Abstract
Adult T‐cell leukemia/lymphoma (ATL) develops in human T‐cell leukemia virus type 1 (HTLV‐1) carriers. Although the HTLV‐1‐encoded HBZ gene is critically involved, HBZ alone is insufficient and additional, cooperative “hits” are required for the development of ATL. Candidate cooperative hits are being defined, but methods to rapidly explore their roles in ATL development in collaboration with HBZ are lacking. Here, we present a new mouse model of acute type ATL that can be generated rapidly by transplanting in vitro‐induced T cells that have been retrovirally transduced with HBZ and two cooperative genes, BCLxL and AKT, into mice. Co‐transduction of HBZ and BCLxL/AKT allowed these T cells to grow in vitro in the absence of cytokines (Flt3‐ligand and interleukin‐7), which did not occur with any two‐gene combination. Although transplanted T cells were a mixture of cells transduced with different combinations of the genes, tumors that developed in mice were composed of HBZ/BCLxL/AKT triply transduced T cells, showing the synergistic effect of the three genes. The genetic/epigenetic landscape of ATL has only recently been elucidated, and the roles of additional “hits” in ATL pathogenesis remain to be explored. Our model provides a versatile tool to examine the roles of these hits, in collaboration with HBZ, in the development of acute ATL.
Keywords: AKT, ATL, BCLxL, HBZ, mouse model
Adult T‐cell leukemia/lymphoma (ATL) is a T‐cell neoplasm with poor clinical outcomes. It develops in roughly 5% of human T‐cell leukemia virus type 1 (HTLV‐1) carriers at an advanced age.1, 2 Such a low penetrance and long latency period suggest that, in addition to HTLV‐1 infection, the accumulation of cooperative genetic or epigenetic “hits” is required for the development of ATL. Among HTLV‐1 genes, Tax and HTLV bZip factor (HBZ) are regarded as central to the development of ATL. However, although Tax has potent oncogenic activity, it is often undetectable in advanced ATL. In contrast, HBZ is expressed in all cases, suggesting a critical role for this gene in the development of ATL. 3
To elucidate the molecular mechanisms underlying ATL development, several mouse models, including Tax‐transgenic mice and HTLV‐1‐infected humanized mice, have been developed; however, these models are not suitable for analyzing the roles of HBZ in ATL.4, 5 HBZ‐transgenic mice are unique in that they develop ATL‐like disease,6 although the disease develops only after a prolonged latency period and with low penetrance, again suggesting the requirement of cooperative “hits.”
To study candidate cooperative genes and their involvement in the development of ATL, models generated by crossing genetically modified mice with HBZ‐transgenic mice are required; however, this approach is laborious and time‐consuming. Therefore, a new mouse model of ATL would facilitate the study of molecular mechanisms underlying the development of ATL.
We have developed a method to rapidly generate mouse models of T‐cell neoplasms by transplanting in vitro‐generated, gene‐transduced T cells.7 We used this method to examine the cooperation of HBZ with Akt and BCLxL and the effect of Ink4a/Arf loss in the development of acute ATL.
Materials and Methods
Induction of T cells in vitro, transduction of retroviral genes, and transplantation
Ink4a/Arf‐null mice (B6.129‐Cdkn2a tm1Rdp/Nci) were obtained from the National Cancer Institute (Frederick, MD, USA). Fetal liver cells isolated from Ink4a/Arf‐null mice (14 days post‐coitum) were depleted of Ter119‐positive cells and cocultured with an X‐irradiated (15 Gy) OP9‐DL1 stromal cell (RIKEN BRC, Tsukuba, Japan) layer in a 6‐well culture plate in Iscove's modified Dulbecco's medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FCS, in the presence of mouse FMS‐like tyrosine kinase 3 (Flt3)‐ligand (5 ng/mL; PeproTech, Rocky Hill, NJ, USA) and 0.5% culture supernatant from the mouse interleukin‐7 (IL7)‐producing cell line J558L‐IL7 (provided by Dr. A.G. Rolink, University of Basel, Basel, Switzerland), as previously described.7 Cells were harvested and seeded at 5 × 104 cells/well onto a fresh OP9‐DL1 layer every 7 days (Fig. 1a). Cells were infected with retrovirus on day 15 and transplanted (5–10 × 106 cells/well) i.v. into irradiated (2.5 Gy) NSG mice (NOD.Cg‐PrkdcscidIl2rgtm1Wjl/SzJ; Jackson Laboratory, Bar Harbor, ME, USA) or irradiated (15 Gy) C57BL6 mice (Charles River, Atsugi, Japan) 28 days after initiation of the culture, together with 1 × 106 fresh bone marrow cells for radioprotection. A total of 5–10 × 106 cells obtained from the thymuses, spleens, or tumors of primary recipient mice were used for secondary transplantations. All animal experiments were carried out according to protocols approved by the Institutional Animal Care and Use Committee at the Aichi Cancer Center (Nagoya, Japan).
