Abstract
Olfactory disorders have been regarded in the past with a sense of therapeutic nihilism. However, there have been remarkable advances in chemosensory research over the past several years. The clinical importance of olfactory disorders is well established, and entities such as presbyosmia have gained considerable broad attention. Powerful basic science experimental approaches have revealed aspects of olfactory neuron physiology, olfactory tissue maintenance and regeneration that provide new potential therapeutic targets for certain forms of olfactory dysfunction. Although many recent advances remain in pre-clinical stages, there is considerable reason for optimism regarding future approaches for treatment of patients with olfactory loss.
Keywords: olfaction, anosmia, hyposmia, presbyosmia, post-viral olfactory disorder, ciliopathy
Introduction
Disorders of the sense of smell are estimated to affect approximately 14 million Americans [1], and may negatively impact quality of life, safety, and nutrition. Remarkably, recent population-based research has also indicated that olfactory decline in the elderly is an independent risk factor for 5-year mortality, although the mechanism underlying this association is unclear [2, 3]. Improved understanding of the pathophysiology underlying various forms of olfactory loss, and novel effective therapeutic options for these conditions, are areas of importance.
A variety of clinical conditions can result in olfactory loss. Broadly, problems may be considered to be conductive or sensorineural, although these are not necessarily mutually exclusive. In conductive losses, pathology blocks inspired odorants from reaching the olfactory cleft in the nasal cavity. In sensorineural losses, the dysfunction is attributed to the olfactory receptor neurons or their central projections. Examples of conductive or mixed losses include nasal polyps or chronic rhinosinusitis. Examples of sensorineural losses include genetic mutations, acquired conditions such as prior head injury, or prior viral upper respiratory infection that has, presumably, damaged the sensory structures. In addition, evidence suggests that presbyosmia, or an age-related olfactory decline, is a sensorineural loss [4–7].
Regarding conductive olfactory losses, such as chronic rhinosinusitis, many clinical studies are including olfactory outcomes in their measures, and are informing our management strategies for this problem [8, 9]. Sensorineural losses, however, remain a clinical challenge, and will be the focus of this review. Considerable attention has been directed recently towards understanding basic aspects of olfactory neuron function. Although new treatments are not yet readily available, these research efforts are revealing mechanisms that may define new therapeutic targets. Here, we will focus on recent advances regarding olfactory neurobiology, specifically regarding ciliopathies, inflammation, presbyosmia, and basal cells, with an emphasis on how these findings are likely to translate into new treatment strategies.
Background
Organization and function of the peripheral olfactory system
The human olfactory system functions as a remarkable chemosensory apparatus, capable of detecting and discriminating a vast array of inspired odorants [10]. Several aspects of the organization of the peripheral olfactory system have direct implications for understanding important pathophysiologic conditions. Therefore, key subjects warrant further elaboration here. Among the most relevant of these are (1) the transduction apparatus used by olfactory neurons; (2) specialized neuronal structures, cilia, that are critical for neuronal function; and (3) the ongoing epithelial self-renewal that is required to maintain a functional neuron population.
