Abstract
Background
Tuberculosis is an important risk factor for chronic respiratory disease in resource poor settings. The persistence of abnormal spirometry and symptoms after treatment are well described, but the structural abnormalities underlying these changes remain poorly defined, limiting our ability to phenotype post-TB lung disease in to meaningful categories for clinical management, prognostication, and ongoing research. The relationship between post-TB lung damage and patient-centred outcomes including functional impairment, respiratory symptoms, and health related quality of life also remains unclear.
Methods
We performed a systematic literature review to determine the prevalence and pattern of imaging-defined lung pathology in adults after medical treatment for pleural, miliary, or pulmonary TB disease. Data were collected on study characteristics, and the modality, timing, and findings of thoracic imaging. The proportion of studies relating imaging findings to spirometry results and patient morbidity was recorded. Study quality was assessed using a modified Newcastle-Ottowa score. (Prospero Registration number CRD42015027958)
Results
We identified 37 eligible studies. The principle features seen on CXR were cavitation (8.3–83.7%), bronchiectasis (4.3–11.2%), and fibrosis (25.0–70.4%), but prevalence was highly variable. CT imaging identified a wider range of residual abnormalities than CXR, including nodules (25.0–55.8%), consolidation (3.7–19.2%), and emphysema (15.0–45.0%). The prevalence of cavitation was generally lower (7.4–34.6%) and bronchiectasis higher (35.0–86.0%) on CT vs. CXR imaging. A paucity of prospective data, and data from HIV-infected adults and sub-Saharan Africa (sSA) was noted. Few studies related structural damage to physiological impairment, respiratory symptoms, or patient morbidity.
Conclusions
Post-TB structural lung pathology is common. Prospective data are required to determine the evolution of this lung damage and its associated morbidity over time. Further data are required from HIV-infected groups and those living in sSA.
Introduction
Chronic respiratory diseases (CRDs) are the fourth leading cause of non-communicable disease (NCD) deaths globally, and pose a particular challenge to low and middle-income countries (LMICs) where risk factors for respiratory damage including poverty-related in-utero and early childhood exposures[1], exposure to acute respiratory infections[2], indoor biomass fuel exposure[3, 4], a rising prevalence of smoking[5], chronic HIV-infection[6], and pulmonary tuberculosis (PTB) intersect. A high prevalence of CRDs has been demonstrated in LMICs, and these are expected to have a substantial impact on population health and health-services in the coming years[7–10]. An improved understanding of the nature of CRDs in LMICs, their natural history, and associated morbidity and mortality is required if we are to design appropriate prevention, diagnostic, and management strategies[11, 12].
Pulmonary TB remains an important cause of chronic respiratory impairment in LMICs. 5.2 million incident cases of PTB were reported globally in 2014[13], and the presence of long term respiratory sequelae following PTB treatment is well established [14, 15]. Pulmonary granuloma formation, tissue necrosis and liquefaction, and aberrant healing responses are known features of TB disease[16, 17]. The persistence of abnormal airway physiology after treatment has been documented in large population-based cross-sectional studies which show 1.37–2.94 higher odds of fixed airways obstruction in those with a history of PTB, compared to those without[15, 18–21]. Previous TB has also been associated with chronic respiratory symptoms in LMICS: previous TB was the strongest predictor of chronic bronchitis within the 1996 South African Demographic & Health Survey [22] and the odds of a medical diagnosis of bronchiectasis were over 3-fold higher in those who had a history of TB, compared to those who had not, in a population based study of 10,811 adults in China [23].
Whilst the evidence for abnormal spirometry and symptoms following PTB disease in LMICS is clear, imaging of patients completing PTB treatment is not routinely performed, and our understanding of the associated patterns of structural lung pathology remains limited. Without these imaging data, we are not yet able to accurately phenotype patterns of post-TB lung disease in to the meaningful categories required for clinical management, prognostication, and ongoing research into the risk factors for post-TB lung damage [24, 25]. In addition, information on the morbidity and mortality associated with post-TB lung damage remains limited, but would be timely given the post-2015 TB agenda, which recognises the need for TB services to mitigate the long-term detrimental impact of TB disease on patients’ lives and livelihoods [26].
In this review we seek to improve our understanding of the nature and impact of post-TB lung disease. We examine the literature on imaging defined structural post-TB lung damage, and its relationship to patient-centred outcomes including functional impairment, respiratory symptoms, and health-related quality of life. We have included studies using both plain chest radiographs (CXR) and more detailed computerised tomography (CT) imaging in our review: CXR is widely available for use in TB screening and diagnosis and may be of programmatic use in the diagnosis and management of post-TB lung damage, but CT provides a higher level of detail and may provide more information on the true nature of this damage.
Methods
A protocol driven literature search was performed following PRISMA guidelines to identify studies in which consecutive participants with pleural, miliary, or pulmonary TB were recruited, where CXR or CT was performed after the completion of a full medical TB treatment regimen, and where the prevalence of abnormal imaging or the severity of residual structural lung damage was reported (S1 File). Cohort studies, cross-sectional studies and randomised control trials (RCTs) were eligible for inclusion. There were no limits on publication date. Only studies published in English were included.
Literature searches were conducted in Medline, Pubmed, Scopus, Web of Science, and the Cochrane Library (July 2016) (Table 1). Reference lists from published reviews and reference and citation lists of papers meeting inclusion criteria were reviewed to identify additional articles.
Table 1. Template for literature search: Pulmonary, pleural or military tuberculosis AND [CXR imaging OR CT imaging].
| Criteria | Search terms |
|---|---|
| Pulmonary, pleural, or miliary tuberculosis | “Tuberculosis, pulmonary”[Mesh] OR “tuberculosis, miliary[Mesh] OR “tuberculosis, pleural”[Mesh] OR "pulmonary TB" OR "pulmonary tuberculosis" |
| CXR Imaging | “thoracic radiography"[MeSH] OR “chest x-ray" OR "chest radiograph" OR "CXR" |
| CT imaging | “computed tomography”[MeSH] OR "CT" OR "comput* tomography" |
The title and abstract of all identified studies were screened by two independent reviewers (JM and HS). Full text review was performed on all selected articles. Studies restricted to paediatric populations, in whom PTB has a varied presentation, or patients with non-HIV related immunosuppression (chemotherapy, malignancy etc.) where imaging was likely to be affected by comorbidities, were excluded. Data from the control arms of trials of adjuvant immunomodulatory therapies, in which patients received medical TB treatment only, were included.
A standardized data extraction form was used by 2 authors (JM and HS) to determine the primary outcome of interest, which was the prevalence of abnormal imaging after TB treatment. Information was collected on the patterns of imaging pathology, study characteristics, participant characteristics, treatment regimens, and the modality and timing of thoracic imaging. We recorded the proportion of studies that presented a measure of association between imaging findings and other clinical parameters including spirometry, functional capacity, respiratory symptoms, or health-related quality of life. Disagreements in study selection and data extraction were resolved by discussion. Subgroup analyses were conducted to explore the effect of different manifestations of disease (pleural vs. pulmonary), imaging modality (CXR vs. CT), and multidrug-resistant (MDR) disease on the primary outcome. A narrative analysis was conducted.
