate macrophages surrounding and contacting lymphatic vessels. (e) Merged images of the lymphatic vessels stained with anti‐podoplanin antibody (green) and anti‐CD163 antibody (red) are shown. White arrows indicate macrophages. (f) The same section was stained with anti‐podoplanin (green) and anti‐lymphatic vessel endothelial hyaluronan receptor (LYVE)1 antibody (red). Podoplanin‐positive cells are coexpressing LYVE1.
Discussion
In this study, we showed that macrophages directly provoked LECs to assemble into capillary‐like structures with lumens. This macrophage induction of capillary morphogenesis of LECs was augmented under the inflammatory condition caused by cancer cells. Our results suggested that macrophages in the tumor microenvironment have a very important role for the progression of gastric cancer through lymphatic vessels.
Experimental studies have shown a direct connection between macrophages and lymphangiogenesis.17 Inhibition of M‐CSF selectively suppressed tumor lymphangiogenesis in a murine model.18 However, little is known about macrophage‐induced tube formation of LECs in lymphangiogenesis in human cancer. In this study, we showed a direct effect of macrophages on the tube formation of LECs in vitro, suggesting that macrophages have the potential to provide LECs with lymphangiogenic characteristics, which involves an increase in the production of VEGF‐C. We showed that genes associated with adhesion molecules and MMPs were upregulated in capillary LECs in direct contact culture with macrophages. Adhesion molecules are involved in integrin‐mediated rolling, activation, and arrest of leukocytes including macrophages.19 Intercellular adhesion molecule‐1expressed on endothelial cells participates in the control of migration and cell polarity.20 VEGF‐A and inflammatory cytokines such as tumor necrosis factor‐α and IL‐1 increase the expression of ICAM‐1 and VCAM‐1 on endothelial cells, resulting in their increased leukocyte adhesiveness.16 CD44 is required for tubular formation of vascular endothelial cells.21 CD44 and osteopontin in endothelial cells are upregulated after stimulation with angiogenic factors and participate in cell–cell and cell–ECM interaction.21, 22, 23 MMPs also have an important role in tumor lymphangiogenesis.24 MMP‐9, which was markedly upregulated in macrophage/LEC coculture, is involved in lymphangiogenesis and promotes dissemination of metastasis.25 These mediators are mainly provided by inflammatory stromal cells including macrophages.8 We showed that blockade of ICAM‐1 and macrophage activation by NF‐κB inhibitor suppressed capillary formation of LECs. Our results indicated that direct interaction of macrophages with LECs through adhesion molecules might trigger an inflammatory condition and induce the production of MMPs in LECs to increase lymphatic vessel formation.
We then examined the characteristics of the macrophages that induce capillary formation of LECs. Classically, macrophages are polarized into M1/M2 phenotypes in response to signals from tissues.26 In general, M1 cells have an IL‐12high/IL‐10low phenotype and M2 cells have an IL‐12low/IL‐10high phenotype.27 Once in tissues, macrophages acquire distinct morphological and functional properties that are directed by the tissue and the immunological microenvironment.28 In addition, macrophages are essential contributors towards the resolution of inflammation.29 These resolution phase macrophages display an alternative M2‐like activated phenotype in which markers of M1 cells are elevated.30 In this study, macrophages in the contact coculture with LECs showed upregulation of various cytokines including IL‐1β, IL‐6, IL‐8, IL‐10, IL‐12, and IL‐18. Our results suggested that the phenotype of these macrophages that induce tubular formation of LECs might be of the “alternative M2 type” and might have the capacity to regulate developmental functions, including remodeling of the ECM, epithelial proliferation, and development of the lymphatic vascular tree. As previously reported, CD11b+ macrophages from the bronchoalveolar lavage fluid of pulmonary fibrosis patients are able to form tube‐like structures in vitro that express the LEC markers LYVE1 and podoplanin, indicating that macrophages can transdifferentiate in a stepwise fashion into LECs, initially forming cell aggregates that integrate into sprouting lymphatic vessels.31, 32 Other investigators have shown that LPS‐activated macrophages recapitulate several features of endogenous LEC progenitors.33 Our findings suggested that the expansion of the lymphatic vasculature is driven by incorporation of transdifferentiated macrophages with local LECs.
