Abstract
Background
Clinical activity and quality of life (QOL) indices assess disease activity in Crohn’s disease (CD) and ulcerative colitis (UC). However, a paucity of data exists on the validity of these indices according to disease characteristics.
Aims
To examine the correlation between QOL and clinical activity indices and endoscopic disease activity according to disease characteristics.
Methods
We used a prospective registry to identify CD and UC patients ≥18 years old with available information on Short Inflammatory Bowel Disease Questionnaire scores (SIBDQ), Harvey–Bradshaw Index (HBI) and simple endoscopic scores for CD (SES-CD), and Simple Clinical Colitis Activity Index (SCCAI) and Mayo endoscopic score for UC. We used Spearman rank correlations to calculate correlations between indices and Fisher transformation to compare correlations across disease characteristics.
Results
Among 282 CD patients, we observed poor correlation between clinical activity and QOL indices to SES-CD with no differences in correlation according to disease characteristics. Conversely, among 226 UC patients, clinical activity and QOL had good correlation to Mayo endoscopic score (r = 0.55 and −0.56, respectively) with better correlations observed with left-sided versus extensive colitis (r = 0.73 vs. 0.45, p = 0.005) and shorter duration of disease (r = 0.61 vs. 0.37, p = 0.04).
Conclusions
Our data suggest good correlation between SCCAI and endoscopic disease activity in UC, particularly in left-sided disease. Poor correlations between HBI or SIBDQ and SES-CD appear to be consistent across different disease phenotypes.
Keywords: Crohn’s disease, Ulcerative colitis, Quality of life, Inflammatory bowel disease
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC), collectively known as inflammatory bowel diseases (IBD), are chronic inflammatory disorders of the gastrointestinal tract with heterogeneous disease presentation and natural history. Genome wide association studies have identified over 160 risk loci associated with IBD [1] with distinct genetic profiles associated with specific IBD phenotypes [2, 3]. In addition, a number of disease characteristics including location and behavior, age of onset, and disease duration are associated with different outcomes and response to therapy [4–6]. As a result, the management of IBD will continue to move toward personalized care accounting for the patient’s unique genetic, clinical, and environmental background [7].
Persistent, active intestinal inflammation in IBD can severely impair quality of life and lead to increased hospitalization and surgical rates [8, 9]. Ideally the goal of therapy is deep remission, a combination of endoscopic and clinical remission, which is associated with improved patient outcomes [10]. However, endoscopic evaluation is not always feasible due to its invasive nature, burden to patients, expense, the risk of complications, and the possibility the site of active disease may not be reached. As a result, noninvasive biochemical markers, clinical activity indices (CAIs), and health-related quality of life (HRQOL) scores have been used as surrogate markers for disease remission.
While a number of prior studies have demonstrated good correlations between clinical activity or HRQOL scores and endoscopic disease activity or inflammatory markers, particularly in UC [11–13], others have shown fair to poor correlation in assessing luminal CD [14–16]. The subjective nature of these indices may explain their poor performance in some studies, but the degree to which disease heterogeneity contributes to this variability is unclear. Although a unique set of CAIs have been developed for pediatric-onset IBD [17, 18], similar disease activity indices are used in adults regardless of age of onset or disease characteristics.
As management of IBD moves toward more personalized care, the validity of current disease activity and HRQOL indices according to various disease phenotypes may be critical in development of unique patient-reported outcome measures. We therefore sought to examine the validity of current CAI and HRQOL indices as they relate to endoscopic disease activity according to various disease phenotypes. We hypothesize that the correlations between clinical activity or HRQOL scores and endoscopic disease activity vary according to disease phenotypes.
Materials and Methods
Study Population
Starting in 2004, adult patients, age ≥18 years, with a diagnosis of CD or UC were recruited in the Prospective Registry in IBD Study at Massachusetts General Hospital. At the time of recruitment, detailed information on disease characteristics according to the Montreal classification, lifestyle factors including smoking, body mass index, and dietary factors, along with other comorbid conditions was collected. For this study, patients with available clinical and endoscopic activity indices measured within 1 month of each other were eligible for primary analyses. The institutional review board at the Massachusetts General Hospital approved this study.
Assessment of Clinical and Endoscopic Activity Indices
In a subset of participants, information on clinical activity indices—the Harvey–Bradshaw Index (HBI) for CD and the Simple Clinical Colitis Activity Index (SCCAI) for UC—and quality of life scores in the form of the Short IBD Questionnaire (SIBDQ) were collected by research coordinators and further confirmed by the treating gastroenterologists. We also retrospectively reviewed endoscopic examinations performed within 1 month of collection of clinical activity indices. Two physicians calculated simple endoscopic scores CD (SES-CD) and Mayo endoscopic score for UC through review of endoscopic images and reports.
