Abstract
Objective
Thiamine-related encephalopathy (Wernicke’s encephalopathy) is a neuropsychiatric syndrome caused by a vitamin B1 (thiamine) deficiency often associated with alcoholism. Cancer predisposes patients to thiamine deficiency unrelated to alcoholism, though many cases are missed clinically. The present report adds to the literature on thiamine as a palliative tool for thiamine-related encephalopathy (TRE) in cancer.
Method
From a larger series of TRE in cancer, we report on three cases with terminal illness.
Results
Case 1
A 61-year old woman with Hodgkin’s lymphoma developed TRE over 13 days. Precipitants included a hypermetabolic state in the background of subacute thiamine deficiency. Diagnosis was supported by abnormal serum thiamine and positive MRI findings. Mental status improved within 36 hours of initiating thiamine 500 mg IV t.i.d.
Case 2
A 68-year-old man with colon cancer metastatic to liver and bone developed TRE precipitated by C. difficile–related diarrhea superimposed on 3 months of low appetite and weight loss. Diagnosis was supported by abnormal serum thiamine, and thiamine 500 mg IV t.i.d. was initiated. Improvements in mental status began within 36 hours.
Case 3
An 80-year-old man with squamous cell carcinoma developed TRE precipitated by systemic infection in the context of three weeks of dysphagia. Antibiotic treatment did not reverse his cognitive symptoms, and a diagnosis of TRE was made based on operationalized criteria. Thiamine 100 mg IV daily did not reverse his symptoms. On his 30th day of admission, thiamine was increased to 500 mg IV t.i.d., resulting in a rapid reversal of altered mental status.
Significance of Results
This report adds to the list of cancer types in which TRE/Wernicke’s encephalopathy has been reported. It supports the use of higher doses of thiamine than are typically recommended in North America. Improvement following treatment allowed patients to engage with family and treatment teams prior to death.
Keywords: Cancer, Thiamine, Wernicke’s encephalopathy, Thiamine-related encephalopathy, Delirium
INTRODUCTION
Wernicke’s encephalopathy (WE) is a neuropsychiatric disorder caused by thiamine deficiency, first described by Carl Wernicke in 1881 (Thomson et al., 2008a). Common myths and misconceptions (Donnino et al., 2007) exist about WE: that it is rare; that it is a syndrome of alcoholics; and that the “classic” triad of signs and symptoms allows for diagnostic reliability. Instead, studies have shown that WE is not rare, that it exists in several nonalcoholic patient populations, and that the classic triad is not a valid diagnostic tool (Donnino et al., 2007). Reports of WE among nonalcoholic cases include HIV/AIDS (Boldorini et al., 1992), post-bariatric surgery (Singh & Kumar, 2007), hyperemesis gravidarum (Palacios-Marques et al., 2012), pancreatitis (Arana-Guajardo et al., 2012), thyrotoxicosis (Bonucchi et al., 2008), cancer (de Reuck et al., 1981), and in the context of total parenteral nutrition (Hahn et al., 1998) and certain medications (Zuccoli et al., 2008). The emergence of WE in terminally ill cancer patients has been reported in one case from New Zealand (Macleod, 2000) and three from Japan (Onishi et al., 2004). To our knowledge, no cases of recovery from WE in terminal delirium have been reported in North America. Through a case series and review of the literature, we will highlight how cancer patients are predisposed to developing WE and how high-dose intravenous thiamine was employed to palliate three patients with terminal cancer who developed WE prior to death. We will also propose the use of thiamine-related encephalopathy (TRE) as a more accurate description of nonalcoholic Wernicke’s encephalopathy.
Historical Perspective
In his first three case descriptions (Thomson et al., 2008a), Carl Wernicke wrote of a novel syndrome manifested by acute gaze palsies, somnolence, and waxing and waning levels of orientation. These symptoms were preceded by a subacute course of fatigue, unsteadiness, poor eyesight, photophobia, mood lability, vomiting, and weight loss. All three of Wernicke’s original cases rapidly deteriorated into stupor and died within a week or two of admission. Thiamine, which was only discovered after Wernicke’s lifetime (Isenberg-Grzeda et al., 2012), would later prove to be the mainstay of treatment.
In spite of Wernicke’s broad clinical descriptions, the medical community of his time focused on a narrow aspect of phenomenology (Lishman, 1981). The notion of Werncike’s triad—altered mental status, ataxia, and nystagmus—was quickly inculcated upon generations of physicians and students to follow (Lishman, 1981). The triad suggests a striking and distinct clinical syndrome and, by implication, a syndrome that could be easily and reliably diagnosed. However, numerous large autopsy studies in the latter half of the 20th century demonstrated that only a minority of cases present with all three classic signs of Wernicke’s triad, and that the triad is not a sensitive diagnostic tool (Isenberg-Grzeda et al., 2012). In practice, up to 80% of cases go undiagnosed (Isenberg-Grzeda et al., 2012). Furthermore, some studies suggest that the true clinical manifestation resembles Wernicke’s broader, original descriptions (Thomson et al., 2008b).