In the present study, the supernatant of OCUM‐12 gastric cancer cells promoted macrophage‐induced tubular formation of LECs. We showed that cancer pretreated macrophages upregulated several genes that have the potential to induce lymphangiogenesis (Table 2). It has been reported that versican binding with fibulin serves as a bridge for the formation of multimolecular structures that are important in the assembly of elastic fibers and angiogenesis.34, 35 Upregulation of versican and hyaluronan synthase 1 are caused by cancer‐induced inflammation and may function in the remodeling of the cancer cell ECM.36, 37 Our results suggested that the production of versican, hyaluronan, integrins, and laminin by macrophages was augmented by cancer inflammation and played an important role in promotion of the interaction of macrophages with LECs to induce tubular formation. Furthermore, our results indicated significant upregulation of pro‐angiogenic and pro‐lymphangiogenic cytokines such as IL‐1β, IL‐6, IL‐8, IL‐10, VEGF‐A, VEGF‐C, CXCL‐1, CXCL‐2, and CXCL‐6, and downregulation of IL‐12 and IL‐18 in macrophages cocultured with OCUM‐12 cells compared to normal macrophages. These results suggested that macrophages cocultured with gastric cancer cells were likely to polarize into the M2 phenotype, consistent with previous reports.38 Our findings indicated that tumor‐induced inflammation changed the phenotype of macrophages to prepare a favorable circumstance for the induction of several lymphangiogenic cytokines.
However, identification of the molecular pathways that participate in the induction of tubular formation of LECs by macrophages will be required to completely understand the regulation of macrophage induction of LEC tubular formation. In addition, it should be investigated whether macrophage‐induced lymphatic vessels have a role in the development of metastasis. The main finding of the present study was that a striking feature of TAMs was their capacity to fuse to LECs and induce differentiation of LECs into tubular structures. To the best of our knowledge, this is the first report to show that direct interaction of macrophages and LECs induced lymphatic vessel formation in gastric cancer, although further experiments will be needed to translate our understanding of cancer‐related inflammation to meaningful therapeutic advances.
In conclusion, our results revealed that macrophages may directly affect the capillary formation of LECs, not only by producing pro‐lymphangiogenic factors but also by triggering phenotypic and functional change of the LECs. Cancer cells could modulate the phenotype of the macrophages and adjust the microenvironment so that it is favorable for the promotion of malignant lymphangiogenesis. The combined data indicate that direct interaction of LECs with macrophages would be an initial step of LN metastasis. Blocking of the interaction between macrophages and LECs might be a useful therapeutic strategy to restrict the metastatic spread of gastric cancer.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- CXCL
chemokine (C‐X‐C motif) ligand
- CXCR
chemokine (C‐X‐C motif) receptor
- ICAM‐1
intercellular adhesion molecule‐1
- IL
interleukin
- LEC
lymphatic endothelial cell
- LN
lymph node
- LYVE1
lymphatic vessel endothelial hyaluronan receptor 1
- M‐CSF
macrophage colony‐stimulating factor
- MMP
matrix metalloproteinase
- NCAM‐1
neural cell adhesion molecule‐1
- NF‐κB
nuclear factor‐κB
- qRT‐PCR
quantitative real‐time RT‐PCR
- TAM
tumor‐associated macrophage
- TGF‐β
transforming growth factor‐β
- VCAM‐1
vascular cell adhesion molecule‐1
- VEGF
vascular endothelial growth factor
Supporting information
Fig. S1. Immunofluorescent staining of macrophages and lymphatic vessels of metastatic lymph nodes of human gastric cancer.
Video S1. Capillary morphogenesis of lymphatic endothelial cells induced by macrophages.
Acknowledgments
This study was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (Grant Nos. 26461990 to H.T. and 26293307 to K.H.).
