The low expression level of Syk in human basophils is likely related to transcription regulation, rather than protein or mRNA instability.
Keywords: signal transduction, miRNA, secretion
Abstract
In human basophils, Syk expression is 10-fold lower than most other types of leukocytes. There are indirect studies that suggest that Syk protein is highly unstable (a calculated half-life less than 15 min) in human peripheral blood basophils. Therefore, in these studies, Syk stability was directly examined. Purified basophils were metabolically labeled and a pulse–chase experimental design showed Syk protein to be stable in the time frame of 12 h (95% likelihood that half-life is more than 12 h). However, its synthetic rate was very slow (∼10-fold slower) compared with CD34-derived basophils, which have been shown to express levels of Syk consistent with other mature circulating leukocytes. Syk mRNA expression was found to be 5–30-fold lower than other cell types (CD34-derived basophils, peripheral blood eosinophils, and plasmacytoid dendritic cells). Syk protein and mRNA levels, across cell types, were relatively concordant. Syk mRNA in basophils showed a half-life of 3.5 h, which was greater than that of interleukin-4 or Fc epsilon receptor I-α mRNA (∼2 h), but somewhat shorter than Fc epsilon receptor I-β mRNA (8 h). A comparison of miR expression between CD34-derived and peripheral blood basophils demonstrated only 1 significant increase, in miR-150 (77-fold). Transfection in human embryonic kidney cells of a stabilized form of miR-150 showed that it modified expression of c-Myb mRNA but not of Syk mRNA or protein. These results suggest that low Syk expression in basophils results, not from protein instability and perhaps not from mRNA stability. Instead, the results point to the transcriptional nature of an important point of regulation.
Introduction
Allergic reactions are characterized by the IgE-dependent secretion of mediators from basophils and mast cells. Studies over the past 3 decades have demonstrated that the tissue allergic reaction is dependent on both basophils and mast cells [1–14]. Although an important component of the response during IgE-mediated secretion is the density of cell surface antigen-specific IgE, several studies have demonstrated that there is also a regulation of the response that appears intrinsic to the signaling pathways for secretion [15–17]. For human basophils, the cellular responsiveness correlates best with the expression level of the tyrosine kinase Syk, although there are several other signaling molecules (e.g., SH2 domain-containing inositol 5-phosphatase-1) that may be modulators in specific basophil phenotypes [16–19]. For basophils, Syk levels are tuned to only marginally support secretion, and a direct comparison with other leukocytes shows levels of 5–30-fold less Syk in basophils than observed for all other leukocytes except the αβ T cell, which utilizes the other Syk family member, ζ-chain-associated protein kinase 70, rather than Syk [16, 20].
This finely tuned expression of Syk in basophils [and to a lesser extent in human lung mast cells (21)] suggests that there are unique mechanisms for its control. There is evidence from studies in patients treated with omalizumab that there may be 2 mechanisms operating to markedly suppress Syk expression in basophils [22, 23]. Treatment causes an increase in Syk expression that thus far is more unique among signaling elements measured, and the increase competes with the down-regulation of cell surface IgE to alter the IgE-dependent functionality of these cells [23, 24]. The following argument is only indirect, but because treatment with omalizumab asymptotically reverses only 20% of the difference in Syk expression between basophils and other leukocytes [20–22], it is proposed that there is a post-translational regulatory path altered by omalizumab and a peritranslational or transcriptional regulation independent of omalizumab. It is hypothesized that as a basophil matures in the bone marrow, it experiences 2 distinct processes that ultimately determine the expression of Syk. It is further hypothesized that one of these processes could be described as regulation upstream of Syk protein, a process that is a part of the genetic program that creates a basophil. The second process is speculated to work post-translationally. One example may be exposure of the cell to FcεRI aggregation during maturation, a process that in mature cells leads to down-regulation of Syk expression [25]. Treatment of patients with omalizumab may diminish this latter process by preventing the binding of IgE to FcεRI, thereby preventing some form of “natural” IgE-mediated aggregation [20]. There are appealing aspects to speculation about a second process, but there remain questions as well, such as whether natural aggregation is sufficiently widespread in the population to account for the distribution of Syk expression.
To move forward in understanding the regulatory role of Syk expression in basophils, it is first necessary to characterize the stability of Syk protein and its mRNA. A published study suggests that Syk protein is highly unstable in basophils [26], with a calculated T1/2 of <15 min (see below). If this were true, then the focus of future efforts to understand Syk regulation in basophils, mast cells, and possibly other leukocytes might be based on an examination of post-translational mechanisms and, in particular, E3 ligases, ubiquitylation, and proteasomal regulation. The published evidence is indirect, however, and not always consistent with this view of instability [25]. The following studies were designed to examine this aspect of the problem. To do so, a context was needed, because absolute levels of synthetic rates are extremely difficult to determine in these cells. The context used was the basophil obtained by IL-3-dependent maturation of CD34+ progenitor cells to a basophil-like cell, where Syk expression is not suppressed to levels observed in mature PBBs [20].
MATERIALS AND METHODS
Materials, Buffers, and Antibodies
The following were purchased: PIPES, BSA, EGTA, EDTA, d-glucose, NaF, Na3VO4, 2-ME, and RPMI 1640 containing 25 mM HEPES and l-glutamine (BioWhittaker, Walkersville, MD, USA); Percoll (Pharmacia, Piscataway, NJ, USA); tris(hydroxymethyl)-aminomethane and Tween-20 (Bio-Rad, Hercules, CA, USA); 4D10 anti-human Syk Ab (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and PAG buffer consisting of 25 mM PIPES, 110 mM NaCl, 5 mM KCl, 0.1% glucose, and 0.003% HSA. PAG-conditioned medium was PAG supplemented with 1 mM CaCl2 and 1 mM MgCl2. Countercurrent elutriation was conducted in PAG containing 0.25% BSA in place of 0.003% HSA (elutriation buffer). Buffers were prepared with RNase- and DNase-free reagents when appropriate for microarray and qPCR studies. ESB (NovexBiotech, Salt Lake City, UT, USA) contained 5% 2-ME. CLB is 20 mM Tris-HCl (pH 7.5), 100 µg/ml aprotinin, 10 mM benzamidine, 1 mM 4-(2-aminoethyl) benzene sulfonyl fluoride hydrochloride, 100 µg/ml leupeptin, 50 mM NaF, 1% Nonidet P-40, and 10% glycerol. ILB is CLB without the protease inhibitors Nonidet P-40, glycerol, or vanadate. For the metabolic labeling studies, in the final protocol, CLB was used without protease inhibitors [benzamidine, 4-(2-aminoethyl) benzene sulfonyl fluoride hydrochloride, leupeptin, and aprotinin].
PBB, PBE, and pDC purification
Basophils were purified from leukophoresis packs or from blood obtained by venipuncture. When used at high purity, they were purified to near homogeneity by sequential application of Percoll gradients, countercurrent elutriation, and negative selection, with the basophil purification kit (StemCell Technologies, Vancouver, BC, Canada) and columns from Miltenyi Biotec (Auburn, CA, USA) [27]. The average purity of these basophils by Alcian blue staining [28] was 99%. The starting viability of these cells was typically >97%. For cells obtained by venipuncture (the protocol was approved by the Johns Hopkins Institutional Review Board), basophils were first enriched on a 2-step Percoll gradient [29] that provides 3 fractions: the top fraction was used to isolate pDCs by positive selection, the middle fraction was used to purify basophils by negative selection (StemSep antibody cocktail for basophils; StemCell Technologies), and the bottom fraction was used to purify eosinophils (anti-CD16 antibody to remove neutrophils) by negative selection. For each cell type, the purity was ≥99%.
Reaction conditions
Purified basophils were typically cultured in medium consisting of RPMI-1640 supplemented with 8 µg/ml gentamicin and 0.03% RNase-free BSA at 2 × 106 cells/ml. Culture periods lasted 2 d in the metabolic labeling studies. After culture, recovery and viability were measured with erythrosin B staining. To extract mRNA for the microarray studies, the purified cells were lysed immediately. RNA was isolated with either the RNeasy or miRNeasy extraction kits (Qiagen, Valencia, CA, USA) according to the manufacturer’s directions.