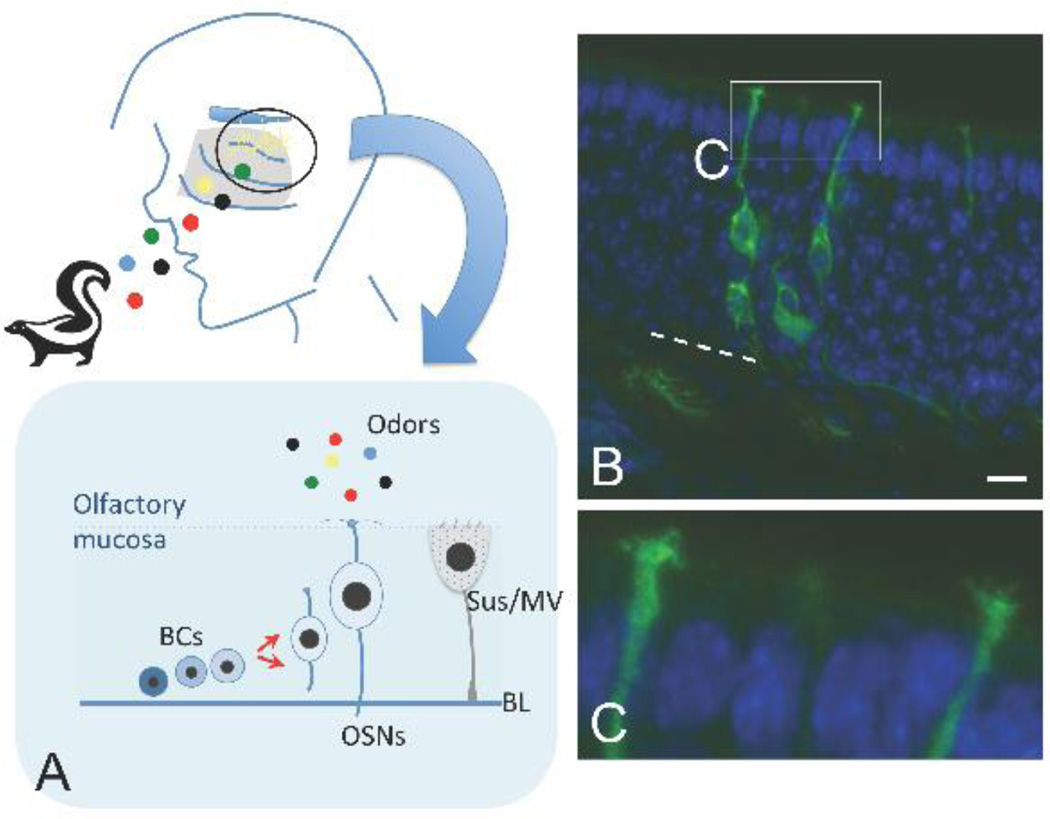
Most of the human nasal cavity is lined by a mucus-secreting non-sensory respiratory mucosa. The exception is the olfactory cleft, or the region along portions of the superior nasal septum and corresponding vertical lamellae of the superior turbinates, and limited areas of the middle turbinates, which is lined by olfactory neuroepithelium [11]. The specialized neuroepithelium houses the bipolar olfactory receptor neurons, which function to transduce inspired odor molecules into a neural signal (Figure 1). How are chemical odorants detected, discriminated, and coded? This is a highly complex problem: for their work elucidating molecular mechanisms involved in chemosensation, the Nobel Prize in Physiology or Medicine was awarded to Buck and Axel in 2004 [12]. They identified a very large family of transmembrane odor receptor genes, revealing that each olfactory neuron expresses one receptor gene, from ≈350 possible choices in humans. Neurons expressing the same receptor are scattered stochastically across broad regions of the neuroepithelium, but their axons converge to synapse with second-order mitral cells at the same positions, or glomeruli, in the olfactory bulbs of the brain [13]. Thus, it is the activation pattern of these glomeruli in the bulb that encodes odor exposure information.
Figure 1.
(A) Schematic diagram of the peripheral olfactory system. Inspired odor molecules (colored dots) reach the olfactory area along the superior turbinate, superior nasal septum, and limited portions of the middle turbinates. Olfactory neurons (yellow) are situated in a specialized epithelium lining this region, and their axon fibers project through the cribriform plate to synapse in the olfactory bulbs of the brain. The lower panel indicates the cell types and organization of the olfactory epithelium in the nose. Note that proliferative basal cells (BCs) produce new neurons (OSNs) to replace them as needed; sustentacular and microvillar cells (Sus/MV) are situated apically and have important supportive functions; BL=basal lamina. (B, C) New olfactory neurons are produced continually in adults under normal conditions. Here, we utilized a mouse genetic approach to visualize olfactory neurons (green) that were produced from a specific basal stem cell, the c-Kit+ basal cell. A membrane-tethered fluorescent protein reveals the morphology of olfactory neurons, situated in a pseudostratified neuroepithelium, with axons exiting the base of the epithelium to project to the olfactory bulbs, and a dendrite extending apically. (C) a higher magnification view of the apical dendrite shows that it extends to the airspace, ending in a dendritic knob that elaborates several cilia. It is at the cilia that inspired odorants interact with their receptors on the neurons. Nuclei are stained with DAPI (blue); bar=10 µm. Dashed white line marks the basal lamina.