Diagnosing Wernicke’s Encephalopathy
The operationalized criteria proposed by Caine and colleagues (1997) offer an approach to diagnosing WE in patients presenting with any two of the following four signs or symptoms: (1) nutritional deficiency, (2) ocular signs, (3) cerebellar signs, and (4) either altered mental status or mild memory impairment. The criteria were left intentionally broad to mirror the breadth of symptoms that were documented about the cases utilized to develop the criteria. The operationalized criteria are 85% sensitive for diagnosing WE. The criteria were developed for use in alcoholic patients and have not been validated in nonalcoholic patient populations. Still, their use in diagnosing nonalcoholic patients has been reported in the literature (Arana-Guajardo et al., 2012; Kleinert-Altamirano & Juarez-Jimenez, 2014; Palacios-Marques et al., 2012; Singh & Kumar, 2007).
A variety of biomarkers have been studied for potential use in identifying WE (Isenberg-Grzeda et al., 2012). Thiamine concentrations in the blood can be inferred by measuring erythrocyte transketolase activity, which is an indirect measure of thiamine, or by directly measuring thiamine diphosphate, one of the isoforms of thiamine in the body (Immadisetty et al., 2009). Because it is not clear if or how the serum levels of thiamine reflect CNS levels, no threshold for diagnosis of WE has been established based on serum thiamine concentrations (Thomson et al., 2010). Thus, the utility of a thiamine blood test is solely for identifying patients at risk. Further complicating matters is the fact that the tests are not routinely available in many hospitals (Thomson et al., 2010). Neuroimaging has been studied in the detection of WE, and MRI findings have been shown to be 93% specific for WE (Antunez et al., 1998). However, its sensitivity is 53%, and thus it is not a reliable tool for ruling out WE (Antunez et al., 1998). Ultimately, WE remains a clinical diagnosis (Isenberg-Grzeda et al., 2012).
Thiamine and Thiamine Deficiency
Thiamine is an essential nutrient and is a cofactor for three enzymes used to produce energy in the Krebs cycle and pentose phosphate pathway (Isenberg-Grzeda et al., 2012). For every 1000 kcal of carbohydrates consumed, 0.33 mg of thiamine is required (Isenberg-Grzeda et al., 2012). Under normal conditions, glucose is converted to pyruvate, which then enters the Krebs cycle to be converted into energy-containing ATP. When an imbalance exists between the amount of glucose and the amount of thiamine, pyruvate is instead shunted away from the Krebs cycle and is converted to lactic acid and reactive oxygen species. This leads to cell death within 7 to 10 days (Sechi & Serra, 2007).
Thiamine deficiency and WE have historically been associated with alcoholism. By being a carbohydrate-rich but thiamine-deficient foodstuff, alcohol, when consumed excessively and at the expense of meals, creates a state of relative thiamine deficiency compared to glucose (Isenberg-Grzeda et al., 2012). Beyond alcoholism, thiamine deficiency can develop in any condition in which thiamine is not absorbed properly, is lacking in the diet, is used excessively by the body, or is low in proportion to the amount of carbohydrates put into the body (Isenberg-Grzeda et al., 2012).
Thiamine Deficiency in Cancer
Cancer is a prototype for the development of thiamine deficiency and WE for several reasons (Isenberg-Grzeda et al., 2012). First, cancer patients can develop malabsorption and malnutrition because of anatomic lesions caused by cancer or secondary to low appetite, nausea, or vomiting. Second, excessive usage or depletion of thiamine occurs in cancer patients because of the higher metabolic demands placed by systemic illness, fevers, infection, steroid use, and high glucose-containing intravenous fluids. Excessive usage or depletion of thiamine is also seen in the fast-growing tumors, including the hematologic malignancies, because of rapid cell turnover. Third, because the majority of the body’s thiamine is stored in the liver, cancer patients may be unable to store thiamine properly. Finally, genomic factors associated with thiamine in cancer have been shown to result in thiamine deficiency in certain cancers (Lu’o’ng & Nguyen, 2013). Still, cases of WE in cancer appear sparingly in the scientific literature. To our knowledge, the use of thiamine as a palliative measure has been reported in only four cases (Macleod, 2000; Onishi et al., 2004).
METHODS
The authors identified 20 cases of TRE in patients admitted to an acute care cancer hospital. Patient were identified and treated as part of routine clinical care. A retrospective chart review was conducted on all patients to determine the breadth of clinical manifestations and associated findings. We report here on three of the cases who were terminally ill and who died shortly after being diagnosed with TRE. This study was approved by the Memorial Sloan Kettering institutional review board.