Study quality was determined using a modified version of the Newcastle-Ottowa score[27], which included assessment of selection bias, adequacy of follow-up, the accuracy with which baseline TB disease and treatment completion were determined, the quality and standardisation of imaging interpretation, and the exclusion of those with structural lung disease preceding TB-disease. A maximum score of 5 was possible for cohort studies, and 4 for cross-sectional studies where no follow-up was required.
Results
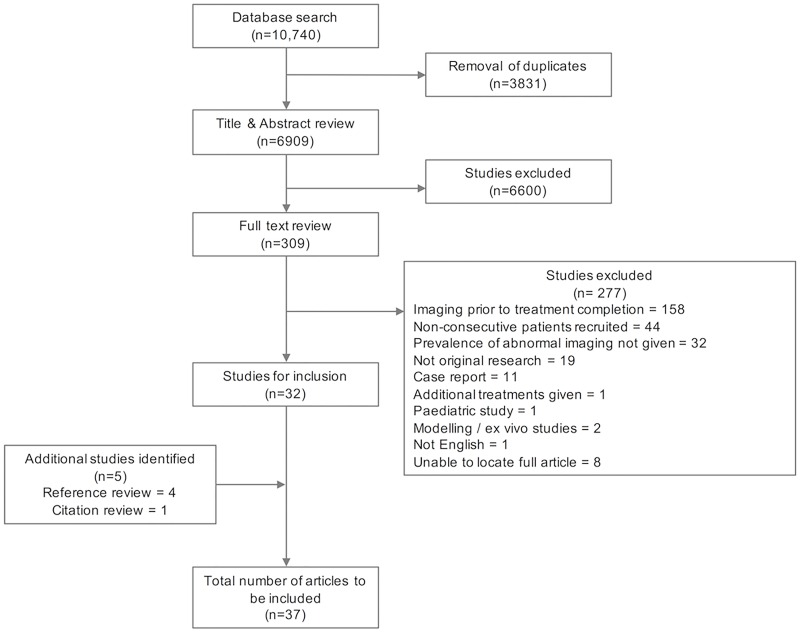
We identified 10,740 articles, with 6909 articles remaining after removal of duplicates. Title and abstract review identified 309 articles for full text review, of which 277 were excluded for reasons including non-consecutive patient recruitment, imaging prior to treatment completion, and failure to report the absolute prevalence or severity of residual lung damage. Reference and citation searches identified 5 further articles for inclusion, giving a total of 37 articles (Fig 1).
Fig 1. PRISMA flow chart.
These papers covered the time period 1973–2015. They included 16 prospective cohort studies with patients recruited at TB diagnosis and imaged upon treatment completion, and one prospective cohort study where imaging was performed 1 year post treatment completion[28]. There were 7 cross-sectional studies, 5 of which performed imaging at various time points after treatment completion, and 7 retrospective cohort studies, which performed imaging upon treatment completion. Data from 6 RCTs were included: data from both study arms were included for two treatment regimen trials [29, 30] and a study investigating the effect of Vitamin-D and L-arginine supplementation[31], but data from the control arms only were included from trials investigating prednisolone use[32, 33], and a trial of M. vaccae immunomodulation[34]. All RCTs performed imaging at treatment completion, and 1 also performed serial imaging 6-months later[32]. Only seven studies used CT imaging to describe structural pathology. Five of these were conducted in the Americas, and all performed imaging at treatment completion. Only one study of pleural disease used both CXR and CT imaging[32].
The total number of patients in all included studies was 4870, with disaggregated data available for 76 HIV-infected individuals. The median number of participants per study was 131 (range 13–1080). Studies were conducted in the Americas (n = 11), South East Asia (n = 9), Europe (n = 6), the Western Pacific region (n = 6), and Africa (n = 2). Both of the studies conducted in Africa were from South Africa and focused on either pleural[32] or MDR disease[35]. 26 studies focused on PTB, 1 on the sequelae of miliary TB, 9 were restricted to pleural TB, and 1 included patients with pulmonary, pleural or mediastinal TB[36]. The marked heterogeneity between studies made meta-analysis of their findings inappropriate.
Few studies specified the pattern of drug sensitivity (12/37), or previous episodes of TB (10/37). Two studies of residual lung damage in patients receiving TB retreatment were included, one of which was restricted to MDR patients[34, 37]. Two additional studies of patients with MDR disease but unspecified histories of TB treatment were identified[35, 38]. The treatment regimens used varied widely; only 7 studies specified use of the gold standard short-course treatment regimen, and only 2 out of 3 studies of MDR disease specified the use of national treatment guidelines[35, 37].
Pulmonary disease
Of the 27 studies reporting the sequelae of pulmonary and miliary TB, prevalence estimates for radiographic pathology were given in 17 CXR (Table 2) and 5 CT studies (Table 3), and varied widely. Twelve CXR studies reported the prevalence of cavitation (8.3–83.7%), 3 reported fibrosis (prevalence 25.0–70.4%) and 4 reported bronchiectasis (prevalence 4.3–11.2%). The CT-based studies generally reported a lower prevalence of cavitation (7.4–34.6%), and a higher prevalence of bronchiectasis (35.0–86.0%) and fibrosis (70.0–92.6%), than studies using CXR imaging. A more diverse range of pathologies was noted on CT imaging: pleural thickening was reported in 3 studies (prevalence 0.1–50.0%, n = 99), features potentially suggestive of ongoing inflammation such as nodules were seen in all 5 studies (prevalence 25.9–55.8%, n = 193), consolidation was reported in 4 studies (3.7–19.2%, n = 119), emphysema was seen in in 2 studies (prevalence 15.4–45.0%, n = 72), and mosaicism was documented in 1 study (prevalence 70%, n = 20). A broad range of severity scores were used to quantify residual damage in 13 studies of PTB sequelae, only one of which was validated for scoring post-TB damage rather than active PTB disease[39] (Table 4).