Figure 1.
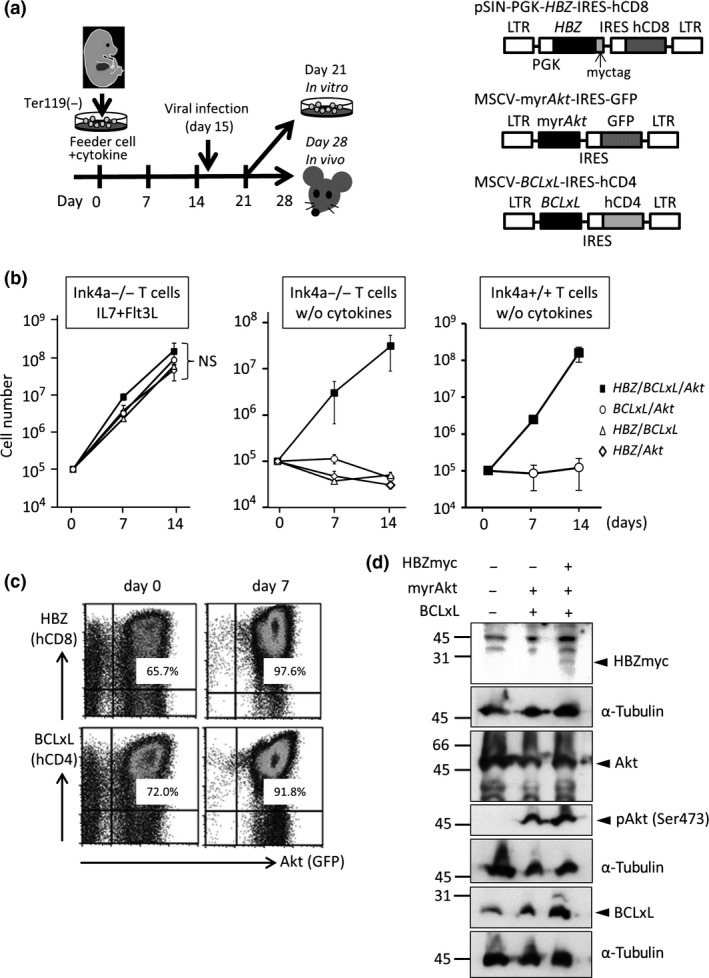
Synergy of HBZ, Akt, and BCLxL in the proliferation of Ink4a/Arf‐null T cells in vitro. (a) Scheme for the induction of T cells and retroviral infection (left), and schematic drawings of the retrovirus vectors for HBZ, myristoylated Akt, and BCLxL (not to scale) that co‐express surrogate markers human (h)CD8, GFP, and hCD4, respectively (right). These markers allow the identification of genes transduced in a given cell. (b) Growth of Ink4a/Arf‐null T cells transduced with the indicated genes in the presence (left) or absence (middle) of cytokines (interleukin‐7 [IL7] and FMS‐like tyrosine kinase 3 [Flt3]‐ligand) in bulk culture on OP9‐DL1 stromal cells. Results using Ink4a/Arf‐proficient T cells in the absence of cytokines are also presented (right). (c) Expression of hCD8, GFP, and hCD4 before (left) and 7 days after (right) the initiation of culture in the absence of cytokines. (d) Expression of transduced genes in T cells. Ink4a/Arf‐null T cells were transduced with the indicated genes as in (a), and subjected to Western blot analysis for the expression of myc‐tagged HBZ, Akt, phospho‐Akt (Ser473), and BCLxL. Anti‐α‐tubulin blots were included as loading controls.
Cell growth assay
In vitro‐induced T cells were grown on an OP9‐DL1 stromal cell layer for 7 days after gene transduction and then subjected to a growth assay. Cells were seeded at a density of 1 × 105 cells/well in a 6‐well culture plate in which OP9‐DL1 cells had been cultured to confluency and irradiated (15 Gy), and were cultured in Iscove's modified Dulbecco's medium supplemented with 10% FCS in the presence or absence of cytokines (5 ng/mL Flt3 ligand and 0.5% culture supernatant of IL‐7‐producing cell line J558L‐IL7). Cells cultured in triplicate were then counted 7 and 14 days after the initiation of culture.
