Abstract
Rationale
Anxiety disorders are the most common mental disorders in the United States. Characterized by feelings of uncontrollable apprehension, they are accompanied by physical, affective, and behavioral symptoms. The neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptor PAC1 (PAC1R) are highly expressed in the central nucleus of the amygdala (CeA) and they have gained growing attention for their proposed role in mediating the body’s response to stress.
Objectives
The aim of this study was to evaluate the anxiogenic effects of PACAP in the CeA and its effects on the hypothalamic-pituitary-adrenal (HPA) axis. Furthermore, the mechanism of action of PACAP in the CeA was investigated.
Methods
PACAP was microinfused into the CeA of rats and its effects in the elevated plus maze (EPM), the defensive withdrawal tests, and plasma corticosterone levels were evaluated. The ability of the melanocortin receptor antagonist SHU9119 to block PACAP effect in the EPM was assessed.
Results
Intra-CeA PACAP exerted a dose-dependent anxiogenic effect and activated the HPA axis. In contrast, PACAP microinfused into the basolateral nucleus of the amygdala (BlA) had no effect. Finally, the anxiogenic effect of intra-CeA PACAP was prevented by SHU9119.
Conclusions
These data prove an anxiogenic role for the PACAP system of the CeA, and reveal that the MC4R system of CeA mediates these effects. Our data provide insights into this neuropeptide system as a mechanism for modulating the behavioral and endocrine response to stress, and suggest that dysregulations of this system may contribute to the pathophysiology of anxiety-related disorders.
Keywords: Stress, anxiety, depression, neuropeptide, animal model, MC4, HPA axis
Introduction
Anxiety disorders are among the most common forms of mental illness in the world, affecting 40 million adults in the United States (Kessler et al. 2005). They are characterized by a state of apprehension, tension, and fear resulting from the anticipation of a real or fantasized threatening event or situation, feelings which impair physical and psychological functioning. Anxiety disorders may arise from both genetic and environmental causes which interact with each other to produce the final pathology (Kessler et al. 2005).
The amygdala is a brain area crucial for the regulation of stress-induced fear responses, release of glucocorticoids, and autonomic nervous system activation (Davis 1992). The CeA in particular is a key region for the regulation of anxiety (Kalin et al. 2004; Tye et al. 2011), with several studies indicating this brain region as a potential target for anxiolytic agents (Carvalho et al. 2012; Kang-Park et al. 2004). The CeA integrates sensory information from the environment and sends projections to various effector regions to trigger the appropriate responses (Davis 1992; Davis and Shi 1999; Pitkänen 2000; Zarrindast et al. 2008). Hyperactivity of the CeA has been hypothesized to be a critical factor in the pathophysiology of anxiety- and trauma-related disorders (Etkin et al. 2009; Etkin and Wager 2007; Jiang et al. 2009).
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a neuropeptide and neurohormone belonging to the secretin glucagon vasoactive intestinal polypeptide (VIP) family; PACAP-38 (here named “PACAP”) represents the major form of PACAP in the brain. PACAP exerts its effects mainly through the activation of PAC1 receptor (PAC1R), which binds PACAP with a 1,000-fold higher affinity than VIP; VPAC1 and VPAC2 receptors, on the other hand, bind both PACAP and VIP with similar affinities (Vaudry et al. 2009).
A key role for the PACAP-PAC1R system in anxiety and stress-related behaviors has been proposed (Dore et al. 2013a; Lezak et al. 2014; Stroth et al. 2011). PACAP is a neurotransmitter at the adrenomedullary synapse and it is required for stress-induced catecholamine release and synthesis (Hamelink et al. 2002; Stroth and Eiden 2010). In the brain, PACAP and its receptor PAC1 are highly expressed in the hypothalamus, particularly in the paraventricular nucleus (PVN) and the supraoptic nucleus, where they stimulate the release of various hypophysiotropic neurohormones, including corticotropin-releasing factor (CRF). PACAP and PAC1R are, however, also abundantly expressed in extrahypothalamic areas such as the central and the basolateral nuclei of the amygdala (CeA, BlA), the bed nucleus of the stria terminalis (BNST), and the brainstem (Joo et al. 2004).
Several pieces of evidence point at PACAP and PAC1R as strong stress response mediators. PAC1R knockout mice exhibit reduced anxiety-like behaviors (Otto et al. 2001), and an association between PACAP/PAC1R (possibly in the amygdala) and posttraumatic stress disorder in heavily traumatized patients has been documented (Ressler et al. 2011). Numerous effects of stress have been shown to be mediated by PACAP, as demonstrated by studies showing that stress is unable to increase CRH synthesis in the PVN or plasma corticosterone levels in PACAP knockout mice (Lehmann et al. 2012; Stroth and Eiden 2010). Central administration of PACAP in rodents has been shown to produce stress-like, HPA-activating, and anorectic effects, and PACAP and PAC1R levels have been reported to be altered in the brain after exposure to stressors (Agarwal et al. 2005; Dore et al. 2013a; Hammack et al. 2009; Legradi et al. 2007; Ressler et al. 2011).
However, the contribution of the different brain regions to the effects observed and importantly the mechanisms by which PACAP exerts its effects are still unclear. Extensive evidence has pointed at the PVN, as well as the BNST, as areas likely involved in the anxiety-modulating effects of PACAP. PACAP indeed increases startled behavior and plasma corticosterone levels when administered into the BNST (Hammack et al. 2009; Lezak et al. 2014); in addition, PACAP administered into the PVN produces elevated face washing, body grooming, decreased locomotor activity, and rearing (Norrholm et al. 2005). Recent studies have begun to propose a role for PACAP in the CeA (Dore et al. 2013a; Missig et al. 2014).