Table 2. Studies reporting prevalence of imaging patterns on CXR imaging following treatment for thoracic tuberculosis.
| Timing of imaging | Author, Year | Country | Study design | TB pattern | Participant HIV status | Treatment episode | Drug sensitivity | Number of participants | Prevalence of pathology (%) | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|
| On completion of TB Treatment | Yu, 1995[40] | Taiwan | Prospective cohort | Pulmonary | Negative | Not specified | Mixed | 22 | Abnormal imaging: 13.6% | 3/5 |
| Al Hajjaj, 2000 [41] | Saudi Arabia | Prospective cohort | Pulmonary | Not specified | Not specified | Not specified | 1080 | Abnormal imaging: 65.9%, Cavitation: 15.0%, Pleural thickening 6.9%, Lung destruction 52.4% | 3/5 | |
| de Valliere, 2004[35] | South Africa | Prospective cohort | Pulmonary | Mixed | Not specified | MDR | 33 | Abnormal imaging: 93.9–100%, Cavitation: 51.5%–69.7% | 2/5 | |
| Buyokoglan, 2007 [42] | Turkey | Prospective cohort | Pulmonary | Negative | Not specified | Not specified | 25 | Cavitation: 28.0% | 3/5 | |
| Swaminathan, 2007 [43] | India | Prospective cohort | Miliary | Positive | Not specified | Not specified | 31 | Abnormal imaging: 22.6%. Lung destruction: 3.2% | 3/5 | |
| Angthong, 2011 [44] | Thailand | Prospective cohort | Pulmonary | Mixed—data disaggregated | First episode | Not specified | 98 HIV+ | Abnormal imaging: 84.7%, Cavitation: 11.2%, Fibrosis: 70.4%, Bronchiectasis: 11.2% | 4/5 | |
| 12 HIV- | Abnormal imaging: 41.7%, Cavitation: 8.3%, Fibrosis: 25.0%, Bronchiectasis: 8.3% | |||||||||
| Small, 1994 [45] | America | Retrospective cohort | Pulmonary | Positive | Not specified | Not specified | 13 | Abnormal imaging: 23.1% | 3/5 | |
| Menon, 2015 [36] | India | Retrospective cohort | Pulmonary, pleural, mediastinal | Not specified | First episode | Not specified | 441 | Abnormal imaging: 40.4, Cavitation: 21.4%, Pleural thickening: 21.2%, Fibrosis: 38.7%, Bronchiectasis: 4.3%, Mediastinal lesions: 23.6% | 2/5 | |
| Kallan, 1988 [46] | India | Cross sectional | Pulmonary | Not specified | Not specified | Not specified | 119 | Abnormal imaging: 100.0%, Cavitation: 42.0%, Bronchiectasis: 7.6% | 1/4 | |
| Anonymous, 1973 [29] | India | RCT–TB treatment regimens* | Pulmonary | Not specified | Not specified | Not specified | 173 | Cavitation: 49.7% | 4/5 | |
| Hamilton, 2008 [30] | America | RC –TB treatment regimens* | Pulmonary | Negative | Not specified | Fully sensitive | 834 | Cavitation: 23.3% | 4/5 | |
| Kenangalem, 2013 [31] | Indonesia | RCT–additional Vit D / L-arginine† | Pulmonary | Mixed | First episode | Mixed | 77 | Cavitation: 18.2% | 2/5 | |
| On completion and at 6m | Corlan, 1997 [34] | Romania | RCT–additional M.vaccae† | Pulmonary | Retreatment | Mixed | Mixed | 43 with imaging on completion | Cavitation: 83.7% | 3/5 |
| 32 with imaging at 6 months | Cavitation: 68.8% | |||||||||
| 6–63 months post completion | Singla, 2009 [38] | India | Cross sectional | Pulmonary | Negative | Not specified | MDR | 45 | Abnormal imaging: 97.8%, Cavitation: 53.3% | 1/4 |
| 14–18 years post completion | Banu Rekha, 2009 [47] | India | Cross sectional | Pulmonary | Not specified | First episode | Not specified | 198 | Abnormal imaging: 85.9% | 1/4 |
| 5 years post completion | Lisha, 2012 [48] | India | Cross sectional | Pulmonary | Not specified | Mixed | Mixed | 224 | Abnormal imaging: 65.6% | 2/4 |
| 0–252 months post completion | Baez-saldana, 2013 [39] | Mexico | Cross sectional | Pulmonary | Mixed | Not specified | Not specified | 127 | Abnormal imaging: 96.9% | 2/4 |
*Data included from both arms
†Data included from placebo arms only
Table 3. Studies reporting prevalence of imaging patterns on CT imaging on completion of treatment for pulmonary tuberculosis.
| Author, Year | Country | Study design | Participant HIV status | Treatment episode | Drug sensitivity | Number of participants | Prevalence of pathology (%) | Quality score |
|---|---|---|---|---|---|---|---|---|
| Poey, 1997[49] | Martinique | Prospective cohort | Negative | Not specified | Not specified | 27 | Cavitation: 7.4%, Bronchiectasis: 85.2%, Fibrosis: 92.6%, Pleural thickening: 4.8%, Nodules: 25.9%, Consolidation: 3.7%, Ground glass pattern: 7.4%, Reticulation: 44.4%, | 3/5 |
| Long, 1998 [50] | Canada | Prospective cohort | Negative | Not specified | Fully sensitive | 20 | Bronchiectasis: 50.0%, Fibrosis: 80.0%, Pleural thickening: 0.1%, Nodules: 55.0%, Consolidation: 15.0%, Emphysema/Bullae: 45.0%, Mosaicism: 70.0% | 3/5 |
| Bombarda, 2003 [51] | Brazil | Prospective cohort | Negative | Not specified | Not specified | 20 | Cavitation: 30.0%, Bronchiectasis: 35.0%, Fibrotic bands: 70.0%, Nodules: 55.0%, Consolidation: 15.0%, Mass lesions: 45% | 3/5 |
| Lee, 2008 [52] | Taiwan | Prospective cohort | Negative | First episode | Fully sensitive | 52 | Cavitation: 34.6%, Bronchiectasis: 44.2%, Fibrosis: 92.3%, Pleural thickening: 50.0%, Nodules: 55.8%, Consolidation: 19.2%, Emphysema/Bullae: 15.4%, Mass lesions: 7.7%, Ground glass pattern: 1.9%, Parenchymal calcification: 11.5% | 1/5 |
| Rufino, 2015[53] | Brazil | Prospective cohort | Not specified | Not specified | Not specified | 74 | Cavitation: 16%, Bronchiectasis: 86%, Nodules: 48%, Parenchymal opacities: 25%, Parenchymal calcifications: 47%, Architectural distortion: 91% | 1/5 |
Table 4. Studies reporting severity scores of residual changes on CXR imaging performed following treatment for pulmonary TB.