As the first step in this process, odors interact with receptor proteins on a specialized portion of the olfactory neuron (Figure 1). The neurons extend a dendrite to the apical surface of the epithelium, at the nasal airspace. Here, multiple cilia extend from a dendritic knob. The odor receptors are localized at the membrane of the neuronal cilia, along with other odor transduction proteins. It is felt that this specialization greatly increases the surface area with which odor molecules can interact with odor receptors [12]. Given the prominent role of olfactory neuronal cilia in odor transduction, it is not surprising that disorders in which cilia function is perturbed can be a cause of anosmia [14–16]. Indeed, strategies to correct cilia dysfunction may lead to new treatments for certain forms of anosmia.
Interestingly, olfactory neurons are relatively short-lived, with an estimated lifespan of only weeks to months [17]. Because of this, mammals have retained the ability to produce new olfactory neurons from adult stem cells situated in the basal layers of the olfactory neuroepithelium (Figure 1). Regulation of self-renewal in the adult olfactory system is an area of active research, revealing the identification of certain categories of basal stem cells capable of generating new neurons [18–22] as well as signaling pathways involved in this process [23, 7, 21]. Problems with olfactory tissue maintenance and/or renewal from basal stem cells appear to be a mechanism contributing to anosmia in conditions such as presbyosmia, post-viral olfactory disorder or chronic rhinosinusitis [7, 24]. Therefore, basal cells are a logical therapeutic target for particular olfactory losses.
Disorders of olfactory neuron function
Anosmia associated with ciliopathy disorders
The presence of nonmotile cilia extending from olfactory receptor neurons at the surface of the olfactory epithelium has long been recognized. However, it is only recently that the critical importance of cilia function in olfactory neurons has been directly demonstrated [14]. Diseases in which the formation and/or function of cilia is altered are termed ciliopathies. Examples include Bardet-Biedel syndrome, Meckel-Gruber syndrome and Joubert syndrome. Since cilia are important in a broad range of cell types and tissues, many ciliopathy mutations involve multiple clinical manifestations, such as renal cysts, polydactyly, vision loss, hearing loss, or cognitive deficits. However, it is felt that anosmia is a pathognomonic feature of ciliopathies. Mechanistically, olfactory neurons must assemble their cilia to properly localize several key transduction proteins at the cilia membrane [16]. If odorant receptor proteins and their accompanying transduction machinery are not localized properly in cilia, or if the cilia are malformed (or absent), odor detection cannot occur, resulting in anosmia. Most of the mutations that have been characterized in ciliopathies are loss-of-function alleles. As such, gene therapies aimed at delivering the normal functional protein represent a plausible treatment strategy. Notably, a mouse model of a ciliopathy disorder has been studied to test adenoviral gene therapy [25]. In this model, the mice are anosmic due to a mutation in an intraflagellar transport protein, which is required for proper cilia assembly. Remarkably, intranasal treatment with adenoviral vectors to deliver the normal transport protein restored both cilia structure and olfactory function. Viral-based gene therapy is a promising approach for anosmia due to ciliopathies, although human clinical trials have not yet been performed. Due to ease of delivery, other forms of olfactory disorders may also be amenable to similar treatment.