RESULTS
Case 1
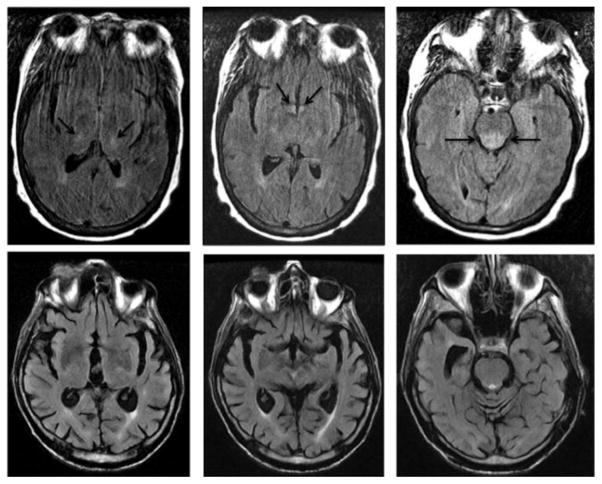
Ms. H was a 61-year-old woman diagnosed with nodular sclerosing Hodgkin’s lymphoma in 2009. She received four cycles of etoposide/vinblastine/coxorubicin, followed by radiation to the right axilla. She was in remission until March of 2013, when she relapsed, and immediately began receiving palliative radiation to the right pelvis. In September of 2013 she developed abdominal distention, and CT of the abdomen and pelvis showed intraabdominal lymphadenopathy, ascites, and infiltration of intraabdominal fat. In October of 2013, she received a dose of brentuximab for palliative purposes, and was discharged home with additional doses scheduled. After 6 days, she began noticing increased abdominal girth, pain, and constipation. She remained at home for 4 days before returning to the hospital with a partial bowel obstruction. She had an additional history of nausea and bilious vomiting as well as dysphagia to liquids and solids occurring intermittently over a chronic time-course of several months. She had a remote history of alcoholism and had been sober for 24 years. She was hypertensive (151/90 mm Hg) and tachycardic (95–114 bpm over 24 hours), had a probable UTI based on urinalysis and microscopy, and an elevated neutrophil count. She was admitted for conservative management including NPO, , surgical evaluation, and treatment with ceftriaxone for presumed UTI. Over the course of 3 days, she developed a fever, as well as altered mental status consisting of hypoarousal, disorientation, and motoric agitation fluctuating with lucid intervals throughout the day. Blood and urine cultures were negative for infections, and chest X-ray showed no evidence of pneumonia. Non-contrast head CT ruled out acute pathology. She was treated empirically with vancomycin, cefepime, ampicillin, and acyclovir, though lumbar puncture results later ruled out bacterial, fungal, and viral meningitis. BUN and Cr were normal. Serum ammonia level was 120 μM/L, and she was started on lactu-lose and rifaximin. After 2 days, her mental status continued to decline with fewer lucid intervals and increased motoric agitation. Despite the onset of bowel movements and normalization of her serum ammonia level within 3 days, her symptoms progressed to the point at which she was disoriented to person, place, and time, and she was not following commands or producing intelligible speech. The patient pulled out her NG tube and was regularly pulling at her intravenous lines, requiring a sitter to help with frequent redirection. An MRI of the brain performed on the 6th day of admission demonstrated an abnormal hyperintense signal within the medial thalami, and mammillary bodies and hypothalamus on the fluid-attenuated inversion recovery (FLAIR) sequence (Figure 1). These findings are considered typical in the spectrum of abnormalities that can be identified on imaging of patients with Wernicke’s encephalopathy. This patient also demonstrated abnormal and fairly symmetric FLAIR hyperintense signals in the posterior pons, which is an atypical imaging finding (Figure 1).
Fig. 1.
Axial FLAIR images from case 1 (top row, left to right) demonstrate abnormal hyperintense signal in the bilateral thalami, mammillary bodies/hypothalamus, and posterior pons. The patient could not hold still for the exam due to her altered mental status, and the images are mildly degraded by motion. The axial FLAIR images from case 3 (bottom row) demonstrate no abnormal signal within the thalami, mammillary bodies, hypothalamus, and posterior pons.
Given the abrupt onset of mental status changes in the context of severe and acute malnutrition, a diagnosis of Wernicke’s encephalopathy was suspected on the 7th day of admission (about 13 days after onset of anorexia). A serum thiamine concentration was drawn and sent to an outside lab, which was later resulted as being abnormally low (<7 nmol/L). Immediately after drawing the thiamine level, administration of high-dose intravenous thiamine (thiamine HCl 500 mg IV q 8 hr) began. On the second day of thiamine repletion, the patient became more alert, was intermittently following simple commands, and oriented to person and place. After 3 days of high-dose intravenous thiamine, dosing was decreased to 100 mg thiamine IV daily. On the 15th day of admission (8 days after starting thiamine), the patient was alert throughout the daytime hours, and oriented to person, place, and time. She was able to produce spontaneous speech and responded to questions with short answers but logically. She was able to engage with her family and able to participate in end-of-life planning. Her serum magnesium remained between 1.8 and 2 mEq/L throughout the duration of treatment. The partial recovery of her mental status symptoms continued for another 14 days until she died from progression of disease 35 days after being admitted.