| Author | Country | Study design | n | Participant HIV status | Treatment episode | Drug sensitivity | Timing of imaging | Source of severity score | Severity score description | Findings | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| de Valliere, 2004 [35] | South Africa | Prospective cohort | 33 | Mixed | Not specified | MDR | On completion | Not specified | CXR split into 6 zones. Involvement of each zone scored 0–3. Total score 18. | Mean score 6.5/18 | 2/5 |
| Ralph, 2010 [54] | Indonesia | Prospective cohort | 152 | Mixed | Not specified | Mixed | On completion | Ralph 2010—diagnostic CXR scoring system | % lung affected + 40 if cavitation seen. Total score 140. | Median score 10/140, Range 0–115 | 3/5 |
| Wang, 2010 [28] | Taiwan | Prospective cohort | 98 | Negative | Not specified | Not specified | 1 year post completion | Not specified | Minimal / Moderate / Advanced fibrosis* | 60.2% Minimal; 14.3% Moderate; 25.5% Advanced | 3/5 |
| Chen, 2011 [55] | Taiwan | Prospective cohort | 51 | Negative | Not specified | Not specified | On completion | McAdams & Erasmus 1995—active TB CXR scoring system | Minimal / Extensive† | 31.4% Extensive | 3/5 |
| Menon, 2015[36]‡ | India | Retrospective cohort | 441 | Not specified | First episode | Fully sensitive | On completion | 1969 National TB associate of the USA—diagnostic CXR scoring system | Minimal / Moderate / Moderately advanced / Far advanced | 55.7% Minimal; 22.8% Moderate; 15.2% Moderately advanced; 6% Advanced | 2/5 |
| How, 2014 [56] | Malaysia | Retrospective cohort | 156 | Mixed | Mixed | Not specified | On completion | 1961 National TB association USA -diagnostic CXR scoring system [57] | Minimal / Moderate / Advanced disease¶ | 26.2% Minimal; 60.8% Moderate; 13% Advanced | 2/5 |
| Singla, 2009 [38] | India | Cross sectional | 45 | Negative | Not specified | MDR | 6-63m post completion | 1961 National TB association USA -diagnostic CXR scoring system[57] | Minimal / Moderate / Advanced disease¶ | 35.6% Minimal; 22.2% Moderate; 40.0% Advanced | 1/4 |
| Lisha, 2012 [48] | India | Cross sectional | 224 | Not specified | Mixed | Mixed | 5 years post completion | 1961 National TB association USA -diagnostic CXR scoring system [57] | Minimal / Moderate / Advanced disease¶ | 34.3% Minimal; 13.4% Moderate; 4.5% Advanced | 2/4 |
| Banu Rekha, 2009 [47] | India | Cross sectional | 198 | Not specified | First episode | Not specified | 14–18 years post completion | Not specified | CXR divided into 6 zones. Number of zones involved counted. | 35.9% ≤2 zones; 50% >2 zones | 1/4 |
| Godoy, 2012 [37] | Brazil | Cross sectional | 18 | Negative | Retreatment | MDR | On completion | Wilcox & Ferguson 1989—diagnostic CXR scoring system [58] | Grade I—III# | 61.1% Grade I; 22.2% Grade II; 16.7% Grade III | 2/4 |
| Baez-saldana, 2013 [39] | Mexico | Cross sectional | 127 | Mixed | Not specified | Not specified | 0–252 months post completion | Created by authors for grading post-TB CXR changes & validated in study | CXR split into 4 quadrants. Involvement of each one scored 0–5. Total score 20. | Mean score 6.46/20, standard deviation 4.14 | 2/4 |
| de la Mora, 2015 [59] | Mexico | Cross sectional | 70** | Not specified | Mixed | Mixed | Post completion. With CAO: 2.7+/- 4.3 yrs. Without CAO: 2.3 +/- 2.1 yrs | Not specified | Number of lung quadrants with fibrocavitatory changes. Total number of cavities | With CAO: 1.8 +/-0.8 affected quadrants, 1.4 +/- 0.8 cavities. Without CAO: 1.3 +/- 0.6 affected quadrants, 0.5 +/-0.7 cavities | 1/4 |
| Kenangalem, 2013 [31] | Indonesia | RCT—Vit D / L-argenine | 77 | Mixed | First episode | Mixed | On completion | Ralph 2010—diagnostic CXR scoring system | % lung affected + 40 if cavitation seen. Total score 140. | Papuans: Median score 6/140, Range 2–15 Non papuans: Median score 12.5/140, Range 4–20.5 |
2/5 |
* Minimal- mild—lung fibrosis <50% of RUL, no change in architecture/ clouding or lung marking or vasculature. Moderate—lung fibrosis >50% of RUL, no change in architecture/ clouding or lung marking or vasculature / lung collapse / tortuous airways / bronchiectasis. Advanced—fibrosis of whole RUL, combined with collapse, bronchiectasis and tortuous airways
†Minimal—slight to moderate density not containing cavitation with total extent not exceeding lung volume on one side above the chondro-sternal junction. Extensive—slight to moderate density extending more than total volume of one lung or equivalent in both lungs
‡Study included patients treated for pulmonary, pleural or mediastinal TB—no disaggregated data available, so all included here
¶Minimal—Unilateral or bilateral. Lesions of slight to moderate density with no cavitation. Involvement should not exceed space above 2nd chondrosternal junction and the spine of the 4th or body of 5th vertebra. Moderate—Unilateral or bilateral. Disseminated lesions of slight-moderate density may extend through total volume of 1 lung or equivalent in both lungs. Dense/confluent lesions limited to 1/3 of one lung. Total diameter of cavitation must be <4cm. Advanced—more extensive than moderate.
#Grade I—minimal change in 1 zone, with no cavities. Grade II—2–3 zones involved, or 1 zone with cavitation. Grade III—severe involvment of >3zones, with or without cavitation
**Results stratified according to the presence of Chronic Airway Obstruction (CAO) on spirometry, as defined by a ratio of the post-bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio <0.7, and the % predicted FEV1< lower limit of normal: patients with CAO (n = 24), without CAO (n = 46). Mean and standard deviation for time since treatment and radiology findings given.
The prevalence of cavitation was higher in studies of re-treatment patients (68.8–83.7%), and those treated for MDR disease (51.5–69.7%), compared to those with fully sensitive, mixed, or unspecified sensitivities (8.3–49.7%). Only 1 study performed repeat imaging; this demonstrated a reduction in the prevalence of cavitation during the 6-month follow-up period from the end of TB treatment, but the findings were limited by a small sample size and 25% loss to follow-up[34]
Pleural disease
The only radiological feature consistently reported in studies of pleural TB sequelae was the presence of residual pleural thickening, but the thoracic area covered by this thickening was not routinely reported. Residual thickening >10mm was seen in 19.6–46.0% of patients in 4 studies (n = 223) (Table 5). One study reported both CXR and CT findings following pleural TB, with mild pleural thickening >2mm seen in 50.0% (18/36) on CXR, and 60.0% (21/35) on CT[32].