Members of another neuropeptide system, consisting of melanocortins (e.g. α-MSH) and melanocortin receptors (mainly MC4 receptor, MC4R), are also highly expressed in the CeA. While historically involved mostly in feeding and energy balance regulation, the role of this system in the modulation of anxiety-like behaviors has started to emerge. Central administration of an MC4R agonist was shown to increase CRF transcription as well as plasma corticosterone levels (Lu et al. 2003), and activation of the α-MSH/MC4R system of the amygdala has been shown to have anxiogenic effects (Kokare et al. 2005; Kokare et al. 2010). Lack of MC4R leads to blunted HPA responses to acute psychological stress (Ryan et al. 2014), and electrical shock increases MC4R mRNA levels in the amygdala and hypothalamus (Yamano et al. 2004). Finally, MC4R antagonism is able to prevent the behavioral consequences of restraint stress and electrical shock (Chaki et al. 2003; Liu et al. 2013; Vergoni et al. 1999), and to reverse isolation -induced anxiety- and depressive-like behavior(Kokare et al. 2010) and single prolonged stress-induced behavioral outcomes (Serova et al. 2013). Whether the PACAP and the melanocortin system interact in the amygdala in the context of anxiety-like behavior is currently unknown. Therefore this study aimed at exploring the role of PACAP in the CeA of rats in the context of anxiety and its mechanism of action.
Experimental Procedures
Subjects
Male Wistar rats, weighing 301–325g at arrival (Charles River, Wilmington, MA), were single-housed in wire-topped, plastic cages in a 12h reverse light cycle, AAALAC-approved, humidity- (60%) and temperature-controlled (22 °C) vivarium. Reverse light cycle allows the testing of rodents during their active phase. Rats had access to corn-based chow (Harlan Teklad LM-485 Diet 7012) and water ad libitum at all times. Subject number per experiment follows: EPM dose-response, n= 54 (CeA), n= 37 (BlA); defensive withdrawal, n= 19 (CeA), EPM blockade experiment, n= 29; corticosterone, n= 47 (CeA), n= 33 (BlA). Animals previously used in the EPM were randomized for the dose previously received and tested again in the Corticosterone experiment (at least 2 weeks later). Rats with incorrect placement of one or both cannulas were not included in the analysis (21 rats for EPM CeA, 14 rats for EPM BlA, 10 rats for the EPM blockade, 21 rats for CeA corticosterone, 15 rats for BlA corticosterone).
Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Principles of Laboratory Animal Care, and were approved by Boston University Medical Campus Institutional Animal Care and Use Committee (IACUC).
Drugs
PACAP (PACAP-38) was purchased from American Peptide Company (Sunnyvale, CA), and the melanocortin antagonist SHU 9119 from R&D Systems Inc. (Pittsburgh, PA). Peptides were dissolved in sterile isotonic saline in the presence of 1% bovine serum albumin (Thermo Fisher Scientific, Waltham, MA). SHU9119 has been described to be a potent melanocortin MC3/4 receptor antagonist and MC5 receptor agonist (IC50 = 0.23 ± 0.02 nM at the hMC3R and IC50 = 0.06 ± 0.01 nM at the hMC4R with no cAMP activity at the receptor at 10–5 M concentration of ligand; IC50 = 0.09 ± 0.02 nM and EC50 = 0.12 ± 0.01 nM with 97% of activity at the hMC5R) (Grieco et al. 2007; Hruby et al. 1995). PACAP doses were chosen based on previous reports from our laboratory using intracranial administration (Dore et al. 2013a; Iemolo et al. 2015). For SHU9119 a sub-threshold dose of SHU9119 was chosen based on previous reports using intracranial administration of the drug in the range of 10–100 pmol (Boghossian et al. 2010; Hagan et al. 1999; Iemolo et al. 2015; Liu et al. 2007; Roseberry 2013). A subthreshold of SHU9119 was chosen to ensure no per se activity at any of the melanocortin receptors bound.
Intracranial surgeries, microinfusion procedure and cannula placement
Intracranial surgeries
Rats were stereotaxically implanted with bilateral cannulas as described previously (Iemolo et al. 2013; Sabino et al. 2007). Stainless steel, guide cannulas (24 gauge, Plastics One, Inc., Roanoke, VA) were lowered 2.0 mm above either the CeA or the BlA. Four stainless steel jeweler’s screws were fastened to the rat’s skull around the cannulas. Dental restorative filled resin (Henry Schein Inc., Melville, NY) and acrylic cement were applied, forming a pedestal firmly anchoring the cannula. The cannula coordinates from the bregma used for the CeA were: AP −2.64, ML ±4.2, DV −6.2 (from skull); the cannula coordinates used for the BlA were: AP −2.64, ML ±4.8, DV −6.5 (from skull), according to (Paxinos and Watson 2007). A stainless steel dummy stylet, which terminated at the end of the guide cannula maintained patency. After surgery, the rats were allowed a 7-day recovery period, during which they were further handled. Supplementary Figure 1 shows CeA and BlA cannula placement (Suppl. Fig. 1A and 1C, respectively) and photomicrographs showing representative injection sites in the CeA and BlA (Suppl. Fig. 1B and 1D, respectively).
Microinfusion procedure
The drugs were microinfused as previously described (Blasio et al. 2013; Dore et al. 2013b; Iemolo et al. 2013). The dummy stylet was removed from the guide and replaced with a 31-gauge stainless steel injector projecting 2 mm beyond the tip of the guide cannula; the injector was connected via PE 20 tubing to a microsyringe (Hamilton Company, Reno, NV) driven by a microinfusion pump (KD Scientific, Holliston, MA). Microinfusions were performed in 0.5 μl volume per side, delivered over 2 min; injectors were left in place for 1 additional min to minimize backflow. SHU9119 was administered 15 min before PACAP, and 30 min after PACAP administration rats were either tested in the behavioral test or their blood was drawn.