Retroviral plasmids
cDNA of the HBZ gene and 35 bp of the 5′‐non‐coding region was amplified by nested PCR using an HTLV‐1 plasmid (a kind gift of Dr. N. Ishida, Tokyo University, Tokyo, Japan) as a template and inserted into the NotI/XhoI site of a pSIN‐PGK‐IRES‐human (h)CD8 plasmid (constructed by inserting a PGK‐IRES‐hCD8 cassette into a pSIN vector purchased from Takara Bio, Kusatsu, Japan). Primer sets used were as follows: 5′‐AGCGGCAGAACGCTTCAACCGGCGTGGATGGCGGCCTCAGGGCTGTT‐3′/5′‐TTATTGCAACCACATCGCCTCCAGCCTC‐3′ and 5′‐CCGGAATTCCTCGAGAGGCGGGGAGCGGCAGAACGCTTCAACCG‐3′/5′‐TTATTGCAACCACATCGCCTCCAGCCTC‐3′. The cDNA for myristoylated Akt 7 was used to express active Akt along with GFP in an MSCV retrovirus vector. The cDNA for BCLxL, kindly provided by Dr. Nunez G, University of Chicago, IL, USA8 was inserted into the EcoRI site of the MSCV‐IRES‐hCD4 vector.7
Flow cytometric analysis and cell sorting
Biotinylated antibodies against hCD8 (HIT8a) and mouse CD4 (GK1.5) in conjunction with streptavidin–allophycocyanin and phycoerythrin‐conjugated anti‐hCD4 (RPA‐T4) and anti‐mouse CD8 (53‐6.7) antibodies (all from eBioscience, San Diego, CA, USA) were used. Flow cytometry was carried out using a FACSCalibur instrument (BD Biosciences, Franklin Lakes, NJ, USA) and FlowJo software version 7.6.5 (Tree Star, Ashland, OR, USA).
Western blot analysis
Rabbit mAb for mouse and human BCLxL (54H6 #2764), rabbit mAb for mouse, rat, and human Akt (C67E7 #4691), rabbit polyclonal antibody for mouse, rat, and human phospho‐Akt (Ser473)(#9271), rabbit mAb for mouse and human glycogen synthase kinase (Gsk)3β (27C10 #9315), rabbit mAb for mouse and human phospho‐Gsk3β (Ser9 5B3 #9323), rabbit mAb for mouse and human p70 S6 kinase (49D7 #2708), rabbit mAb for mouse and human phospho‐p70 S6 kinase (108D2 #9234) (all from Cell Signaling Technology, Danvers, MA, USA), rabbit polyclonal antibody against a myc tag (A14; from Santa Cruz Biotechnology, Dallas, TX, USA), and mouse mAb reactive to mouse and human α‐tubulin (DM1A #T9026; Sigma‐Aldrich, St. Louis, MO, USA) were used.
Analysis of clonality of cells
Clonality was assessed by PCR amplification of the T‐cell receptor β gene (Dβ2‐Jβ fragment), as described previously.7
Statistical analyses
Statistical analyses were carried out using EZR software.9
Results
HBZ facilitates cytokine‐independent growth of Akt/BCLxL‐transduced T cells in vitro
We searched for genes and other factors that might function in cooperation with HBZ, and chose Akt and the loss of Ink4a/Arf for further evaluation. Akt is activated in most ATL cells, in part due to epigenetic silencing of NDRG2 10 and mutations in CCR4.11 Ink4a/Arf is often lost in acute ATL, and this loss is associated with disease progression.12, 13 T cells were induced from fetal liver cells of Ink4a/Arf‐null mice on OP9‐DL1 stromal cells in the presence of cytokines (Flt3‐ligand and IL‐7). Flow cytometric analysis of cells in culture (Fig. S1a) revealed that, although T cells were barely detectable at the beginning of culture, almost all cells 21 days after the initiation of culture were Thy1‐positive T cells, with CD4+CD8−, CD4+CD8+, and CD4−CD8+ T cells accounting for approximately 20%, 10%, and 3% of cells, respectively. The bulk T cells in culture were then retrovirally transduced with genes (Fig. 1a, left panel). Our initial attempt to confer a growth advantage to Ink4a/Arf‐null T cells by expressing HBZ and Akt was unsuccessful. In the presence of cytokines (FL and IL‐7), the cells grew comparably with control cells. In the absence of cytokines, the cell number declined over time as did that of the control cells, suggesting cell death. We therefore searched for anti‐apoptotic genes whose expression was elevated in ATL cells compared with normal T cells. Examination of our gene expression data revealed significantly (P < 0.05) elevated expression of BCLxL among the BCL2 family genes (BCL2, BCLxL, MCL1, BCL‐W, and BCL2A1) in acute ATL cells relative to that in normal CD4 T cells (Fig. S2).12 Next, therefore, we examined the effects of a combination of HBZ, Akt, and BCLxL, as well as Ink4a/Arf loss, on the growth of T cells in vitro. Retrovirus vectors