Table 5. Studies of residual pleural thickening (RPT) on completion of treatment for TB pleural effusion.
| Imaging modality | Author, Year | Country | Study design | Number of participants | Participant HIV status | Prevalence of pathology (%) | Quality score |
|---|---|---|---|---|---|---|---|
| CXR | Kunter, 2002[60] | Turkey | Retrospective cohort | 47 | Not specified | RPT>2mm: 63.8% RPT>10mm: 25.5% | 2/5 |
| CXR | Uskal, 2005[61] | Turkey | Retrospective cohort | 121 | Not specified | RPT>2mm: 52.1% | 3/5 |
| CXR | Wong, 2005[62] | Hong Kong | Retrospective cohort | 70 | Not specified | RPT>10mm: 41.4% | 2/5 |
| CXR | Barbas, 1991[63] | Brazil | Prospective cohort | 44 | Not specified | RPT>2mm: 52.3% | 2/5 |
| CXR | de Pablo, 1997 [64] | Spain | Prospective cohort | 56 | Mixed | RPT>2mm: 42.9% RPT>10mm: 19.6% | 3/5 |
| CXR | Frye, 1997[65] | America | Retrospective cohort | 20 | Positive | Any RPT: 65.0% | 3/5 |
| CXR | Galarza, 1995 [33] | Spain | RCT—steroid use* | 60 | Negative | Any RPT: 8.3% | 2/5 |
| CXR | Wyser, 1996 [32] | South Africa | RCT—steroid use* | 36 | Negative | RPT>2mm: 50.0% | 4/5 |
| CT | Wyser, 1996 [32] | South Africa | RCT—steroid use* | 35 | Negative | RPT>2mm: 60.0% | 4/5 |
| CT | Seiscento, 2007 [66] | Brazil | Prospective cohort | 50 | Not specified | RPT>10mm: 46.0% | 3/5 |
*Date from study arm including steroids excluded
Relationship of imaging changes and other respiratory parameters
Although several studies described spirometry following PTB disease, only 2 studies directly related physiological impairment to imaging findings. The first showed a statistically significant inverse correlation between both FEV1 (forced expiratory flow in 1-second) and FVC (forced vital capacity), and the extent of radiographic abnormality on CXR in 127 adults who were a median of 11 months (IQR 6–18 months) post completion of TB treatment[39]. The second described imaging findings in patients with (n = 24) and without (n = 46) fixed airway obstruction on spirometry following treatment completion. Those with airway obstruction had had more previous episodes of TB (1.9+/-0.7 vs. 1.4+/-0.6, p = 0.009), but had more fibrocavitatory changes evident on CXR imaging[59].
The only study to relate imaging findings to functional capacity included 18 patients completing treatment for MDR-TB, and found a higher level of impairment amongst those with more marked radiographic damage: 64% of those with Grade I damage (7/11) failed to reach an expected 6-minute walking distance, compared to 100% of patients with Grade III damage (3/3). However, the findings of this study were limited by a small sample size and a lack of statistical testing[37]. Only one study described the relationship between imaging findings and patient quality of life. This was a cross-sectional study of 198 patients who had been treated for TB a mean of 16.5 years previously, and found no statistically significant difference in the symptoms, activity, impact, or overall St George’s Respiratory Questionnaire scores between those with pathology affecting ≤2 vs. >2 CXR zones[47].
Discussion
This review suggests a high prevalence of residual structural lung pathology following PTB treatment, and highlights the contribution that CT imaging may make to our understanding of this pathology. Several gaps in the literature have been identified, including a paucity of prospective data on the evolution of post-TB lung damage over time, limited geographical coverage of studies, little data from HIV-infected adults, and little information on the relationship between structural pathology, physiological abnormalities, and patient centered outcomes.
Despite the increasing focus on long-term patient outcomes within the post-2015 TB agenda, international targets for the management of PTB disease remain microbiologically and mortality driven: standard short course treatment for fully sensitive disease is provided for 6-months with discharge from health services on completion and no routine follow-up thereafter. Thoracic imaging is not routinely performed at the conclusion of treatment, and national TB treatment programmes are required to report data on treatment completion and survival only. Our review describes a high prevalence of residual abnormalities on imaging following medical treatment for PTB, in keeping with existing evidence on the high burden of abnormal airway physiology following PTB[15, 21]. This is particularly high amongst patients treated for MDR disease, which is of concern given the increasing prevalence of drug resistance in many high TB prevalence settings[67]. Our review suggests that there may be a substantial burden of undetected lung damage amongst patients completing TB treatment, but that our understanding of the combined structural/physiological nature of this damage, and the consequences of this for patients is limited. There remains insufficient evidence on which to base any changes to imaging and follow-up practices within existing TB programs.
Our review demonstrates differences in the prevalence and patterns of lung damage detected using CXR and CT imaging. CXR studies focused on cavitation, bronchiectasis, and fibrosis, and reported cavitation as the most common finding. In CT studies the prevalence of bronchiectasis was higher than that of cavitation, with possible features of ongoing inflammation (nodules and consolidation), emphysema, and mosaicism which may reflect small airways disease also reported. Whilst access to CT imaging is unlikely to be available in LMICs for routine assessment of patients after PTB treatment, limited use within research studies may have a role in accurately phenotyping disease, and identifying pathologies that could otherwise be missed. Studies performing paired CXR and CT imaging after pulmonary TB will then be required to determine the sensitivity of CXR imaging used within National Treatment Programs in LMICS for this CT defined structural damage.
Efforts to better define post-TB lung pathology will need to include an understanding of its evolution over time if we are to improve long-term patient outcomes. We identified a paucity of prospective data on the trajectory of imaging defined post-TB lung disease. This limitation is also seen in our understanding of the evolution of lung function following PTB, which is based on cross-sectional studies[68]. Uncertainty therefore remains about the rate and drivers of recovery/deterioration in those with post-TB lung damage. Factors influencing the progression of lung damage may include modifiable exposures such as smoking, biomass fuel[69, 70], and ongoing respiratory tract infections[71]. Further research in this area may inform strategies to prevent decline in this group.
Few studies attempted to examine the relationship between lung damage and patient functional capacity, symptom burden, and quality of life. The available data suggest that exercise capacity may decrease with increasing extents of structural pathology, but sample sizes in the one study to assess this were low[37]. The only study relating structural pathology to quality of life was vulnerable to selection bias, and the nature of this relationship therefore remains unclear[47]. A cohort study of patients with extensively destroyed lung after TB disease has confirmed a high incidence of infective exacerbations and mortality in this group[72], but further prospective data are required on outcomes amongst those with residual respiratory damage of a broader range of patterns and severity, from the time of treatment.
We identified a paucity of data from sSA and from HIV-positive patients. Given the convergence of a high TB prevalence and multiple risk factors for disease progression in sSA, data from this region is critical to our understanding of the public health impact of residual lung damage following TB treatment. HIV-positive individuals have a higher risk of developing TB disease, so should also be a focus of research. Although this group have atypical PTB presentations, with less structural damage seen at diagnosis[73], they may experience a differential pattern of progression of lung disease during or after treatment due to the effects of opportunistic infections, immune reconstitution syndrome and HIV-related immune activation on the lung[67, 74–76]. HIV-positive patients may also be particularly vulnerable to decreased quality of life and impaired functional capacity related to residual damage, and further analysis of respiratory outcomes in this group is urgently required[77].