Case 2
Mr. G was a 68-year-old man with adenocarcinoma of the colon metastatic to liver, lung, and peritoneum, who presented to the acute care setting after 3 months of persistent nausea, vomiting, and decreased oral intake. The cancer diagnosis had been made 3 months earlier, when he was hospitalized for newly developing ascites. He had immediately begun chemotherapy with FOLFOX, but after four cycles his disease progressed, and he was switched to irinotecan 1 week prior to presenting to our hospital. After his first dose of irinotecan, he developed nausea and vomiting that did not respond to oral antiemetics. After 6 days, he decided to present to our hospital because of worsening weight loss and anorexia. Since his initial diagnosis of cancer 3 months earlier, he had lost 40 pounds in total, about 22% of his body weight.
Mr. G was married, had two children, and worked as an accountant at a large business. He had a 50 pack-year smoking history and continued to smoke 2–4 cigarettes per day. He denied drinking alcohol or using any recreational drugs. His pertinent medical history included non-insulin-dependent diabetes mellitus (no prescribed medications) with evidence of end-organ damage (toe amputation in 2008), mild hypertension (well controlled with metoprolol and lisinopril), and a history of a transient ischemic attack 8–10 years prior. Additional medications included fondaparinux, atorvastatin, finasteride, and tamsulosin. He had no past psychiatric history. At the time of presentation, Mr. G was hemodynamically stable. He appeared cachectic and chronically ill. He had bitemporal muscle wasting, lower extremity edema, and difficulty ambulating due to generalized weakness. He was alert and oriented, and had no evidence of mental status changes. Laboratory abnormalities included leukocytes 0.7 K/μL, hemoglobin 7.9 g/dL, albumin 2.6 g/dL, alkaline phosphatase 798 units/L, potassium 3.2 mEq/L, phosphorus 2.2 mEq/L, and magnesium 1.3 mEq/L.
Mr. G was admitted, his electrolytes were repleted, and intravenous ondansetron and orally disintegrating olanzapine were used to try to control nausea and vomiting. C. difficile PCR was returned positive on the second day of admission, and Mr. G was treated with metronidazole. On the fourth day of admission, Mr. G began experiencing brief episodes of confusion and disorientation, which waxed and waned throughout the day. Because he was already receiving olanzapine 5 mg at bedtime for nausea, no additional antipsychotic medication was added. His nausea, vomiting, and poor intake continued, and after 9 days of admission, metoclopramide was added without efficacy. His delirium progressed, and by the 12th day of admission he seemed to have more confused periods than lucid ones. His periods of confusion were now sometimes accompanied by climbing out of bed, which required a 24-hour sitter. On the 14th day of admission, percutaneous endoscopic gastrosotomy (PEG) tube placement was attempted. Due to compression of the stomach in multiple areas, a PEG feeding tube could not be placed; however, a PEG drainage tube was placed, which resulted in relief of vomiting and some relief from nausea.
At this point, Mr. G’s delirium had progressed to where he believed he was on a boat, was unable to consistently identify family members in photos, exhibited frequent paraphasic errors, frequent derailments in his thought process, executive dysfunction, and semi-purposeful movements. He was unable to maintain coherent conversations and thus unable to participate in planning his treatment. His sodium was 134 mEq/L, Hgb remained low at 8.8 g/dL, and alkaline phosphatase remained high at 707 units/L. Potassium, magnesium, phosphate, AST, ALT, BUN, creatinine, TSH, and folate were all within normal ranges. Vitamin B12 was supratherapeutic. He had no clinical evidence of infections and continued to be treated with metronidazole. He was normotensive but tachycardic (BP 113/76; HR 97–111 bpm); oxygen saturation 96%; temperature 36.2°C. On exam, mild slurring of speech was noted, and he was not able to follow commands to perform testing for cerebellar signs. No nystagmus was noted. Wernicke’s encephalopathy was suspected because of the change in mental status in the context of a likely thiamine deficiency. Serum thiamine, drawn on the 14th day of admission, was abnormally low (<7 nmol/L), and thiamine 500 mg IV t.i.d. was administered for 3 days. Within 36 hours of initiating thiamine, the patient displayed less motor agitation overnight. Some 12 hours later, he was able to speak in full sentences, though he still appeared confused. Four days after initiating thiamine, Mr. G was lucid, able to converse with his family and participate in end-of-life planning. He remained lucid an additional 3 days until being transferred to an inpatient hospice, where he died one day later.
Case 3
Mr. A was an 80-year-old man with recurrent squamous cell carcinoma of the right eyebrow. He was initially diagnosed in 2012 and treated with Mohs surgery of the right eyebrow. His cancer recurred in July of 2013, for which he had an additional Mohs surgery. He was admitted in December of 2013 due to 3 weeks of progressive weakness, difficulty swallowing food and liquids, decreased energy, and low engagement in daily activities. The symptoms began while receiving daily radiation therapy to the right supraorbital tumor, V1, VII, in-transit lymphatics, preauricular and cervical IB–III lymph nodes (5600 cGy in 28 fractions), and right supraorbital tumor boost (3000 cGy in 15 fractions). Psychiatric consult was requested to evaluate for depression.
