Abstract
Objective:
To compare implant stability, survival, and soft tissue reactions for a novel (test) and previous generation (control) percutaneous auditory osseointegrated implant for bone conduction hearing at long-term follow-up of 5 years.
Study Design:
Single follow-up visit of a previously completed multicenter, randomized, controlled trial.
Patients:
Fifty-seven of the 77 participants of a completed randomized controlled trial on a new auditory osseointegrated implant underwent a single follow-up visit 5 years after implantation, which comprised implant stability measurements and collection of Holgers scores. Additionally, implant survival was recorded for all 77 patients from the original trial.
Results:
The test implant showed significantly higher implant stability quotient (ISQ) values compared with the control implant throughout the 5-year follow-up. Mean area under the curve of ISQ high from baseline to 5 years was 71.6 (standard deviation [SD] ±2.0) and 66.7 (SD ±3.4) for the test and control implant, respectively (p < 0.0001). For both implants, the mean ISQ value recorded at 5 years was higher compared with implantation (test group +2.03 [SD ±2.55, within group p < 0.0001] and control group +2.25 [SD ±4.95, within group p = 0.12]). No difference was noticed in increase from baseline between groups (p = 0.64). Furthermore, evaluation of soft tissue reactions continued to show superiority of the test implant. At the 5-year follow-up visit, one patient (2.5%) presented with a Holgers grade 2 in the test group, compared with four patients (23.5%) in the control group (p = 0.048); no patient presented with more severe soft tissue reactions. Excluding explantations, the survival rate was 95.8% for the test group and 95.0% for the control group. The corresponding rates including explantations were 93.9 and 90.0%.
Conclusion:
The test implant showed superiority in terms of higher mean ISQ values and less adverse soft tissue reactions, both at the single 5-year follow-up visit and during the complete follow-up. In addition, both implants showed an equally high implant survival.
Keywords: Auditory osseointegrated implant, Baha, Bone-anchored hearing aid, Bone conduction, Hearing loss, Implant loss, Implant stability, ISQ, Soft tissue reactions
Since Tjellström reported on the fitting of the first patient with a bone-anchored hearing device using a temporal bone implant in 1977 (1), many improvements have been made to auditory osseointegrated implant systems (also referred to as bone conduction hearing implant systems). Hearing rehabilitation through direct bone conduction via an implant anchored in the temporal bone is nowadays an established method to overcome pure conductive hearing loss and also for mixed hearing loss as well as single-sided sensorineural deafness (2). The original auditory osseointegrated implant was a titanium implant with an as-machined surface, designed by Brånemark in the late 1970s and later made commercially available as the Cochlear™ Baha® flange fixture. In 2009, a new implant design was introduced, with a wider diameter aimed to increase implant stability (3) and a moderately roughened surface to increase bone response (i.e., remodeling) after implantation (4). Moreover, a new rounded shape and conical connection that provides a tighter seal to the percutaneous abutment were chosen to reduce soft tissue reactions. Previously, Dun et al. (5) and Nelissen et al. (6) reported 6 month and 3-year results from a randomized controlled trial of this new (test) implant and previous generation (control) implant. Implant stability measurements showed higher mean implant stability quotient (ISQ) values during the complete follow-up period for the test implant compared with the control implant. An initial decrease in stability was recorded 10 days after surgery in both study groups, while ISQ values remained relatively stable above baseline scores across the 6, 12, and 24 months visits. However, a statistically significant decrease towards baseline was noticed for both implants at the last follow-up visit at 3 years. Better soft tissue outcomes were observed with the test implant, while implant survival after 3 years was comparably high for both implants.
While formally a separate study, the current clinical investigation is a continuation of the previously completed and reported trial with a single follow-up visit 5 years after implantation (5,6). The aim of the current study was to measure long-term implant stability and explore the development of the decreasing ISQ values seen at the 3-year follow-up visit, and to confirm good implant survival and abutment tolerability at long-term follow-up. The current results comprise the first 5-year clinical data collected prospectively on percutaneous auditory osseointegrated implants.
METHODS
Study Design and Participants
The aim of the current study was to show superiority of the test implant compared with a control implant in terms of implant stability (primary outcome measure), and to evaluate long-term implant survival, and soft tissue reactions (secondary outcome measures).