Cannula placement
Cannula placement was verified at the conclusion of all testing. Subjects were sacrificed under anesthesia (isoflurane, 2–3% in oxygen) and injected with 0.5 μl/side of India ink. Brains were removed, frozen in a methyl butane/dry ice bath, and stored at −80°C. Coronal sections of 40 μm were collected using a cryostat and placements were verified under a microscope. Only subjects with correct placements were included in the analyses.
Elevated Plus-Maze Test
The elevated plus-maze test was performed as previously described (Cottone et al. 2009a; Sabino et al. 2009). The black Plexiglas plus-maze apparatus (Blasio et al. 2013; Cottone et al. 2007; 2009b) consisted of four arms (10 cm × 50 cm) positioned at right angles, 50 cm above the floor. Two “closed” arms had 40-cm high walls; two “open” arms had 0.5-cm high ledges. Testing occurred in a dim room with 1.5–2.0 lx of open arm illumination and <1 lx in the closed arms. Rats were kept in a dark anteroom with white noise present (70 dB) for ≥1 h before testing, and white noise was also present during testing. Rats were placed individually onto the center of the maze for a 5-min period. The primary measures were the percent of open arm time [i.e., 100 × open arm/(open arm + closed arm)], a validated index of anxiety-related behavior (Fernandes and File 1996), and the number of closed arm entries, an index of motor activity (Cruz et al. 1994). The test was scored by raters blind to the treatment conditions.
Defensive Withdrawal Test
Rats were acclimated to a room next to the testing for at least 60 min, in the presence of a white noise. The defensive withdrawal test (Cottone et al. 2009b; Zorrilla et al. 2002) apparatus was a walled, black/gray polyvinylchloride open field (106 cm × 92 cm × 77 cm) containing a cylindrical “withdrawal” chamber (2L Pyrex beaker wrapped in black tape). The chamber was located 15 cm from a corner facing the open arena, and testing occurred under room light (~300 lx). Rats were kept in a dark anteroom with white noise present (70 dB) for ≥1 h before testing. For the 10-min test, rats were placed into the withdrawal chamber facing the rear, and behavior was video recorded. The total duration of withdrawal was scored. The test was scored by raters blind to the treatment conditions.
Plasma corticosterone measurement
Plasma levels of corticosterone were determined as previously described (Cottone et al. 2009a; Dore et al. 2013a). Blood was sampled from the rats’ tails and collected in tubes containing 0.5M EDTA pH 8.0 (Gibco, Thermo Fisher Scientific, Cambridge, MA). Plasma was obtained after blood centrifugation, and it was stored at −80 °C until levels of corticosterone-like immunoreactivity were determined using a commercially available radioimmunoassay kit according to the manufacturer’s instructions (MP Biomedicals, Inc., Santa Ana, CA). Intra- and inter-assay coefficients of variation were <10%.
Statistical analysis
PACAP dose-response curve data were analyzed using one-way analyses of variance (ANOVAs). The antagonist blockade experiment was analyzed using a two-way ANOVA with PACAP and Antagonist as between-subject factors. ANOVAs were followed by post-hoc comparisons (Dunnett in dose-responses, Fisher’s LSD in the antagonist blockade). Significance was set at p<0.05. The software/graphic packages used were Systat 11.0, SigmaPlot 11.0, InStat 3.0, and Statistica 7.0.
RESULTS
PACAP produces anxiety-like behavior when administered into the CeA
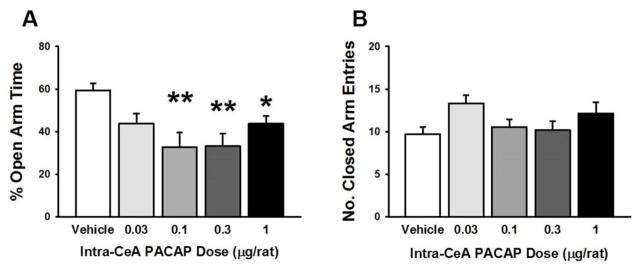
As shown in Fig. 1A, intra-CeA administration of PACAP significantly affected the % time the rats spent in the open arms of an elevated plus maze (EPM) (F(4,49)= 6.32, p<0.0001); post-hoc analysis showed that the doses of 0.1, 0.3, and 1 μg/rat were all effective in reducing the % open arm time. As shown in Fig. 1B, PACAP had no effects on the number of closed arm entries (Fig. 1B), an index of motor activity (F(4,49)= 1.75, not significant (n.s.)). In addition, as Supplementary Fig. 2 shows, rats administered PACAP intra-CeA (0.3 μg/rat) withdrew more into the sheltered chamber of a defensive withdrawal test than did vehicle-treated rats (~75% reduction; t(17)=2.02, p<0.05), which is another measure of anxiety-like behavior. Suppl. Table 1 shows the drug effects (in this EPM experiment as well as the following ones) on other measures, such as % open arm entries.
Figure 1.
Effects of PACAP (0–1 μg/rat) microinfused bilaterally into the central nucleus of the amygdala (CeA) on the percent (%) of open arm time (A) and the number of entries in the closed arms (B) of an elevated plus maze. N=6–16/group. Data represent Mean ± SEM. * p<0.05, ** p<0.01 vs. vehicle group.