Assessment of Disease Characteristics and Other Covariates
Information on age of diagnosis, disease behavior (inflammatory, penetrating, stricturing, and perianal disease), disease location (ileal, colonic, and ileocolonic) for CD or disease extent (proctitis, left-sided, and extensive) for UC, previous surgeries for CD, duration of disease, and ever use of IBD-related medications (steroids and biologics) were collected and confirmed by review of medical records and further verified by primary gastroenterologists. The retrospective nature of the study prevented accurate assessment of medication use at the time of clinical activity and quality of life scoring. In addition, we obtained inflammatory markers (ESR and CRP) at the time of ascertainment of clinical activity indices.
Statistical Analysis
We used Spearman rank correlation to calculate the relatedness between endoscopic scores and CAI scores, quality of life (QOL) scores, and CRP and ESR levels. We defined correlations <0.3 as poor, 0.3–0.7 as good, and >0.7 as excellent [19]. We also calculated correlations according to the strata defined by age of diagnosis (17–39, 40–59, ≥60), disease behavior, disease location or extent, disease duration (<10 or ≥10 years), inflammatory markers (above or below upper the limit of normal), and surgical history. The stratified correlation coefficients were then compared using a Fisher r-to-z transformation [20, 21]. All analyses were conducted using R statistical software. All p values were two-sided, and the threshold for statistical significance was set at 0.05. However, we used a Bonferroni correction to adjust for multiple comparisons in analyses that compared correlations according to disease phenotype with the threshold for statistical significance set at 0.017.
Results
In total, 282 CD and 226 UC cases with available information on endoscopic disease activity and CAIs were eligible for our study (Table 1). The mean age at diagnosis for CD and UC patients was 30 and 33 years, respectively. At the time of enrollment, the mean duration of disease for CD and UC was 9 and 10 years, respectively. Among CD patients, 72 % had some ileal involvement while 28 % had isolated colonic disease. Over half of CD participants had either stricturing or penetrating disease at the time of enrollment. Among UC patients, 55 % had extensive disease and just 11 % had proctitis.
Table 1.
Clinical characteristics of participants according to disease type
| Disease type | ||
|---|---|---|
| Crohn’s disease N = 283 |
Ulcerative colitis N = 227 |
|
| Age of diagnosis, mean (std) | 30 (11) | 34 (14) |
| Gender, n (%) | ||
| Female | 166 (59) | 97 (43) |
| Race, n (%) | ||
| White | 269 (95) | 213 (94) |
| Duration at endoscopy, mean (std) | 10 (9) | 9 (9) |
| Location, n (%) | ||
| TI | 80 (28) | N/A |
| Colon | 77 (27) | N/A |
| Ileocolonic | 126 (45) | N/A |
| Extent, n (%) | ||
| Proctitis | N/A | 25 (11) |
| Left-sided | N/A | 76 (33) |
| Extensive | N/A | 126 (56) |
| Behavior, n (%) | ||
| Inflammatory | 137 (48) | N/A |
| Stricturing | 61 (22) | N/A |
| Penetrating | 85 (30) | N/A |
| Perianal | 60 (21) | N/A |
| Steroid use, n (%) | ||
| Ever | 224 (79) | 177 (78) |
| Biologic use, n (%) | ||
| Ever | 135 (48) | 69 (30) |
HBI Harvey–Bradshaw Index, SIBDQ Short IBD Questionnaire, CRP C-reactive protein, ESR erythrocyte sedimentation rate
In a small validation study (N = 20) of endoscopic scoring, the inter-observer correlations for Mayo endoscopic score and SES-CD were 0.95 and 0.98, respectively. In addition, in a subset of patients where endoscopic activity scores also were documented by treating gastroenterologists, the correlations between endoscopic activity scores reported by the treating gastroenterologists and those reported by the two reviewers were consistently greater than 0.90.
There was poor correlation between CD simple endoscopic score (SES-CD) to SIBDQ (r = −0.16) and HBI (r = 0.18) and good correlation to ESR (r = 0.30) and CRP (r = 0.39) (Table 2). We observed good correlation between the Mayo endoscopic score to SIBDQ (r = −0.56), SCCAI (r = 0.55), ESR (r = 0.33), and CRP (r = 0.32).
Table 2.