The study was designed as a single prospective follow-up visit 5 years after implantation for the patients who participated in the completed 3-year multicenter, randomized, controlled trial conducted at Radboud University Medical Centre Nijmegen (Nijmegen, The Netherlands), Salford Royal Hospital (Salford, UK), Sahlgrenska University Hospital (Göteborg, Sweden), and Manchester Royal Infirmary (Manchester, UK). All patients who participated in the original trial were invited to participate in the current study. To be included in the original trial, the patients had to be at least 18 years old, have a bone thickness at the implant site of at least 4 mm, and no disease or treatment known to compromise the bone quality at the implant site. Exclusion criteria for the current study were inability to follow investigational procedure and any factor, at the discretion of the investigator, that was considered to contraindicate participation, for example, mental or physical disability or traveling plans not compliant with the study protocol. For patients who had lost or removed the implant placed in the original trial, only time to implant loss was recorded. Patients who for other reasons did not attend the 5-year visit were also included in the implant survival analysis; the last available information regarding implant survival was obtained verbally from the patient, from medical records, or from information captured in the original investigation.
Randomization for the original investigation was fixed in proportions 2:1 (test:control), stratified for each site, and was realized by means of numbered blinded envelopes. Both patients and surgeons were blinded until implantation, but because of differences in implant design, no blinding could be applied thereafter. Surgery was performed between April and December 2009. A single-stage surgical procedure with reduction of subcutaneous soft tissue was applied in all centers; a linear incision technique was used in Nijmegen, the flap technique in Manchester and Salford, and the dermatome technique in Göteborg. At each site, the same technique was used for test and control implants. Loading of the implants with sound processors was performed from 6 weeks after implantation. Follow-up visits in the previous study were completed at 10 days, at 4, 6, 8, and 12 weeks, and at 6, 12, 24, and 36 months.
Implants
The test implant was the novel titanium implant (diameter 4.5 mm; length 4 mm) with a 6 mm rounded, apically converging titanium abutment developed by Cochlear Bone Anchored Solutions AB (Mölnlycke, Sweden). This system was later commercialized under the name Cochlear™ Baha® BIA300 Implant with abutment with an additional minor change to the internal abutment connection design. The control implant was the previous generation as-machined titanium flange fixture (diameter 3.75 mm; length 4 mm) with a 6 mm conically shaped abutment from the same manufacturer. Aside from the difference in abutment shape, the test implant incorporates a wider diameter, small-sized threads at the implant neck, and the moderately rough TiOblast™ (Dentsply, Mölndal, Sweden) surface on the intraosseous part of the implant (Fig. 1).
FIG. 1.
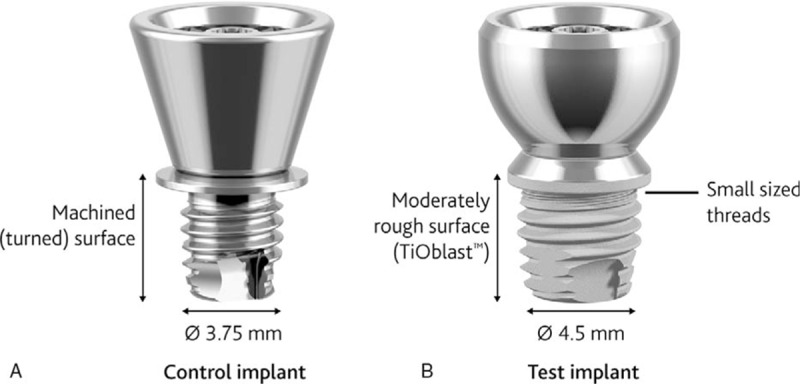
Control (A) and test (B) implants with abutments.
Outcomes of the 5-year Follow-up Visit
For all patients who attended the single visit, demographics, baseline variables (date of birth, sex, ethnical background, use of nicotine), and relevant medical history since the previous study were recorded. ISQ values were measured using resonance frequency analysis (RFA) at the abutment level with the Osstell Mentor or Osstell ISQ and a SmartPeg (type 43) (Osstell AB, Göteborg, Sweden). The ISQ score ranges from 1 to 100, with increasing scores presenting a more rigid implant-bone interface. As this score is also a representation of other implant variables, assessment of changes over time is consequently more sensible than evaluation of absolute values at a given time point (7,8). The highest (ISQ high) and lowest value (ISQ low) obtained from perpendicular measurements were recorded. Soft tissue status was assessed according to the Holgers soft tissue classification on a 5-point scale from 0, no signs of soft tissue reaction, to 4, an infection requiring implant removal (9). Holgers grade 2 or higher is considered an adverse soft tissue reaction in need of (local) treatment and the distinction is consequently of clinical importance. Furthermore, implant survival/loss was recorded, including the reason of implant loss or explantation (active removal of the implant).