The quality of studies identified in this review varied substantially. None included imaging prior to TB diagnosis, thus the presence of structural damage present prior to TB disease was not truly excluded. The majority of studies failed to specify how the radiological abnormalities reported on imaging were defined, and did not use gold-standard methods of reporting, making them vulnerable to misclassification of outcomes. Selection bias was a common issue; many studies were unclear about the reference population from which participants were drawn. The cross-sectional studies imaging patients sometime after treatment completion consistently struggled to locate eligible patients, and were limited by survival bias.
The strengths of our review include the use of several databases with no limitation on study dates, and the use of reference and citation review to identify additional papers. The study is limited by the inclusion of English language articles only. The heterogeneity of patient populations, treatment regimens, and the timing and modality of imaging meant that we were unable to perform a meta-analysis.
Conclusion
This systematic review identified a high burden of structural pathology after PTB treatment. A better understanding of the nature of this pathology, evolution of disease over time, factors driving deterioration, and the impact of respiratory pathology on patients’ lives and livelihoods is needed to guide clinical management and health-service planning. Data on the sensitivity of CXR and spirometric indices for structural changes seen on CT imaging are required, together with studies examining post-TB lung damage in HIV-infected individuals and those from sSA. Further investigation of these areas is required if we are to meet the post-2015 TB aim of reducing the long-term detrimental impact of TB disease on patients’ lives and livelihoods.
Supporting Information
(DOCX)
(DOC)
Acknowledgments
JM conceived of the idea of the systematic review, and designed the protocol. JM and HS performed the review and extracted the data. JM drafted the article, which was reviewed by HS and KM. Both KM and BS provided senior supervision for the systematic review and manuscript authorship. All authors have approved the final version of the manuscript. JM is the guarantor of the content of the article.
Data Availability
Data for this systematic review are drawn from the papers included. These are referenced in the manuscript and are accessible in the public repositories described in the attached protocol.
Funding Statement
This study was supported by a Wellcome Trust Clinical PhD Fellowship Grant, awarded to Dr Jamilah Meghji (106065/Z/14/A). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1(9):728–42. 10.1016/s2213-2600(13)70118-8 [DOI] [PubMed] [Google Scholar]
- 2.Pasteur MC H S, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, Flower CD, et al. An Investigation into Causative Factors in Patients with Bronchiectasis. Am J Respir Crit Care Med. 2000;162(4):1277–84. [DOI] [PubMed] [Google Scholar]
- 3.Salvi S, Barnes PJ. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138(1):3–6. 10.1378/chest.10-0645 [DOI] [PubMed] [Google Scholar]
- 4.Haines A, McMichael AJ, Smith KR, Roberts I, Woodcock J, Markandya A, et al. Public health benefits of strategies to reduce greenhouse-gas emissions: overview and implications for policy makers. Lancet. 2009;374(9707):2104–14. 10.1016/s0140-6736(09)61759-1 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organisation. Global Status Report on noncommunicable diseases, 2014. Geneva, Switzerland: World Health Organisation, 2014. [Google Scholar]
- 6.Fitzpatrick M, Crothers K, Morris A. Future directions: lung aging, inflammation, and human immunodeficiency virus. Clin Chest Med. 2013;34(2):325–31. 10.1016/j.ccm.2013.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–50. 10.1016/s0140-6736(07)61377-4 . [DOI] [PubMed] [Google Scholar]
- 8.Finney LJ, Feary JR, Leonardi-Bee J, Gordon SB, Mortimer K. Chronic obstructive pulmonary disease in sub-Saharan Africa: a systematic review. Int J Tuberc Lung Dis. 2013;17(5):583–9. 10.5588/ijtld.12.0619 [DOI] [PubMed] [Google Scholar]
- 9.Meghji J, Nadeau G, Davis KJ, Wang D, Nyirenda MJ, Gordon SB, et al. Non-communicable Lung Disease in Sub Saharan Africa: a Community-based Cross-sectional Study of Adults in Urban Malawi. Am J Respir Crit Care Med. 2016;194(1):67–76. 10.1164/rccm.201509-1807OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organisation. Global Action Plan for the prevention and control of noncommunicable diseases, 2013–2020. Geneva, Switzerland: World Health Organisation, 2013. [Google Scholar]
- 11.Beran D, Zar HJ, Perrin C, Menezes AM, Burney P. Burden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low-income and middle-income countries. Lancet Respir Med. 2015;3(2):159–70. 10.1016/s2213-2600(15)00004-1 [DOI] [PubMed] [Google Scholar]
- 12.Marks GB, Billo NE. Chronic respiratory disease: the forgotten NCD? Int J Tuberc Lung Dis. 2014;18(11):1261 10.5588/ijtld.14.0707 [DOI] [PubMed] [Google Scholar]
- 13.World Health Organisation. Global tuberculosis report, 2015. Geneva, Switzerland: World Health Organisation, 2015. [Google Scholar]
- 14.Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015;32:138–46. 10.1016/j.ijid.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 15.Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86(1):76–85. 10.1159/000350917 [DOI] [PubMed] [Google Scholar]
- 16.Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA. Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005;192(7):1201–9. 10.1086/444545 [DOI] [PubMed] [Google Scholar]
- 17.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61(3):259–66. 10.1136/thx.2005.051979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caballero A, Torres-Duque CA, Jaramillo C, Bolivar F, Sanabria F, Osorio P, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study). Chest. 2008;133(2):343–9. 10.1378/chest.07-1361 [DOI] [PubMed] [Google Scholar]
- 19.Menezes AM, Hallal PC, Perez-Padilla R, Jardim JR, Muino A, Lopez MV, et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J. 2007;30(6):1180–5. 10.1183/09031936.00083507 [DOI] [PubMed] [Google Scholar]
- 20.Lam KB, Jiang CQ, Jordan RE, Miller MR, Zhang WS, Cheng KK, et al. Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest. 2010;137(3):593–600. 10.1378/chest.09-1435 [DOI] [PubMed] [Google Scholar]
- 21.Ehrlich RI, Adams S, Baatjies R, Jeebhay MF. Chronic airflow obstruction and respiratory symptoms following tuberculosis: a review of South African studies. Int J Tuberc Lung Dis. 2011;15(7):886–91. 10.5588/ijtld.10.0526 [DOI] [PubMed] [Google Scholar]