Cancer Sci 107 (2016) 1101–1109
Funding Information
Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Kumagai K, Tanaka T, Yamagata K, Yokoyama N, Shimizu K. Liver metastasis in gastric cancer with particular reference to lymphatic advancement. Gastric Cancer 2001; 4: 150–5. [DOI] [PubMed] [Google Scholar]
- 2. Ji RC. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: new insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev 2006; 25: 677–94. [DOI] [PubMed] [Google Scholar]
- 3. Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014; 14: 159–72. [DOI] [PubMed] [Google Scholar]
- 4. Watanabe M, Tanaka H, Ohira M et al Intranodal lymphangiogenesis precedes development of lymph node metastasis and accelerates progression of gastric cancer. J Gastrointest Surg 2014; 18: 481–90. [DOI] [PubMed] [Google Scholar]
- 5. Noy R, Pollard JW. Tumor‐associated macrophages: from mechanisms to therapy. Immunity 2014; 41: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122: 787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol 2014; 5: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010; 141: 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Go Y, Tanaka H, Tokumoto M et al Tumor‐Associated Macrophages Extend Along Lymphatic Flow in the Pre‐metastatic Lymph Nodes of Human Gastric Cancer. Ann Surg Oncol 2016; 23 Suppl 2: S230–5. [DOI] [PubMed] [Google Scholar]
- 10. Fantin A, Vieira JM, Gestri G et al Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF‐mediated endothelial tip cell induction. Blood 2010; 116: 829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood 2011; 118: 3436–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tokumoto MW, Tanaka H, Tauchi Y et al Identification of tumour‐reactive lymphatic endothelial cells capable of inducing progression of gastric cancer. Br J Cancer 2015; 113: 1046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kato Y, Yashiro M, Noda S et al Establishment and characterization of a new hypoxia‐resistant cancer cell line, OCUM‐12/Hypo, derived from a scirrhous gastric carcinoma. Br J Cancer 2010; 102: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paschoalin T, Carmona AK, Rodrigues EG et al Characterization of thimet oligopeptidase and neurolysin activities in B16F10‐Nex2 tumor cells and their involvement in angiogenesis and tumor growth. Mol Cancer 2007; 6: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Springer TA. Adhesion receptors of the immune system. Nature 1990; 346: 425–34. [DOI] [PubMed] [Google Scholar]
- 16. Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), and E‐selectin through nuclear factor‐kappa B activation in endothelial cells. J Biol Chem 2001; 276: 7614–20. [DOI] [PubMed] [Google Scholar]
- 17. Zumsteg A, Christofori G. Myeloid cells and lymphangiogenesis. Cold Spring Harb Perspect Med 2012; 2: a006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubota Y, Takubo K, Shimizu T et al M‐CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med 2009; 206: 1089–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7: 678–89. [DOI] [PubMed] [Google Scholar]
- 20. Kevil CG, Orr AW, Langston W et al Intercellular adhesion molecule‐1 (ICAM‐1) regulates endothelial cell motility through a nitric oxide‐dependent pathway. J Biol Chem 2004; 279: 19230–8. [DOI] [PubMed] [Google Scholar]
- 21. Olofsson B, Porsch H, Heldin P. Knock‐down of CD44 regulates endothelial cell differentiation via NFkappaB‐mediated chemokine production. PLoS One 2014; 9: e90921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dai J, Peng L, Fan K et al Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009; 28: 3412–22. [DOI] [PubMed] [Google Scholar]
- 23. Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 2007; 27: 2302–9. [DOI] [PubMed] [Google Scholar]
- 24. Bruyere F, Melen‐Lamalle L, Blacher S et al Modeling lymphangiogenesis in a three‐dimensional culture system. Nat Methods 2008; 5: 431–7. [DOI] [PubMed] [Google Scholar]
- 25. Nakamura ES, Koizumi K, Kobayashi M, Saiki I. Inhibition of lymphangiogenesis‐related properties of murine lymphatic endothelial cells and lymph node metastasis of lung cancer by the matrix metalloproteinase inhibitor MMI270. Cancer Sci 2004; 95: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M‐1/M‐2 macrophages and the Th1/Th2 paradigm. J Immunol 2000; 164: 6166–73. [DOI] [PubMed] [Google Scholar]
- 27. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229: 176–85. [DOI] [PubMed] [Google Scholar]
- 28. Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol 2009; 9: 259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kataru RP, Jung K, Jang C et al Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 2009; 113: 5650–9. [DOI] [PubMed] [Google Scholar]
- 30. Bystrom J, Evans I, Newson J et al Resolution‐phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood 2008; 112: 4117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El‐Chemaly S, Malide D, Zudaire E et al Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci U S A 2009; 106: 3958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maruyama K, Ii M, Cursiefen C et al Inflammation‐induced lymphangiogenesis in the cornea arises from CD11b‐positive macrophages. J Clin Invest 2005; 115: 2363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hall KL, Volk‐Draper LD, Flister MJ, Ran S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One 2012; 7: e31794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res 2005; 15: 483–94. [DOI] [PubMed] [Google Scholar]
- 35. Zheng PS, Wen J, Ang LC et al Versican/PG‐M G3 domain promotes tumor growth and angiogenesis. FASEB J 2004; 18: 754–6. [DOI] [PubMed] [Google Scholar]
- 36. Yang W, Yee AJ. Versican V2 isoform enhances angiogenesis by regulating endothelial cell activities and fibronectin expression. FEBS Lett 2013; 587: 185–92. [DOI] [PubMed] [Google Scholar]
- 37. Siiskonen H, Oikari S, Pasonen‐Seppanen S, Rilla K. Hyaluronan synthase 1: a mysterious enzyme with unexpected functions. Front Immunol 2015; 6: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O'Brien FJ. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater Today 2015; 18: 313–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Immunofluorescent staining of macrophages and lymphatic vessels of metastatic lymph nodes of human gastric cancer.
Video S1. Capillary morphogenesis of lymphatic endothelial cells induced by macrophages.