co‐expressed the extracellular domains of human (h)CD8, hCD4, and GFP as surrogate markers for HBZ, BCLxL, and Akt, respectively (Fig. 1a, right panel), to facilitate the identification of gene(s) transduced in every single cell by flow cytometry. Cells were then cultured on stromal cells and monitored for growth. In the presence of cytokines, the total number of bulk T cells transduced with the three genes (HBZ, Akt, and BCLxL) was not significantly different than those transduced with any combination of two genes (Fig. 1b, left panel). In contrast, in the absence of cytokines, the total number of bulk T cells transduced with the three genes (HBZ, Akt, and BCLxL) increased over 14 days, whereas the number of T cells transduced with any two of the three genes (Fig. 1b, middle panel), as well as the vector‐only control and uninfected control cells (Fig. S1b), decreased over time. This suggests cooperation between the three genes in cytokine‐independent growth. Flow cytometric analysis showed that, whereas HBZ/Akt/BCLxL triply transduced cells accounted for, at most, approximately 66% of cells in culture on day 0, they accounted for 90% or more on day 7, which is further evidence of cooperation between these three genes (Fig. 1c). Expression of HBZ, elevated expression of BCLxL, and the presence of activated Akt in cultured T cells were confirmed by Western blotting (Fig. 1d). Activation of Akt was additionally confirmed by the fact that Gsk3β, a direct target of Akt, was phosphorylated, although phosphorylation of a downstream, indirect target of Akt, p70s6k, was not appreciably augmented (Fig. S3a).10, 14 HBZ/Akt/BCLxL triply transduced T cells proliferated more in a Ink4a/Arf −/− than in a Ink4a/Arf +/+ genetic background (Fig. S3b), and were more viable than HBZ/Akt doubly transduced T cells (Fig. S3d), although inhibition of caspase 3 cleavage was minimal (Fig. S3c) in the culture conditions used.
Although the combination of HBZ/Akt/BCLxL was required for cytokine‐independent growth, loss of Ink4a/Arf seemed dispensable, as the use of Ink4a/Arf‐proficient wild‐type T cells generated in vitro for the transduction of HBZ, Akt, and BCLxL yielded a similar result to that obtained using Ink4a/Arf‐null T cells (Fig. 1b, right panel).
Development of acute ATL‐like disease in mice transplanted with T cells transduced with HBZ, Akt, and BCLxL
Next, we examined tumorigenic activity associated with the combination of HBZ/Akt/BCLxL and Ink4a/Arf‐loss in vivo. To this end, Ink4a/Arf‐null T cells were transduced with the three genes in vitro, and bulk T cells were transplanted into sublethally irradiated NSG mice. All NSG recipient mice (n = 7) developed leukemia (n = 4) or died (n = 3) within 104 days of transplantation (Fig. 2a, Table 1). Although all five mice transplanted with Akt/BCLxL doubly transduced Ink4a/Arf‐null bulk T cells died, latency was significantly prolonged compared to that of mice transplanted with HBZ/Akt/BCLxL triply transduced Ink4a/Arf‐null T cells (P = 0.0014). NSG mice transplanted with Ink4a/Arf‐proficient, HBZ/Akt/BCLxL triply transduced T cells (n = 6) developed leukemia (n = 3) or died (n = 3), with latency comparable to that observed in Ink4a/Arf‐null, HBZ/Akt/BCLxL triply transduced T cells (Fig. 2a), again suggesting a non‐essential role for Ink4a/Arf loss in the development of disease in our experimental conditions (see also Fig. 1b).
Figure 2.

Development of adult T‐cell leukemia‐like disease in NSG mice. (a) Kaplan–Meier analysis of the probability of disease‐free survival. Ink4a/Arf‐null or ‐proficient T cells were transduced with the indicated genes and transplanted into irradiated NSG mice. The difference in probabilities was evaluated statistically using the log–rank test. N.S., not significant. (b) Flow cytometric analysis of cells before and after transplantation for the expression of Akt, BCLxL, and HBZ (represented by GFP, human [h]CD4, and hCD8, respectively) and for the expression of mouse CD4 and mouse CD8. PB, peripheral blood of mouse #4; Thy, thymus. (c) “Flower cell”‐like T cells in the thymus and peripheral blood. (d) Histology of the indicated organs by HE staining.
Table 1.