Mr. A had previously been employed in the service industry, but had lost his job about six months earlier. Since then, he recalled being less physically active and eating less; however, upon initiation of radiation therapy in December of 2013, he became increasingly fatigued. He also began having difficulty swallowing and, in addition, little appetite. He denied cognitive difficulties or disorientation, and was able to relate both his remote and recent history accurately. He denied all other psychiatric symptoms initially, including other symptoms of depression, anxiety, and psychosis. His medical history included coronary artery disease requiring stent placements and an ejection fraction of 37%, both recently diagnosed. He had no past psychiatric history.
On mental status examination, he appeared lethargic, chronically ill, cachectic, psychomotor retarded, and had poor eye contact. He described his mood as “good,” but affect appeared constricted. Thought process was linear and logical, though content was somewhat superficial. He denied perceptual disturbances, and denied suicidality. Cognitive testing was significant for being fully oriented, with 3/3 registration, 1/3 recall, intact naming. In a test of his attention, he was able to name months of the year in reverse from December to June, but then refused to go any further, citing fatigue. Labs were significant for mild hypernatremia (Na 147), acute renal insufficiency (BUN 52, Cr 1.5, baseline Cr 1.3), and hypoalbuminemia (alb 2.9). He was initially felt to have an adjustment disorder with depressed mood in the setting of his progressing cancer, with a rule out of mood disorder due to general medical condition, as well as possible mild cognitive impairment.
Over the next two days, Mr. A became increasingly hypoaroused, less interactive, and weaker. He continued to endorse feeling “good,” denied all depressive symptoms, but on cognitive testing was oriented only to person and setting, and was perseverative on the name of the hospital to the point of being unable to participate any further with cognitive tasks. Sodium had normalized, but BUN was 50 and Cr remained elevated at 1.4. CBC was unremarkable. He was felt to be delirious, and workup was recommended to assess for infectious, metabolic, or neurological etiologies. A thyroid panel was normal, and B12 and folate were normal, AM cortisol level was normal, FSH/LH were normal, and prolactin was normal. Testosterone was low (55). Blood cultures were negative. Urinalysis and urine cultures were initially positive for Klebsiella but repeat culture showed coagnegative staph, and this was felt to be a contaminant. Head CT showed mild brain parenchymal volume loss, no evidence of acute infarct, hydrocephalus, mass effect, or acute intracranial hemorrhage. Olanzapine 5 mg was recommended at bedtime.
Based on the operationalized criteria, the patient was diagnosed with Wernicke’s encephalopathy. Thiamine levels were recommended but not drawn, and he received thiamine IV 100 mg daily for 3 days. He remained minimally interactive and lethargic. Methylphenidate and modafinil were considered but were felt to present unacceptable cardiac risk by the cardiology service given his CHF. After an additional 7 days, blood cultures were positive for MRSA, and he was started on vancomycin. Olanzapine was changed to aripiprazole 5 mg PO daily, with some improvement in alertness during the day, though he remained perseverative and intermittently disoriented. He had forward flexed posture with decreased step length, decreased cadence, and gait unsteadiness. There was no rigidity or tremor, and motor and sensory exams were noted to be normal. Rapid alternating movements were not tested. The patient’s brain MRI demonstrated moderate diffuse parenchymal volume loss and multiple asymmetric FLAIR hyper-intensities that were more consistent with chronic microvascular ischemic disease than Wernicke’s encephalopathy (Figure 1). In contrast to case 1, this patient did not demonstrate abnormal signal in the thalami, mammillary bodies, and hypothalamus (Figure 1). Another 7 days passed, after which point his blood cultures were cleared of infection, but he remained minimally interactive, perseverative, and hypoaroused. On day 30 of his admission, thiamine dosing was increased to 500 mg IV three times daily.
Within 24 hours of administering high-dose intravenous thiamine, the patient appeared more alert, had a brighter affect, was fully conversant, and used humor appropriately. He described having felt “sicker than I ever felt before,” and having been aware of time passing but being unable to interact with people around him. He reported feeling clearer cognitively, his attention and memory had improved, and he was alert and oriented, and he was no longer perseverative. For the first time in 28 days, he was able to converse with his daughters, one of whom was his healthcare proxy. The following day, he developed a rapidly progressive infection and died of sepsis later that day. His wishes, confirmed by his daughter, were not to be resuscitated.
DISCUSSION
This study describes the clinical presentations of Wernicke’s encephalopathy in three patients with cancer whose symptoms improved following administration of high-dose intravenous thiamine. It adds to the relatively sparse literature on WE in terminally ill cancer patients (Macleod, 2000; Onishi et al., 2004; Yae et al., 2005). Diagnosis was made based on the operationalized Caine criteria in all three patients. The prompt response to high-dose intravenous thiamine further supported the diagnosis. Multiple risk factors for developing thiamine deficiency were identified in each of the three cases (Table 1). Thiamine deficiency may not translate into the full clinical syndrome in every individual, but it is a necessary ingredient in developing WE.