Differentiation of basophils from CD34+ progenitors
Frozen GM-CSF mobilized peripheral blood CD34+ progenitors were purchased from the laboratory of Dr. Shelly Heimfeld, Fred Hutchinson Cancer Research Center (Seattle, WA, USA). After thawing, cells were resuspended at a density of 0.3–0.7 × 106 cells/ml in StemPro serum-free medium (Thermo Scientific-Gibco, Grand Island, NY, USA), which contained the provided nutrient supplement, 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 5 ng/ml recombinant human IL-3 (complete StemPro-34). Cells were kept at 37°C with 5% CO2. Cell counts and viability were checked each week by erythrosin B staining, and freshly prepared medium was added so that the total volume was doubled. After 3 wk, the cells were used for metabolic labeling experiments, as described for PBBs.
qPCR for mRNA expression
Total RNA was determined both by the RiboGreen RNA Quantitation Kit (Thermo Scientific-Molecular Probes, Eugene, OR, USA) and by PicoSeries RNA analysis (Pico Series II; Agilent, Santa Clara, CA, USA). Integrity of the samples was also measured on the Agilent Bioanalyzer; the RNA integrity numbers ranged from 6.6 to 8.3. A pilot survey established that an RNA integrity number >4 did not alter the measurement of Syk mRNA. Samples were analyzed with a TaqMan primer/probe set (Thermo Scientific-Applied Biosystems, Foster City, CA, USA) with primers for the human Syk cDNA sequence [30]: forward, 5′-AGCAGAAGCAAATGTCATGCA-3′; reverse 5′-CCTCGCATATCCCGATCATC-3′. The probe sequence was 5′-CAGCTGGACAACCCGTACATCGTGC-3′. The remaining primers were as follows: FcεRIα forward 5′-GTGAACCTGTGTACCTGGAAGTCTT-3′, reverse CATCCCAGTTCCTCCAACCA (position 524); FcεRIβ forward 5′-CCAGGAAGTATCTTCAGGCAGACT-3′, reverse 5′-TCAAAACTGTCAGCCATGTATGC-3′. The probe sequences were 5′-TGACTGGCTGCTCCTTCAGGCCTC-3′ and 5′-TTGAAGTCGGCCTCATCCCCACC-3′ for FcεRIα and -β respectively. The primers for IL-4 were 5′-CGACTGCACAGCAGTTCCA-3′ and 5′-CAGGCCCCAGAGGTTCC-3′, respectively, and the probe was 5′-TCCGATTCCTGAAACGGCTCGACA-3′. For the HEK studies, a SYBR green–based qPCR was used to analyze Syk and c-Myb mRNA: Syk forward 5′-TGCACTATCGCATCGACAAAG-3′, reverse 5′-CATTTCCCTGTGTGCCGATTT-3′; and c-Myb forward 5′-GAAAGCGTCACTTGGGGAAAA-3′, reverse 5′-TGTTCGATTCGGGAGATAATTGG-3′. For the analysis of miRNA by qPCR, Taqman primer/probe sets were purchased from Thermo Scientific-Applied Biosystems designed for miR-150, -181c, -101, -26b, -221, and -1225-5p.
Syk mRNA measurements by microarray analysis
Samples (250 ng) were concentrated to a uniform volume for analysis using the human HT12v4 microarray (Illumina, San Diego, CA, USA). This study will report only the results from these microarrays, which were part of another study performed in this laboratory [31], that pertained to Syk mRNA expression. That study showed that microarray results closely parallel qPCR results for Syk and other species. To normalize the readings between microarray slides, the samples were grouped. Group 1 consisted of PBBs, PBEs, and pDCs from the same subject preparation, which were run on the same microarray slide. In this way, mRNA results were expressed as relative to the PBBs, which allowed averaging across 5 subjects. On separate slides, PBBs, with or without 24 h of IL-3 treatment, and CD34B preparations were run together, with the results again compared with levels in PBBs. The data from the different data sets were then joined for a comparison of the 5 cell types (PBBs±IL3, CD34Bs, PBEs, pDC, with PBBs acting as the common element for different microarray slides). In addition, the data were normalized for the number of cells obtained before mRNA extraction and after accounting for the total RNA isolated and the fraction used to input 250 ng into the microarray analysis. miRNA from the same samples was analyzed by the Exiqon miRCURY-LNA-microRNA array by the Johns Hopkins University Genomics Core. The results were normalized by finding the average of spot intensities for each experiment and using these values to normalize the individual spot values for interexperiment data.
Metabolic labeling
Cells (10–20 × 106 purified PBBs or 2–3 × 106 CD34Bs) were preincubated for 30 min at 37°C in methionine-free RPMI-1640 medium and then labeled with 0.1 mCi/ml [35S]Met (NEN Life Science, Boston, MA, USA) in methionine-free RPMI-1640 medium containing 40 µg/ml gentamicin and 5 ng/ml IL-3 for 18 h. For the chase component of the pulse–chase design, the cells were washed twice to free them of labeling medium and resuspended in RPMI-1640 without [35S]Met (containing IL-3). The cells were collected and lysed in ice-cold lysis buffer (see below). For pilot studies of the pulse–chase design, KU812F cells [a basophil-like cell line (32)] were examined, and Syk was found to be synthesized at rates up to 100-fold faster than PBBs (see below), and there was a measurable loss of Syk during the chase period. These pilot studies established that the procedure could detect loss of Syk in this design.
Western blot analysis
For immunoprecipitation, cell pellets were lysed in CLB by vortexing and incubating on ice for 10 min. The lysates were then centrifuged for 3 min at 16,000 g and precleared with protein G Sepharose beads for 30 min at 4°C. The clarified lysates were incubated with antibodies (Syk or SH2 domain-containing inositol 5-phosphatase-1) prebound to protein G-Sepharose beads (1–5 µg antibody/20 µl beads) for 1 h at 4°C. The beads were washed 3 times, and the immunoprecipitated proteins were eluted by boiling for 5 min in ESB. Samples were run in an 8% Tris-glycine gel–coated 15-well plate with molecular weight markers with transblotting to nitrocellulose membranes. Nitrocellulose membranes were first analyzed by autoradiography, and then the membranes were exposed to high-performance autoradiography film (GE Life Sciences, Pittsburgh, PA, USA) with intensifying screens (Eastman Kodak, Rochester, NY, USA) at −80°C for 24–100 h. Samples of CD34Bs and PBBs were always run in pairs when the experiment was the pulse component alone. For the pulse–chase design, CD34Bs were not analyzed. After radiography, the membranes were blotted with 4D10 to detect Syk.
miR-150 transfection of HEK cells
HEK-293 cells (American Type Culture Collection, Manassas, VA, USA) were grown to confluence in DMEM containing 10% heat-inactivated FCS and penicillin/streptomycin (and supplemented with Glutamax; Thermo Scientific-Gibco). After the cells were dissociated and counted (with viability), they were resuspended in Opti-MEM reduced-serum medium (with no antibiotics; Thermo Scientific-Gibco); 8 × 104 viable cells per well were placed into a 12-well culture plate that had been pretreated with mirVana iRNA (Thermo Scientific-Ambion, Austin, TX, USA) stabilized mimic as a negative control sequence or with miR-1 or miR-150 sequences (as designed by the manufacturer) according to the manufacturer’s guidelines, using 90 picomoles of miRNA reagent per well and 3.5% (final) siPORT NeoFx transfection reagent (Thermo Fisher-Ambion). These reagents were mixed according the manufacturer’s guidelines and diluted in Opti-MEM). The cells were cultured for 24 or 42 h, but for the 42 h time point, an equal volume of DMEM with 10% FCS was added at the 24 h time point. For the measurement of mRNA, the medium in the well was transferred to a tube for centrifugation of nonadherent cells, Qiazol reagent (Qiagen) was added to the well to lyse cells, and the same Qiazol reagent was transferred to the centrifuged cell pellet, to capture all cells associated with a given well. (This approach was used because it was not clear a priori whether treatment would lead to weaker adherence of HEK cells.) After extraction with the miRNeasy extraction reagents, the RNA was quantified by Ribogreen assay (ThermoFisher Scientific, Waltham, MA, USA), and equal RNA content was used in an initial RT step followed by qPCR. In some experiments, the cells were harvested from the well by dissociation with 4 mM EDTA-DMEM, counted, and pelleted for lysis with 1× ESB for analysis by Western blot.