Features of NSG mice transplanted with HBZ/Akt/BCLxL triply transduced Ink4a/Arf‐null T cells
| Mouse ID | Dpt | HBZ/Akt/BCLxL triple‐positive cells (%) | CD4+CD8− †, % | CD4−CD8+ †, % | CD4+CD8+ †, % | Spl. weight, mg | Flower cell‡ | Observation | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BM | Thy | Spl | PB | ||||||||
| #1 | 104 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | N.E. | Found dead |
| #2 | 104 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | N.E. | Found dead |
| #3 | 49 | n.d. | 97.5 | 83.0 | 40.3 | 10.8 | <0.1 | 89.2 | 216 | + | Submandibular tumor§ |
| #4 | 75 | 0.3 | 99.0 | N.E. | 23.0 | 80.8 | <0.1 | <0.1 | N.E. | + | No discernible spleen |
| #5 | 41 | 0.9 | <0.1 | <0.1 | 26.4 | 8.3 | 20.5 | 60.2 | n.d. | + | |
| #6 | 21 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | N.E. | Found dead |
| #7 | 49 | 99.9 | 98.6 | 61.0 | 92.4 | <0.1 | 83.4 | <0.1 | 229 | − | |
†Percentages of CD4/CD8 represent those of cells in submandibular tumor (#3), thymus (Thy) (#4), peripheral blood (PB) (#5), and bone marrow (BM) (#7). ‡Flower cell‐like cells were present (+) in submandibular tumor (#3), Thy and PB (#4), and PB (#5). §Percentage of HBZ/Akt/BCLxL triple‐positive cells in the tumor was 98.5%. −, Not present; Dpt, days post‐transplantation; n.d., not done; N.E., not evaluable; Spl, spleen.
Flow cytometric analysis of cells from a diseased mouse (#4 in Table 1) revealed the predominance of HBZ/Akt/BCLxL triply transduced cells in the thymus (>99%) and peripheral blood (~23%), most of which were CD4+CD8− cells (80.8% in the thymus and 71.1% in the peripheral blood) (Fig. 2b). Of particular note, there were atypical lymphocytes with multiply cleaved nuclei that resembled “flower cells” typically seen in acute ATL in humans (Fig. 2c).15 The cells infiltrated into various organs, including the lungs and liver (Fig. 2d). Skin appearance was not evidently affected. The flower cell‐like cells were also noted in mice #3 and #5, but not in #7 (Table 1). CD4+CD8− cells were not predominant in mice #3 (Fig. S4), #5, or #7, but this is consistent with the observation that human ATL cases can show CD4+CD8+ and/or CD4−CD8+ phenotypes.16 NSG mice transplanted with Ink4a/Arf‐proficient, HBZ/Akt/BCLxL triply transduced T cells also presented with the flower cell‐like cells (Table 2; Fig. S5).
Table 2.
Features of NSG mice transplanted with HBZ/Akt/BCLxL triply transduced Ink4a/Arf +/+ T cells
| Mouse ID | Dpt | HBZ/Akt/BCLxL triple‐positive cells, % | CD4+CD8− †, % | CD4−CD8+ †, % | CD4+CD8+ †, % | Spl weight, mg | Flower cell | Observation | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BM | Thy | Spl | PB | ||||||||
| #1N | 64 | 64.2 | n.d. | 68.6 | 61.2 | 12.4 | 1.0 | 85.4 | 77 | + | |
| #2N | 64 | 76.2 | n.d. | 45.4 | 67.7 | 28.9 | 1.4 | 61.8 | n.d. | + | |
| #3N | 20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 216 | N.E. | Found dead |
| #4N | 35 | <0.1 | 78.8 | 0.3 | 0.4 | 89.9 | 0.4 | 9.5 | N.E. | + | |
| #5N | 32 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | N.E. | Found dead |
| #6N | 67 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | N.E. | Found dead |
†Percentages of CD4/CD8 represent those of cells in spleen (Spl) (#1N and #2N) and thymus (Thy) (#4N). ‡Flower cell‐like cells were present (+) in Spl (#1N and #2N) and Thy (#4N). BM, bone marrow; Dpt, days post‐transplantation; n.d., not done; N.E., not evaluable; PB, peripheral blood.
To examine the leukemia‐propagating activity of HBZ/Akt/BCLxL triply transduced T cells in primary recipient mice, submandibular tumor cells obtained from mouse #3 (Table 1) and thymocytes from mouse #4 (Table 1) were transplanted into C57BL6 mice (n = 2 and n = 3, respectively). The secondary recipient mice rapidly succumbed to leukemia within 25 days (Fig. 3a). Although four mice were found dead, we were able to analyze the single remaining mouse (a recipient of cells from mouse #3), and found that the leukemia cells massively infiltrated various organs, including the bone marrow, thymus, spleen, lungs, and liver (Fig. 3b–d). Taken together, these findings reveal the leukemia‐propagating activity of HBZ/Akt/BCLxL triply transduced T cells.
Figure 3.