Comparisons of clinical activity indices, quality of life scores, and inflammatory makers with endoscopic activity indices
| Disease type | ||
|---|---|---|
| Crohn’s disease SES |
Ulcerative colitis Mayo |
|
| SIBDQ, n | 207 | 161 |
| Spearman correlation, rho (p value) | −0.16 (0.02) | −0.56 (<0.001) |
| HBI, n | 247 | N/A |
| Spearman correlation, rho (p value) | 0.18 (0.005) | |
| SCCAI, n | 199 | |
| Spearman correlation, rho (p value) | N/A | 0.55 (<0.001) |
| ESR, n | 150 | 121 |
| Spearman correlation, rho (p value) | 0.30 (<0.001) | 0.34 (<0.001) |
| CRP, n | 148 | 124 |
| Spearman correlation, rho (p value) | 0.39 (<0.001) | 0.46 (<0.001) |
Bold values indicate statistical significance (p < 0.05)
SCCAI Simple Colitis Clinical Activity Index, SIBDQ Short IBD Questionnaire, CRP C-reactive protein, ESR erythrocyte sedimentation rate
Disease Location and Behavior
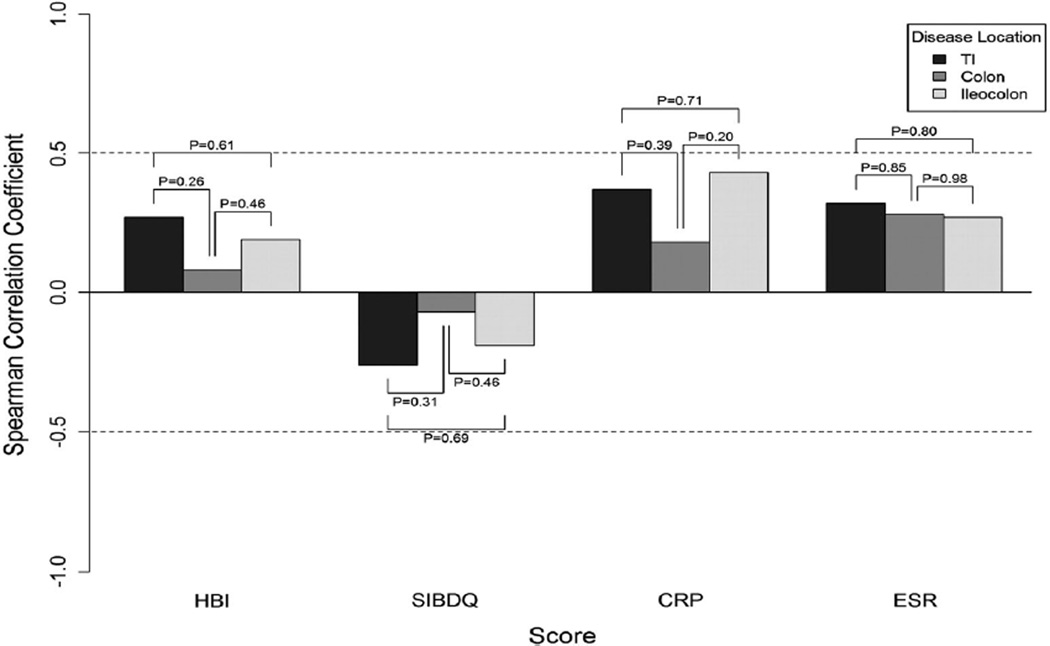
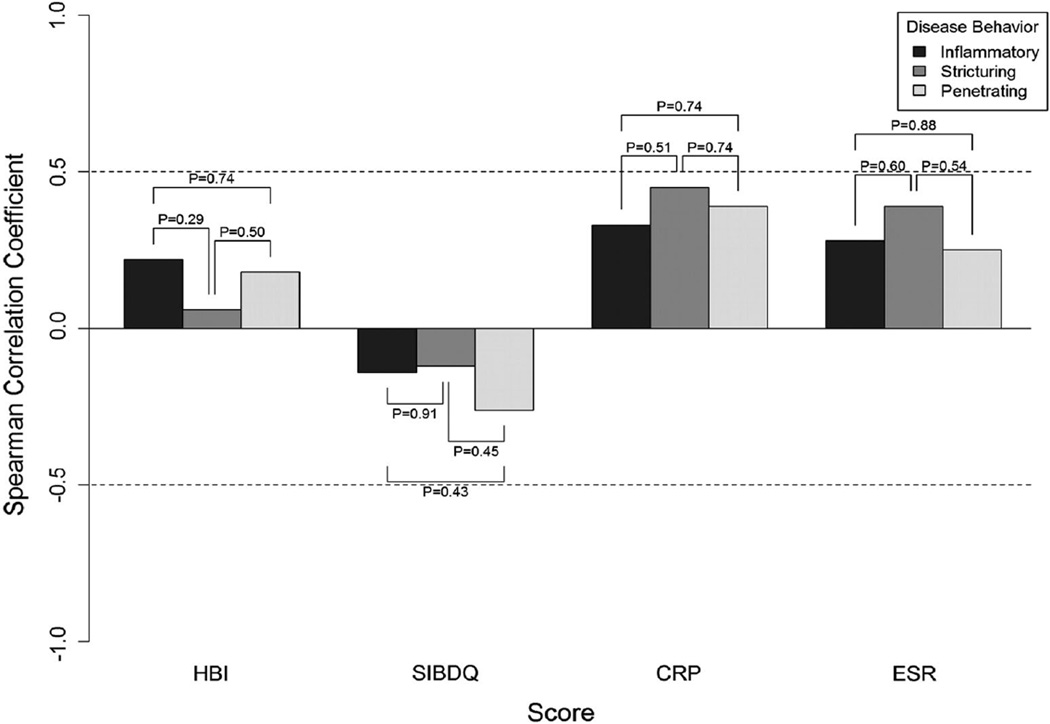
Although the correlations between HBI and SES-CD appeared to be better in ileal CD compared to colonic or ileocolonic disease, these comparisons did not reach statistical significance (Fig. 1). Similarly, the correlations between SES-CD and SIBDQ according to disease behavior were not significantly different (Fig. 2). We observed a similar pattern when comparing SES-CD to inflammatory markers according to disease location.