Anosmia associated with chronic rhinosinusitis
Although blockage of inspired air due to congestion associated with edema and infection can cause a conductive olfactory loss, there is considerable evidence that ongoing hyposmia or anosmia associated with some forms of chronic rhinosinusitis is due to effects of inflammation on olfactory neurons. A clue that such a mechanism exists is that some chronic rhinosinusitis patients appear to have, by endoscopy or CT imaging, an unobstructed olfactory cleft yet remain objectively hyposmic. Furthermore, such patients may exhibit a steroid-responsive hyposmia. Recent basic science work has provided direct evidence that inflammation can impair or even kill olfactory neurons [24]. A mouse model in which inflammatory cytokines can be inducibly expressed by the sustentacular cells of the olfactory epithelium has been shown to initially cause impaired olfactory neuron function [24]. Over time, with continued over-production of the cytokines, olfactory neurons die, leading to pronounced histologic changes in the neuroepithelium. Importantly, if the inflammatory cytokine production is halted, the basal stem cells within the epithelium can produce new olfactory neurons resulting in a restoration of olfactory function [26]. Taken together, these findings demonstrate directly that inflammation can cause a sensorineural hyposmia, and that new strategies aimed at eliminating specific inflammatory mediators may be effective treatments for some forms of sinusitis-associated hyposmia.
Disorders of olfactory neuron maintenance
Because the olfactory epithelium is a self-renewing tissue, it stands to reason that processes that impair neuroepithelial renewal or maintenance may result in sensorineural olfactory loss. The basal cell populations in the olfactory epithelium contain stem and progenitor cells that function to produce new olfactory neurons as needed [27]. Therefore, modulation or replenishment of basal cells may be a focus for future sensorineural anosmia treatment strategies. Recent work has provided new insights into models of olfactory neuron maintenance dysfunction, such as aging-related olfactory decline (presbyosmia), as well as an increased understanding regarding the biology of olfactory progenitors.
Presbyosmia
A decline in olfactory function with aging has been well-documented [28, 1]. However, the cellular or molecular basis for this decline, or for clinically important presbyosmia conditions, has not been fully understood. Furthermore, the broader possible significance of presbyosmia as a clinical entity has not been well-documented until recently. Evidence that presbyosmia may have important broad health consequences was provided recently, from a population-based study demonstrating an association with olfactory impairment in older adults and decreased 5-year survival [2]. More recently, an additional large study has examined this association [3]. The authors used a database of over 3000 adults aged 57–85 to explore for biomarkers of mortality. Anosmia in older adults was found to increase risk of 5-year mortality four times that of normosmic subjects. Controlling for other leading causes of death such as smoking and other factors such as cognitive function, alcohol abuse, or mental health, the study indicates that olfactory function is a strong predictor of 5-year mortality. Presently, it is not clear why presbyosmia is a strong biomarker for mortality, and further research is needed to elucidate the underlying mechanisms.
Using human olfactory specimens as well as animal models, there is evidence that maintenance of an intact, healthy olfactory receptor neuron population may fail in presbyosmia [6, 29]. Tissue studies reveal that there is often a patchy replacement of olfactory neuroepithelium with intervening areas of respiratory non-sensory epithelium. Respiratory metaplasia is felt to be a reflection of failed olfactory tissue maintenance, which may be a consequence of exogenous damage and/or limitations related to the reparative abilities of remaining basal stem and progenitor cells. Mechanisms that contribute to the regulation of olfactory maintenance have begun to be understood, providing possible candidate therapies. For instance, a recent report identifies Neuropeptide Y as an olfactory epithelium regulatory factor that may decline with aging [7]. In that work, the investigators examined specific mechanisms that are involved in declines in olfactory neuron maintenance in aging mice, and found that Neuropeptide Y signaling may be a therapeutic target. Neuropeptide Y is released from olfactory microvillar cells, and has been found to stimulate basal neural progenitor cell proliferation previously [30]. Indeed, basal cell proliferation has been demonstrated to decline with aging in a rat model [5]. Taken together, evidence indicates that olfactory basal cells are critically important to olfactory tissue maintenance in aging or disease.