Statistics
For most of these studies, paired analysis was possible due to the nature of the experimental design. Figures show error bars for sem. In some cases, statistics were performed on log-transformed results. Where necessary, ANOVA with Tukey post-hoc analysis was used to isolate differences in unique categorical comparisons.
RESULTS
Protein stability
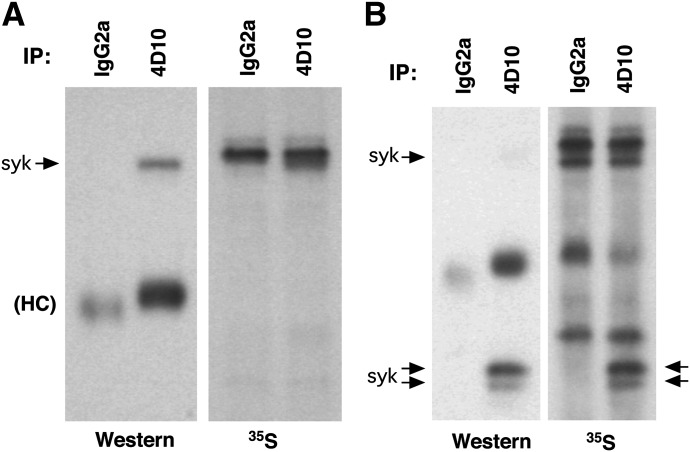
The published literature provides conflicting viewpoints on the stability of Syk protein in human basophils, although previous studies have only indirectly investigated the issue. Studies from this laboratory using cycloheximide to block synthesis suggested that Syk protein is very stable [25], whereas Youssef et al. [26], using proteasomal inhibitors, suggested that it is highly unstable. To resolve the question, basophils were metabolically labeled with [35S]Met to label Syk protein in a pulse–chase experimental design. Because these studies compared mature PBBs with CD34Bs and metabolic labeling required culture of PBBs, it was important to normalize conditions. CD34Bs are produced by culture in IL-3-containing medium, and IL-3 is necessary to sustain PBBs in culture. Therefore, both cell types could be compared, with IL-3 being shared in both experimental conditions. Because IL-3 is known to significantly modify PBB behavior, it is relevant to note that studies have shown that IL-3 increases Syk protein expression only a modest 1.35-fold [16]. The experimental design was to stabilize the PBBs in IL-3 for 24 h before the addition of [35S]Met, culture for an additional 18 h to measure the rate of uptake into Syk and then wash the cells free of medium and [35S]Met and monitor the loss of [35S]Met-labeled Syk in PBBs. However, in the current study there were molecules that were highly labeled in basophils whose migration on gel electrophoresis was only slightly slower than p72Syk (Fig. 1A). The intensity of labeling and its proximity to Syk confounds measurement of changes in [35S] Met-labeled Syk. It was found that this molecule probably bound to the carbohydrate moieties of the Sepharose used to perform the IP (data not shown) and was therefore present, whether or not 4D10 was used for IP (even if additional preclearing steps were used). It should also be noted that there are no radiographic bands without [35S]Met labeling (data not shown). However, exclusion of protease inhibitors from the lysis buffer allowed Syk to be cleaved (as others have shown previously) into 1 or 2 p38–42-sized products that retain their ability to be detected with the anti-Syk Ab 4D10. (There was some variability in the presence of 1 or 2 bands on electrophoresis, and in Figs. 1–3, examples of both can be observed). This experimental maneuver moves [35S]Met-labeled Syk to a clearer region of the radiographic blot (Fig. 1B).
Figure 1. Metabolic labeling of cellular proteins in basophils.
Purified human basophils were incubated for 24 h with 5 ng/ml IL-3, and the cells were pulsed with [35S]Met for 18 h and then lysed for IP with IgG2a or 4D10 (subclass, IgG2a). Immunoblot (Western) or radiography was performed. (A) Lysis of the cells including a standard mix of proteolytic inhibitors. (B) Lysis of cells excluding proteolytic inhibitors.
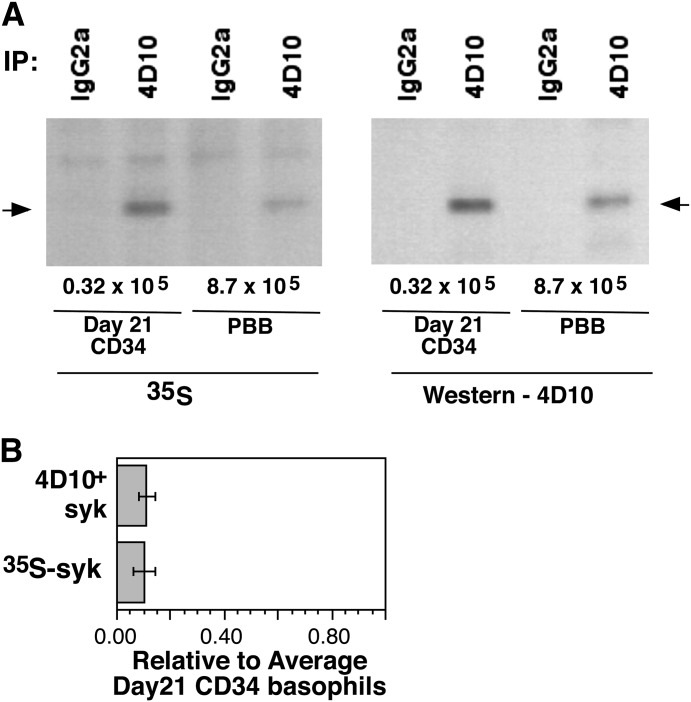
Figure 3. Pulse component of [35S]Met labeling of PBBs and CD34Bs.
Purified PBBs were cultured with 5 ng/ml IL-3 for 24 h (CD34-derived basophils were already in medium with 5 ng/ml IL-3), [35S]Met added to the medium, and the cells lysed after 18 h. Cells were counted before and after the cultures, to normalize the results to and equivalent number of cells and cells lysates of PBBs and CD34Bs run on the same electrophoresis gels. (A) Example of 1 experiment; (B) average of 3 experiments.
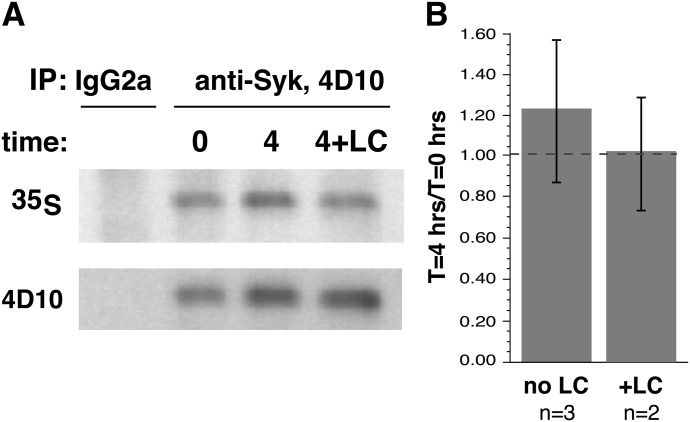
Figure 2 shows the chase results of the pulse–chase experiments. During a 4 h incubation in the chase portion of the experiment (4 h was chosen to minimize the off-target effects of lactacystin A), there was no discernible decrease in the presence of [35S]Met-labeled Syk. This finding was true in the autoradiographs and the Western blot for total Syk levels. Lactacystin was also included in the protocol. This proteasome inhibitor had somewhat less influence on the general viability of PBBs than did other proteasomal inhibitors, and the concentration chosen was based on pilot studies of this class of inhibitors (Table 1). Its presence did not alter the decay rate of Syk (as would be expected if there were no decay). The pulse phase of the experiment could be used to estimate the relative synthetic rate of Syk in CD34Bs and PBBs, with the caveats that there was not significant degradation and that the specific activity of the [35S]Met in the cells were similar. Figure 3 shows an example radiograph and Western blots; Fig. 3B shows the average level of incorporation (normalized to cell number equivalency). PBBs incorporated 10-fold less [35S]Met into Syk than did CD34Bs during an 18 h culture. These results indicate that Syk is a very stable protein in PBB and that the rate of Syk synthesis is probably 10-fold slower in PBBs than in CD34Bs, a result consistent with steady-state levels of Syk in the 2 cell types.