Fig. 1.
Correlations between endoscopic activity and indirect disease measures according to Crohn’s disease location
Fig. 2.
Correlations between endoscopic activity and indirect disease measures according to Crohn’s disease behavior
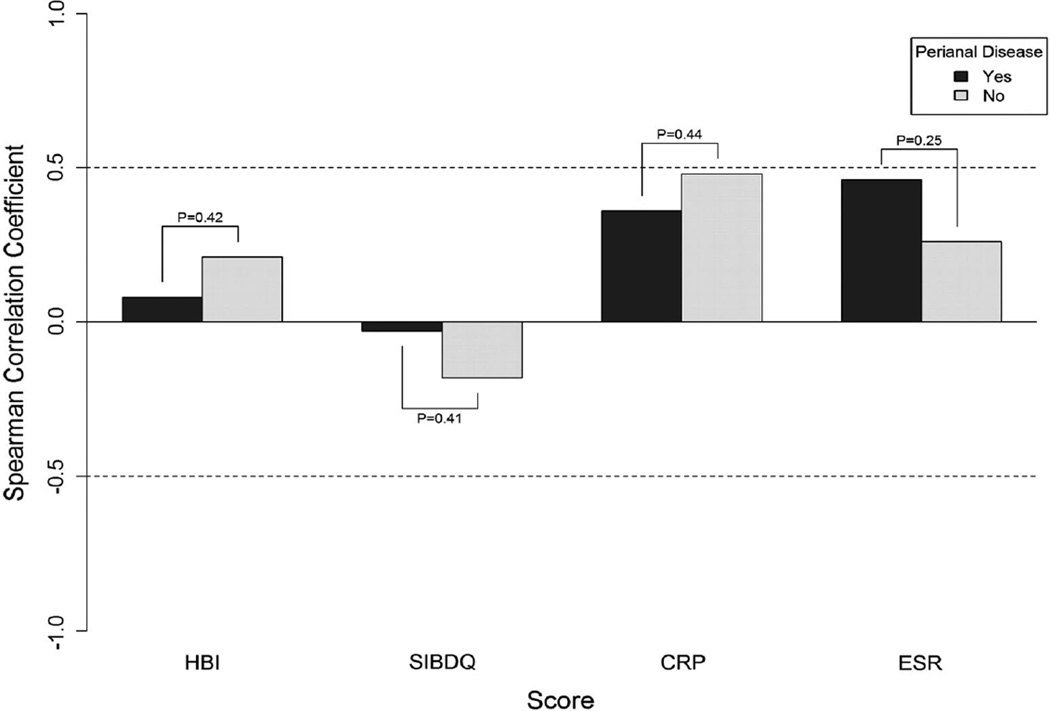
We also explored the correlations between CAI or QOL indices and inflammatory markers to SES-CD according to disease behavior and observed no significant differences (Fig. 2). Specifically, the SES-CD had poor correlations with HBI, SIBDQ, and ESR and good correlations to CRP regardless of disease behavior. Finally, presence of perianal disease was not associated with any differences in the correlations between CAIs, SIBDQ, or ESR/CRP and SES-CD (Fig. 3).
Fig. 3.