Table 1.
Mechanisms of thiamine deficiency in cancer patients
| Pathophysiological Mechanism Causing Thiamine Deficiency |
Clinical Correlates Frequently Seen in Cancera |
Specific Clinical Features: Case 1b |
Specific Clinical Features: Case 2c |
Specific Clinical Features: Case 3d |
|---|---|---|---|---|
| ↓ Availability of thiamine | Low intake |
|
|
|
| Poor absorption |
|
|
||
| ↓ Storage capacity (e.g., liver disease, muscle wasting) |
|
|
||
| ↑ Usage of thiamine | ↑ Cell turnover Fast-growing tumors |
|
|
|
| ↑ Metabolic rate (Systemic illness, corticosteroids, infections) |
|
|
|
|
| Impaired activity of thiamine-dependent enzymes | Cofactor deficiency (e.g., magnesium) |
|
||
| Direct effects of Medications (e.g. metronidazole, 5-fluorouracil) |
|
|||
| ↑ Losses | Hemodialysis | |||
| Diabetes |
|
Pathophysiological mechanisms causing thiamine deficiency in cancer patients.
Clinical correlates related to each pathophysiological mechanism of thiamine deficiency.
Specific clinical features from each case, which may have predisposed the patient to developing thiamine deficiency. Clinical features are grouped by pathophysiological mechanism.
The utility of serum thiamine levels and MRI in detecting thiamine deficiency and WE, respectively, is exemplified by these three cases. In cases 1 and 2, serum thiamine levels were in the abnormally low range, indicating thiamine deficiency, though not diagnostic for WE. At our hospital, serum thiamine is analyzed at an outside laboratory, and results are returned after 7–10 days. This is too long to withhold thiamine if thiamine deficiency is suspected based on clinical history and presentation. Therefore, the sole, albeit important, utility of the blood test in these cases was to retrospectively confirm the suspicion of thiamine deficiency. Whereas serum thiamine levels detect thiamine deficiency, MRI findings, when present, are highly specific for WE. In case 1, the MRI was ordered prior to suspicion of WE in order to rule out other intracranial pathology. The initial results did not indicate WE-type findings, and it was only after clinical improvement from thiamine that one author (EIG) requested a second reading from another author (VH). With the new and additional clinical information, the MRI findings compatible with WE were noted as above. Thus, the use of MRI in the evaluation of WE seems to necessitate a thorough description of WE in the clinical information provided to the radiologist. Still, given the cost and low sensitivity of MRI, it does not seem to warrant routine use in the diagnosis of WE. Furthermore, delaying treatment for the purpose of ordering an MRI is not warranted. Ultimately, WE is a clinical diagnosis, as demonstrated by case 3: serum thiamine was not ordered; MRI did not detect changes consistent with WE; the diagnosis was made solely on clinical grounds according to the operationalized Caine criteria; suspicion of thiamine deficiency (see Table 1) and prompt response to thiamine both supported the diagnosis.
In all three cases, the outcome of using thiamine was a rapid improvement in mental status. The first signs of improvement were noted within 24 to 36 hours, improvements in mental status continued over 3–5 days, and clinical improvements were maintained until death. In all three cases, 500 mg IV thiamine HCl t.i.d. was administered. While high-dose thiamine appears to be universally recommended in the literature, little consensus exists on what actually constitutes a high dose. Recommendations for high-dose thiamine in the treatment of alcoholic patients with WE is 500 mg IV t.i.d. in the United Kingdom (Thomson et al., 2002) and 200 mg t.i.d. in Europe (Galvin et al., 2010). In both cases, the emphasis is on intravenous use, multiple times daily, and for several days. A review by Isenberg-Grzeda et al. (2012) noted that 100 mg thiamine is insufficient to treat WE. A recent study found that nearly all inpatients with alcoholism were receiving inadequate doses of thiamine (Isenberg-Grzeda et al., 2014), though no similar studies in cancer patients exist. Case 3 did not improve on 100 mg thiamine but did improve with a higher dose.