Statistical Analysis and Data Management
No new sample size calculations were performed; all patients from the previous investigation were asked to participate. For the original study a power calculation was conducted on the primary outcome variable ISQ (6). For comparisons between test and control groups, Mann–Whitney U test was used for all continuous variables, Mantel–Haenszel chi-square test for all ordered categorical variables, and Fisher exact test for dichotomous variables. Wilcoxon signed-rank test was used for change within groups for continuous variables. A weighted average of ISQ during the entire study period was obtained by mean area under the curve (AUC) calculations. Implant survival probability was analyzed using a Kaplan–Meier survival curve with log-rank test; the last available information regarding implant survival was used as the censoring date for the implant survival analysis.
A significance level of 0.05 was adopted and all tests were two-tailed. No corrections were made for multiple comparisons. For the primary outcome variable, in case of missing baseline value the value at the second visit was used as baseline value instead; furthermore, no imputation with last observation carried forward was used.
Data management was performed by external data managers (dSharp, Göteborg, Sweden; and Statistiska Konsultgruppen, Göteborg, Sweden), and statistical analysis was realized by external biostatisticians (Statistiska Konsultgruppen, Göteborg, Sweden) according to a predefined statistical analysis plan using SAS® v9.4 (Cary, NC).
Ethical Consideration
The investigation was conducted in accordance with the Declaration of Helsinki and the international standard for “Clinical investigation of medical devices for human subjects—Good clinical practice (ISO 14155:2011).” Local ethics committees and competent authorities in all participating countries gave approval or a declaration of no objection for this single follow-up visit after 5 years.
The current study was registered at ClinicalTrials.gov and assigned the identifier NCT02092610.
RESULTS
Patient Characteristics
Out of the 77 patients in the original study, 57 patients (37 in Nijmegen, 11 in Salford, and 9 in Göteborg) signed the informed consent to participate in this follow-up trial and attended the 5-year follow-up visit. While the study protocol indicated a visit window of 60 ± 3 months, the actual visit dates ranged from 60 to 71 months post implantation. The patients from Manchester Royal Infirmary (Manchester, UK) could not visit the clinic, but were included in the implant survival population.
Twenty patients were lost to follow-up, had lost their implant, or were not able to visit the clinic. The baseline characteristics of the 57 patients who attended the study visit (“5-year follow-up population”) and the 77 patients in the original trial (who constituted the “implant survival population” in the current investigation) are shown in Table 1. A slightly older patient population was seen for the control implant, which was more evident in the 5-year follow-up population. There were no other significant or important differences in baseline characteristics between the two study groups.
TABLE 1.
Baseline characteristics
| Five-year Follow-up Population (n = 57)a | Implant Survival Population (n = 77)a | |||
| Characteristics | Test Group (n = 40) | Control Group (n = 17) | Test Group (n = 52) | Control Group (n = 25) |
| Sex | ||||
| Male | 19 (47.5%) | 10 (58.8%) | 23 (44.2%) | 15 (60.0%) |
| Female | 21 (52.5%) | 7 (41.2%) | 29 (55.8%) | 10 (40.0%) |
| Age at baselineb | ||||
| Years | 55.4 (SD 12.8; range, 22.1–78.8) | 64.2 (SD 9.4; range. 43.2–83.3) | 55.5 (SD 13.8; range, 22.1–80.1) | 61.7 (SD 13.5; range, 25.4–84.2) |
| Smoking at baseline | ||||
| No | 36 (90.0%) | 16 (94.1%) | 46 (88.5%) | 22 (88.0%) |
| Yes | 4 (10.0%) | 1 (5.9%) | 6 (11.5%) | 3 (12.0%) |
| Indication | ||||
| Conductive | 12 (30.0%) | 5 (29.4%) | 14 (26.9%) | 7 (28.0%) |
| Mixed | 14 (35.0%) | 9 (52.9%) | 20 (38.5%) | 13 (52.0%) |
| SSD | 13 (32.5%) | 2 (11.8%) | 17 (32.7%) | 4 (16.0%) |
| Other | 1 (2.5%) | 1 (5.9%) | 1 (1.9%) | 1 (4.0%) |
| Study site | ||||
| Nijmegen | 26 (65.0%) | 11 (64.7%) | 28 (53.8%) | 14 (56.0%) |
| Salford | 7 (17.5%) | 4 (23.5%) | 12 (23.1%) | 6 (24.0%) |
| Göteborg | 7 (17.5%) | 2 (11.8%) | 9 (17.3%) | 4 (16.0%) |
| Manchester | – | – | 3 (5.8%) | 1 (4.0%) |
a“Five-year follow-up population” includes all patients who were able to visit the clinic 5 years after implantation. “Implant survival population” includes all patients from the original trial and was used to determine the implant survival/loss during the complete follow-up.
bThe age at baseline was significantly different between the two treatment groups within the 5-year follow-up population (p = 0.03). There were no other significant or important differences between groups.