Correlations between endoscopic activity and indirect disease measures according to presence or absence of perianal disease
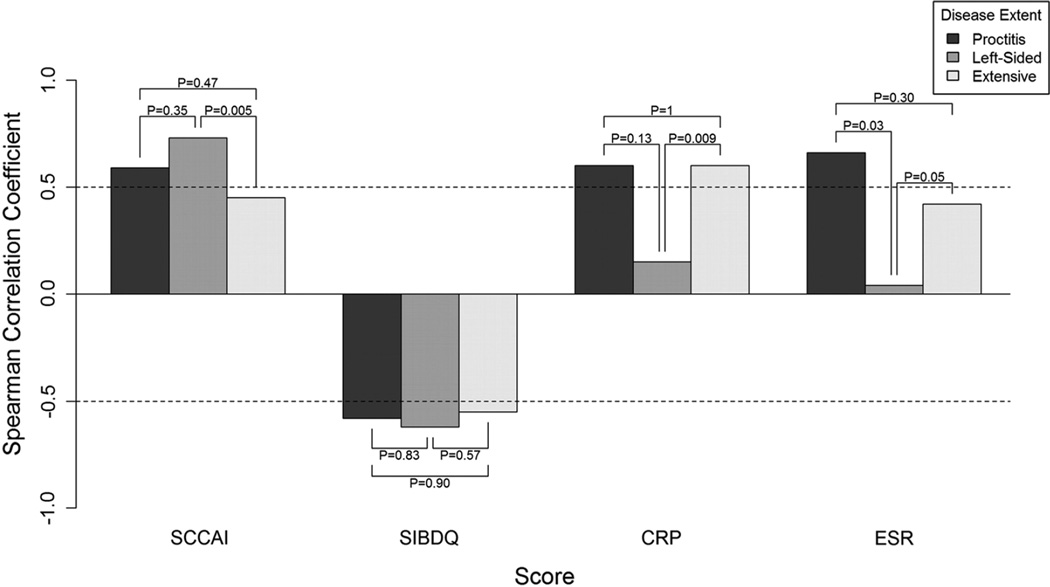
In UC, compared to extensive disease, we observed a better correlation between SCCAI and Mayo endoscopic score in left-sided colitis (r = 0.73 vs. 0.45, pcomparison = 0.005) (Fig. 4). The correlations between SIBDQ and Mayo endoscopic score were good across different disease locations with no statistically significant difference between the correlations. CRP and ESR values appeared to correlate better with endoscopic score in extensive colitis compared to left-sided colitis (pcomparison = 0.006 and 0.05, respectively).
Fig. 4.
Correlations between endoscopic activity and indirect disease measures according to ulcerative colitis extent
Age at Diagnosis
In UC, SCCAI and SIBDQ had good correlations with the Mayo endoscopic score and were not significantly different according to age of onset (S1). In CD, we observed consistently poor correlations between clinical and HRQOL to endoscopic activity indices for patients <60 years old and good correlation for those ≥60 years with all comparisons being statistically insignificant (S1).
Surgical History and Disease Duration
In CD, the correlations between CRP and SES-CD were good regardless of surgical history (S2). Similarly, correlation between HBI and SIBDQ to SES-CD was poor regardless of prior surgery (S2).
We also explored whether the correlation between clinical and endoscopic activity indices differ according to disease duration (S3). HBI and SIBDQ had poor to good correlation to SES-CD, while inflammatory markers correlated well with SES-CD regardless of disease duration. In UC, SCCAI correlates better among patients with less than 10 years of disease duration versus longer duration of disease (r = 0.61 vs. 0.37, pcomparison = 0.04). SIBDQ had good correlation with endoscopic disease activity, and this was independent of disease duration. Inflammatory markers had good correlation with endoscopic disease activity, irrespective of disease duration.
Discussion
In this large cross-sectional study, we show that in CD, HBI and SIBDQ overall correlate poorly to endoscopic disease activity regardless of age at diagnosis, disease location and behavior, duration of disease, and surgical history. In UC, although there is a good correlation between SCCAI and SIBDQ to endoscopic disease activity overall, the correlations appeared to be stronger with left-sided UC and individuals with shorter duration of disease. This is the first study to substantiate the use of HRQOL and clinical activity indices among different IBD phenotypes according to previously validated endoscopic activity markers.
Despite the variability of clinical presentations and outcomes of CD and UC patients, for the most part in our study the validity of noninvasive measures of disease activity as they relate to endoscopic disease activity is not significantly different according to disease behavior and location and age of onset. This may suggest a couple of different possibilities. First, clinical activity and HRQOL scores in IBD may be insufficiently sensitive to distinguish among different IBD phenotypes. Second, endoscopic disease activity may not be an accurate measure of complete disease remission, particularly with regard to CD where active disease may be located beyond the reach of endoscopic evaluation. In UC, histological remission is associated with less disease relapse and lower colorectal cancer risk when compared to endoscopic remission, suggesting that microscopic remission may be a more accurate predictor of disease outcome [22]. In CD, a transmural process, mucosal biopsies may not be a sufficient indication of “true remission.”