PACAP does not affect anxiety-like behavior when administered into the BlA
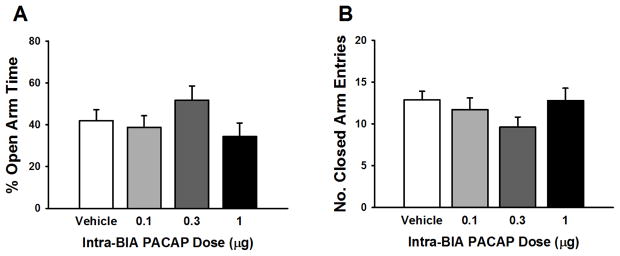
Fig. 2A shows that PACAP, administered at doses which were effective intra-CeA, did not affect the % of open arm time when given into the BlA (F(3,33)= 1.54, n.s.). Closed arm entries (Fig. 2B) were also unaffected by intra-BlA PACAP treatment (F(3,33)= 1.31, n.s.).
Figure 2.
Effects of PACAP (0–1 μg/rat) microinfused bilaterally into the basolateral nucleus of the amygdala (BlA) on the percent (%) of open arm time (A) and the number of entries in the closed arms (B) of an elevated plus maze. N=7–10/group. Data represent Mean ± SEM.
PACAP activates the HPA axis when administered into the CeA but not the BlA
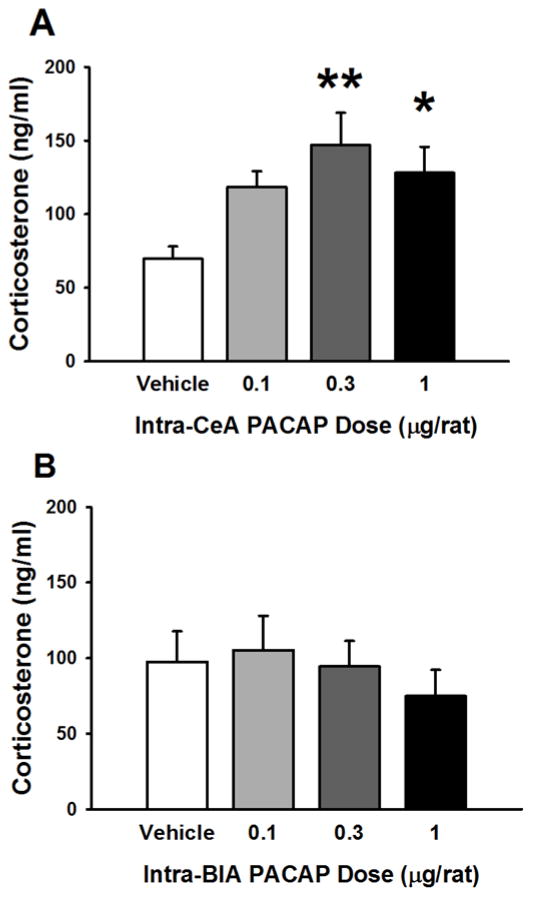
Intra-CeA PACAP treatment caused an increase in plasma levels of corticosterone 30 min after administration (F(3,43)= 3.92, p=0.015), as shown in Fig. 3A; post-hoc analysis showed that the doses of 0.3 and 1 μg/rat were effective in increasing corticosterone levels.
Figure 3.
Effects of PACAP (0–1 μg/rat) microinfused bilaterally either into the central nucleus of the amygdala (CeA, A) or into the basolateral nucleus of the amygdala (BlA, B) on plasma corticosterone levels. N=8–14/group (A), 8–9/group (B). Data represent Mean ± SEM. * p<0.05, ** p<0.01 vs. vehicle group.
In contrast, as shown in Fig. 3B, intra-BlA PACAP treatment did not affect plasma levels of corticosterone 30 min after drug administration (F(3,29)= 0.44, n.s.).
The MC3R/MC4R antagonist SHU-9119 blocks intra-CeA PACAP-induced anxiety-like behavior
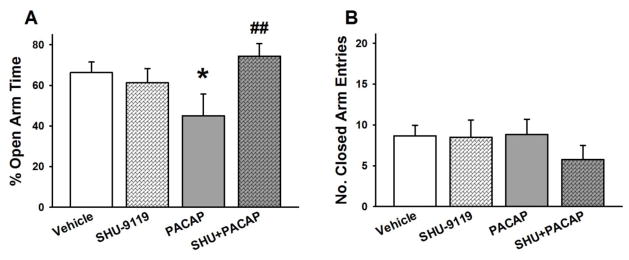
Fig. 4A shows that the reduction of % open arm time (anxiogenic effect) produced by intra-CeA PACAP, which was administered at the most effective dose (0.3 μg/rat), was blocked by intra-CeA pretreatment with the MC3R/MC4R antagonist SHU-9119 (50 pmol/rat) (PACAP x SHU-9119 F(1,25)= 5.75, p<0.05). Post-hoc analysis showed that PACAP-treated animals spent significantly less % time in the open arms compared to the vehicle-treated, while the SHU+PACAP-treated animals did not differ from vehicle-treated animals. As shown in Fig. 4B, PACAP, SHU-9119 or their combination had no effect on the number of closed arm entries (PACAP (F(1,25)= 0.55, n.s.; PACAP × SHU-9119 (F(1,25)= 0.71, n.s.).
Figure 4.
Effects of PACAP (0, 0.3 μg/rat) and the MC3R/MC4R antagonist SHU 9119 (0, 50 pmol/rat, −45 min) microinfused bilaterally into the CeA on the percent (%) of open arm time (A), and the number of closed arm entries (B). N=6–9/group. Data represent Mean ± SEM. * p<0.05 vs. vehicle group; # # p<0.01 vs. PACAP group.