SSD indicates single sided deafness.
ISQ
The ISQ values for the test implant were significantly higher compared with those of the control implant at all visits. The mean AUC for ISQ high between baseline and 5 years was 71.6 (SD ±2.0) and 66.7 (SD ±3.4) for the test and control implant, respectively (p < 0.0001) (Fig. 2). The corresponding values for ISQ low were 69.9 (SD ±2.0) and 64.9 (SD ±3.3) (p < 0.0001).
FIG. 2.
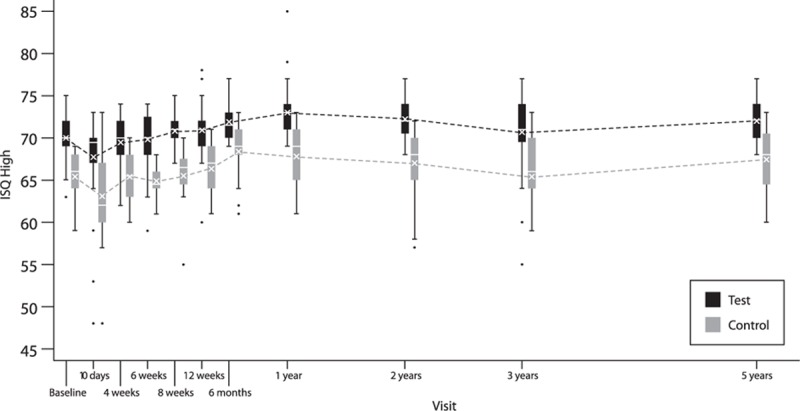
Box-and-whisker plot of ISQ high—lines represent ISQ high for patients who attended the 5-year follow-up. Mean (cross) and median (horizontal line) are defined within the boxplot. The box represents the interquartile range, the whiskers the 95% confidence interval and the single dots the outliers.
Mean ISQ high at 5 years was 72.1 (SD ±2.2) for the test implant compared with 67.4 (SD ±4.0) for the control implant (p < 0.0001). ISQ low resulted in similar results, with absolute numbers on average one to two points lower. An increase in ISQ values was recorded between the last visit at 3 years in the original trial and the 5-year visit in the current study for both implants. The change in ISQ high from baseline to 5 years was 2.03 (SD ±2.55, within group p < 0.0001) for the test implant and 2.25 (SD ±4.95, within group p = 0.12) for the control implant. No difference was noticed in increase from baseline between groups (p = 0.64). All outcome variables are shown in more detail in Table 2.
TABLE 2.
Outcome variables
| Five-year Follow-up Population (n = 57) | |||
| Outcome | Test Group (n = 40) | Control Group (n = 17) | Statistical Analysis Between Groups |
| ISQ AUC 0–5 yra | |||
| High | 71.6 (SD 2.0; range, 65.6–75.8) | 66.7 (SD 3.4; range, 61.0–71.8) | p < 0.0001 |
| Low | 69.9 (SD 2.0; range, 65.1–73.9) | 64.9 (SD 3.3; range, 58.3–70.1) | p < 0.0001 |
| ISQ at 5 yrs | |||
| High | 72.1 (SD 2.2; range, 68–77) | 67.4 (SD 4.0; range, 60–73) | p < 0.0001 |
| Low | 70.9 (SD 2.3; range, 66.0–75.0) | 65.9 (SD 4.3; range, 57.0–71.0) | p < 0.0001 |
| Change in ISQ 0–5 yra | |||
| High | 2.03 (SD 2.55; range, −4 to 10) | 2.25 (SD 4.95; range, −7 to 11) | p = 0.64 |
| Low | 3.69 (SD 3.6; range, −3 to 12) | 4.06 (SD 4.89; range, −5 to 13) | p = 0.59 |
| Holgers at 5 yrs | |||
| Grade 0 | 36 (90%) | 9 (52.9%) | |
| Grade 1 | 3 (7.5%) | 4 (23.5%) | |
| Grade 2 | 1 (2.5%) | 4 (23.5%) | |
| Grade 3 | 0 | 0 | |
| Grade 4 | 0 | 0 | p = 0.0013b |
| Maximum Holgers 0–5 yra | |||
| Grade 0 | 10 (25%) | 2 (11.8%) | |
| Grade 1 | 22 (55%) | 5 (29.4%) | |
| Grade 2 | 7 (17.5%) | 9 (52.9%) | |
| Grade 3 | 1 (2.5%) | 1 (5.9%) | |
| Grade 4 | 0 | 0 | p = 0.015b |
| Implant Survival Population (n = 77) | ||
| Outcome | Test Group (n = 52) | Control Group (n = 25) |
| Implant loss 0–5 yra | ||
| Including explantations | 3 (6.1%) | 2 (10%) |
| Excluding explantations | 2 (4.2%) | 1 (5%) |
a0–5 year included all measurements from surgery till 5-year follow-up for ISQ, and all measurements from first postoperative visit till 5-year follow-up for soft tissue reactions.