Traditionally, biochemical parameters and other clinical activity scores have validated HRQOL and other clinical activity scores [23, 24], though more recently correlation with mucosal assessment has become standard [25, 26]. This appears to be a more clinically relevant comparison as the goal of IBD therapy is mucosal healing, which in turn is associated with durable remission and decreased risk of surgery [27]. Consistent with previous work [14, 15, 25], in our study, SIBDQ and HBI correlated poorly with endoscopic disease activity in CD. When examining the relationship between SIBDQ and endoscopic disease activity, Casellas et al. [25] reported a correlation of −0.31, similar to the −0.17 in this study. However, they did not use validated endoscopic disease scores for either CD or UC. The SES-CD and the Mayo endoscopic score in UC, used in the current study, appear to be most reliable measures of endoscopic disease assessment [28, 29]. In UC, our finding was consistent with others showing good correlation between SIBDQ and SCCAI with endoscopic disease activity [30].
HRQOL scores and CRP have been evaluated according to various disease characteristics in CD. A European study of 189 CD patients found no difference between patients based on the Vienna classification when measured by the Psychological General Well-Being Index, the EuroQol, and the IBD Questionnaire [31]. SIBDQ has been measured according to disease phenotype in patients developing CD after ileal-pouch anal anastomosis for UC, and no difference was found between various phenotypes [32]. In CD, after excluding fibrostenotic disease, elevated CRP levels have been more commonly associated with colonic and ileocolonic disease than ileal disease [33], though this finding has not been consistent in all IBD studies [34]. Additionally, there was no statistically significant difference between high sensitivity-CRP and disease behavior [33]. Our study did not show that patient heterogeneity effected HRQOL and CRP. Additionally, unlike previous analysis, we also evaluated the validity of CRP and HRQOL scores among older-onset (age ≥60) disease as recent data suggests that the elderly may have a less aggressive disease course [35]. Variations in prior studies in CRP may be related to the wide ranges of cutoff values used in IBD [36, 37], and currently there is no accepted cutoff value. A cutoff value of 10 mg/L has been proposed for high sensitivity-CRP at diagnosis as a predictor of disease exacerbation [33].
Recently, the FDA has mandated that clinical trial endpoints move away from using CAIs and toward endoscopic disease assessment and patient-reported outcome measures [38]. These measures are expected to address the gaps of previously utilized outcomes and highlight the patient experience. Although several IBD patient-reported outcome measures have been proposed [39, 40] or served as endpoints in clinical trials [10], none have been validated or correlated with endoscopic disease activity. Kappelmen et al. used the patient-reported outcome measure information system (PROMIS), developed by the National Institute of Health, in a longitudinal and cross-sectional study. In analyzing over 10,000 patients, they found a statistically significant association between PROMIS and increasing SIBDQ, short Crohn’s disease activity indices, and SCCAI. Additionally, short disease duration (<1 year) was associated with the highest anxiety and depression in CD and the highest anxiety and fatigue in UC independent of disease activity. Also, for most outcome measures, age >60 was associated with better outcomes than patients between the ages of 18–30 [40]. In this study, often the differences between outcomes among the different phenotypes were small suggesting patient-reported outcome measures may be more sensitive to subtle differences between patient phenotypes than currently used HRQOL and disease activity measures.
Various PROMIS scores are associated with disease activity and QOL scores in IBD [40]. However, since QOL and disease activity scores often do not correlate well with endoscopic findings, the accuracy of patient outcome measures in predicting active intestinal inflammation is unclear. If further studies reveal little to no correlation between patient-reported outcomes and mucosal healing, appropriate medical management in patients will require an effective balance between these measures.
We acknowledge several limitations. First, our participants were from a single tertiary IBD center and therefore our results may not be generalizable to other populations. However, the characteristics of participants in our study including age of diagnosis, disease location, and rates of complications were similar to previous natural history studies of large population-based cohorts [41, 42]. Second, the number of participants in some of the categories of disease location, behavior, and age of diagnosis was small limiting the precision of our estimates. However, to date our study represents one of the largest and most comprehensive studies attempting to examine the validity of clinical indices according to various disease characteristics. In addition, we were able to examine the validity of clinical indices in older-onset patients (age ≥60 years), an advantage over prior studies [43]. Further studies should be done to confirm our findings. Third, over 90 % of patients in the study were Caucasian, so our results may not extend to other races or ethnicities. Though a more aggressive disease course in African-Americans was suggested previously [44], further analysis has revealed that these differences were likely due to socioeconomic status and access to health care [45]. A recent study showed no difference in perioperative complication rates between Hispanics and non-Hispanics [46]. Furthermore, HRQOL scores and the HBI in CD have been validated in African-Americans and remain similar to Caucasians during the course of disease [45]. Finally, we acknowledge the subjective nature of retrospective evaluation of endoscopic disease activity. However, in our small validation study, the correlations between the two reviewers and between the reviewers and the endoscopists were excellent.