- 22.Ehrlich RI, White N, Norman R, Laubscher R, Steyn N, Lobard C, et al. Predictors of chronic bronchitis in South African adults. Int J Tuberc Lung Dis. 2004;8(5):369–76. [PubMed] [Google Scholar]
- 23.Zhou YM, Wang C, Yao WZ, Chen P, Kang J, Huang SG, et al. The prevalence and risk factors of bronchiectasis in residents aged 40 years old and above in seven cities in China. Chinese J Int Med. 2013;52(5):379–82. [PubMed] [Google Scholar]
- 24.Wardlaw AJ, Silverman M, Siva R, Pavord ID, Green R. Multi-dimensional phenotyping: towards a new taxonomy for airway disease. Clin Exp Allergy. 2005;35(10):1254–62. 10.1111/j.1365-2222.2005.02344.x [DOI] [PubMed] [Google Scholar]
- 25.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. 10.1164/rccm.200912-1843CC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organisation. Global Tuberculosis Report: Post-2015 global tuberculosis strategy framework. Geneva: World Health Organisation, 2013. [Google Scholar]
- 27.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottowa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis [cited 2015 24th November]. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 28.Wang CH, Lin HC, Lin SM, Huang CD, Liu CY, Huang KH, et al. MMP-1(-1607G) polymorphism as a risk factor for fi brosis after pulmonary tuberculosis in Taiwan. Int J Tuberc Lung Dis. 2010;14(5):627–34. [PubMed] [Google Scholar]
- 29.Tuberculosis Chemotherapy Centre, Madras. Controlled comparison of oral twice-weekly and oral daily isoniazid plus PAS in newly diagnosed pulmonary tuberculosis. BMJ. 1973;2:7–11. [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton CD, Stout JE, Goodman PC, Mosher A, Menzies R, Schluger NW, et al. The value of end-of-treatment chest radiograph in predicting pulmonary tuberculosis relapse. Int J Tuberc Lung Dis. 2008;12(9):1059–64. [PMC free article] [PubMed] [Google Scholar]
- 31.Kenangalem E, Waramori G, Pontororing GJ, Sandjaja, Tjitra E, Maguire G, et al. Tuberculosis outcomes in Papua, Indonesia: the relationship with different body mass index characteristics between papuan and non-Papuan ethnic groups. PloS one. 2013;8(9):e76077 10.1371/journal.pone.0076077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyser C, Walzl G, Smedema JP, Swart F, van Schalkwyk EM, van de Wal BW. Corticosteroids in the treatment of tuberculous pleurisy. A double-blind, placebo-controlled, randomized study. Chest. 1996;110(2):333–8. [DOI] [PubMed] [Google Scholar]
- 33.Galarza I, Canete C, Granados A, Estopa R, Manresa F. Randomised trial of corticosteroids in the treatment of tuberculous pleurisy. Thorax. 1995;50(12):1305–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corlan E, Marica C, Macavei C, Stanford JL, Stanford CA. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosisis Romania. Respir Med. 1997;91(1):21–9. 10.1016/S0954-6111(97)90133-5 [DOI] [PubMed] [Google Scholar]
- 35.de Valliere S, Barker RD. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8(6):767–71. [PubMed] [Google Scholar]
- 36.Menon B, Nima G, Dogra V, Jha S. Evaluation of the radiological sequelae after treatment completion in new cases of pulmonary, pleural, and mediastinal tuberculosis. Lung India. 2015;32(3):241–5. 10.4103/0970-2113.156233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godoy MD, Mello FC, Lopes AJ, Costa W, Guimaraes FS, Pacheco AG, et al. The functional assessment of patients with pulmonary multidrug-resistant tuberculosis. Respir Care. 2012;57(11):1949–54. 10.4187/respcare.01532 [DOI] [PubMed] [Google Scholar]
- 38.Singla N, Singla R, Fernandes S, Behera D. Post treatment sequelae of multi-drug resistant tuberculosis patients. Indian J Tuberc. 2009;56(4):206–12. [PubMed] [Google Scholar]
- 39.Baez-Saldana R, Lopez-Areaga Y, Bizarron-Muro A, Ferreira-Guerrero E, Ferreyra-Reyes L, Delgado-Sanchez G, et al. A novel scoring system to measure radiographic abnormalities and related spirometric values in cured pulmonary tuberculosis. PloS one. 2013;8(11). 10.1371/journal.pone 10.1371/journal.pone.0078926.t001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu CT, Wang CH, Huang TJ, Lin HC, Kuo HP. Relation of bronchoalveolar lavage T lymphocyte subpopulations to rate of regression of active pulmonary tuberculosis. Thorax. 1995;50(8):869–74. 10.1136/thx.50.8.869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Hajjaj MS, Joharjy IA. Predictors of radiological sequelae of pulmonary tuberculosis. Acta Radiol. 2000;41(6):533–7. [DOI] [PubMed] [Google Scholar]
- 42.Buyukoglan H, Gulmez I, Kelestimur F, Kart L, Oymak FS, Demir R, et al. Leptin levels in various manifestations of pulmonary tuberculosis. Mediators of Inflammation. 2007;2007:1–6. 10.1155/2007/64859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swaminathan S, Padmapriyadarsini C, Ponnuraja C, Sumathi CH, Rajasekaran S, Amerandran VA, et al. Miliary tuberculosis in human immunodeficiency virus infected patients not on antiretroviral therapy: Clinical profile and response to short-course chemotherapy. Journal of Postgraduate Medicine. 2007;53(4):228–31. [DOI] [PubMed] [Google Scholar]
- 44.Angthong W, Angthong C, Varavithya V. Pretreatment and posttreatment radiography in patients with pulmonary tuberculosis with and without human immunodeficiency virus infection. Japanese Journal of Radiology. 2011;29(8):554–62. 10.1007/s11604-011-0597-3 [DOI] [PubMed] [Google Scholar]
- 45.Small PM, Hopewell PC, Schecter GF, Chaisson RE, Goodman PC. Evolution of chest radiographs in treated patients with pulmonary tuberculosis and HIV infection. Journal of Thoracic Imaging. 1994;9(2):74–7. [DOI] [PubMed] [Google Scholar]
- 46.Kallan BM, Kishan J. Clinicoradiological Status of Patients of Pulmonary Tuberculosis after Adequate Treatment. Indian Journal of Medical Sciences. 1988;42(1):4–8. [PubMed] [Google Scholar]
- 47.Banu Rekha VV, Ramaschandran R, Kuppu Rao KV, Rahman F, Adhilakshmi AR, Kalaiselvi D, et al. Assessment of Long-term status of sputum positive pulmonary TB patients successfully treated with short course chemotherapy. Indian J Tuberc. 2009;56:132–40. [PubMed] [Google Scholar]
- 48.Lisha PV, James PT, Ravindran C. Morbidity and mortality at five years after initiating Category I treatment among patients with new sputum smear positive pulmonary tuberculosis. Indian J Tuberc. 2012;59(2):83–91. [PubMed] [Google Scholar]
- 49.Poey C, Verhaegen F, Giron J, Lavayssiere J, Fajadet P, Duparc B. High resolution chest CT in tuberculosis: evolutive patterns and signs of activity. J Comput Assist Tomogr. 1997;21(4):601–7. [DOI] [PubMed] [Google Scholar]