bWhen comparing Holgers 0–1 with Holgers 2–4 statistical analysis between groups results in p = 0.048 for Holgers at 5 years and p = 0.011 for maximum Holgers 0–5 yrs.
AUC indicates area under the curve; ISQ, implant stability quotient.
Soft-tissue Reactions
The classification of soft-tissue reactions using Holgers index showed continued improvement for the test implant compared with the control implant, with less type 1 and type 2 soft-tissue reactions, as shown in Figure 3. At the 5-year follow-up visit, one patient (2.5%) presented with a Holgers grade 2 in the test group, compared with four patients (23.5 %) in the control group (p = 0.048). No patients presented with Holgers grade 3 or 4. The distribution of soft-tissue reactions over all Holgers grades (i.e., grade 0 to grade 4) was also significantly different between groups (p = 0.0013). When comparing the maximum severity of soft tissue reactions per patient across all visits (i.e., highest Holgers grade during complete study), a significant difference in favor of the test implant was also recorded (p = 0.015) (Table 2).
FIG. 3.
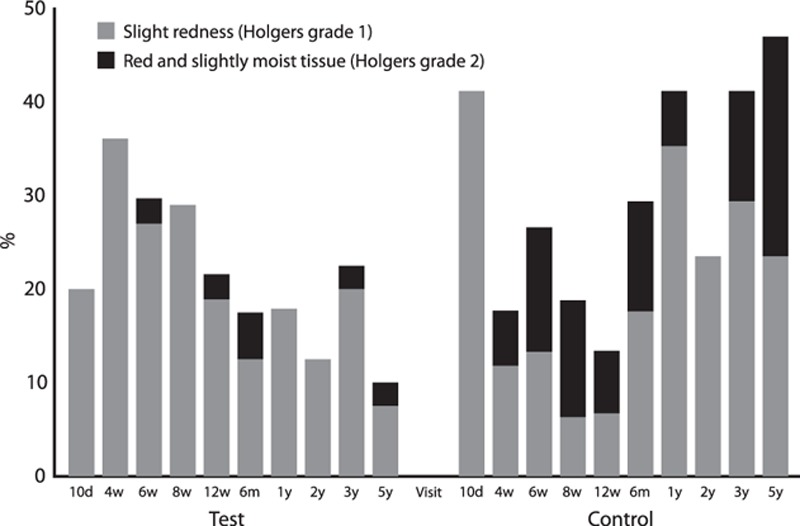
Soft-tissue reactions according to Holger classification—bars represent the percentage of patients with a soft tissue reaction in patients who attended the 5 years follow-up visit.
Implant Survival
In the test group, during the first 3 years of the study, one implant was explanted (chronic pain around abutment) and one implant was lost (8 weeks after surgery, at time of sound processor fitting, attributed to failure of osseointegration); in the control group no implants were explanted or lost during this period. Between the 3- and 5-year visits, another implant was lost in the test group (51 months after implantation). In the control group, one implant was explanted after 60 months and one implant was lost after 58 months (possibly related to radiotherapy at the implant site in the months before implant loss). Excluding explantations, the implant survival rate was 95.8 and 95.0% for the test and control group, respectively (Fig. 4). The corresponding rates including explantations were 93.9 and 90.0%.
FIG. 4.