Olfactory basal cells
Given the importance of basal cells in olfactory neuron maintenance, there has been considerable interest in studying this cell population. The presence of proliferative basal cells capable of replacing adult olfactory neurons as needed, and of responding to experimental damage to reconstitute the neuroepithelium, has been recognized for decades [27]. However, recent investigations have utilized powerful approaches to define the identities, differentiation lineages, and regulatory mechanisms for these stem and progenitor cells [20, 22, 31, 21]. For instance, genetic lineage tracing and culture work indicates that certain olfactory basal cells are regulated by Wnt signaling [21, 23]. Understanding how olfactory neurons are generated from their precursor cells may lead to new drug therapies for olfactory neuron loss: these findings suggest that modulation of Wnt signaling could be therapeutically useful for certain clinical situations.
In a variety of non-olfactory disorders, cell-based therapies, delivering appropriate stem cells to repair or replace damaged tissue, are being utilized or studied in clinical trials (for review, see [32]). The gold-standard cell type for stem cell therapies has been the bone marrow mesenchymal stem cell, which has been recognized for over three decades and has been the subject of highly meticulous study. However, the presence of adult neural stem cells in the olfactory epithelium has raised the prospect of utilizing this cell therapeutically, or of treating certain forms of olfactory loss with a cell-based therapy. While there is a growing body of knowledge regarding olfactory basal stem cells, rigorous study will be required prior to safely embarking on a cell-based olfactory treatment. Preliminary animal studies do suggest the feasibility of manipulating and engrafting basal cells [33, 34]. Our lab has identified a population of mouse olfactory basal cells, defined by expression of the surface receptor c-Kit, that is important in the production of adult olfactory neurons [22]. Experiments using a genetic approach to selectively delete c-Kit+ cells demonstrate that this population is required for adult olfactory tissue maintenance. Therefore, this and similar basal cell populations are of particular interest for further study. It is important to note that many reports have also focused on non-basal cell populations obtained from nasal turbinate connective tissue, and these cells, while potentially useful, are a distinct “mesenchymal-like” population of unknown in vivo function, that have not been shown to produce olfactory receptor neurons [35]. Precise use of proper terminology to describe cell populations under study will be necessary to avoid confusion as stem cell research continues to evolve.
Current treatment strategies, controversies, and future directions
At present, disorders of olfaction remain a difficult clinical problem. Ruling out treatable conditions or serious pathology with appropriate imaging is often very helpful to patients. Multiple studies over recent decades have examined potential treatments. Unfortunately, there is insufficient evidence for the effectiveness in humans for therapies such as zinc, theophylline, minocycline, vitamins, lipoic acid, acupuncture, and others [36]. As such, it is important to avoid offering ineffective options. Considering available evidence, recommendations and treatment controversies for specific diagnoses are discussed here (see Table 1).
Table 1.
Causes of olfactory impairment, treatment options and possible therapeutic targets.
| Cause | Current treatment options |
Potential therapeutic targets |
|---|---|---|
| Rhinosinusitis | Maximal medical therapy; endoscopic sinus surgery |
Olfactory cleft inflammation |
| Genetic ciliopathies | None | Gene replacement via viral gene therapy |
| Post-viral olfactory disorder |
Olfactory training therapy | Inflammation; Basal cell signaling pathways |
| Head injury | Supportive | Neuron degeneration; Glial scarring |
| Presbyosmia | Supportive | Basal cell signaling pathways; Basal cell replacement |
Sinusitis: olfactory loss in the setting of chronic rhinosinusitis may be considered to be due to obstruction of the olfactory cleft and/or may be associated with sensorineural dysfunction due to inflammation. Improvement may be achieved via maximal medical therapy (which varies, but often includes oral steroids), with surgery employed to treat recalcitrant disease, including addressing the olfactory cleft carefully, if involved with polyps [9]. New selective inflammatory inhibitors, and/or new topical delivery methods, may provide future options.