DISCUSSION
The main findings of the present study were as follows: i) PACAP exhibits a dose-dependent anxiogenic-like effect when microinfused into the CeA, but not the BlA, in male rats; ii) PACAP microinfused into the CeA, but not the BlA, elevates plasma corticosterone levels; iii) The anxiogenic effect of intra-CeA PACAP is prevented by local infusion of the melanocortin receptor 3/4 antagonist SHU9119; iv) PAC1R is highly expressed in the CeA where it partially co-localizes with MC4R.
In the elevated plus maze test, rats treated with increasing doses of PACAP injected into the CeA spent a significantly lower percent of time in the open arms compared with vehicle-treated rats, suggesting that drug treatment has an anxiogenic-like effect. Interestingly, the 0.3 μg/rat dose was the most effective one in inducing anxiety-like behavior (44% reduction), which suggests that PACAP at higher doses may lose selectivity. The number of entries in the closed arms, commonly inferred as a measure of motor activity (Cruz et al. 1994), was unaffected by PACAP treatment; this observation confirms that the anxiety-like behavior following PACAP administration into the CeA was not due to a general suppression of motor activity, as we have already shown previously analyzing the number of beam breaks (Iemolo et al. 2015). Importantly, intra-CeA PACAP was able to induce anxiety-like behavior also using a different behavioral task, the defensive withdrawal test. On the other hand, microinfusion of PACAP into the BlA was unable to elicit anxiety-like behavior, suggesting the regional specificity of the observed effects. Even though the hypothesis that doses of PACAP higher than 1 μg/rat may still induce an anxiogenic effect in the BlA cannot be completely ruled out, we consider it unlikely based on published observations that doses of 1 and 3 μg/rat are sufficient to induce anxiety-like and anti-rewarding effects when administered in the entire brain by i.c.v. injection (Seiglie et al. 2015; Telegdy and Adamik 2015). We consider it improbable that the differences in baseline % open arm time between the CeA and the BlA cohorts (~60% vs. ~40%, respectively) may be responsible for the lack of effect of PACAP injected into the BlA; indeed, the BlA % open arm time values under vehicle conditions were still high enough to allow potential reductions to be detected (i.e. a floor effect seems quite unlikely).
Our results are in line with previous studies showing that PACAP and PAC1R receptor knockout mice are less anxious than the wild-type counterparts and show an anxiolytic profile (Hashimoto et al, 2001; Otto et al, 2001). Pharmacological studies have reported that PACAP produces stress-like effects in rats, such as face washing, body grooming, and wet-dog shakes, when injected either intracerebroventricularly (i.c.v.) or into the PVN (Agarwal et al, 2005; Norrholm et al, 2005). When administered into the BNST, PACAP also increases the startle response (Hammack et al, 2009); recently a nociceptive effect of intra-CeA PACAP has been shown, which was associated with an anxiogenic effect (Missig et al. 2014). However, a higher dose of PACAP was used in that study compared to this one.
Altogether, this evidence strongly indicates a critical role for the PACAP system of the CeA in the behavioral response to stress. A high number of PACAP fibers and PAC1R positive cells are found in the CeA (Joo et al. 2004); whether the source of PACAP into the CeA is local or not is currently unknown, even though lesions of the lateral parabrachial nucleus have been shown to attenuate PACAP immunoreactivity in the CeA, suggesting that projections from this region could be responsible for part of the PACAP released in the CeA (Missig et al. 2014). The observation that PAC1R gene expression is elevated in the amygdala of rats following fear-conditioning (Ressler et al, 2011) further suggests a putative role of the endogenous amygdalar PACAP system in anxiety and fear responses. Interestingly, PACAP has been shown to potentiate excitatory transmission in projections from the BlA to the CeA, although those effects were likely mediated by VPAC1 receptor (Cho et al. 2012). Since the present study involved an exogenous administration of PACAP, subsequent studies involving the use of pharmacological antagonists or viral knockdown of the PAC1R will be needed to establish the role of the endogenous system.
Importantly, we found that administration of PACAP into the CeA (but not the BlA) dose-dependently elevated corticosterone levels in the plasma of rats, suggesting that the activation of extrahypothalamic areas can stimulate the HPA axis. Interestingly, while all three doses of intra-CeA PACAP (0.1, 0.3, and 1 μg) produced anxiogenic effects, only the two higher (0.3 and 1 μg) were effective at increasing the levels of corticosterone. The fact that a significant increase of corticosterone could not be detected at the 0.1 μg dose suggests that variability may have played a role in the apparently differential effect observed, even though the hypothesis that CeA PACAP may just be more potent at inducing its behavioral -compared to endocrine- effects cannot be excluded. I.c.v. administration of PACAP is known to activate the HPA axis (Agarwal et al. 2005; Dore et al. 2013a; Norrholm et al. 2005); more recently intra-BNST PACAP was shown to also increase plasma corticosterone levels, although higher doses appear to be necessary to induce a significant increase (Lezak et al. 2014), suggesting that the CeA may be more sensitive compared to the BNST to the HPA axis-activating effects of PACAP.
PACAP has been proposed as a “master regulator” of the stress response, due to its ability to regulate the HPA axis at multiple levels, including the hypothalamic, pituitary, and adrenal glands (Stroth et al. 2011). Indeed, in PACAP knockout mice, restraint stress is unable to increase CRH mRNA in the PVN or corticosterone levels, and in the same animals social defeat induces less PVN activation and a smaller corticosterone rise compared to wild-type mice (Lehmann et al. 2012; Stroth and Eiden 2010). Noteworthy, the CeA is known to regulate the HPA axis function and glucocorticoid release; electrical stimulation of the CeA increases ACTH release, while lesions of CeA attenuate HPA axis responses to immobilization stress (Beaulieu et al. 1987) and the increase in ACTH secretion following adrenalectomy (Allen and Allen 1974), proving that the CeA participates in the regulation of the HPA axis function in response to stressors.