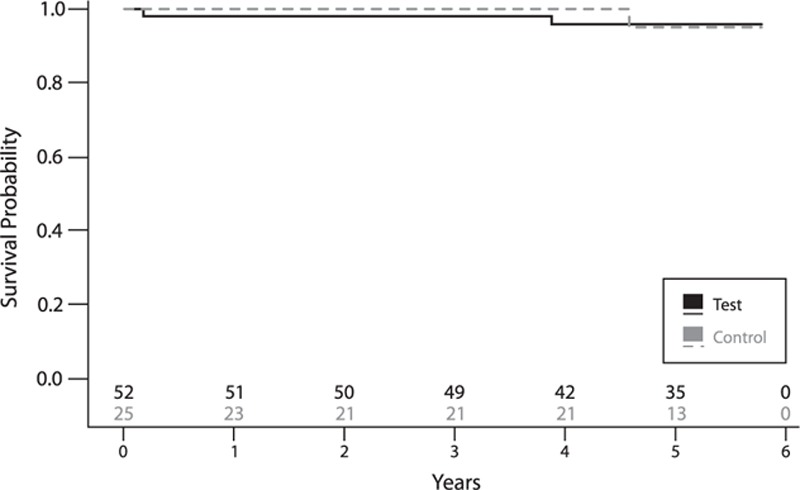
Implant survival, excluding explantations—lines represent the survival curve for both study groups. Numbers above x axis represent the numbers of patients at risk at the specific time point.
DISCUSSION
Principal Findings
The aim of the current study was to compare clinical outcomes of a novel and a previous generation auditory osseointegrated implant system at long-term follow-up. The study showed superiority of the test implant compared with the control implant regarding ISQ measurements during the complete follow-up. The decrease in ISQ values recorded between 2 and 3 years of follow-up returned to higher ISQ values at the 5-year follow-up. The test implant continued to show superior soft tissue outcomes at 5 years, with less adverse soft tissue reactions in the test group. Implant survival of both study groups was slightly lower at 5 years of follow-up, however, still at high levels compared with previously reported numbers (10–12).
Strengths and Limitations
The current investigation provides the first 5-year evidence on novel, wide implants in bone conduction hearing in a controlled approach. The original randomized controlled trial with multiple participating centers already provided very strong evidence for a high implant survival and good soft tissue outcome at 3-year follow-up. With the additional long-term follow-up in a prospective manner and with the original multicenter set-up, we were able confirm these good outcomes and showed reassuring results for future follow-up with increasing ISQ scores since last follow-up, continued high implant survival and good soft tissue outcomes for the test group.
One of the limitations of the current study is the loss to follow-up of some patients for the 5-year visit compared with the original study sample. Twenty patients, including five patients who had lost their implant or were explanted during the past 5 years and five patients who were already lost to follow-up/withdrew consent during the original trial, could not be included in the 5-year follow-up analysis of implant stability and soft tissue reactions. Consequently, a selection bias for this last follow-up visit cannot be excluded, even more since the current visit was a distinct investigation for which patients had to give separate informed consent. However, mostly minor differences in baseline characteristics between the 5-year follow-up population and original study sample were observed. A difference in inclusion proportion between centers compared with the original trial and a small difference in age at baseline was noticed. All 77 patients of the original study population were included in the implant survival analysis; however, for the patients who could not be contacted, survival information was censored from a date before the 5-year follow-up and was based on patient files and/or information collected in the original investigation. The non-blinded follow-up and analysis is another limitation, as was already discussed in the previous reports (5,6).
Interpretation and Comparison With Other Studies
The available literature reporting on the same type of implant generally shows good results in terms of implant stability and soft tissue outcomes; however, the majority of the investigations are retrospective cohort studies without a control group or small pilot studies (13–18). For other wide auditory osseointegrated implants, similarly higher ISQ values compared with smaller diameter implants have been reported in short-term follow-up (19). To obtain more evidence on clinically important outcomes like implant survival, it would be highly desirable to have additional well-designed studies on wider implants in bone conduction hearing. Long-term follow-up, which was one of the major strengths of the current investigation, would be expedient for these studies.
Nelissen et al. (6) previously hypothesized that the dip in mean ISQ between 2 and 3 years (for both types of implants) could be the result of marginal bone loss around the implant. With the current results showing increasing values at the 5-year follow-up (with ISQ values comparable to the 2-year results), and with another investigation of the same implant showing no stability dip at 3 years any biological explanation of the previous decrease in stability seems unlikely (6). An alternative reason for the dip could be a measurement error. Studies in dental implantology show conflicting results on intra-rater and inter-rater reliability of RFA (20–22). Importantly, the small decrease detected by the stability measurements did not translate into clinical instability.
Implant stability as measured by RFA was chosen as the main outcome measure of the current study. This outcome measure should be interpreted with caution, as it is influenced by many factors in implant, abutment design, and surgery (8). It should additionally be emphasized that implant stability measures are a surrogate measure for implant survival, which is ultimately the most important for patients. Implant survival rates were shown to be high and equal for both study groups.