Many case reports of WE in cancer have established WE as the sole cause of altered mental status. Our diagnostic and conceptual approach was different, and more consistent with the notion that up to 25% of confusional states in cancer have multiple etiologies (Kuo et al., 2009). In case 1, readily identifiable factors that were likely contributing to the delirium included infection and hepatic encephalopathy. In case 3, factors might have included infection and uremia. In every case of thiamine deficiency, the possibility of other vitamin and nutrient deficiencies should be considered as possible contributing factors to the delirium. The possibility of leptomeningeal spread and brain metastasis should also be considered in appropriate cases. In all three cases, other possible causes of delirium were treated either sequentially or concurrently. Because thiamine is safe, inexpensive, and effective, and because the risks of untreated WE include significant morbidity and mortality, the benefits seem to greatly outweigh the risks in the context of the acute inpatient setting. While the Caine criteria helped made great strides in improving diagnostic sensitivity to 85%, many cases will still be missed, including among nonalcoholic patients for whom the criteria have not been validated. Thus, to avoid the devastating consequences of missed cases, the emphasis should be on overdiagnosing and overtreating, rather than the opposite. In this case series, we used thiamine as a tool to treat WE in three patients with terminal cancer who died shortly after treatment. Thiamine was found to be an effective tool for palliation of delirious symptoms, and allowed patients to regain enough lucidity to partake in end-of-life discussions and decision making with their families and treatment providers.
The limitations of our study include its retrospective design and small sample size. Prospective studies are needed to help further characterize the clinical manifestations of TRE in cancer patients. Studies should include larger numbers of cancer patients and use standardized delirium assessments and neurological exams. Studies aiming to improve the diagnostic criteria for TRE in cancer are needed, as are comparative efficacy studies for thiamine dosing strategies in cancer.
CONCLUSION
Much of what is known about Wernicke’s encephalopathy comes from studies in alcoholic patients, and it is still unclear if or how the phenomenology, clinical course, and treatment outcomes differ. The fact that WE is so strongly associated with alcoholism implies that as a field, we know even less about WE as it pertains to cancer. It seems reasonable, therefore, to conceptualize the syndrome reported here as a thiamine-related encephalopathy (TRE) in cancer. Future studies will need to validate the Caine criteria in cancer patients, or create a new set of diagnostic criteria for diagnosing TRE in cancer. While thiamine is safe and inexpensive, vitamin shortages have served as a reminder that medication supplies are limited, and thus future studies should also clarify dosing strategies for thiamine repletion among cancer patients with TRE in order to maximize efficacy and prevent while limiting the potential for shortages. As more studies emerge about the prevalence of thiamine deficiency and TRE in cancer, TRE may become increasingly identified by cancer care providers. An understanding of the risk factors, prompt diagnosis, and evidence-based treatment strategies will be crucial in managing TRE and avoiding significant morbidity and mortality.
References
- Antunez E, Estruch R, Cardenal C, et al. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke’s encephalopathy. AJR. American Journal of Roentgenology. 1998;171(4):1131–1137. doi: 10.2214/ajr.171.4.9763009. [DOI] [PubMed] [Google Scholar]
- Arana-Guajardo AC, Camara-Lemarroy CR, Rendon-Ramirez EJ, et al. Wernicke encephalopathy presenting in a patient with severe acute pancreatitis. Journal of the Pancreas. 2012;13(1):104–107. [PubMed] [Google Scholar]
- Boldorini R, Vago L, Lechi A, et al. Wernicke’s encephalopathy: Occurrence and pathological aspects in a series of 400 AIDS patients. Acta Bio-Medica de l’Ateneo Parmense. 1992;63(1–2):43–49. [PubMed] [Google Scholar]
- Bonucchi J, Hassan I, Policeni B, et al. Thyrotoxicosis-associated Wernicke’s encephalopathy. Journal of General Internal Medicine. 2008;23(1):106–109. doi: 10.1007/s11606-007-0438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine D, Halliday GM, Kril JJ, et al. Operational criteria for the classification of chronic alcoholics: Identification of Wernicke’s encephalopathy. Journal of Neurology Neurosurgery, & Psychiatry. 1997;62(1):51–60. doi: 10.1136/jnnp.62.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reuck J, Sieben G, De Coster W, et al. Prospective neuropathologic study on the occurrence of Wernicke’s encephalopathy in patients with tumours of the lymphoid–hemopoietic systems. Acta Neuropathologica Supplementum. 1981;7:356–358. doi: 10.1007/978-3-642-81553-9_101. [DOI] [PubMed] [Google Scholar]