Post-viral olfactory disorder: at present, there are no accepted treatments. Is this damage that has reduced the basal stem cell pool so that neuron replacement is no longer maintained? Or is there some level of ongoing inflammatory damage impairing neuron and/or basal cell function? New topical intranasal therapies aimed at treating these etiologies need to be developed and tested. One treatment option that is currently offered is olfactory training therapy [36–38]. Olfactory training therapy involves intentional repeated exposure to odorants in an effort to stimulate recovery of function. A typical protocol has subjects use 4 different odors and practice sniffing each for about 15 seconds twice daily for several weeks [36]. The precise mechanism of action is unclear, but there is ample basic science evidence supporting activity-dependent mechanisms of neuronal survival or synaptic remodeling that might result in functional improvement.
Post head-trauma olfactory loss: at present, there are no accepted treatments. Pathophysiology is thought to involve stretching or shearing of olfactory fibers projecting from the nose across the cribriform to connect to the olfactory bulbs, with subsequent neuron death. However, olfactory basal stem cells normally can replace neuron loss by generating new neurons. Ongoing sensory loss following head trauma must, therefore, involve other pathology, such as damage to the olfactory bulb or central pathways, or reactive glial scarring intracranially, which might prevent new axons from establishing synaptic connections within the olfactory bulb glomeruli. In a mouse model of head trauma, the anti-inflammatory/anti-apoptotic agent minocycline showed promise at preventing olfactory loss, if administered at the time of damage [39]. However, studies so far have not shown efficacy in humans [40]. Novel drugs, perhaps aimed at reducing degeneration or preventing glia scar formation, are needed. Olfactory training therapy may be of benefit as well [37].
Presbyosmia: although the etiology remains unclear, age-related olfactory decline is likely to be a basal stem cell problem, at least in part. Future strategies aimed at stimulating or replacing basal stem cells may be useful, as discussed. For instance, evidence for reduced NPY in aged mice, or the role for other signaling pathways in basal cell regulation, suggest directed use of appropriate agonists may be helpful. Alternatively, cell-based therapies might be plausible, if an appropriate cell source and pre-clinical trials prove feasibility. As with other sensorineural losses, olfactory training therapy may be considered, although data for this specific indication are not available.
Conclusions
Olfactory disorders represent a significant clinical challenge. Considerable research has been directed at understanding olfactory physiology, including cellular and molecular mechanisms involved in odor transduction, olfactory neuron function, olfactory tissue maintenance and basal stem cells responsible for olfactory renewal. Furthermore, research has provided new insights into the pathophysiology contributing to certain olfactory disorders, including ciliopathies, sinusitis, and presbyosmia. By maximizing therapies for treatable causes of hyposmia, such as sinusitis and inflammation, excluding other serious pathology via imaging studies, providing supporting adjuncts such as olfactory training therapy, and discouraging ineffective treatments, patients can benefit. Finally, basic research exploring viral gene therapy and stem cell work suggest the feasibility of other novel treatments for sensorineural anosmia.
Acknowledgments
Dr. Bradley J. Goldstein reports grants from NIH (DC013556), during the conduct of the study.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Dr. Stefania Goncalves declares that she has no conflicts of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 2.Gopinath B, Sue CM, Kifley A, Mitchell P. The association between olfactory impairment and total mortality in older adults. J Gerontol A Biol Sci Med Sci. 2012;67(2):204–209. doi: 10.1093/gerona/glr165. [DOI] [PubMed] [Google Scholar]
- 3. Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PloS One. 2014;9(10):e107541. doi: 10.1371/journal.pone.0107541. Presbyosmia is a strong predictor of 5-year mortality. In a large database sample of older US. adults, olfactory loss was identified as a biomarker for mortality.