Accurate identification of active intestinal disease in patients with varying phenotypes is a key to early and effective therapy. While quality of life and clinical activity scores reflect endoscopic disease activity in UC, they perform poorly in CD. The utility of HBI, SCCAI, and SIBDQ in patients appears to be the same according to various disease phenotypes. Further studies are necessary to validate other indices and newly developed IBD patient-reported outcome measures based on various phenotypes.
Supplementary Material
Acknowledgments
ST was involved in the study concept and design, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the manuscript. KOS was involved in the statistical analysis and drafting of the manuscript. DKL and PS were involved in acquisition of data. DSP was involved in the study concept and design and critical revision of the manuscript for important intellectual content. HCS, DN, VY, ANN were involved acquisition of data and critical revision of the manuscript for important intellectual content. HK was involved in the study concept and design, analysis and interpretation of the data, and critical revision of the manuscript.
Funding This work is supported by a career development award from the American Gastroenterological Association (AGA) and the National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK099681 to HK) along with a grant from the National Institutes of Health (K23 DK097142 to ANN).
Dr. Ananthakrishnan is a member of the scientific advisory board for Exact Sciences, AbbVie, and Cubist pharmaceuticals. Dr. Khalili has received consultant fee from AbbVie. Dr. Yajnik has received consulting fees from NPS, Janssen Pharmaceuticals, and UCB.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10620-016-4180-8) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest None of the authors had any personal disclosures.
References
- 1.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso A, Domenech E, Julia A, et al. Identification of risk loci for Crohn’s disease phenotypes using a genome-wide association study. Gastroenterology. 2015;148:794–805. doi: 10.1053/j.gastro.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 3.Ho GT, Nimmo ER, Tenesa A, et al. Allelic variations of the multidrug resistance gene determine susceptibility and disease behavior in ulcerative colitis. Gastroenterology. 2005;128:288–296. doi: 10.1053/j.gastro.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Veloso FT, Ferreira JT, Barros L, Almeida S. Clinical outcome of Crohn’s disease: analysis according to the Vienna classification and clinical activity. Inflamm Bowel Dis. 2001;7:306–313. doi: 10.1097/00054725-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J Crohn’s Colitis. 2013;7:213–221. doi: 10.1016/j.crohns.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Hoie O, Wolters F, Riis L, et al. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007;102:1692–1701. doi: 10.1111/j.1572-0241.2007.01265.x. [DOI] [PubMed] [Google Scholar]
- 7.Gerich ME, McGovern DP. Towards personalized care in IBD. Nat Rev Gastroenterol Hepatol. 2014;11:287–299. doi: 10.1038/nrgastro.2013.242. [DOI] [PubMed] [Google Scholar]
- 8.Casellas F, Lopez-Vivancos J, Casado A, Malagelada JR. Factors affecting health related quality of life of patients with inflammatory bowel disease. Qual Life Res. 2002;11:775–781. doi: 10.1023/a:1020841601110. [DOI] [PubMed] [Google Scholar]
- 9.Pineton de Chambrun G, Peyrin-Biroulet L, Lemann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 10.Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:414–422. doi: 10.1016/j.cgh.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 12.Voiosu T, Bengus A, Dinu R, et al. Rapid fecal calprotectin level assessment and the SIBDQ score can accurately detect active mucosal inflammation in IBD patients in clinical remission: a prospective study. J Gastrointest Liver Dis JGLD. 2014;23:273–278. doi: 10.15403/jgld.2014.1121.233.thv. [DOI] [PubMed] [Google Scholar]
- 13.Dhanda AD, Creed TJ, Greenwood R, Sands BE, Probert CS. Can endoscopy be avoided in the assessment of ulcerative colitis in clinical trials? Inflamm Bowel Dis. 2012;18:2056–2062. doi: 10.1002/ibd.22879. [DOI] [PubMed] [Google Scholar]
- 14.af Björkesten CG, Nieminen U, Turunen U, Arkkila P, Sipponen T, Färkkilä M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol. 2012;47:528–537. doi: 10.3109/00365521.2012.660542. [DOI] [PubMed] [Google Scholar]
- 15.Falvey JD, Hoskin T, Meijer B, et al. Disease activity assessment in IBD: clinical indices and biomarkers fail to predict endoscopic remission. Inflamm Bowel Dis. 2015;21:824–831. doi: 10.1097/MIB.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 16.Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 17.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447. [PubMed] [Google Scholar]
- 18.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–432. doi: 10.1053/j.gastro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. 5th. Boston, MA: Houghton Mifflin; 2003. [Google Scholar]