Figure 2. Chase component of the [35S]Met labeling of basophils.
Purified human basophils were incubated for 24 h with 5 ng/ml, and the cells were pulsed with [35S]Met for 18 h, washed, and placed back into similar medium without [35S]Met. In 2 experiments, conditions included ±100 μM lactacystin A. Samples at 0 and 4 h were removed and lysed for IP. (A) An example of the Western blot and autoradiographs. (B) Average of the experiments.
TABLE 1.
Effect of lysosomal or proteasomal inhibitors on cell viability
| Drug | n | Viability (vehicle) (%) | Viability (drug) (%) |
|---|---|---|---|
| MG132 | 5 | 90 ± 3 | 64 ± 9 (P = 0.0227) |
| Proteasome inhibitor I | 3 | 88 ± 3 | 69 ± 6 (P = 0.0457) |
| Lactacystin A | 3 | 97 ± 3 | 81 ± 1 (P = 0.036) |
| Bafilomycin A | 3 | 96 ± 3 | 92 ± 4 (ns) |
Basophils were cultured in medium + 10 ng/ml IL-3 for 24 h, with or without the drugs shown. MG-132 was at 5 µM, proteasome inhibitor I at 5 µM, lactacystin A at 40 µM, and bafilomycin A at 200 nM. Viability was determined with erythrosin B staining.
mRNA stability
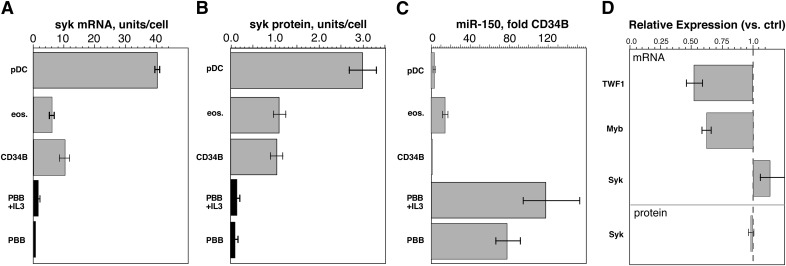
In preliminary experiments, mRNA levels were compared in CD34Bs, PBBs, PBEs, and pDCs. These cells were chosen for comparison because they represent various extremes in the continuum of Syk expression in leukocytes [20]. There are anecdotal reports that, during the maturation of CD34 cells in IL-3, early-culture cell phenotypes resemble eosinophils, and in humans, the lineage of basophils probably more closely aligns with eosinophils than with mast cells or other granulocytes [33–36]. Therefore, is it interesting that CD34 progenitors, CD34Bs, and eosinophils have nearly identical levels of Syk protein [20]. At one extreme of the continuum are PBBs, with very low levels of Syk, and at the other extreme are pDCs, with 3 times the level of Syk as found in PBEs [20]. Under somewhat different conditions, Syk mRNA levels were compared between CD34Bs and PBBs [20]. In the current study, the results include the additional cell types as well as PBBs cultured with IL-3, to normalize the PBB and CD34B comparison. Figure 4 shows that there is a rough concordance in the levels of Syk mRNA in the 4 cell types and the level of Syk protein (abstracted from a previous study [20]), suggesting that control of Syk synthesis occurs at the level of Syk mRNA expression.
Figure 4. A comparison of Syk mRNA and protein levels in 4 cell types (including 2 culture conditions for PBBs).
(A) eos, eosinophils (n = 4), pDCs (n = 4), CD34B (n = 3), PBBs, basophils with or without IL-3 (n = 4). The results were normalized to number of cells used in the microarray and are expressed relative to expression in PBB (1 U/cell). (B) The results for Syk protein are abstracted from an earlier study [20]. (C) Relative levels of expression of miR-150 for the same cell types. (D) Changes in TWF1 mRNA (using miR-1 positive control reagent for transfection), c-Myb or Syk mRNA (using miR-150 for transfection) relative to transfection with negative control (scrambled) miR-treated cells. The bottom bar shows the absence of change in Syk protein.
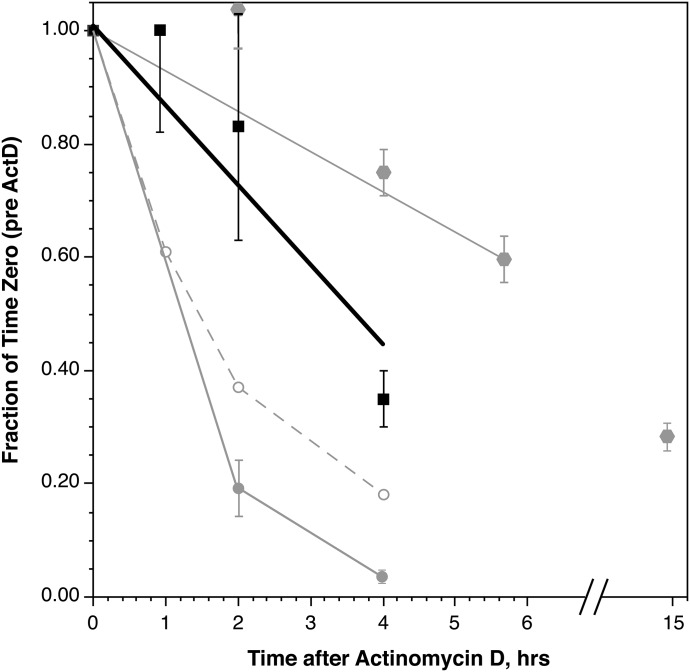
The steady-state level of Syk mRNA in PBBs may be the result of the highly unstable nature of the species. To provide a context for understanding the significance of a particular level of stability for Syk mRNA, a comparison was made to 3 additional mRNAs, 2 of which have been examined in another study [37], although under slightly different conditions. IL-4 mRNA could be considered a transient species, given that it is part of the transient mediator release profile of PBBs during IgE-mediated stimulation. Its stability could therefore represent one end of the spectrum. The stability of 2 of the subunits of FcεRI was also examined to provide context, although, a priori, there were no outright expectations for the β subunit. To determine mRNA stability, the level of mRNA for each species was examined during culture of the cells in actinomycin D-containing medium. In preliminary studies, it was found that actinomycin D introduces instability into PBB viability if the cells were not cultured first in IL-3 to stabilize apoptosis. Therefore, the protocol was to culture the cells in IL-3 for 4 h, introduce actinomycin D, and lyse the cells at different times after its introduction. Unlike previous studies conducted in this laboratory, in these studies, the unstimulated (resting) level of IL-4 mRNA was examined. Likewise, the IL-3 step was not part of the prior study of FcεRIα. The decay of FcεRIβ mRNA was found to be slow, and the measurement time was therefore extended to 15 h for this species. Figure 5 shows a composite of the experiments, not all of which were performed with the same basophil preparations. Syk mRNA stability was intermediate between that of IL-4/FcεRIα and FcεRIβ. There was no change in Syk mRNA without actinomycin D (data not shown).