- 4.Paik SI, Lehman MN, Seiden AM, Duncan HJ, Smith DV. Human olfactory biopsy. The influence of age and receptor distribution. Arch Otolaryngol Head Neck Surg. 1992;118(7):731–738. doi: 10.1001/archotol.1992.01880070061012. [DOI] [PubMed] [Google Scholar]
- 5.Weiler E, Farbman AI. Proliferation in the rat olfactory epithelium: age-dependent changes. J Neurosci. 1997;17(10):3610–3622. doi: 10.1523/JNEUROSCI.17-10-03610.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loo AT, Youngentob SL, Kent PF, Schwob JE. The aging olfactory epithelium: neurogenesis, response to damage, and odorant-induced activity. Int J Dev Neurosci. 1996;14(7–8):881–900. doi: 10.1016/s0736-5748(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 7. Jia C, Hegg CC. Effect of IP3R3 and NPY on age-related declines in olfactory stem cell proliferation. Neurobiol Aging. 2015;36(2):1045–1056. doi: 10.1016/j.neurobiolaging.2014.11.007. Neuropeptide Y, which is a known basal cell mitogen, is found to decrease with age in mouse olfactory epithelium. This decrease may play a role in presbyosmia development.
- 8.DeConde AS, Mace JC, Alt JA, Schlosser RJ, Smith TL, Soler ZM. Comparative effectiveness of medical and surgical therapy on olfaction in chronic rhinosinusitis: a prospective, multi-institutional study. Int Forum Allergy Rhinol. 2014;4(9):725–733. doi: 10.1002/alr.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuperan AB, Lieberman SM, Jourdy DN, Al-Bar MH, Goldstein BJ, Casiano RR. The effect of endoscopic olfactory cleft polyp removal on olfaction. AM J Rhinol Allergy. 2015;29(4):309–313. doi: 10.2500/ajra.2015.29.4191. [DOI] [PubMed] [Google Scholar]
- 10.Bushdid C, Magnasco MO, Vosshall LB, Keller A. Humans can discriminate more than 1 trillion olfactory stimuli. Science. 2014;343(6177):1370–1372. doi: 10.1126/science.1249168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121(8):1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 13.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, et al. Visualizing an olfactory sensory map. Cell. 1996;87(4):675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 14.Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36(9):994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- 15.Tadenev AL, Kulaga HM, May-Simera HL, Kelley MW, Katsanis N, Reed RR. Loss of Bardet-Biedl syndrome protein-8 (BBS8) perturbs olfactory function, protein localization, and axon targeting. PNAS. 2011;108(25):10320–10325. doi: 10.1073/pnas.1016531108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams CL, McIntyre JC, Norris SR, Jenkins PM, Zhang L, Pei Q, et al. Direct evidence for BBSome-associated intraflagellar transport reveals distinct properties of native mammalian cilia. Nat Comm. 2014;5:5813. doi: 10.1038/ncomms6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr VM, Farbman AI. The dynamics of cell death in the olfactory epithelium. Exp Neurol. 1993;124(2):308–314. doi: 10.1006/exnr.1993.1201. [DOI] [PubMed] [Google Scholar]
- 18.Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400(4):469–486. [PubMed] [Google Scholar]
- 19.Goldstein BJ, Schwob JE. Analysis of the globose basal cell compartment in rat olfactory epithelium using GBC-1, a new monoclonal antibody against globose basal cells. J Neurosci. 1996;16(12):4005–4016. doi: 10.1523/JNEUROSCI.16-12-04005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10(6):720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- 21.Chen M, Tian S, Yang X, Lane AP, Reed RR, Liu H. Wnt-responsive Lgr5(+) globose basal cells function as multipotent olfactory epithelium progenitor cells. J Neurosci. 2014;34(24):8268–8276. doi: 10.1523/JNEUROSCI.0240-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldstein BJ, Goss GM, Hatzistergos KE, Rangel EB, Seidler B, Saur D, et al. Adult c-Kit(+) progenitor cells are necessary for maintenance and regeneration of olfactory neurons. J Comp Neurol. 2015;523(1):15–31. doi: 10.1002/cne.23653. The cell surface protein c-Kit is expressed by basal stem cells needed to produce new olfactory neurons in adult olfactory epithelium.