The implants in the present investigation were loaded with the sound processor from 6 weeks after surgery, which at the time of study initiation was not common practice. At that time, mostly loading protocols allowing 3 months of unloaded implants were reported. With the high implant survival rate and good soft tissue outcomes at 5 years, earlier loading seems to be safe at long-term follow-up. Nowadays, even earlier loading is frequently reported and considered to be safe (23,24). These early loading protocols allow patients to use their device as soon as possible with an improved patient satisfaction as a result.
Regarding one of the other secondary outcome measures, the decrease in soft tissue reactions is an important advantage of the new implant-abutment system. Percentages of adverse soft tissue reactions were reduced to 20% for the new implant versus 58.8% for the previous generation implant during the complete follow-up, representing an essential reduced need for postoperative treatment. Both the rounded shape of the abutment and the conical connection between the new implant and abutment that provides a tighter seal, have been proposed as explanations for this reduction (5,6).
CONCLUSION
The new auditory osseointegrated implant design showed superiority compared with the previous implant design in terms of long-term implant stability as measured by RFA. Furthermore, although auditory osseointegrated implant surgery is a relatively safe procedure already, an important and persistent reduction in soft tissue reactions was noticed for the new implant. These good outcomes at longest follow-up reported to date in a prospective controlled study, support the replacement of the previous generation implants by the new BIA300 implant with abutment.
Acknowledgments
The following persons are acknowledged for their contributions throughout the original study: Catharina A. J. Dun (Radboud University Medical Center), Maarten J. F. de Wolf (Radboud University Medical Center), Cor W. R. J. Cremers (Radboud University Medical Center), Michael P. Rothera (Salford Royal Hospital), Corina Ichim (Salford Royal Hospital).
Footnotes
Cochlear Bone Anchored Solutions AB assumed the role as sponsor of the current study in accordance with ISO 14155:2011. In collaboration with all authors, Cochlear, designed and managed the study and were responsible for data analysis and report writing. Data were recorded by the investigators and monitored by Cochlear. Data management and statistical analyses were completed by external data managers and biostatisticians. All authors had full access to the results. The sponsor and authors had final responsibility for the content of the publication.
CB, RN, EM, MH report financial support to their authors institution for conducting two clinical studies from Oticon Medical AB (Askim, Sweden) and from Cochlear Bone Anchored Solutions AB (Mölnlycke, Sweden), outside the submitted work.
REFERENCES
- 1.Tjellström A, Lindström J, Hallén O, Albrektsson T, Brånemark PI. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol 1981; 2:304–310. [PubMed] [Google Scholar]
- 2.Snik AFM, Mylanus EAM, Proops DW, et al. Consensus statements on the BAHA system: where do we stand at present? Ann Otol Rhinol Laryngol Suppl 2005; 195:2–12.16619473 [Google Scholar]
- 3.Lee J-H, Frias V, Lee K-W, Wright RF. Effect of implant size and shape on implant success rates: a literature review. J Prosthet Dent 2005; 94:377–381.doi:10.1016/j.prosdent.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Albrektsson T, Sennerby L, Wennerberg A. State of the art of oral implants. Periodontol 2000 2008; 47:15–26.doi:10.1111/j.1600-0757.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- 5.Dun CAJ, de Wolf MJF, Hol MKS, et al. Stability, survival, and tolerability of a novel baha implant system: six-month data from a multicenter clinical investigation. Otol Neurotol 2011; 32:1001–1007.doi:10.1097/MAO.0b013e3182267e9c. [DOI] [PubMed] [Google Scholar]
- 6.Nelissen RC, Stalfors J, de Wolf MJF, et al. Long-term stability, survival, and tolerability of a novel osseointegrated implant for bone conduction hearing: 3-year data from a multicenter, randomized, controlled, clinical investigation. Otol Neurotol 2014; 35:1486–1491.doi:10.1097/MAO.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 7.Sennerby L, Meredith N. Implant stability measurements using resonance frequency analysis: biological and biomechanical aspects and clinical implications. Periodontol 2000 2008; 47:51–66.doi:10.1111/j.1600-0757.2008.00267.x. [DOI] [PubMed] [Google Scholar]