Figure 5. Stability of mRNA in purified PBBs in the presence of actinomycin D.
(▪) Syk (n = 3), (grey circles) IL-4 (n = 3), (○) FcεRIα (n = 2), and (grey hexagons) FcεRIβ (n = 5).
miRNA in CD34Bs and PBBs
The intermediate stability of Syk mRNA and its low level of expression in basophils suggests that there are peritranslational influences that prevent translation or induce degradation of Syk mRNA or that regulation is imposed at the level of transcription. Regulatory RNAs such as miRNA are one means of influencing the levels of mRNA but the number of possible miRNAs to examine is large. An in silico analysis using various web-based tools (e.g., TargetScanHuman; available at http://www.targetscan.org/) have not been revealing; there is no known miRNA that has been shown to influence Syk mRNA translation or stability, nor is there an miRNA calculated from sequence information. To assist in guiding a study of miRNAs that might be involved in regulating Syk mRNA, CD34B and PBB miRNA expression profiles were compared. As this laboratory recently published, PBBs appear to express ∼160 miRNAs measurable by microarray [31]. Table 2 is a compilation of the miRNA differences when comparing PBBs (n = 5) with CD34Bs (n = 2). (Note: a third CD34B was analyzed but the method involved an older array chip, and whereas the results were similar in pattern, the numerical values were not readily normalized with the newer array chip used for the remaining experiments; see Supplemental Table 1 for further details of these experiments.) The results for the PBBs were reasonably consistent, whereas the results for CD34Bs could be highly variable. Table 2 shows differences >2.5-fold in either direction for normalized and nonnormalized results. The amount of variance limited interpretation of the ratios observed, and there was often considerable overlap between CD34Bs and PBBs. With this consideration, there were ∼10 changes that appeared readily separable, but microarrays for analyzing miRNA expression profiles are not as quantitatively rigorous as mRNA arrays, and, for differences that appeared potentially significant in the miRNA microarray results, the comparisons were re-evaluated by qPCR of the same samples, for 6 different miRNAs. Table 3 shows that for microarray differences that were less than ∼10-fold, qPCR indicated that the microarray results were not valid. If this spot check is valid, the results for the microarray studies could be reduced to only 5 candidates, based on the magnitude of the change (4 decreases and 1 increase). The most interesting of these differences was miR-150: (1) the extreme difference between CD34Bs and PBBs; (2) an increase in expression in PBBs, a direction of change consistent with a potential direct effect on Syk mRNA stability; and (3) its loosely consistent expression levels vs. Syk expression in eosinophils and pDCs For the last point, PBBs, PBEs, and pDCs were 77-fold (95% CI, 56–105 fold), 13-fold (95% CI, 10–19-fold), and 3-fold (95% CI, 2.1–3.5-fold) greater than CD34Bs (Fig. 4C). The miR-1225-5p changes were also replicated with qPCR and differ when comparing PBBs with eosinophils and pDCs (PBBs, PBEs, and pDCs were 0.02-, 1.22-, and 1.02-fold, respectively, relative to CD34Bs). Because miR-1225-5p decreases in PBBs, however, the effect would be expected to be indirect (e.g., down-modulating a protein that up-regulates Syk). For the remaining 4 species in Table 3, there were no differences in PBBs, PBEs, and pDCs—that is, they showed no pattern consistent with Syk expression levels, in contrast to the pattern shown in Fig. 4C.
TABLE 2.
Comparison of miRNA levels in CD34B and PBB
| miRNA | Ratio | Normalized Averages |
Nonnormalized Averages |
||
|---|---|---|---|---|---|
| CD34B | PBB | CD34B ± SD | PBB ± SD | ||
| Hsa-miR-1246 | 0.04 | 34,993 | 1282 | 33,443 ± 32,013 | 711 ± 317 |
| Hsa-miR-34a | 0.06 | 12,010 | 726 | 10,933 ± 4843 | 413 ± 89 |
| Hsa-miR-1225-5p | 0.07 | 89,139 | 6110 | 85,197 ± 81,623 | 3819 ± 2495 |
| Hsa-miR-1207-5p | 0.08 | 43,088 | 3234 | 41,038 ± 37,821 | 1990 ± 1097 |
| hsv1-miR-[1H] | 0.10 | 3058 | 294 | 2971 ± 3340 | 154 ± 76 |
| Hsa-miR-134 | 0.11 | 4426 | 481 | 4216 ± 3895 | 266 ± 150 |
| Hsa-miR-1249 | 0.12 | 4791 | 595 | 4521 ± 3738 | 370 ± 215 |
| Hsa-miR-320c | 0.12 | 45,995 | 5587 | 43,201 ± 33,549 | 3345 ± 1026 |
| Hsa-miR-574-5p | 0.13 | 13,789 | 1858 | 13,013 ± 10,749 | 1056 ± 406 |
| Hsa-miR-575 | 0.13 | 5129 | 670 | 4933 ± 5043 | 422 ± 301 |
| Hsa-miR-1202 | 0.15 | 27,757 | 4185 | 26,352 ± 23,404 | 2593 ± 1468 |
| Hsa-miR-1268 | 0.15 | 10,020 | 1527 | 9472 ± 7988 | 908 ± 234 |
| Hsa-miR-923 | 0.15 | 389,344 | 60,062 | 356,859 ± 184,338 | 37,767 ± 26,439 |
| Hsa-miR-146b-5p | 0.17 | 4995 | 826 | 3984 ± 4340 | 478 ± 134 |
| Hsa-miR-483-5p | 0.20 | 2479 | 505 | 2347 ± 2018 | 208 ± 130 |
| Hsa-miR-1228 | 0.21 | 3128 | 668 | 2953 ± 2444 | 403 ± 148 |
| Hsa-miR-886-3p | 0.21 | 3580 | 766 | 3049 ± 921 | 434 ± 147 |
| Hsa-miR-1224-5p | 0.26 | 1570 | 407 | 1494 ± 1357 | 245 ± 90 |
| Hsa-miR-1275 | 0.26 | 17,918 | 4576 | 16,806 ± 12,808 | 2831 ± 1624 |
| Hsa-miR-638 | 0.27 | 11,137 | 3017 | 10,380 ± 7215 | 1818 ± 688 |
| Hsa-let-7g | 3.20 | 19,441 | 62,653 | 16,313 ± 7774 | 36,148 ± 2377 |
| Hsa-miR-130a | 3.20 | 2346 | 7454 | 2032 ± 226 | 4359 ± 864 |
| Hsa-miR-192 | 3.20 | 477 | 1512 | 395 ± 246 | 862 ± 107 |
| Hsa-miR-766 | 3.20 | 1011 | 3246 | 885 ± 7 | 1950 ± 645 |
| Hsa-miR-193a-5p | 3.40 | 201 | 684 | 186 ± 113 | 245 ± 240 |
| Hsa-miR-30e* | 3.40 | 395 | 1330 | 330 ± 180 | 768 ± 51 |
| Hsa-miR-551b | 3.40 | 576 | 1973 | 495 ± 101 | 1191 ± 469 |
| Hsa-miR-652 | 3.50 | 3192 | 11,033 | 2727 ± 725 | 6551 ± 1699 |
| Hsa-miR-142-3p | 3.60 | 24,852 | 90,533 | 20,529 ± 13,592 | 52,964 ± 8959 |
| Hsa-miR-142-5p | 3.60 | 6305 | 22,734 | 5235 ± 3141 | 13,263 ± 1806 |
| Hsa-miR-148a | 4.20 | 5311 | 22,406 | 4544 ± 1142 | 12,985 ± 1283 |
| Hsa-miR-128 | 4.50 | 610 | 2746 | 508 ± 281 | 1621 ± 417 |
| Hsa-miR-26b | 4.50 | 4988 | 22,303 | 4134 ± 2578 | 12,827 ± 1791 |
| Hsa-miR-101 | 4.60 | 732 | 3394 | 638 ± 28 | 1999 ± 486 |
| Hsa-miR-181c | 5.20 | 856 | 4468 | 746 ± 30 | 2653 ± 789 |
| Hsa-miR-150 | 79.00 | 1837 | 144,271 | 1544 ± 714 | 82,705 ± 12,354 |
Hsa, Homo sapiens.