The CeA, like other limbic structures, has a limited number of direct connections with the hypophysiotropic neurons of the PVN, but it is hypothesized to contribute to the disinhibition of the PVN also via intermediary neurons in the BNST and the lateral septum (Sawchenko et al. 1993). An important question is whether the HPA axis activation produced by intra-CeA administration of PACAP is responsible for the observed anxiogenic effect or vice versa whether the endocrine effects are secondary to the effects of the peptide on behavior. We have previously shown that, while the CRF receptor antagonist D-Phe-CRF(12–41) is able to prevent i.c.v. PACAP-induced anxiogenic effect, it however does not block PACAP-induced HPA axis activation, suggesting that PACAP-induced endocrine effects are likely not responsible for its anxiogenic effects (Dore et al. 2013a). Since the present study does not help clarify this aspect (as both HPA axis activation and anxiety-like behavior were investigated 30 min after administration), future studies involving a precise time course after PACAP administration and/or using adrenalectomized animals will be needed to fully answer this question.
Pretreatment with the MC3R/MC4R antagonist SHU9119 successfully prevented the reduction in % open arm time induced by intra-CeA PACAP. The melanocortin system, and in particular the MC4R of the amygdala, plays a key role in the regulation of anxiety-like behavior: activation of the α-MSH/ MC4R system of the amygdala has been shown to produce anxiogenic effects, and the blockade of MC4R to prevent stress-induced anxiety-like behavior (Kokare et al. 2005; Kokare et al. 2010). Interestingly, it has been demonstrated that manipulation of the MC4R in the medial nucleus of the amygdala (MeA) affects anxiety-like behavior and corticosterone levels (Liu et al. 2013). In this regard, because of the volume of PACAP microinfused into the CeA (0.5 μl/side), we cannot rule out that the solution may have spread into neighbor areas, such as the MeA, to exert its effects; future in vivo PACAP studies targeting specifically the MeA will therefore be needed to definitely answer this question. However, it should be noted that lower doses of SHU9119, compared to those described to be required in the MeA, were here able to block the PACAP effects (0.05 nmol vs. 1 nmol), strengthening the notion that the CeA is the site of action.
The melanocortin receptor antagonist used in this study, SHU9119, is widely used as an MC4 receptor antagonist, despite not binding MC4R exclusively; indeed, SHU9119 acts also as an antagonist of MC3R and as an agonist of MC5R (Grieco et al. 2007; Hruby et al. 1995). However, a putative involvement of MC3R in the anxiogenic effects of PACAP can be considered highly unlikely because of the negligible levels of this receptor subtype in the CeA (undetectable by real time PCR, personal observations), in line with previous immunohistochemical and in situ hybridization studies showing that the expression of MC3R is restricted to hypothalamus and brainstem (Liu et al. 2003; Roselli-Rehfuss et al. 1993). In addition, we can also rule out that the SHU9119 blockade of PACAP effects reflects a putative physiological antagonism through activation of MC5R, since in our experiments SHU9119 had per se no effect. Therefore, despite the lack of selectivity of the antagonist used represents a limitation of the current study, based on the above we can confidently conclude that MC4R, rather than MC3R and MC5R, mediates the anxiogenic effects of PACAP.
This mechanism is in line with previous observations showing that central administration of PACAP (but not VIP) activates melanocortinergic neurons, and that the effects of PACAP, both in the hypothalamus and in the CeA, on food intake are mediated by MC4R (Iemolo et al. 2015; Mounien et al. 2006; Mounien et al. 2009). PACAP administered into the CeA has indeed been shown to reduce food intake (Iemolo et al. 2015). One can hypothesize that PACAP-induced hypophagia may be the consequence of the anxiety-like state; however, since the hypophagia shown previously is not evident until three hours after PACAP treatment while anxiety is already 30 min later, we can exclude this explanation.
In this study, we did not confirm that the anxiogenic effects of PACAP are mediated by the PACAP-selective PAC1R rather than VPAC receptors. However, the anxiolytic, rather than anxiogenic, profile of VIP may suggest the exclusive involvement of PAC1R in PACAP-induced anxiety (Ivanova et al. 2014), even though the large experimental differences between this study and the VIP study make any conclusion uncertain. Future experiments will be needed to directly ascertain that the effects of PACAP on anxiety-like behavior and the HPA axis are indeed mediated by PAC1R. In addition, follow-up studies will need to confirm that the PACAP-induced HPA axis activation, like the anxiety-like behavior, is mediated by MC4R activation.
In summary, our results prove that activation of the PACAP system of the CeA induces anxiety-like behavior via MC4R, providing novel insights into this neuropeptide system as a mechanism for modulating the behavioral and endocrine response to stress.
Supplementary Material
Suppl. Fig. 1. Drawing of coronal rats’ brain slices. Dots represent the injection sites in either the CeA (A) or the BlA (C) included in the data analysis. The photomicrographs show coronal sections of rat brains with a representative injection site in either the CeA (B) or the BlA (D).
Suppl. Table 1. Effects of PACAP and SHU9119 (on other measures of the elevated plus maze test. No. OA entries: number of entries in the open arms; No. CA entries: number of entries in the closed arms; % OA entries: % of entries in the open arms compared to total arm time; % C time: % of time spent in the center of the maze. Data represent Mean ± SEM. * p<0.05, ** p<0.01, *** p<0.001 vs. vehicle group.