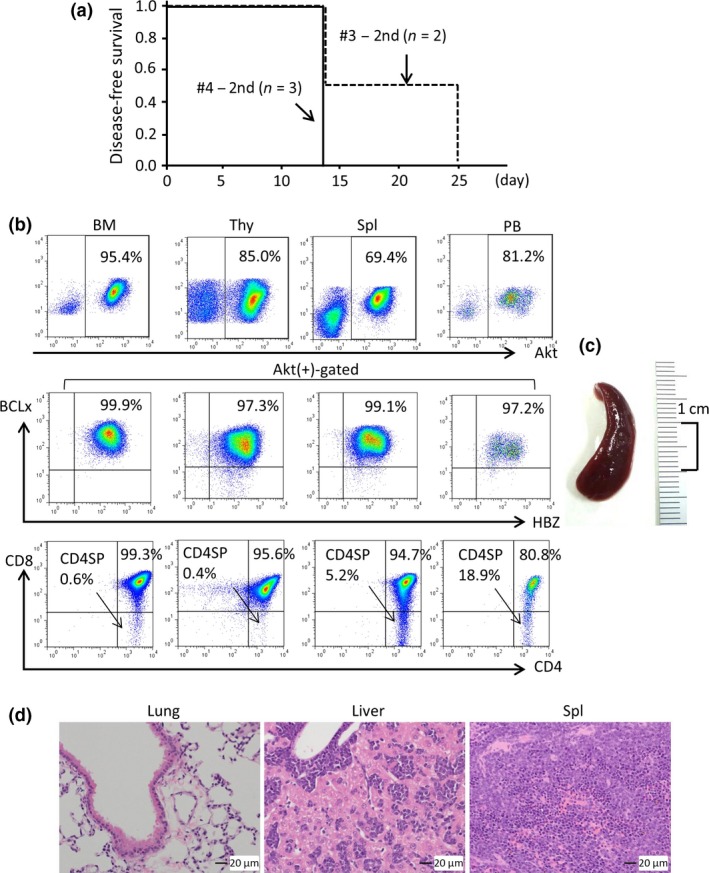
Analysis of mice secondarily transplanted with tumor cells. Cells obtained from a submandibular tumor (mouse #3) and thymus (mouse #4) were secondarily transplanted into two and three C57BL6 mice, respectively. (a) Kaplan–Meier plot for the probability of disease‐free survival. (b) Flow cytometric analysis of cells obtained from the indicated organs of a mouse that received a transplant of tumor cells from mouse #3. (c, d) Splenomegaly (c) and histology of the indicated organs by HE staining (d) of the mouse analyzed in (b) are shown.
The expression of exogenously transduced HBZ, phosphorylated Akt, and BCLxL was evident in tumor cells (Fig. 4a). Analysis of the clonality of tumors by PCR amplification of the Dβ2‐Jβ fragment of the T‐cell receptor β revealed the mono‐ or oligoclonal nature of the tumors (Fig. 4b), suggesting that a combination of HBZ, Akt, BCLxL, and loss of Ink4a/Arf may not be sufficient, and that additional factors are likely at play in tumor development. Elucidation of these factors awaits further investigation.
Figure 4.

Analysis of protein expression and clonality of neoplastic T cells. (a) Western blot analysis of cells obtained from the indicated organs of the indicated mice for the expression of myc‐tagged HBZ, phospho‐Akt, and BCLxL. α‐Tubulin served as a loading control. Note that exogenously transduced genes were expressed in neoplastic T cells. BM, bone marrow; B6 #3 – 2nd, C57BL6 mouse transplanted with tumor cells from NSG mouse #3; Spl, spleen; Thy, thymus; Tumor, submandibular tumor mass. (b) Genomic DNAs extracted from the indicated organs of the indicated mice were subjected to a PCR‐mediated clonality analysis of T‐cell receptor β. The monoclonal (NSG mouse #4) and oligoclonal (NSG mouse #3 and a C57BL6 mouse transplanted with tumor cells from NSG mouse #3) natures of the tumors are depicted. G, germ line.
Although we used NSG mice as the primary recipients, the use of C57BL6 mice yielded a comparable result, but with longer latencies (Fig. S6a). Although a detailed analysis was precluded by the death of most of the mice, one particular mouse that became moribund on day 64 was found to have flower cell‐like cells in the thymus (Fig. S6b) and HBZ/Akt/BCLxL triply transduced T cells accounted for >80% of cells (Fig. S6c), again supporting the notion that HBZ, Akt, and BCLxL cooperatively induce ATL‐like disease in mice. Another mouse that became moribund on day 30 had HBZ/Akt/BCLxL triply transduced T cells that comprised 55% of thymocytes, but the cells did not have a flower cell‐like appearance (data not shown). HBZ‐only transduced T cells did not engraft in C57BL6 recipient mice, as no HBZ + (hCD8+) cells were detectable in the peripheral blood, bone marrow, spleen, or thymus following euthanasia on days 34 (n = 1) or 56 (n = 2) (data not shown).