TABLE 3.
Comparison of selected miRNA differences between CD34B and PBBs
| miRNA | Array | qPCR |
|---|---|---|
| miR-150 | 79.00 | 77.00 |
| miR-1225-5p | 0.07 | 0.02 |
| miR-181c | 5.2 | 0.67 |
| miR-101 | 4.6 | 1.06 |
| miR-26b | 4.5 | 1.33 |
| miR-221 | 2.2 | 0.4 |
Values are the average fold change for PBB/CD34B total RNA normalized levels, as determined by microarray or qPCR. The average ratios result from PBBs (n = 4) and CD34Bs (n = 3).
Although the effect of IL-3 on Syk protein is modest (1.35-fold), it induces larger changes (3.7-fold) in Syk mRNA [31]. In this context, it is worth noting that the effect of IL-3 on miR-150 was equivocal. The microarray results suggested a slight decrease in miR-150 after 1 d of IL-3 treatment (10 ng/ml) of PBBs (data not shown), but qPCR showed a log-fold increase in miR-150 that was 0.61 ± 0.225 (n = 5; P = 0.0528; 1.52-fold), and after 3 d of IL-3 treatment, a log-fold increase of 1.06 ± 0.525 (n = 3; P = 0.18; 2.08-fold)—that is, there was no evidence of a decrease in miR-150. Stimulation with anti-IgE Ab is not known to alter Syk mRNA levels, and the alteration of miR-150 was not statistically significant (log-fold change of −0.45 ± 0.263; n = 4; P = 0.22).
There is no indication in the literature or by in silico matching that miR-150 binds to Syk mRNA. To directly test this possibility, HEK cells were used as a test bed for transfection of miR-150 (attempts were made with the basophil-like cell line KU812F, but these cells did not transfect well). In pilot studies (not shown) HEK cells were demonstrated to express Syk at concentrations only somewhat greater than that in basophils (Syk levels were 7-fold greater on a per cell basis, but the cells were 10 times the volume of basophils). Additional pilot studies demonstrated that transfection of positive control miRNA (miR-1) that modulates TWF1 protein was shown to down-regulate TWF1 mRNA by ∼50% (0.52 ± 0.07-fold; n = 3; P = 0.02) (Fig. 4D), indicating that the cell line can be transfected with a stabilized miRNA that results in a measurable change in its target mRNA. The miR-150 sequence has been shown to induce down-regulation of c-Myb [38]; therefore, in addition to measuring Syk mRNA, the same samples were examined for Myb mRNA by qPCR. In 3 experiments (which included 2 time points, 24 and 42 h after transfection), miR-150 (compared to a scrambled miRNA) induced a modest down-regulation of Myb mRNA of 0.62-fold (95% CI; 0.58–0.65; P = 0.003), while showing no change in Syk mRNA (1.14-fold; 95% CI, 0.96–1.35) (Fig. 4D). There was no significant difference between the 24 and 42 h time points, although the average decrease at 42 h was 0.42-fold for c-Myb and 1.20-fold for Syk. There was an indirect indication that the down-regulation of c-Myb (or other unmeasured proteins) resulted in slower growth of the HEK cells; the total RNA isolated from the miR-150-treated 24 h cultures was equal, but by 42 h, it was 0.86-fold less than scrambled miR (note also that for the qPCR measurements, an equal amount of total RNA was added to reactions, normalizing for the difference in total input RNA). In 2 experiments, Syk protein was also measured at the 24 h time point and found to be unchanged in the miR-150-treated cells (0.97 ± 0.08-fold).
DISCUSSION
In previous reports, the stability of Syk protein in freshly isolated PBBs has been inferred from experiments that could be considered indirect. Youssef et al. [26] suggested that it was highly unstable, based on an increase (10–40-fold) in Syk expression observed during culture of basophils with proteasomal inhibitors. The inference was drawn from these experiments under the assumption that the only effect of a proteasome inhibitor would be the accumulation of proteins unable to complete degradation through the proteasome. There were two concerns about this interpretation. First, the experience in this laboratory with proteasomal inhibitors shows that, when Syk degradation is altered during IgE-mediated down-regulation of Syk, proteasomal inhibitors do not affect the p72Syk band but do affect the presence of the ubiquitinylated species of Syk (>p72), as one would expect for not inhibiting ubiquitinylation but inhibiting the processing of ubiquitinylated species by the proteasome [25]. Studies in the author’s laboratory also have found that proteasomal inhibitors have a variety of nonspecific effects on PBBs that culminate in death, so that concentrations of the inhibitors have to be chosen to limit collateral behaviors. With careful choice, some proteasomal inhibitors have no effect on Syk levels. Second, the degradation rate of Syk that could be inferred from the study by Youssef et al. [26] would be so fast that the normal steady-state would have to be the result of a very fast rate of synthesis, replacing the steady-state level of Syk with an equivalent amount of Syk every 15 min (and therefore losing an equivalent amount every 15 min). Our own results found that inclusion of cycloheximide to prevent synthesis of Syk had no effect on the steady-state level of Syk [25]. If the results found by Youssef et al. were taken at face value, it should have been obvious that Syk was being lost rapidly in the presence of cycloheximide. The current study supports previous indirect results obtained in this laboratory. First, labeled Syk was not lost in a 4 h period, indicating that it is stable, with a half-life of at least 12 h. (Based on the sd of the results in these experiments, one can estimate a T1/2 of >12.5 h with 95% confidence.) In addition, the synthetic rate of Syk is 10 times slower than that of CD34Bs. Although this is not readily translated to absolute numbers, it suggests that synthesis and loss do not occur rapidly and are unlikely to represent total replacement or loss every 15 min. These results therefore suggest that 1 point of post-translation control, the degradation of Syk protein, is not as viable a candidate for explaining the variability in Syk expression among different subject’s basophils, as it would have been if Syk protein were highly unstable. It remains possible that by performing the metabolic labeling studies in IL-3, protein stability is enhanced. To change from a half-life of less than 15 min to >12 h would suggest a remarkable influence by IL-3, but the possibility cannot be wholly discounted. However, studies using cycloheximide are consistent with the results of this study, and they were not performed with IL-3.
The stability of Syk protein is consistent with a slow synthetic rate and therefore low steady-state levels. In addition, the slow synthetic rate is consistent with low steady-state levels of mRNA. Recent studies have shown that steady-state levels of mRNA are only loosely concordant with protein levels, so that there is no strong a priori expectation for this concordance between Syk protein and Syk mRNA among the cell types studied [39]. Because the rough concordance occurs and a weak correlation has also been found between Syk mRNA and Syk protein when comparing different subjects’ basophils (R2 = 0.25) [17], the results suggest that average Syk protein expression is largely under the control of steady-state levels of Syk mRNA. It should be stressed that the average low Syk expression of basophils may be under the control of Syk mRNA levels but that the large variance (variance limited to the range of 0–20% of the Syk levels observed in CD34 progenitors or CD34B) around this average could still result from post-translational processes.