- Donnino MW, Vega J, Miller J, et al. Myths and misconceptions of Wernicke’s encephalopathy: What every emergency physician should know. Annals of Emergency Medicine. 2007;50(6):715–721. doi: 10.1016/j.annemergmed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Galvin R, Brathen G, Ivashynka A, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. European Journal of Neurology. 2010;17(12):1408–1418. doi: 10.1111/j.1468-1331.2010.03153.x. [DOI] [PubMed] [Google Scholar]
- Hahn JS, Berquist W, Alcorn DM, et al. Wernicke encephalopathy and beriberi during total parenteral nutrition attributable to multivitamin infusion shortage. Pediatrics. 1998;101(1):E10. doi: 10.1542/peds.101.1.e10. [DOI] [PubMed] [Google Scholar]
- Immadisetty V, Cant T, Thyarappa P, et al. Bio-markers for detecting thiamine deficiency: Improving confidence and taking a comprehensive history are also important. Alcohol and Alcoholism. 2009;44(6):634. doi: 10.1093/alcalc/agp074. [DOI] [PubMed] [Google Scholar]
- Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke–Korsakoff syndrome: Under-recognized and under-treated. Psychosomatics. 2012;53(6):507–516. doi: 10.1016/j.psym.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Isenberg-Grzeda E, Chabon B, Nicolson SE. Prescribing thiamine to inpatients with alcohol use disorders: How well are we doing? Journal of Addiction Medicine. 2014;8(1):1–5. doi: 10.1097/01.ADM.0000435320.72857.c8. [DOI] [PubMed] [Google Scholar]
- Kleinert-Altamirano AP, Juarez-Jimenez H. Wernicke’s encephalopathy and Caine criteria: Report of six cases. Revista Médica del Instituto Mexicano del Seguro Social. 2014;52(1):104–107. [PubMed] [Google Scholar]
- Kuo SH, Debnam JM, Fuller GN, et al. Wernicke’s encephalopathy: An underrecognized and reversible cause of confusional state in cancer patients. Oncology. 2009;76(1):10–18. doi: 10.1159/000174951. [DOI] [PubMed] [Google Scholar]
- Lishman WA. Cerebral disorder in alcoholism: Syndromes of impairment. Brain. 1981;104(Pt. 1):1–20. doi: 10.1093/brain/104.1.1. [DOI] [PubMed] [Google Scholar]
- Lu’o’ng KV, Nguyen LT. The role of thiamine in cancer: Possible genetic and cellular signaling mechanisms. Cancer Genomics & Proteomics. 2013;10(4):169–185. [PubMed] [Google Scholar]
- Macleod AD. Wernicke’s encephalopathy and terminal cancer: Case report. Palliative Medicine. 2000;14(3):217–218. doi: 10.1191/026921600668593115. [DOI] [PubMed] [Google Scholar]
- Onishi H, Kawanishi C, Onose M, et al. Successful treatment of Wernicke encephalopathy in terminally ill cancer patients: Report of three cases and review of the literature. Supportive Care in Cancer. 2004;12(8):604–608. doi: 10.1007/s00520-004-0637-y. [DOI] [PubMed] [Google Scholar]
- Palacios-Marques A, Delgado-Garcia S, Martin-Bayon T, et al. Wernicke’s encephalopathy induced by hyperemesis gravidarum. BMJ Case Reports. 2012 doi: 10.1136/bcr-2012-006216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechi G, Serra A. Wernicke’s encephalopathy: New clinical settings and recent advances in diagnosis and management. The Lancet Neurology. 2007;6(5):442–455. doi: 10.1016/S1474-4422(07)70104-7. [DOI] [PubMed] [Google Scholar]
- Singh S, Kumar A. Wernicke encephalopathy after obesity surgery: A systematic review. Neurology. 2007;68(11):807–811. doi: 10.1212/01.wnl.0000256812.29648.86. [DOI] [PubMed] [Google Scholar]
- Thomson AD, Cook CC, Touquet R, et al. The Royal College of Physicians report on alcohol: Guidelines for managing Wernicke’s encephalopathy in the accident and emergency department. Alcohol and Alcoholism. 2002;37(6):513–521. doi: 10.1093/alcalc/37.6.513. [DOI] [PubMed] [Google Scholar]
- Thomson AD, Cook CC, Guerrini I, et al. Wernicke’s encephalopathy revisited: Translation of the case history section of the original manuscript by Carl Wernicke, “Lehrbuch der Gehirnkrankheiten für Aerzte und Studirende” (1881), with a commentary. Alcohol and Alcoholism. 2008a;43(2):174–179. doi: 10.1093/alcalc/agm144. [DOI] [PubMed] [Google Scholar]
- Thomson AD, Cook CC, Guerrini I, et al. Wernicke’s encephalopathy: “Plus ca change, plus c’est la meme chose. Alcohol and Alcoholism. 2008b;43(2):180–186. doi: 10.1093/alcalc/agm149. [DOI] [PubMed] [Google Scholar]
- Thomson AD, Marshall EJ, Guerrini I. Bio-markers for detecting thiamine deficiency: Improving confidence and taking a comprehensive history are also important. Alcohol and Alcoholism. 2010;45(2):213. doi: 10.1093/alcalc/agq004. [DOI] [PubMed] [Google Scholar]
- Yae S, Okuno S, Onishi H, et al. Development of Wernicke encephalopathy in a terminally ill cancer patient consuming an adequate diet: A case report and review of the literature. Palliative & Supportive Care. 2005;3(4):333–335. doi: 10.1017/s1478951505050509. [DOI] [PubMed] [Google Scholar]
- Zuccoli G, Pipitone N, Santa Cruz D. Metronidazole-induced and Wernicke encephalopathy: Two different entities sharing the same metabolic pathway? AJNR. American Journal of Neuroradiology. 2008;29(9):E84. doi: 10.3174/ajnr.A1142. author reply E85. [DOI] [PMC free article] [PubMed] [Google Scholar]