- 8.Nelissen RC, Wigren S, Flynn MC, Meijer GJ, Mylanus EAM, Hol MKS. Application and interpretation of resonance frequency analysis in auditory osseointegrated implants: a review of literature and establishment of practical recommendations. Otol Neurotol 2015; 36:1518–1524.doi:10.1097/MAO.0000000000000833. [DOI] [PubMed] [Google Scholar]
- 9.Holgers KM, Tjellström A, Bjursten LM, Erlandsson BE. Soft tissue reactions around percutaneous implants: a clinical study of soft tissue conditions around skin-penetrating titanium implants for bone-anchored hearing aids. Am J Otol 1988; 9:56–59. [PubMed] [Google Scholar]
- 10.Dun CAJ, Faber HT, de Wolf MJF, Mylanus EAM, Cremers CWRJ, Hol MKS. Assessment of more than 1,000 implanted percutaneous bone conduction devices: skin reactions and implant survival. Otol Neurotol 2012; 33:192–198.doi:10.1097/MAO.0b013e318241c0bf. [DOI] [PubMed] [Google Scholar]
- 11.Larsson A, Tjellström A, Stalfors J. Implant losses for the bone-anchored hearing devices are more frequent in some patients. Otol Neurotol 2015; 36:336–340.doi:10.1097/MAO.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 12.Kiringoda R, Lustig LR. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol 2013; 34:790–794.doi:10.1097/MAO.0b013e318291c651. [DOI] [PubMed] [Google Scholar]
- 13.McLarnon CM, Johnson I, Davison T, et al. Evidence for early loading of osseointegrated implants for bone conduction at 4 weeks. Otol Neurotol 2012; 33:1578–1582.doi:10.1097/MAO.0b013e31826dba5f. [DOI] [PubMed] [Google Scholar]
- 14.D’Eredità R, Caroncini M, Saetti R. The new Baha implant: a prospective osseointegration study. Otolaryngol Head Neck Surg 2012; 146:979–983.doi:10.1177/0194599812438042. [DOI] [PubMed] [Google Scholar]
- 15.Wróbel M, Gawęcki W, Szyfter W. New insight into Baha® implant stability measurements: observations on resonance frequency analysis results. Otol Neurotol 2013; 34:1018–1020.doi:10.1097/MAO.0b013e31827e5fc4. [DOI] [PubMed] [Google Scholar]
- 16.McLarnon C, Johnson I, Davison T, et al. Resonance frequency analysis of osseo-integrated implants for bone conduction in a pediatric population—a novel approach for assessing stability for early loading. Int J Pediatr Otorhinolaryngol 2014; 78:641–644.doi:10.1016/j.ijporl.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 17.H⊘gsbro M, Agger A, Johansen LV. Successful loading of a bone-anchored hearing implant at two weeks after surgery: randomized trial of two surgical methods and detailed stability measurements. Otol Neurotol 2015; 36:e51–e57.doi:10.1097/MAO.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 18.Marsella P, Scorpecci A, D’Eredità R, Volpe Della A, Malerba P. Stability of osseointegrated bone conduction systems in children: a pilot study. Otol Neurotol 2012; 33:797–803.doi:10.1097/MAO.0b013e318255dd73. [DOI] [PubMed] [Google Scholar]
- 19.Nelissen RC, Besten den CA, Mylanus EAM, Hol MKS. Stability, survival, and tolerability of a 4.5-mm-wide bone-anchored hearing implant: 6-month data from a randomized controlled clinical trial. Eur Arch Otorhinolaryngol 2016; 273:113–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geckili O, Bilhan H, Cilingir A, Mumcu E, Bural C. A comparative in vitro evaluation of two different magnetic devices detecting the stability of osseo-integrated implants. J Periodont Res 2012; 47:508–513.doi:10.1111/j.1600-0765.2011.01462.x. [DOI] [PubMed] [Google Scholar]
- 21.Brouwers JEIG, Lobbezoo F, Visscher CM, Wismeijer D, Naeije M. Reliability and validity of the instrumental assessment of implant stability in dry human mandibles. J Oral Rehabil 2009; 36:279–283.doi:10.1111/j.1365-2842.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- 22.Lachmann S, Kimmerle-Müller E, Axmann D, Gomez-Roman G, Weber H, Haas R. Reliability of findings around healthy implants in association with oral hygiene measures: a clinical, microbiological, and immunological follow-up in edentulous patients. Clin Oral Implants Res 2007; 18:686–698.doi:10.1111/j.1600-0501.2007.01399.x. [DOI] [PubMed] [Google Scholar]
- 23.Faber HT, Dun CAJ, Nelissen RC, Mylanus EAM, Cremers CWRJ, Hol MKS. Bone-anchored hearing implant loading at 3 weeks: stability and tolerability after 6 months. Otol Neurotol 2013; 34:104–110.doi:10.1097/MAO.0b013e318277a282. [DOI] [PubMed] [Google Scholar]
- 24.Nelissen RC, Besten den CA, Faber HT, Dun CAJ, Mylanus EAM, Hol MKS. Loading of osseointegrated implants for bone conduction hearing at 3 weeks: 3-year stability, survival, and tolerability. Eur Arch Otorhinolaryngol 2016; 273:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]