If Syk mRNA were highly stable, one might extrapolate that Syk mRNA levels were therefore under the control of Syk gene transcription. However, the results suggest that Syk mRNA shows an intermediate level of stability raising the possibility of peritranslational control as an explanation for low steady-state Syk mRNA levels in basophils. In addition, some peritranslational control mechanisms, like miRNA-dependent regulation, do not always alter stability but simply inhibit translation. Also, the relative stability of Syk mRNA was determined in the presence of IL-3, and previous studies of ours suggested that it was somewhat less stable in the absence of IL-3 [20]. Although it was not explicitly addressed in the methods and results, the overall design of these studies was derived from initial experience measuring the decay rate of the FcεRIβ subunit mRNA. Studies have shown that IL-3 increases expression of FcεRIβ mRNA [40], and this can confound attempts to assess decay rates. Therefore, the protocol requires stabilization/preconditioning of cells in IL-3 before making the measurement. In addition, it is sometimes observed that using actinomycin D without IL-3 can alter mRNA decay studies. Studies of FcεRIβ mRNA without IL-3 preconditioning are difficult because mRNA levels are very low, but results suggest that decay is somewhat faster, the opposite of the historical difference for Syk mRNA decay measurements. But the effects are variable among mRNAs and there is no hard-and-fast rule. We have speculated that combining the dynamics of apoptosis with measurements like these can be problematic. There are several other indicators that suggest that simple isolation of basophils (or other polymorphonuclear cells) leads to a variety of changes that can confound mechanistic studies [31, 40]. It was with these concerns in mind, as well as attempting to equalize the conditions of CD34 culture with PBB culture, that the studies were performed with cells that were first exposed to IL-3 for extended periods. It cannot be excluded that use of IL-3 alters cellular dynamics in ways that poorly reflect conditions in vivo.
To address the possibility that Syk mRNA was made unstable by a miRNA species, a survey was conducted to identify the miRNA species that are expressed in PBBs vs. CD34Bs. Studies of miRNAs are complicated by the inability to work backward accurately from the known sequence of an mRNA to the miRNA that regulates its stability or translation. There are several in silico methods available to attempt this calculation, but by and large, the best information is dependent on experimental observations. It is interesting that the in silico algorithms find no significant matches for Syk with known miRNAs (and not with any of those shown in Table 2). Therefore, the approach taken was to compare miRNA expression profiles for CD34-derived basophils vs. PBBs, reasoning that the large difference in Syk expression in the 2 otherwise similar cell types may provide guidance for which miRNA species to examine. The microarray methods for miRNAs are not as quantitatively robust as for mRNAs, and so the results for this comparison were re-examined by qPCR for some of the species showing the greatest difference between CD34Bs and PBBs. This added step winnowed the choices considerably and left only 1 species showing significant up-regulation in PBBs. There were a couple of down-regulated species, but for these to explain the differences in Syk expression, their influence would have to be indirect, and determining these differences was considered a difficult path of study. A future study of these differences may be warranted. In addition, the targets for the few down-regulated species are largely unknown. The one large positive difference was for miR-150 and its expression pattern was loosely concordant with Syk expression in the cell types shown in Fig. 4. This kind of pattern was not observed for the other species shown in Table 3. However, although transfection of HEK cells with a stabilized version of miR-150 resulted in a previously described effect on c-Myb, there was no effect on either Syk mRNA or protein. Therefore, at this time, there are no indications, either from in silico predictions in general or from experimental observations for the only miRNA species showing a significant change between CD34Bs and PBBs, for a miRNA species that modulates Syk expression at the peritranslational stage of Syk generation. However, these studies did uncover several significant differences in miRNA species that differentiate CD34Bs from mature PBBs, but further study is necessary to determine the functional significance of these differences.
Therefore, conclusions regarding the most relevant points of control are restricted to noting that steady-state regulation does not occur at the level of protein turnover, but is likely related to regulation of steady-state Syk mRNA levels and that the absence of evidence for an miRNA to regulate Syk mRNA suggests that regulation occurs at the transcriptional level.
AUTHORSHIP
D.M .designed, performed the studies, and wrote the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The author thanks Dr. John Schroeder, Anja Bienenman, and Sherry Hudson for assistance in the preparation of the PBEs and pDCs and Valerie Alexander for excellent technical assistance. This work was support in part by U.S. National Institutes of Health National Institute of Allergy and Infectious Disease Grants AI100952 and AI070345.
Glossary
- CD34B
CD34-derived basophil
- CLB
complete lysis buffer
- ESB
electrophoresis sample buffer
- FcεRI
Fc epsilon receptor I
- HEK
human embryonic kidney (cell)
- IP
immunoprecipitation
- miR
microRNA
- PAG
PIPES-albumin-glucose
- PBB
peripheral blood basophil
- PBE
peripheral blood eosinophil
- pDC
plasmacytoid dendritic cell
- qPCR
quantitative PCR
- Syk
spleen tyrosine kinase
- T1/2
half-life
- TWF
twinfilin actin binding protein
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURE
The author declares no conflicts of interest.
REFERENCES
- 1.Kimura I., Tanizaki Y., Saito K., Takahashi K., Ueda N., Sato S. (1975) Appearance of basophils in the sputum of patients with bronchial asthma. Clin. Allergy 5, 95–98. [DOI] [PubMed] [Google Scholar]
- 2.Naclerio R. M., Proud D., Togias A. G., Adkinson N. F. J. Jr., Meyers D. A., Kagey-Sobotka A., Plaut M., Norman P. S., Lichtenstein L. M. (1985) Inflammatory mediators in late antigen-induced rhinitis. N. Engl. J. Med. 313, 65–70. [DOI] [PubMed] [Google Scholar]
- 3.Peters S. P., Naclerio R. M., Freeland H. S., Togias A., Schleimer R. P., MacGlashan D. W. Jr., Kagey-Sobotka A., Fox C. C., Adkinson N. F. J. Jr., Lichtenstein L. M. (1985) Mast cells and mast cell mediators in models of airway disease. Prog. Clin. Biol. Res. 199, 153–161. [PubMed] [Google Scholar]
- 4.Bascom R., Wachs M., Naclerio R. M., Pipkorn U., Galli S. J., Lichtenstein L. M. (1988) Basophil influx occurs after nasal antigen challenge: effects of topical corticosteroid pretreatment. J. Allergy Clin. Immunol. 81, 580–589. [PubMed] [Google Scholar]
- 5.Charlesworth E. N., Hood A. F., Soter N. A., Kagey-Sobotka A., Norman P. S., Lichtenstein L. M. (1989) Cutaneous late-phase response to allergen. Mediator release and inflammatory cell infiltration. J. Clin. Invest. 83, 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlesworth E. N., Kagey-Sobotka A., Schleimer R. P., Norman P. S., Lichtenstein L. M. (1991) Prednisone inhibits the appearance of inflammatory mediators and the influx of eosinophils and basophils associated with the cutaneous late-phase response to allergen. J. Immunol. 146, 671–676. [PubMed] [Google Scholar]
- 7.Liu M. C., Hubbard W. C., Proud D., Stealey B. A., Galli S. J., Kagey-Sobotka A., Bleecker E. R., Lichtenstein L. M. (1991) Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes. Am. Rev. Respir. Dis. 144, 51–58. [DOI] [PubMed] [Google Scholar]
- 8.Irani A. A., Huang C., Zweiman B., Schwartz L. B. (1997) Immunohistochemical detection of basophil infiltration in the skin during the IgE-mediated late phase reaction. J. Allergy Clin. Immunol. 99, S92. [Google Scholar]