- 20.Fisher RA. Biometrika. Eugen Rev. 1916;8:62–64. [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher RA. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika. 1925;10:507–521. [Google Scholar]
- 22.Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol. 2014;12:929–934. doi: 10.1016/j.cgh.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s Relapse Prevention Trial Study Group. Gastroenterology. 1994;106:287–296. doi: 10.1016/0016-5085(94)90585-1. [DOI] [PubMed] [Google Scholar]
- 24.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 25.Casellas F, Alcalá M-J, Prieto L, Miró J-RA, Malagelada J-R. Assessment of the influence of disease activity on the quality of life of patients with inflammatory bowel disease using a short questionnaire. Am J Gastroenterol. 2004;99:457–461. doi: 10.1111/j.1572-0241.2004.04071.x. [DOI] [PubMed] [Google Scholar]
- 26.Seo M, Okada M, Maeda K, Oh K. Correlation between endoscopic severity and the clinical activity index in ulcerative colitis. Am J Gastroenterol. 1998;93:2124–2129. doi: 10.1111/j.1572-0241.1998.00607.x. [DOI] [PubMed] [Google Scholar]
- 27.Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 28.Khanna R, Bouguen G, Feagan BG, et al. A systematic review of measurement of endoscopic disease activity and mucosal healing in Crohn’s disease: recommendations for clinical trial design. Inflamm Bowel Dis. 2014;20:1850–1861. doi: 10.1097/MIB.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 29.Samaan MA, Mosli MH, Sandborn WJ, et al. A systematic review of the measurement of endoscopic healing in ulcerative colitis clinical trials: recommendations and implications for future research. Inflamm Bowel Dis. 2014;20:1465–1471. doi: 10.1097/MIB.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 30.Ricanek P, Brackmann S, Perminow G, et al. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011;46:1081–1091. doi: 10.3109/00365521.2011.584897. [DOI] [PubMed] [Google Scholar]
- 31.Casellas F, Vivancos JL, Sampedro M, Malagelada J-R. Relevance of the phenotypic characteristics of Crohn’s disease in patient perception of health-related quality of life. Am J Gastroenterol. 2005;100:2737–2742. doi: 10.1111/j.1572-0241.2005.00360.x. [DOI] [PubMed] [Google Scholar]
- 32.Shen B, Fazio VW, Remzi FH, et al. Clinical features and quality of life in patients with different phenotypes of Crohn’s disease of the ileal pouch. Dis Colon Rectum. 2007;50:1450–1459. doi: 10.1007/s10350-007-0284-8. [DOI] [PubMed] [Google Scholar]
- 33.Kiss LS, Papp M, Lovasz BD, et al. High-sensitivity C-reactive protein for identification of disease phenotype, active disease, and clinical relapses in Crohn’s disease: a marker for patient classification? Inflamm Bowel Dis. 2012;18:1647–1654. doi: 10.1002/ibd.21933. [DOI] [PubMed] [Google Scholar]
- 34.Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–1523. doi: 10.1136/gut.2007.146357. [DOI] [PubMed] [Google Scholar]
- 35.Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014;63:423–432. doi: 10.1136/gutjnl-2012-303864. [DOI] [PubMed] [Google Scholar]
- 36.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 37.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 38.Williet N, Sandborn WJ, Peyrin-Biroulet L. Patient-reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:1246–1256. doi: 10.1016/j.cgh.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Bodger K, Ormerod C, Shackcloth D, et al. Development and validation of a rapid, generic measure of disease control from the patient’s perspective: the IBD-Control questionnaire. Gut. 2014;63:1092–1102. doi: 10.1136/gutjnl-2013-305600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kappelman MD, Long MD, Martin C, et al. Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:1315–1323. doi: 10.1016/j.cgh.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loftus EV, Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn’s disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 42.Loftus EV, Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Ulcerative colitis in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gut. 2000;46:336–343. doi: 10.1136/gut.46.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang D-H, Yang S-K, Park SH, et al. Usefulness of C-reactive protein as a disease activity marker in Crohn’s disease according to the location of disease. Gut Liver. 2015;9:80. doi: 10.5009/gnl13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldman CD, Kodner IJ, Fry RD, MacDermott RP. Clinical and operative experience with non-Caucasian patients with Crohn’s disease. Dis Colon Rectum. 1986;29:317–321. doi: 10.1007/BF02554120. [DOI] [PubMed] [Google Scholar]
- 45.Ghazi LJ, Lydecker AD, Patil SA, Rustgi A, Cross RK, Flasar MH. Racial differences in disease activity and quality of life in patients with Crohn’s disease. Dig Dis Sci. 2014;59:2508–2513. doi: 10.1007/s10620-014-3141-3. [DOI] [PubMed] [Google Scholar]
- 46.Yarur AJ, Abreu MT, Salem MS, Deshpande AR, Sussman DA. The impact of Hispanic ethnicity and race on post-surgical complications in patients with inflammatory bowel disease. Dig Dis Sci. 2014;59:126–134. doi: 10.1007/s10620-013-2603-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.