Discussion
Adult T‐cell leukemia is an intractable T‐cell neoplasm and the only retrovirus‐induced cancer known in humans. Although ATL originates in HTLV‐1‐infected T cells, in which the viral products Tax and HBZ play indispensable roles, ATL cells lack Tax expression whereas HBZ expression is retained,3, 17 suggesting a central role for HBZ in the development of ATL. Indeed, HBZ‐transgenic mice develop ATL‐like disease,6 albeit with low penetrance and only after a long latency period, similar to what is seen in humans. A recent integrated molecular analysis of ATL revealed many genetic mutations and genomic amplifications and deletions.17 Epigenetic abnormalities were also noted that are likely involved in ATL development.17, 18 However, the roles of these genetic/epigenetic abnormalities in the development and maintenance of ATL in collaboration with HBZ are not well defined. Our mouse model of ATL allows genes/mutated genes and potentially microRNAs to be easily tested for their roles in ATL development, in collaboration with HBZ. This use for the model was illustrated in the present study to determine collaborations between HBZ, Akt, and BCLxL in the development of ATL‐like neoplasms. With our model system, one can test a constellation of candidate genes, using them as a retrovirus “library” for their roles in ATL development in collaboration with HBZ. Although genes/mutated genes found to collaborate with HBZ in tumorigenicity need to be validated for their involvement in ATL through multiple alternative approaches, our model will provide insight into the roles of specific genes/mutated genes in ATL.
Despite our findings in the present study, we do not exclude the possibility that combinations of genes other than HBZ, Akt, and BCLxL are tumorigenic and produce ATL‐like disease in mice. However, other combinations of genes we tested (HBZ/Akt/NIK, HBZ/Akt/Notch1, HBZ/Akt/Ccnd1, and HBZ/Akt/Ccnd3) were not tumorigenic. NIK expression is elevated in ATL through evasion of miR‐31‐mediated suppression.18 Notch1 is activated through mutations in the corresponding gene in some ATL cases.17, 19 Ccnd1 facilitates the development of Myc/Bcl2‐driven T‐cell neoplasms in our mouse model.7 In our preliminary experiments using the above combinations of genes, we found that a combination of HBZ, Akt, and NIK did not provoke cytokine‐independent growth of T cells. A combination of Akt and an active Notch1 provoked cytokine‐independent growth, and HBZ had no additional effects. Although combinations of HBZ, Akt, and either Ccnd1 or Ccnd3 provoked cytokine‐independent growth, the cells did not result in leukemia/lymphoma in transplanted mice; Akt and Ccnd1 or Ccnd3, without HBZ, did not provoke cytokine‐independent growth in vitro. Thus, among the combinations of genes we tested, HBZ/Akt/BCLxL was the only one that allowed cytokine‐independent growth of T cells in vitro and that was tumorigenic in mice following transplantation. The molecular mechanisms underlying the collaboration between HBZ, Akt, and BCLxL are not clear from the present study, and await further investigation.
Our observation that the presence or absence of the Ink4a/Arf locus did not affect tumorigenicity of HBZ/Akt/BCLxL‐transduced T cells in our mouse model was unexpected, given that the loss of the locus is associated with ATL disease progression.12, 13 Although the reasons for this inability of Ink4a/Arf loss to facilitate ATL‐like disease in our model are not clear, the loss of Ink4a/Arf locus in humans involves the neighboring DNA elements, such as that encoding hsa‐miR‐31, the downregulation of which has been implicated in ATL development. 18
In summary, we have established a new method for rapidly generating a mouse model of acute ATL and have shown cooperation between HBZ, Akt, and BCLxL in the development of acute ATL. Thus, our method provides a versatile tool to explore the roles of additional “hits” in cooperation with HBZ in the development of acute ATL.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Flow cytometric analysis of bulk T cells induced in culture and their growth after gene transduction.
Fig. S2. mRNA expression of the BCL2 family of anti‐apoptotic genes.
Fig. S3. Analysis of the activation status of Akt, cell cycle, and apoptosis.
Fig. S4. Analysis of an NSG mouse that received HBZ/BCLxL/Akt triply transduced Ink4a/Arf‐null T cells.
Fig. S5. Analysis of an NSG mouse that received HBZ/BCLxL/Akt triply transduced Ink4a/Arf‐proficient T cells.
Fig. S6. Development of adult T‐cell leukemia‐like disease in C57BL6 mice.
Acknowledgments
We thank Seiko Sato for help with animal husbandry. This work was supported by a grant from the Ministry of Health, Labor, and Welfare of Japan (H26‐Kakushingann‐Ippan‐010 to K.O., M.S., and S.T.), and a grant from the Japan Agency for Medical Research and Development (15ck0106015h0102 to S.T.).
Cancer Sci 107 (2016) 1072–1078
Funding Information
Ministry of Health, Labor, and Welfare of Japan (H26‐Kakushingann‐Ippan‐010); Japan Agency for Medical Research and Development (15ck0106015h0102).