- 23.Wang YZ, Yamagami T, Gan Q, Wang Y, Zhao T, Hamad S, et al. Canonical Wnt signaling promotes the proliferation and neurogenesis of peripheral olfactory stem cells during postnatal development and adult regeneration. J Cell Sci. 2011;124(Pt 9):1553–1563. doi: 10.1242/jcs.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci. 2010;30(6):2324–2329. doi: 10.1523/JNEUROSCI.4507-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McIntyre JC, Davis EE, Joiner A, Williams CL, Tsai IC, Jenkins PM, et al. Gene therapy rescues cilia defects and restores olfactory function in a mammalian ciliopathy model. Nat Med. 2012;18(9):1423–1428. doi: 10.1038/nm.2860. A mouse model of cilia disorder is proven to be anosmic. The anosmia is rescued by using viral-based gene therapy, providing proof-of-concept that some forms of sensorineural anosmia can be treated with viral gene therapy.
- 26.Turner JH, May L, Reed RR, Lane AP. Reversible loss of neuronal marker protein expression in a transgenic mouse model for sinusitis-associated olfactory dysfunction. Am J Rhinol Allergy. 2010;24(3):192–196. doi: 10.2500/ajra.2010.24.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graziadei GA, Graziadei PP. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979;8(2):197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- 28.Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- 29.Holbrook EH, Leopold DA, Schwob JE. Abnormalities of axon growth in human olfactory mucosa. Laryngoscope. 2005;115(12):2144–2154. doi: 10.1097/01.MLG.0000181493.83661.CE. [DOI] [PubMed] [Google Scholar]
- 30.Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410(6831):940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- 31.Goss GM, Chaudhari N, Hare JM, Nwojo R, Seidler B, Saur D, et al. Differentiation potential of individual olfactory c-Kit+ progenitors determined via multicolor lineage tracing. Dev Neurobiol. 2015 doi: 10.1002/dneu.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein BJ, Fang H, Youngentob SL, Schwob JE. Transplantation of multipotent progenitors from the adult olfactory epithelium. Neuroreport. 1998;9(7):1611–1617. doi: 10.1097/00001756-199805110-00065. [DOI] [PubMed] [Google Scholar]
- 34.Jang W, Lambropoulos J, Woo JK, Peluso CE, Schwob JE. Maintaining epitheliopoietic potency when culturing olfactory progenitors. Exp Neurol. 2008;214(1):25–36. doi: 10.1016/j.expneurol.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murrell W, Wetzig A, Donnellan M, Feron F, Burne T, Meedeniya A, et al. Olfactory mucosa is a potential source for autologous stem cell therapy for Parkinson's disease. Stem Cells. 2008;26(8):2183–2192. doi: 10.1634/stemcells.2008-0074. [DOI] [PubMed] [Google Scholar]
- 36.Damm M, Pikart LK, Reimann H, Burkert S, Goktas O, Haxel B, et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 2014;124(4):826–831. doi: 10.1002/lary.24340. [DOI] [PubMed] [Google Scholar]
- 37.Konstantinidis I, Tsakiropoulou E, Bekiaridou P, Kazantzidou C, Constantinidis J. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope. 2013;123(12):E85–E90. doi: 10.1002/lary.24390. [DOI] [PubMed] [Google Scholar]
- 38.Altundag A, Cayonu M, Kayabasoglu G, Salihoglu M, Tekeli H, Saglam O, et al. Modified olfactory training in patients with postinfectious olfactory loss. Laryngoscope. 2015;125(8):1763–1766. doi: 10.1002/lary.25245. [DOI] [PubMed] [Google Scholar]
- 39.Siopi E, Calabria S, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Minocycline restores olfactory bulb volume and olfactory behavior after traumatic brain injury in mice. J Neurotrauma. 2012;29(2):354–361. doi: 10.1089/neu.2011.2055. [DOI] [PubMed] [Google Scholar]
- 40.Reden J, Herting B, Lill K, Kern R, Hummel T. Treatment of postinfectious olfactory disorders with minocycline: a double-blind, placebo-controlled study. Laryngoscope. 2011;121(3):679–682. doi: 10.1002/lary.21401. [DOI] [PubMed] [Google Scholar]