Suppl. Fig. 2. Effects of PACAP (0–0.3 μg/rat) microinfused bilaterally into the central nucleus of the amygdala (CeA) on the time spent in the withdrawal chamber during the defensive withdrawal test. N= 9–10/group. Data represent Mean ± SEM. * p<0.05 vs. vehicle group.
Acknowledgments
We thank Stephen St. Cyr, Carlos Santiago, Diane Tang, and Angela Tung for technical assistance, Lillian Huang for editorial assistance. This publication was made possible by grant numbers MH093650, MH091945, DA030425, T32GM008541, from the National Institute of Health, by the Peter Paul Career Development Professorship (PC), the Peter McManus Charitable Trust (VS), and by Boston University’s Undergraduate Research Opportunities Program (UROP). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosures
The authors declare no conflict of interest.
References
- Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain research Molecular brain research. 2005;138:45–57. doi: 10.1016/j.molbrainres.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Allen CF. Role of the amygdaloid complexes in the stress-induced release of ACTH in the rat. Neuroendocrinology. 1974;15:220–30. doi: 10.1159/000122310. [DOI] [PubMed] [Google Scholar]
- Beaulieu S, Di Paolo T, Cote J, Barden N. Participation of the central amygdaloid nucleus in the response of adrenocorticotropin secretion to immobilization stress: opposing roles of the noradrenergic and dopaminergic systems. Neuroendocrinology. 1987;45:37–46. doi: 10.1159/000124701. [DOI] [PubMed] [Google Scholar]
- Blasio A, Steardo L, Sabino V, Cottone P. Opioid system in the medial prefrontal cortex mediates binge-like eating. Addiction biology. 2013 doi: 10.1111/adb.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boghossian S, Park M, York DA. Melanocortin activity in the amygdala controls appetite for dietary fat. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298:R385–93. doi: 10.1152/ajpregu.00591.2009. [DOI] [PubMed] [Google Scholar]
- Carvalho MC, Moreira CM, Zanoveli JM, Brandao ML. Central, but not basolateral, amygdala involvement in the anxiolytic-like effects of midazolam in rats in the elevated plus maze. J Psychopharmacol. 2012;26:543–54. doi: 10.1177/0269881110389209. [DOI] [PubMed] [Google Scholar]
- Chaki S, Ogawa S, Toda Y, Funakoshi T, Okuyama S. Involvement of the melanocortin MC4 receptor in stress-related behavior in rodents. European journal of pharmacology. 2003;474:95–101. doi: 10.1016/s0014-2999(03)02033-8. [DOI] [PubMed] [Google Scholar]
- Cho JH, Zushida K, Shumyatsky GP, Carlezon WA, Jr, Meloni EG, Bolshakov VY. Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:14165–77. doi: 10.1523/JNEUROSCI.1402-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proceedings of the National Academy of Sciences of the United States of America. 2009a;106:20016–20. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. FG 7142 specifically reduces meal size and the rate and regularity of sustained feeding in female rats: evidence that benzodiazepine inverse agonists reduce food palatability. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:1069–81. doi: 10.1038/sj.npp.1301229. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2009b;34:38–49. doi: 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–6. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual review of neuroscience. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Annals of the New York Academy of Sciences. 1999;877:281–91. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V. CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013a;38:2160–9. doi: 10.1038/npp.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V. CRF Mediates the Anxiogenic and Anti-Rewarding, but not the Anorectic Effects of PACAP. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013b doi: 10.1038/npp.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of general psychiatry. 2009;66:1361–72. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American journal of psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- Grieco P, Cai M, Han G, Trivedi D, Campiglia P, Novellino E, Hruby VJ. Further structure-activity studies of lactam derivatives of MT-II and SHU-9119: their activity and selectivity at human melanocortin receptors 3, 4, and 5. Peptides. 2007;28:1191–6. doi: 10.1016/j.peptides.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Schwartz MW, Yagaloff KA, Burn P, Woods SC, Seeley RJ. Role of the CNS melanocortin system in the response to overfeeding. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:2362–7. doi: 10.1523/JNEUROSCI.19-06-02362.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, Eiden LE. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:461–6. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–43. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, al-Obeidi FA, Hadley ME, Cone RD. Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7,Lys10] alpha-melanocyte-stimulating hormone-(4–10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. Journal of medicinal chemistry. 1995;38:3454–61. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- Iemolo A, Blasio A, St Cyr SA, Jiang F, Rice KC, Sabino V, Cottone P. CRF-CRF1 Receptor System in the Central and Basolateral Nuclei of the Amygdala Differentially Mediates Excessive Eating of Palatable Food. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:2456–66. doi: 10.1038/npp.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Ferragud A, Cottone P, Sabino V. Pituitary Adenylate Cyclase-Activating Peptide in the Central Amygdala Causes Anorexia and Body Weight Loss via the Melanocortin and the TrkB Systems. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova M, Belcheva S, Belcheva I, Stoyanov Z, Tashev R. Modulatory effect of VIP injected into hippocampal CA1 area on anxiety in olfactory bulbectomized rats. Acta neurobiologiae experimentalis. 2014;74:317–27. doi: 10.55782/ane-2014-1997. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xing G, Yang C, Verma A, Zhang L, Li H. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:410–23. doi: 10.1038/npp.2008.71. [DOI] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. The Journal of comparative neurology. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:5506–15. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park MH, Wilson WA, Moore SD. Differential actions of diazepam and zolpidem in basolateral and central amygdala nuclei. Neuropharmacology. 2004;46:1–9. doi: 10.1016/s0028-3908(03)00340-x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokare DM, Dandekar MP, Chopde CT, Subhedar N. Interaction between neuropeptide Y and alpha-melanocyte stimulating hormone in amygdala regulates anxiety in rats. Brain research. 