- 9.Maruyama N., Tamura G., Aizawa T., Ohrui T., Shimura S., Shirato K., Takishima T. (1994) Accumulation of basophils and their chemotactic activity in the airways during natural airway narrowing in asthmatic individuals. Am. J. Respir. Crit. Care Med. 150, 1086–1093. [DOI] [PubMed] [Google Scholar]
- 10.Irani A. A., Leung D. Y. M., Boguniewicz M., Strickland I., Baran M. L., Schwartz L. B. (2001) Acute atopic dermatitis is associated with increased infiltration of basophils. J. Allergy Clin. Immunol. 107, S237. Available at http://eurekamag.com/research/034/351/034351864.php. [Google Scholar]
- 11.Kepley C. L., McFeeley P. J., Oliver J. M., Lipscomb M. F. (2001) Immunohistochemical detection of human basophils in postmortem cases of fatal asthma. Am. J. Respir. Crit. Care Med. 164, 1053–1058. [DOI] [PubMed] [Google Scholar]
- 12.Kleinjan A., Buhring H.-J., McEuen A. R., Walls A. F., Fokkens W. J. (2001) Alterations in mast cell phenotype and basophil numbers in the nasal mucosa following repeated low dose allergen challenge in allergic rhinitis. J. Allergy Clin. Immunol. 107, S284. Available at http://eurekamag.com/research/034/379/034379295.php. [Google Scholar]
- 13.Gauvreau G. M., Lee J. M., Watson R. M., Irani A. M., Schwartz L. B., O’Byrne P. M. (2000) Increased numbers of both airway basophils and mast cells in sputum after allergen inhalation challenge of atopic asthmatics. Am. J. Respir. Crit. Care Med. 161, 1473–1478. [DOI] [PubMed] [Google Scholar]
- 14.Eckman, J. A., Sterba, P. M., Kelly, D., Alexander, V., Liu, M. C., Bochner, B. S., Macglashan, D. W., Jr., Saini, S. S. (2010) Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J. Allergy Clin. Immunol.125, 889–895 e7. [DOI] [PMC free article] [PubMed]
- 15.Nguyen K. L., Gillis S., MacGlashan D. W. Jr (1990) A comparative study of releasing and nonreleasing human basophils: nonreleasing basophils lack an early component of the signal transduction pathway that follows IgE cross-linking. J. Allergy Clin. Immunol. 85, 1020–1029. [DOI] [PubMed] [Google Scholar]
- 16.MacGlashan D. W., Jr (2007) Relationship between Syk and SHIP expression and secretion from human basophils in the general population. J. Allergy Clin. Immunol. 119, 626–633. [DOI] [PubMed] [Google Scholar]
- 17.Ishmael S., MacGlashan D. Jr (2009) Early signal protein expression profiles in basophils: a population study. J. Leukoc. Biol. 86, 313–325. [DOI] [PubMed] [Google Scholar]
- 18.Vonakis B. M., Gibbons S. Jr., Sora R., Langdon J. M., MacDonald S. M. (2001) Src homology 2 domain-containing inositol 5′ phosphatase is negatively associated with histamine release to human recombinant histamine-releasing factor in human basophils. J. Allergy Clin. Immunol. 108, 822–831. [DOI] [PubMed] [Google Scholar]
- 19.Vonakis B. M., Vasagar K., Gibbons S. P. Jr., Gober L., Sterba P. M., Chang H., Saini S. S. (2007) Basophil FcepsilonRI histamine release parallels expression of Src-homology 2-containing inositol phosphatases in chronic idiopathic urticaria. J. Allergy Clin. Immunol. 119, 441–448. [DOI] [PubMed] [Google Scholar]
- 20.Ishmael S. S., MacGlashan D. W. Jr (2010) Syk expression in peripheral blood leukocytes, CD34+ progenitors, and CD34-derived basophils. J. Leukoc. Biol. 87, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havard S., Scola A. M., Kay L. J., Ishmael S. S., MacGlashan D. W. Jr., Peachell P. T. (2011) Characterization of syk expression in human lung mast cells: relationship with function. Clin. Exp. Allergy 41, 378–388. [DOI] [PubMed] [Google Scholar]
- 22.Zaidi, A. K., Saini, S. S., Macglashan, D. W., Jr. (2010) Regulation of Syk kinase and FcRbeta expression in human basophils during treatment with omalizumab. J. Allergy Clin. Immunol. 125, 902–908 e7. [DOI] [PMC free article] [PubMed]
- 23.Macglashan, D. W., Jr. Saini, S. S. (2013) Omalizumab increases the intrinsic sensitivity of human basophils to IgE-mediated stimulation. J. Allergy Clin. Immunol. 132, 906–911 e1–4. [DOI] [PMC free article] [PubMed]
- 24.MacGlashan, D. W., Jr., Savage, J. H., Wood, R. A., Saini, S. S. (2012) Suppression of the basophil response to allergen during treatment with omalizumab is dependent on 2 competing factors. J. Allergy Clin. Immunol. 130, 1130–1135 e5. [DOI] [PMC free article] [PubMed]
- 25.MacGlashan D., Miura K. (2004) Loss of syk kinase during IgE-mediated stimulation of human basophils. J. Allergy Clin. Immunol. 114, 1317–1324. [DOI] [PubMed] [Google Scholar]
- 26.Youssef L. A., Wilson B. S., Oliver J. M. (2002) Proteasome-dependent regulation of Syk tyrosine kinase levels in human basophils. J. Allergy Clin. Immunol. 110, 366–373. [DOI] [PubMed] [Google Scholar]
- 27.Lavens-Phillips S. E., MacGlashan D. W. Jr (2000) The tyrosine kinases p53/56lyn and p72syk are differentially expressed at the protein level but not at the messenger RNA level in nonreleasing human basophils. Am. J. Respir. Cell Mol. Biol. 23, 566–571. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert H. S., Ornstein L. (1975) Basophil counting with a new staining method using Alcian blue. Blood 46, 279–286. [PubMed] [Google Scholar]
- 29.Warner J. A., Yancey K. B., MacGlashan D. W. Jr (1987) The effect of pertussis toxin on mediator release from human basophils. J. Immunol. 139, 161–165. [PubMed] [Google Scholar]
- 30.Law C. L., Sidorenko S. P., Chandran K. A., Draves K. E., Chan A. C., Weiss A., Edelhoff S., Disteche C. M., Clark E. A. (1994) Molecular cloning of human Syk. A B cell protein-tyrosine kinase associated with the surface immunoglobulin M-B cell receptor complex. J. Biol. Chem. 269, 12310–12319. [PubMed] [Google Scholar]
- 31.MacGlashan D., Jr (2015) Expression profiling of human basophils: modulation by cytokines and secretagogues. PLoS One 10, e0126435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda T., Kishi K., Ohnishi Y., Shibata A. (1987) Bipotential cell differentiation of KU-812: evidence of a hybrid cell line that differentiates into basophils and macrophage-like cells. Blood 70, 612–619. [PubMed] [Google Scholar]
- 33.Denburg, J. A., Tanno, Y., Bienenstock, J. (1986) Growth and differentiation of human basophils, eosinophils, and mast cells. In: Mast Cell Differentiation and Heterogeneity (Befus A.D., Bienenstock J., Denburg, J.A., eds.), Raven Press, New York, 71-83. [Google Scholar]
- 34.Denburg J. A., Otsuka H., Ohnisi M., Ruhno J., Bienenstock J., Dolovich J. (1987) Contribution of basophil/mast cell and eosinophil growth and differentiation to the allergic tissue inflammatory response. Int. Arch. Allergy Appl. Immunol. 82, 321–326. [DOI] [PubMed] [Google Scholar]
- 35.Denburg J. A., Dolovich J., Harnish D. (1989) Basophil mast cell and eosinophil growth and differentiation factors in human allergic disease. Clin. Exp. Allergy 19, 249–254. [DOI] [PubMed] [Google Scholar]
- 36.Grundström J., Reimer J. M., Magnusson S. E., Nilsson G., Wernersson S., Hellman L. (2012) Human cord blood derived immature basophils show dual characteristics, expressing both basophil and eosinophil associated proteins. PLoS One 7, e48308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacGlashan D. W. Jr., Xia H. Z., Schwartz L. B., Gong J. P. (2001) IgE-regulated loss, not IgE-regulated synthesis, controls expression of FcepsilonRI in human basophils. J. Leukoc. Biol. 70, 207–218. [PubMed] [Google Scholar]
- 38.Barroga C. F., Pham H., Kaushansky K. (2008) Thrombopoietin regulates c-Myb expression by modulating micro RNA 150 expression. Exp. Hematol. 36, 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwanhäusser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J., Chen W., Selbach M. (2011) Global quantification of mammalian gene expression control. Nature 473, 337–342. [DOI] [PubMed] [Google Scholar]
- 40.Saini S., Richardson J. J., Wofsy C., Lavens-Phillips, Bochner, B., MacGlashan, D. W., Jr. (2001) Expression and modulation of FcepsilonRIalpha and FcepsilonRIbeta in human blood basophils. J. Allergy Clin. Immunol. 107, 832–841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.