- 50.Long R, Maycher B, Dhar A, Manfreda J, Hershfield E, Anthonisen N. Pulmonary tuberculosis treated with directly observed therapy: serial changes in lung structure and function. Chest. 1998;113(4):933–43. [DOI] [PubMed] [Google Scholar]
- 51.Bombarda S, Figueiredo CM, Seiscento M, Terra Filho M. Pulmonary tuberculosis: tomographic evaluation in the active and post-treatment phases. Sao Paulo Medical Journal. 2003;121(5):198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JJ, Chong PY, Lin CB, Hsu AH, Lee CC. High resolution chest CT in patients with pulmonary tuberculosis: characteristic findings before and after antituberculous therapy. Eur J Radiol. 2008;67(1):100–4. 10.1016/j.ejrad.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 53.Rufino RL, Capone R, Capone D, Costa CHD, Uerj. Pattern of chest computed tomography before and after treatment in patients with proven pulmonary tuberculosis. Am J Respir Crit Care Med. 2015;191(A3309). [Google Scholar]
- 54.Ralph AP, Ardian M, Wiguna A, Maguire GP, Becker NG, Drogumuller G, et al. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax. 2010;65(10):863–9. 10.1136/thx.2010.136242 [DOI] [PubMed] [Google Scholar]
- 55.Chen YC, Chin CH, Liu SF, Wu CC, Tsen CC, Wang YH, et al. Prognostic values of serum IP-10 and IL-17 in patients with pulmonary tuberculosis. Dis Markers. 2011;31(2):101–10. 10.3233/DMA-2011-0808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.How SH, Kuan YC, Ng TH, Razali MR, Fauzi AR. Monitoring treatment response in sputum smear positive pulmonary tuberculosis patients: comparison of weight gain, sputum conversion and chest radiograph. Malays J Pathol. 2014;36(2):91–6. [PubMed] [Google Scholar]
- 57.National Tuberculosis Association of the USA. Diagnostic standards and classification of tuberculosis. 11th ed New York: 1961. [Google Scholar]
- 58.Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med. 1989;83(3):195–8. [DOI] [PubMed] [Google Scholar]
- 59.de la Mora IL, Martinez-Oceguera D, Laniado-Laborin R. Chronic airway obstruction after successful treatment of tuberculosis and its impact on quality of life. Int J Tuberc Lung Dis. 2015;19(7):808–10. 10.5588/ijtld.14.0983 [DOI] [PubMed] [Google Scholar]
- 60.Kunter E, Ilvan A, Kilic E, Cerrahoglu K, Isitmangil T, Capraz F, et al. The effect of pleural fluid content on the development of pleural thickness. Int J Tuberc Lung Dis. 2002;6(6):516–22. [DOI] [PubMed] [Google Scholar]
- 61.Uskul B, Turker H, Ulman C, Ertugrul M, Selvi A, Kant A, et al. The relation of the pleural thickening in tuberculosis pleurisy with the activity of adenosine deaminase. Monaldi Arch Chest Dis. 2005;63(2):101–7. [DOI] [PubMed] [Google Scholar]
- 62.Wong CF, Leung SK, Yew WW. Percentage reduction of pleural effusion as a simple predictor of pleural scarring in tuberculous pleuritis. Respirology. 2005;10(4):515–9. 10.1111/j.1440-1843.2005.00743.x [DOI] [PubMed] [Google Scholar]
- 63.Barbas CS, Cukier A, de Varvalho CR, Barbas Filho JV, Light RW. The relationship between pleural fluid findings and the development of pleural thickening in patients with pleural tuberculosis. Chest. 1991;100(5):1264–7. . [DOI] [PubMed] [Google Scholar]
- 64.de Pablo A, Villena V, Echave-Sustaeta J, Encuentra AL. Are pleural fluid parameters related to the development of residual pleural thickening in tuberculosis? Chest. 1997;112(5):1293–7. [DOI] [PubMed] [Google Scholar]
- 65.Frye MD, Pozsik CJ, Sahn SA. Tuberculous pleurisy is more common in AIDS than in non-AIDS patients with tuberculosis. Chest. 1997;112(2):393–7. [DOI] [PubMed] [Google Scholar]
- 66.Seiscento M, Vargas FS, Antonangelo L, Acencio MM, Bombarda S, Capelozzi VL, et al. Transforming growth factor beta-1 as a predictor of fibrosis in tuberculous pleurisy. Respirology. 2007;12(5):660–3. 10.1111/j.1440-1843.2007.01135.x [DOI] [PubMed] [Google Scholar]
- 67.Drummond MB, Kirk GD. HIV-associated obstructive lung diseases: insights and implications for the clinician. Lancet Respir Med. 2014;2(7):583–92. 10.1016/s2213-2600(14)70017-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chung KP, Chen JY, Lee CH, Wu HD, Wang JY, Lee LN, et al. Trends and predictors of changes in pulmonary function after treatment for pulmonary tuberculosis. Clinics. 2011;66(4):549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bishwakarma R, Kinney WH, Honda JR, Mya J, Strand MJ, Gangavelli A, et al. Epidemiologic link between tuberculosis and cigarette/biomass smoke exposure: Limitations despite the vast literature. Respirology. 2015;20(4):556–68. 10.1111/resp.12515 . [DOI] [PubMed] [Google Scholar]
- 70.Lin HH, Suk CW, Lo HL, Huang RY, Enarson DA, Chiang CY. Indoor air pollution from solid fuel and tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2014;18(5):613–21. 10.5588/ijtld.13.0765 [DOI] [PubMed] [Google Scholar]
- 71.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. 2014;384(9944):691–702. 10.1016/s0140-6736(14)61136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryu YJ, Lee JH, Chun EM, Chang JH, Shim SS. Clinical outcomes and prognostic factors in patients with tuberculous destroyed lung. Int J Tuberc Lung Dis. 2011;15(2):246–50. [PubMed] [Google Scholar]
- 73.Munthali L, Khan PY, Mwaungulu FD, Chilongo F, Floyd S, Kayange M, et al. The effect of HIV and antiretroviral therapy on characteristics of pulmonary tuberculosis in northern Malawi: a cross-sectional study. BMC Infect Dis. 2014;14(107):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen C, Walaza S, Moyes J, Groome M, Tempia S, Pretorius M, et al. Epidemiology of Severe Acute Respiratory Illness (SARI) among Adults and Children Aged ≥5 Years in a High HIV-Prevalence Setting, 2009–2012. PLoS One. 2015;10(2):e0117716 10.1371/journal.pone.0117716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feikin DR, Njenga MK, Bigogo G, Aura B, Aol G, Audi A, et al. Etiology and Incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007–2010. PloS one. 2012;7(8):e43656 10.1371/journal.pone.0043656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crothers K. Chronic obstructive pulmonary disease in patients who have HIV infection. Clin Chest Med. 2007;28(3):575–87. 10.1016/j.ccm.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 77.Drummond MB, Kirk GD, McCormack MC, Marshall MM, Ricketts EP, Mehta SH, et al. HIV and COPD: Impact of risk behaviors and diseases on quality of life. Qual Life Res. 2010;19(9):1295–302. 10.1007/s11136-010-9701-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
Data for this systematic review are drawn from the papers included. These are referenced in the manuscript and are accessible in the public repositories described in the attached protocol.