2005;1043:107–14. doi: 10.1016/j.brainres.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Dandekar MP, Singru PS, Gupta GL, Subhedar NK. Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology. 2010;58:1009–18. doi: 10.1016/j.neuropharm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM. Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast. 2007;2007:79102. doi: 10.1155/2007/79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Mustafa T, Eiden AM, Herkenham M, Eiden LE. PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak KR, Roelke E, Harris OM, Choi I, Edwards S, Gick N, Cocchiaro G, Missig G, Roman CW, Braas KM, Toufexis DJ, May V, Hammack SE. Pituitary adenylate cyclase-activating polypeptide (PACAP) in the bed nucleus of the stria terminalis (BNST) increases corticosterone in male and female rats. Psychoneuroendocrinology. 2014;45:11–20. doi: 10.1016/j.psyneuen.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:7143–54. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Garza JC, Li W, Lu XY. Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. The international journal of neuropsychopharmacology/ official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:105–20. doi: 10.1017/S146114571100174X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. 2007;148:5531–40. doi: 10.1210/en.2007-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:7863–72. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missig G, Roman CW, Vizzard MA, Braas KM, Hammack SE, May V. Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain. Neuropharmacology. 2014;86:38–48. doi: 10.1016/j.neuropharm.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounien L, Bizet P, Boutelet I, Gourcerol G, Fournier A, Vaudry H, Jegou S. Pituitary adenylate cyclase-activating polypeptide directly modulates the activity of proopiomelanocortin neurons in the rat arcuate nucleus. Neuroscience. 2006;143:155–63. doi: 10.1016/j.neuroscience.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J, Vaudry H, Jegou S. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:424–35. doi: 10.1038/npp.2008.73. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Das M, Legradi G. Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN) Regulatory peptides. 2005;128:33–41. doi: 10.1016/j.regpep.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain research Molecular brain research. 2001;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat brain in stereotaxic coordinates. 6. Academic press; 2007. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. In: Connectivity of the rat amygdaloid complex. JPA, editor. The Amygdala: Oxford University Press; 2000. [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–7. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseberry AG. Altered feeding and body weight following melanocortin administration to the ventral tegmental area in adult rats. Psychopharmacology (Berl) 2013;226:25–34. doi: 10.1007/s00213-012-2879-6. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8856–60. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Mul JD, Clemmensen C, Egan AE, Begg DP, Halcomb K, Seeley RJ, Herman JP, Ulrich-Lai YM. Loss of melanocortin-4 receptor function attenuates HPA responses to psychological stress. Psychoneuroendocrinology. 2014;42:98–105. doi: 10.1016/j.psyneuen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Steardo L, Schmidhammer H, Zorrilla EP. 14-Methoxymetopon, a highly potent mu opioid agonist, biphasically affects ethanol intake in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2007;192:537–46. doi: 10.1007/s00213-007-0746-7. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L, Jr, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP. The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:1482–93. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Foundation symposium. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. discussion 21–9. [DOI] [PubMed] [Google Scholar]
- Seiglie MP, Smith KL, Blasio A, Cottone P, Sabino V. Pituitary adenylate cyclase-activating polypeptide induces a depressive-like phenotype in rats. Psychopharmacology (Berl) 2015;232:3821–31. doi: 10.1007/s00213-015-4045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165:1025–30. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Holighaus Y, Ait-Ali D, Eiden LE. PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response. Annals of the New York Academy of Sciences. 2011;1220:49–59. doi: 10.1111/j.1749-6632.2011.05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telegdy G, Adamik A. Neurotransmitter-mediated anxiogenic action of PACAP-38 in rats. Behavioural brain research. 2015;281:333–8. doi: 10.1016/j.bbr.2014.12.039. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–62. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Vergoni AV, Bertolini A, Wikberg JE, Schioth HB. Selective melanocortin MC4 receptor blockage reduces immobilization stress-induced anorexia in rats. European journal of pharmacology. 1999;369:11–5. doi: 10.1016/s0014-2999(99)00045-x. [DOI] [PubMed] [Google Scholar]
- Yamano Y, Yoshioka M, Toda Y, Oshida Y, Chaki S, Hamamoto K, Morishima I. Regulation of CRF, POMC and MC4R gene expression after electrical foot shock stress in the rat amygdala and hypothalamus. The Journal of veterinary medical science/ the Japanese Society of Veterinary Science. 2004;66:1323–7. doi: 10.1292/jvms.66.1323. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Solati J, Oryan S, Parivar K. Effect of intra-amygdala injection of nicotine and GABA receptor agents on anxiety-like behaviour in rats. Pharmacology. 2008;82:276–84. doi: 10.1159/000161129. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain research. 2002;952:188–99. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1. Drawing of coronal rats’ brain slices. Dots represent the injection sites in either the CeA (A) or the BlA (C) included in the data analysis. The photomicrographs show coronal sections of rat brains with a representative injection site in either the CeA (B) or the BlA (D).
Suppl. Table 1. Effects of PACAP and SHU9119 (on other measures of the elevated plus maze test. No. OA entries: number of entries in the open arms; No. CA entries: number of entries in the closed arms; % OA entries: % of entries in the open arms compared to total arm time; % C time: % of time spent in the center of the maze. Data represent Mean ± SEM. * p<0.05, ** p<0.01, *** p<0.001 vs. vehicle group.
Suppl. Fig. 2. Effects of PACAP (0–0.3 μg/rat) microinfused bilaterally into the central nucleus of the amygdala (CeA) on the time spent in the withdrawal chamber during the defensive withdrawal test. N= 9–10/group. Data represent Mean ± SEM. * p<0.05 vs. vehicle group.