References
- 1. Uchiyama T, Yodoi J, Sagawa K et al Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood 1977; 50: 481–92. [PubMed] [Google Scholar]
- 2. Swerdlow SH, Campo E, Harris NL et al World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues, 4th edn Lyon, France: IARC Press, 2009; 281–4. [Google Scholar]
- 3. Satou Y, Yasunaga J, Yoshida M et al HTLV‐I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci USA 2006; 103: 720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hasegawa H, Sawa H, Lewis MJ et al Thymus‐derived leukemia‐lymphoma in mice transgenic for the Tax gene of human T‐lymphotropic virus type I. Nat Med 2006; 12: 466–72. [DOI] [PubMed] [Google Scholar]
- 5. Tezuka K, Xun R, Tei M et al An animal model of adult T‐cell leukemia: humanized mice with HTLV‐1‐specific immunity. Blood 2014; 123: 346–55. [DOI] [PubMed] [Google Scholar]
- 6. Satou Y, Yasunaga J, Zhao T et al HTLV‐1 bZIP factor induces T‐cell lymphoma and systemic inflammation in vivo. PLoS Pathog 2011; 7: e1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arita K, Maeda‐Kasugai Y, Ohshima K et al Generation of mouse models of lymphoid neoplasm using retroviral gene transduction of in vitro‐induced germinal center B and T cells. Exp Hematol 2013; 41: 731–41.e9. [DOI] [PubMed] [Google Scholar]
- 8. Boise LH, González‐García M, Postema CE et al bcl‐x, a bcl‐2‐related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993; 74: 597–608. [DOI] [PubMed] [Google Scholar]
- 9. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakahata S, Ichikawa T, Maneesaay P et al Loss of NDRG2 expression activates PI3K‐AKT signalling via PTEN phosphorylation in ATLL and other cancers. Nat Commun 2014; 5: 3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakagawa M, Schmitz R, Xiao W et al Gain‐of‐function CCR4 mutations in adult T cell leukemia/lymphoma. J Exp Med 2014; 211: 2497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshida N, Karube K, Utsunomiya A et al Molecular characterization of chronic‐type adult T‐cell leukemia/lymphoma. Cancer Res 2014; 74: 6129–38. [DOI] [PubMed] [Google Scholar]
- 13. Hasegawa H, Yamada Y, Iha H et al Activation of p53 by nutlin‐3a, an antagonist of MDM2, induces apoptosis and cellular senescence in adult T‐cell leukemia cells. Leukemia 2009; 23: 2090–101. [DOI] [PubMed] [Google Scholar]
- 14. Blume‐Jensen P, Hunter T. Oncogenic kinase signaling. Nature 2001; 411: 355–65. [DOI] [PubMed] [Google Scholar]
- 15. Shimoyama M, Minato K, Tobinai K et al Atypical adult T‐cell leukemia‐lymphoma: diverse clinical manifestations of adult T‐cell leukemia‐lymphoma. Jpn J Clin Oncol 1983; 13(Suppl. 2): 165–87. [PubMed] [Google Scholar]
- 16. Kamihira S, Sohda H, Atogami S et al Unusual morphological features of adult T‐cell leukemia cells with aberrant immunophenotype. Leuk Lymphoma 1993; 12: 123–30. [DOI] [PubMed] [Google Scholar]
- 17. Kataoka K, Nagata Y, Kitanaka A et al Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet 2015; 47: 1304–15. [DOI] [PubMed] [Google Scholar]
- 18. Yamagishi M, Nakano K, Miyake A et al Polycomb‐mediated loss of miR‐31 activates NIK‐dependent NF‐κB pathway in adult T cell leukemia and other cancers. Cancer Cell 2012; 17: 121–35. [DOI] [PubMed] [Google Scholar]
- 19. Pancewicz J, Taylor JM, Datta A et al Notch signaling contributes to proliferation and tumor formation of human T‐cell leukemia virus type 1‐associated adult T‐cell leukemia. Proc Natl Acad Sci USA 2010; 107: 16619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Flow cytometric analysis of bulk T cells induced in culture and their growth after gene transduction.
Fig. S2. mRNA expression of the BCL2 family of anti‐apoptotic genes.
Fig. S3. Analysis of the activation status of Akt, cell cycle, and apoptosis.
Fig. S4. Analysis of an NSG mouse that received HBZ/BCLxL/Akt triply transduced Ink4a/Arf‐null T cells.
Fig. S5. Analysis of an NSG mouse that received HBZ/BCLxL/Akt triply transduced Ink4a/Arf‐proficient T cells.
Fig. S6. Development of adult T‐cell leukemia‐like disease in C57BL6 mice.


